Abstract
Background:
The use of exercise is a potential treatment option to modulate pain (exercise-induced hypoalgesia). The pain threshold (PT) response is a measure of pain sensitivity that may be a useful marker to assess the effect of physical exercise on pain modulation.
Aim:
The aim of this systematic review and meta-analysis is to evaluate the PT response to exercise in healthy subjects.
Methods:
We searched in MEDLINE, EMBASE, Web of Science, Lilacs, and Scopus using a search strategy with the following search terms: “exercise” OR “physical activity” AND “Pain Threshold” from inception to December 2nd, 2019. As criteria for inclusion of appropriate studies: randomized controlled trials or quasi-experimental studies that enrolled healthy subjects; performed an exercise intervention; assessed PT. Hedge’s effect sizes of PT response and their 95% confidence intervals were calculated, and random-effects meta-analyses were performed.
Results:
For the final analysis, thirty-six studies were included (n=1326). From this we found a significant and homogenous increase in PT in healthy subjects (ES=0.19, 95% CI= 0.11 to 0.27, I2=7.5%). According to subgroup analysis the effect was higher in studies: with women (ES=0.36); performing strength exercise (ES=0.34), and with moderate intensity (ES=0.27), and no differences by age were found. Confirmed by the meta-regression analysis.
Conclusion:
This meta-analysis provides evidence of small to moderate effects of exercise on PT in healthy subjects, being even higher for moderate strength exercise and in women. These results support the idea of modulation of the endogenous pain system due to exercise and highlight the need of clinical translation to chronic pain population.
Keywords: exercise-induced analgesia, pain threshold [MeSH Term], healthy volunteers [MeSH Term]
1. Introduction
Physical exercise is a beneficial intervention for many conditions and affects overall health and quality of life. Numerous studies have shown that physical exercise is an important component in the treatment of patients with chronic pain (Edmonds, McGuire, & Price, 2004). Furthermore, exercise studies including healthy subjects resulted in a period of hypoalgesia, characterized by a reduction in sensitivity to a painful stimulus – the exercise-induced hypoalgesia (Rice et al., 2019). The exercise modalities vary among these studies, as well as the pain induction techniques and measurement procedures. However, these results in general suggest that exercise could be used as an alternative or adjunct therapy to modulate pain (Geneen et al., 2017).
Studies have suggested that exercise can induce hypoalgesia by peripherally modulating the transduction, transmission and processing of noxious stimuli (Jones, Taylor, Booth, & Barry, 2016). Some other hypothesis include the systemic activation of the endogenous opioid, serotoninergic and immune system, and the central activation of the cortico-thalamic descending inhibitory pathways (K. F. Koltyn, 2000), assuming that these physiological mechanisms could be assessed by quantitative sensory testing, such as paint threshold (PT). Even though these mechanisms are not entirely understood, there is strong evidence that showed not only the benefits in bouts of exercise but also in long-term exercise, leading to sustained hypoalgesia effects in healthy adults (Anshel & Russell, 1994; Ellingson, Koltyn, Kim, & Cook, 2014; Jones, Booth, Taylor, & Barry, 2014; Lemming et al., 2015; Naugle & Riley, 2014; Umeda, Newcomb, & Koltyn, 2009). Recent studies (Agnew, Hammer, Roy, & Rahmoune, 2018; Kelli F. Koltyn, Brellenthin, Cook, Sehgal, & Hillard, 2014; Krüger, Khayat, Hoffmeister, & Hilberg, 2016; Lee, 2014; Kathy J Lemley, Hunter, & Bement, 2015; K. J. Lemley, Senefeld, Hunter, & Hoeger Bement, 2016; Lewis & Sullivan, 2018; Kelly Marie Naugle, Keith E. Naugle, Roger B. Fillingim, Brian Samuels, & Joseph L. Riley, 3rd, 2014; Naugle & Riley, 2014) have aimed to analyze and standardize the effects of exercise on nociception using pain threshold (PT) response with mixed results. Besides, the only available meta-analysis (Naugle, Fillingim, & Riley, 2012) have shown a significant improvement of pain, but they combined PT and pain rating measures hampering the interpretation of the results, also the outdated search (till 2012) does not included the remarkable production in the field in the past years. Based on that, it is still not clear how exercise modulates the pain threshold and how different types of physical exercise would induce that effect. Therefore, this study aims to quantitatively evaluate the updated published literature on exercise pain perception modulation indexed by a pain threshold changes in healthy subjects.
2. METHODS
A systematic review of the literature and meta-analysis was conducted following the recommendation of the Cochrane handbook (Higgins et al., 2011), including the PRISMA guidelines (Appendix A)(Moher, Liberati, Tetzlaff, Altman, & The, 2009).
2.1. Literature search and study selection
We have searched in MEDLINE, EMBASE, Web of Science, Lilacs and Scopus from inception to December 2nd, 2019 using a search strategy with the following search terms: “exercise” OR “physical activity” AND “Pain Threshold.” The full research strategy is shown in Appendix B. Duplicates were eliminated before selection, and previous to the title and abstract selection, two experienced reviewers agreed on a standard approach. Afterward, the citations were independently screened by the two reviewers in terms of titles and abstracts. Discrepancies between reviewers were resolved by a third reviewer. Then, the two main reviewers independently assessed full text of selected studies, and again the third reviewer resolved discrepancies.
2.2. Eligibility criteria
We have searched for full-text articles restricted to English and included articles that had: a) enrolled healthy subjects (participants without any prior pain condition or chronic disease, including athletes or non-athletes; b) performed exercise intervention regardless intensity or duration; the exercise interventions performed to produce exercise-induce pain were excluded; c) assessed the pain threshold (PT) without any restriction of type of stimulus; d) been designed as randomized controlled trials (RCTs) – included parallel-group, crossover designs and pilot studies – and quasi-experimental studies, since we were focus in the within-studies changes (pre vs. post) after exercise to evaluate intrinsic properties of the intervention to modulate pain thresholds.
2.3. Data extraction
For each study, we extracted independently by two researchers in a standardized spreadsheet the following: i) participant characteristics (sample size, age, sex, and drop-outs), if the study included data from both patients and healthy subjects, just the last one were extracted; ii) exercise intervention protocol characteristics, and iii) outcomes of interest (pain threshold). In case of missing or unclear information, we requested the data to authors by email and, to extract data from relevant graphs, we have used WebPlotDigitizer v.3.11 (Rogatgi, 2011). If we were unable to contact the authors or extract the data graphically, we excluded the study from the quantitative analysis. Some of the included studies measured multiple variables to assess the PT outcome within-subjects (more than one body location for PT assessments – left arm, right arm, left leg, etc.). We were aware that computing different effect sizes for the same sample or overlapping sets of participants and treating them as completely unrelated effect sizes violate the basic assumptions of the traditional meta-analytic method. In those cases, we calculated a weight mean of the multiple variables to compute a unique measurement of the outcome of interest, in order to not loss relevant information.
Pain Threshold (PT) corresponds to the smallest stimulus that is reported by subjects as painful. This can be measured by different stimuli such as pressure with an algometer, heat, cold, or electrical stimulus . We have extracted and analyzed changes in stimulus units (kilopascals, centigrade degrees, and others) pre and post-exercise intervention and their standard deviations (SD) as a measurement of PT response, similar to previous literature (O’Brien, Deitos, Trinanes Pego, Fregni, & Carrillo-de-la-Pena, 2018; O’Brien et al., 2019).
2.4. Risk of bias assessment method
The risk of bias of the selected studies was evaluated by two reviewers using the Methodological Index for Non-randomized Studies (MINORS) tool (Slim et al., 2003) because the effect size was obtained from a pre-post comparison, meaning we were focus on the with-in group changes, instead of the between group comparison. The MINORS tool has 8 domains to assess one-arm studies: 1) a clearly stated aim, 2) inclusion of consecutive patients, 3) prospective collection of data, 4) endpoints appropriate to the aim of the study, 5) unbiased assessment of the study endpoint, 6) follow-up period appropriate to the aim of the study, 7) loss to follow up less than 5%, and 8) prospective calculation of the study size. This tool considered three possible scores for each item from 0 to 2: 0 for not reported information, 1 for information reported inadequately, and 2 for well-reported information. We considered that scores less than 16 points indicated a high risk of bias and from 16 to 24 points indicated low risk of bias (Zafra-Tanaka, Pacheco-Barrios, Tellez, & Taype-Rondan, 2019).
We assessed the certainty of our pooled estimates applying the grading of recommendation, assessment, development, and evaluation (GRADE) approach (Balshem et al., 2011). This assessment is based on five domains: study limitations (risk of bias of the studies included), imprecision (sample sizes and CI), indirectness (generalizability), inconsistency (heterogeneity), and publication bias as stated in the GRADE handbook (Schunemann, 2008). The certainty of the evidence was characterized as high, moderate, low, or very low (Balshem et al., 2011).
2.5. Data Synthesis
The within-studies effect sizes of PT estimates and their 95% confidence intervals (95% CI) were calculated. We adjusted Cohen’s d to Hedge’s g by applying a correction factor as Cohen’s d has a slight bias to overestimate in small sample sizes. Then, an exploratory meta-analysis was performed. Since we were focus in the within-studies changes (pre vs. post) after exercise, we considered appropriated to pooled estimates from RCTs and quasi-experimental studies, when the intervention, population, and PT measurement were comparable. We assessed heterogeneity using an I2 statistic, and we considered low heterogeneity when I2 <40% (Higgins et al., 2011). We consider it appropriate to use random-effects models due to the overall heterogeneity evaluation(DerSimonian R Fau - Laird & Laird). Moreover, we performed subgroup analysis (by exercise type [aerobic and strength], exercise intensity [mild, moderate, intense] and duration, sex, and age), sensitivity analysis (to test consistency of the results by analyzing the effects of risk of bias and study design categories [randomized control trials or quasi-experimental studies]). Furthermore, we conducted univariate meta-regression to test the influence of study level moderators on the PT estimates. Each co-variate was tested on a minimum of eight included studies in the meta-analysis. To select the best random-effects model, we assessed the residual percentage of variation due to heterogeneity and the proportion of between-study variance explained in addition to the significant criterion of p<0.05 per each moderator. The publication bias was evaluated by visual assessment (funnel plot) and by the Egger test. The data were analyzed using Stata v15.1 software (StataCorp LLC).
3. RESULTS
3.1. Overview
The search retrieved 4429 results; after removing duplicates, 2390 titles and abstracts were screened, and, of these, 2146 were excluded. 244 studies were evaluated in full-text, 208 studies were excluded (Appendix C). One article was retrieved from the citations. And finally, 36 were included (Agnew et al., 2018; Alsouhibani, Vaegter, & Hoeger Bement, 2019; Arroyo-Morales, Rodriguez, Rubio-Ruiz, & Olea, 2012; Bartholomew, Lewis, Linder, & Cook, 1996; Burrows, Booth, Sturnieks, & Barry, 2014; Falla, Gizzi, Tschapek, Erlenwein, & Petzke, 2014; Fingleton, Smart, & Doody, 2017; Focht & Koltyn, 2009; Gajsar, Titze, Hasenbring, & Vaegter, 2016; Gomolka et al., 2019; Harris, Sterling, Farrell, Pedler, & Smith, 2018; K. F. Koltyn & Arbogast, 1998; K. F. Koltyn, M. R. Trine, A. J. Stegner, & D. A. Tobar, 2001; K. F. Koltyn & M. Umeda, 2007; E. Kosek, Ekholm, & Hansson, 1996; Krüger et al., 2016; Lee, 2014; K. J. Lemley, Drewek, Hunter, & Hoeger Bement, 2014; K. J. Lemley et al., 2016; Lewis & Sullivan, 2018; Löfgren et al., 2018; Meeus, Roussel, Truijen, & Nijs, 2010; Micalos & Arendt-Nielsen, 2016; Kelly Marie Naugle et al., 2014; K. M. Naugle, K. E. Naugle, R. B. Fillingim, B. Samuels, & J. L. Riley, 3rd, 2014; Naugle, Naugle, & Riley, 2016; Ohlman, Miller, Naugle, & Naugle, 2018; Persson, Hansson, Kalliomaki, Moritz, & Sjolund, 2000; Slater, Theriault, Ronningen, Clark, & Nosaka, 2010; Staud, Robinson, Weyl, & Price, 2010; Treseler, Bixby, & Nepocatych, 2016; Vaegter, Handberg, Jorgensen, Kinly, & Graven-Nielsen, 2015; Vaegter et al., 2017; Vaegter et al., 2019; van Weerdenburg et al., 2017). A flow diagram of the literature that was searched and evaluated is presented in Figure 1. Descriptive information on each article included in the meta-analysis is provided in Table 1.
Figure 1.
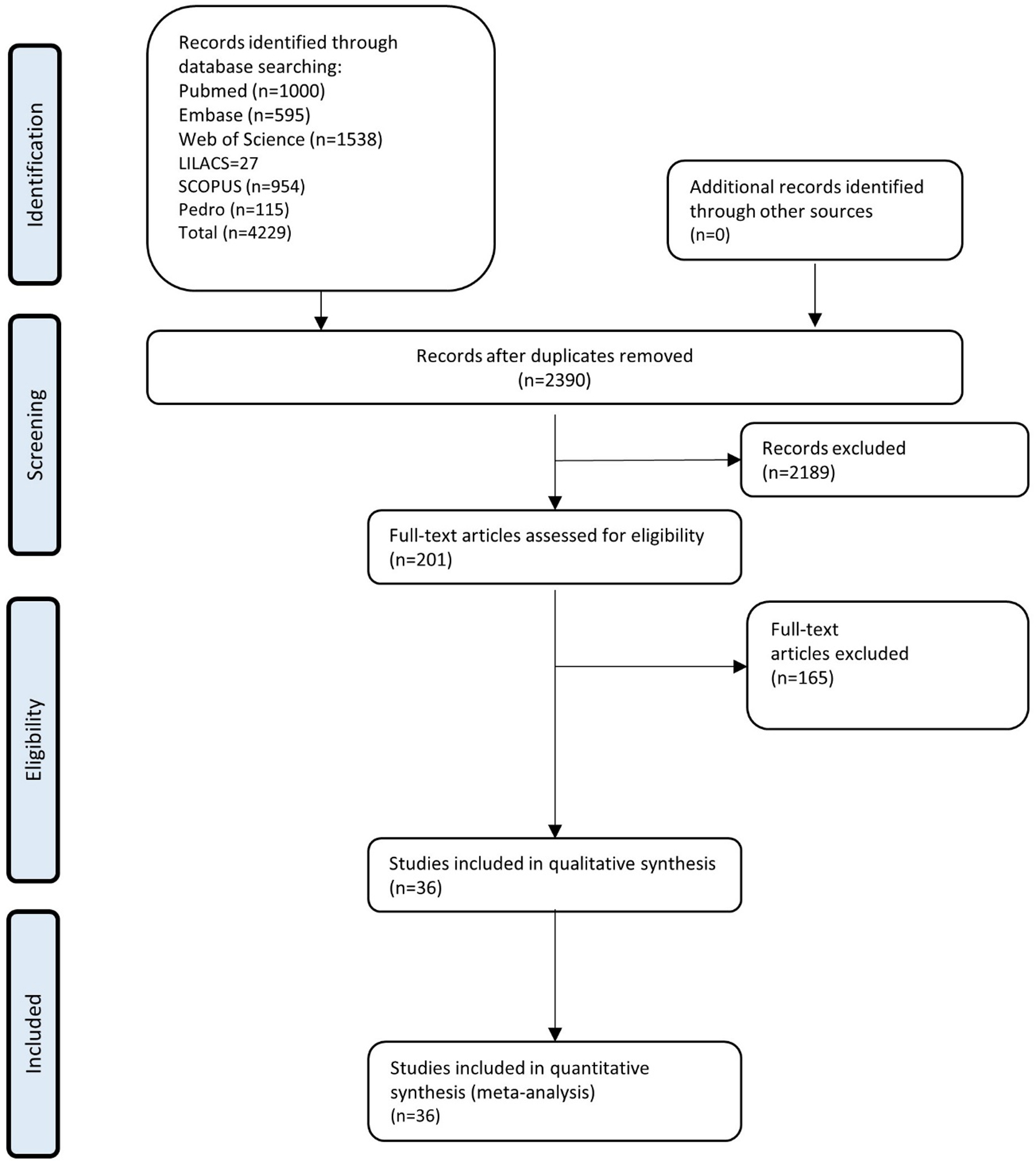
Flow diagram of the study selection based on inclusion and exclusion criteria
Table 1.
Included studies characteristics
| Study | Study Design | Participants that completed the study (n, male/female) |
Age (years, mean ± SD) |
Details of Exercise | Type of exercise | Intensity of exercise | Outcomes for pain | Assessment timing | Conclusion | MINORS Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Ohlman et al. 2018 | Quasi-experiment | 20/32 | Male=67.6±5.3 Female=67.2±4.9 |
A submaximal isometric handgrip exercise at 25% of MVC by the left arm | Strength | Light | PPT 30-seconds of continuous heat pain test |
Before and immediately after exercise | Older adults did not exhibit EIH after submaximal isometric exercise. However, those who did more MVPA per week experienced a greater magnitude of pain inhibition after acute exercise. | 22 |
| Persson et al. 2000 | Quasi-experiment | 0/25 | Younger: 44±10.18 | A weight belt of 1 kg was wrapped around the wrist of the subject’s hand. In the resting position, the subject held her forearms and hands on a pillow in her lap. During test contractions for EMG recording (each lasting 15 seconds) and during the endurance test, the subject’s right arm was abducted 90[degrees] in the scapular plane, with a slightly flexed (20[degrees]) elbow, pronated with the thumb pointing downward | Strength | Intense | PPT | Before and after the endurance test | The mechanisms of recovery from fatigue and nociception are independent of each other. The bilateral PPT increases might be explained by central antinociceptive mechanisms activated by static muscle work. | 16 |
| Koltyn K. F. et al. 1998 | RCT | 7/6 | 23±5 | Exercise group:45 minutes of lifting three sets of 10 repetitions at 75% of the individual’s one repetition maximum. Control group: Quiet rest consisted of 45 minutes of sitting quietly in a room free from distractions. |
Strength | Moderate | PPT Pain ratings |
Before and after (5 and 15 mins) exercise and quiet rest | A single bout of resistance exercise is capable of modifying the sensation of experimentally induced pain. | 20 |
| Koltyn K. F. et al. 2001 | Quasi-experiment | 15/16 | Male=22±5 Female=21.5±2 |
2 sessions: 1)Squeezed a hand dynamometer with their right hand (dominant hand) as hard as they could for 5 s, rested for 2 min, and then squeezed the hand dynamometer again for 5 s; 2) Submaximal isometric exercise consisted of squeezing the hand dynamometer with the right hand between 40% and 50% of maximum for 2 min. | Strength | Moderate and intense | PPT Pain ratings |
Before and immediately after exercise | It is concluded that: 1) men and women differed in PT, SBP, and DBP before ISO EX; and 2) analgesia after ISO EX is observed more consistently in women. | 17 |
| Koltyn K. F. et al. 2007 | Quasi-experiment | 0/14 | 19.5±1 | 2 sets of submaximal (40% to 50% of max) isometric exercise consisting of squeezing a dynamometer for 2 minutes with the dominant hand. | Strength | Moderate | PPT Pain ratings |
Before and immediately after exercise | Submaximal isometric exercise performed for 2 minutes resulted in ipsilateral and contralateral hypoalgesia responses. | 17 |
| Kruger S. 2016 | RCT | Healthy control group=29 No other demographic characteristics data provided |
Control group=48±13 | Walk for a maximum of 30 min with a self-chosen velocity. | Aerobic | Moderate | PPT | Before and immediately after exercise | Subjects were able to perform an endurance exercise with self-chosen velocity for 30 min as recommended, without increasing the acute pain condition. | 18 |
| Focht B.C. et al. 2009 | Quasi-experiment | 21/0 | 21.4±2.5 | 2 RE sessions, each session consists of leg extension, torso-arm pull-down, chest press, and overhead press, 3 sets of 10 repetitions 75% of each individual’s 1-repetition maximum.1st session at 6:00–8:00am and repeat at 6:00–8:00 pm. | Strength | Moderate | PPT Pain ratings |
Before and after (1 and 15 mins) each bout of RE. | Acute RE results in alterations in the perception of experimentally induced pressure pain and that this hypoalgesic response is not influenced by the time of day that RE is performed. | 17 |
| Bartholomew et al. 1996 | Quasi-experiment | 17/0 | Younger (no more details) | 20 min of self-selected exercise | Strength | Moderate | PPT Pain tolerance |
Before and immediately after exercise | The analgesic effect of exercise is not limited to controlled experimental conditions but generalizes to naturally occurring stimulations. | 11 |
| Falla D. et al. 2014 | RCT | Control group:9/8 | Control group:29.4±7.4 | Subjects were asked to repetitively move a box with hole-shaped handles, loaded with a weight of 5 kg. | Strength | Light | PPT | Before and immediately after exercise | LBP alters the normal adaptation of lumbar erector spinae muscle activity to exercise, which occurs in the presence of exercise-induced hyperalgesia. Reduced variability of muscle activity may have important implications for the provocation and recurrence of LBP due to repetitive tasks. | 15 |
| Kosek E. et al. 1995 | Quasi-experiment | 0/14 | 36.8±9.96 | The maximum isometric knee extensor strength at a 90° of knee flexion was determined 3 times with I min of rest between trials. The best value was used for calculating 25% of MVC. | Strength | Moderate | PPT | Before, during rest, during contraction and following contraction. | The results suggest that input from cutaneous and deeper tissues interacts with nociceptive activity set up by the pressure stimulus. Determining the degree of sensory modulation in muscle and skin in different chronic pain syndromes could become a functional method of patient assessment important for differential diagnosis, treatment evaluation, and follow-up. | 18 |
| Arroyo-Morales M. et al. 2012 | Quasi-experiment | 25/25 | 22.4±3.42 | A standardized light warm-up protocol, followed by three 30-s Wingate tests on an ergometer cycle separated by 3-min recovery periods. | Aerobic | Intense | PPT | Before and immediately after exercise | High-intensity interval exercise induces a worse psychoneuroimmunological state in males than in females. | 13 |
| Agnew J. W. et al. 2018 | Quasi-experiment | 25 miles group=5/1 50 miles group =9/13 100 miles group =9/8 |
25 miles group =37.2±12.9 50 miles group =42.1±6.9 100 miles group =41.8±7.8 |
Complete 25 miles, 50 miles or 100 miles marathon | Aerobic | Intense | PPT CPM |
Before and immediately after completion of 25°miles, 50°miles and 100°miles. | An increased peripheral and/or central pain sensitization starting at 25° miles and continuing throughout an ultra-marathon competition run in these conditions. | 21 |
| Burrows N. J. et al. 2018 | RCT | Old group=5/6 Young group=4/7 |
Old group=61.3±8.2 Young group=25±4.9 |
Three sets of 10 repetitions were performed at 60% of the individuals’ 1RM. One-minute rest was given between sets. | Strength | Intense | PPT Pressure pain tolerance |
Before and immediately after exercise | An acute bout of upper or lower body exercise evoked a systemic decrease in pain sensitivity in healthy individuals irrespective of age. The decreased pain sensitivity following resistance exercise can be attributed to changes in pain thresholds, not pain tolerance. | 22 |
| Lee H. S. et al. 2014 | RCT | Aerobic exercise group(AG)=5 Strengthening exercise group(SG)=5 Control group(CG)=5 No other demographic characteristics data provided |
AG=25.2±0.8 SG=71.2±5.3 CG=24.3±0.5 |
AG=walked on a treadmill for 10 and 40 min at 6.5 km/h; SG= performed 10 and 40 min of circulate training exercises that included a bench press, lateral pulldown, biceps curl, triceps extension, and shoulder press based on the perceived exertion. CG= rested in a quiet room without exercising for 10 and 40 min. |
Aerobic Strength |
Light Light |
PPT | Before and immediately after 10 and 40 min of exercise | 40 min is a more appropriate exercise time, although the efficacy of controlling pain did not differ be-tween strengthening exercise and aerobic exercise. | 20 |
| Lemley K. J.et al. 2016 | Quasi-experiment | 33/31 | Young women=20.6 ±1.5 Young men=21.7 ±3.7 Old women=71.3±7.6 Old men=71.3 ±5.1 |
Before and after maximal velocity concentric contractions of knee extensors or elbow flexors (separate days). | Strength | Intense | PPT | Before and immediately after exercise | Under controlled conditions where muscle fatigue is similar, sex differences in EIH occur in young and older adults that is site specific (upper extremity). Only women experience EIH following acute single limb high-velocity contractions. | 11 |
| Lofgren M. et al. 2018 | RCT | Control group=4/16 | Control group=60±6 | A right-leg isometric knee extension contraction and to maintain it until they were unable to sustain 30% of their MVC. | Strength | Moderate | PPT EIH |
Before and immediately after exercise | A generally increased pain sensitivity but normal function of EIH among persons with RA and offer one possible explanation for pain reduction observed in this group of patients following clinical exercise programs. | 19 |
| Micalos P. S. et al. 2016 | Quasi-experiment | 10/0 | 21.2±3.4 | Aerobic cycling exercise performed at 70 and 30°% of VO2peak on two separate visitations in a counterbalanced order. | Aerobic | Light/Moderate | PPT | Before and 5 min after exercise | Aerobic activity attenuates pressure pain sensitivity locally at the exercise muscle site following cycling exercise at 70% of peak oxygen uptake, however, may facilitate pain sensitivity following exercise at 30% of VO2peak. | 14 |
| Meeus M. et al. 2010 | RCT | Control group=10/21 | Control group=39.88±12.63 | A submaximal aerobic exercise protocol on a bicycle ergometer: each plateau phase at a certain workload lasted for 60 s. Each exercise bout consisted of 2 plateau phases at incremental workloads. | Aerobic | Moderate | PPT VAS |
Before and immediately after exercise | Hyperalgesia and abnormal central pain processing during submaximal aerobic exercise in chronic fatigue syndrome, but not in chronic low back pain. Nitric oxide appeared to be unrelated to pain processing. | 20 |
| Lewis Z. et al. 2018 | Quasi-experiment | 24/9 | 22.75±3.98 | Rowed two 20-min time trials under two counterbalanced conditions-paired and large group | Aerobic | Moderate | PPT | Before, immediately post, 5-min post, and 10-min post each session. | Pain threshold were affected by a wide variety of synchronous activities. There was a significantly higher pain threshold in the large group than in the paired condition after 10 min of exercise. | 13 |
| Lemley K. J. et al. 2014 | Quasi-experiment | 24 No other demographic characteristics data provided |
72.2±6.2 | Isometric contractions of the left elbow flexor muscles of the following doses: 1) three brief MVC; 2) 25% MVC held for 2 min; 3) 25% MVC held to task failure. | Strength | Moderate and intense | PPT | Before and immediately after exercise | Older adults experienced similar reductions in pain after several different intensities and durations of isometric contractions. Both older men and women experienced increases in pain threshold, but only older women experienced reductions in pain ratings. | 16 |
| Fingleton C. et al. 2017 | RCT | Control group=11/9 | Control group=62±7.9 | Aerobic exercise in a sitting position on a cycle ergometer and doing the Aerobic Power Index test. Isometric exercise: extend knee as far as could be achieved without pain >3/10. And then hold an isometric knee extension contraction until exhaustion. |
Aerobic and strength | Intense | PPT CPM EIH |
Before and immediately after exercise | Dysfunctional EIH in response to aerobic and isometric exercise in knee OA patients with abnormal CPM, and normal function of EIH in knee OA patients with an efficient CPM response. | 18 |
| Alsouhibani et al. 2018 | RCT | 15/15 | 19.3±1.5 | Isometric exercise group: a submaximal (30% MVIC) iso-metric contraction of the right knee extensor muscles that was held for three minutes while seated upright on the edge of a plinth table. Control group: quiet rest |
Strength | Moderate | CPM PPT |
Before, during, and after ice water immersions. | Isometric exercise decreased CPM in individuals who reported systemic EIH, suggesting activation of shared mechanisms between CPM and systemic EIH responses. | 21 |
| Harris S. et al. 2018 | Quasi-experiment | 19/16 | 23.6±6.6 | 5 min knee extension isometric contraction at 20–25% MVC | Strength | Moderate | PPT OffA |
Before and immediately after exercise | Five minutes of 20–25% MVC lower limb isometric exercise provided non-pharmacological pain modulation in young, active adults. | 16 |
| Gajsar H. et al. 2016 | Quasi-experiment | 12/17 | 29.97±6.06 | 120 seconds of the isometric Biering-Soerensen back extension test | Strength | Moderate | PPT | Before and immediately after exercise | Isometric back exercise produces local and remote hypoalgesia. | 15 |
| Naugle K. M. et al. 2014 | Quasi-experiment | 12/15 | 21.78±4.14 | Vigorous aerobic exercise: 5-minute warm-up period and then cycled for 20 minutes at an intensity of 70% HRR. Moderate intensity aerobic exercise: 5-minute warm-up period and then cycled for 20 minutes at an intensity of 50–55% HRR. |
Aerobic | Moderate and intense | PPT Suprathreshold pressure pain test. Static continuous heat test. Repetitive pulse heat pain test |
Before and immediately after exercise | MAE is capable of producing a hypoalgesic effect using continuous and repetitive pulse heat stimuli. However, a dose-response effect was evident as VAE produced larger effects than MAE. | 23 |
| Naugle K. M. et al. 2014 | Quasi-experiment | 12/15 | Younger (no more details) | A 3-minute trial of submaximal isometric handgrip exercise at 25% of MVC. | Strength | Moderate | PPT Suprathreshold pressure pain test. Static prolonged heat test. Temporal summation of heat pain |
Before and immediately after exercise | The hypoalgesic response to submaximal isometric exercise is partially a function of sex and experimental pain test. | 23 |
| Naugle. K. M. et al. 2016 | Quasi-experiment | Young adults=11/14 Old adults=9/9 |
Young adults: 21.7±4.1 Old adults: 63.7±6.6 |
Vigorous aerobic exercise: 5-minute warm-up period and then cycled for 20 minutes at an intensity of 70% HRR. Moderate intensity aerobic exercise: 5-minute warm-up period and then cycled for 20 minutes at an intensity of 50–55% HRR. Submaximal isometric exercise: the dominant arm contract at 25% of their MVC. |
Strength Strength Aerobic Aerobic |
Light Moderate Moderate Moderate |
PPT Pain rating Temporal summation of heat pain |
Before and immediately after exercise | Age differences in EIH following isometric and aerobic exercise, with younger adults experiencing greater EIH compared to older adults. | 23 |
| Gomolka S. et al. 2019 | Quasi-experiment | 15/15 | 24.4±1.8 | 15 minutes of heart rate–controlled aerobic cycling in two sessions. | Aerobic | Moderate | PPT | Before, immediately after, and 15 min after exercise. | Fair test–retest reliability of EIH after aerobic cycling for local and semi local body parts, but only in men. | 16 |
| Slater et al. 2009 | RCT | 6/7 | 27.1±1.4 | Eccentric-only exercise: 5 sets of 20 arm contractions at 30% maximal wrist extension force for 4 weeks. Concentric–eccentric exercise: 5 sets of 10 eccentric/10 concentric contractions at 30% maximal wrist extension force for 4 weeks. |
Strength | Intense | PPT | Before and immediately after exercise at each session | Mechanical hypoalgesia is induced by repeated low load exercises regardless of exercise mode, and this may prove beneficial if replicated clinically. | 21 |
| Vaegter et al. 2015 | Quasi-experiment | 28/28 | 23.5±2.19 | 2 min warm-up and then 3 min to achieve the ATHR and then continued bicycling for additional 10 minutes. | Aerobic | Moderate | PPT | Before, immediately after, and 15 min after conditioning and exercise | Cold pressor stimulation and aerobic exercise caused comparable multisegmental increases in PPT in active and inactive men and women. | 20 |
| Vaegter et al. 2016 | Quasi-experiment | 20/0 | 24.4±2.0 | Session 1: 15 min quiet rest Session 2 and 3: 12 min rest and then 3 min submaximal isometric knee extension at 30% of MVC with the dominant leg. |
Strength | Moderate | PPT PTT HPT |
Before and immediately after exercise and rest | Hypoalgesia after submaximal isometric exercise is primarily affecting tolerance of pressure pain compared with the pain threshold. | 21 |
| Treseler et al. 2016 | Quasi-experiment | 0/19 | 20±1 | Two 5-km performance time trials with CS or regular socks in a counterbalanced order separated by 1 week. | Aerobic | Moderate | PPT Muscle soreness |
Before and immediately after exercise at each session | There were no significant improvements in average 5-km running time, heart rate, or perceived calf MS. | 20 |
| Staud et al. 2010 | RCT | Control group=0/36 | Control group: 44.7±11.0 | Rotate the 1 kp flywheel consistently at 60 rpm until exhaustion, repeat twice alternating with 15-minute rest periods. | Aerobic | Moderate | VAS PPT |
Before and after each rest-and-exercise period | Alternating strenuous exercise with brief rest periods not only decreased overall clinical pain of FM subjects but also their mechanical hyperalgesia. | 20 |
| Vaegter et al. 2018 | RCT | 21/13 | 25.8±3.4 | 2 sessions, each session consist of 15 min rest and 15 min bicycling | Strength | Moderate | PPT | Before and immediately after exercise and rest | Incremental bicycling exercise in-creased PPTs with fair relative and absolute reliability of the EIH response. | 19 |
| Van Weerdenburg et al. 2017 | RCT | 9/6 | 25±6.5 | 3 interventions consisting of 20 min of aerobic cycling, 12 minutes of isometric knee extension and a deep breathing procedure | Aerobic and strength | Moderate | PPT. Cold pressor test |
Before and after each intervention | No hypoalgesic effect of aerobic and isometric exercise was found. | 20 |
PPT: pressure pain threshold; CPM: Conditioned Pain Modulation; EIH: Exercise-induced analgesia; CPP: cold pressor pain; VAS: Visual Analogue Scale; MVC: maximum voluntary contraction; PTT: pain tolerance; HPT: Heat pain threshold; HRR: heart rate reserve; RM: repetition maximum; CS: compression stockings; OffA: offset analgesia; MVIC: maximal voluntary isometric contractions; ATHR: age-related target heart rate; PwH: patients with hemophilia; LBP: low back pain; SBP: Systolic blood pressure ; DBP: diastolic blood pressure; ISO EX: isometric handgrip exercise; RA: Rheumatoid arthritis.
3.2. Effects on pain threshold
We conducted a random-effects model meta-analysis on 67 comparisons (36 studies, 1326 participants) and found a significant and homogeneous pooled effect: ES=0.19 95% CI= 0.11 to 0.27, I2=7.5%. The positive ES favors PT increasing after exercise. The forest plot in Appendix D shows individual effect sizes that ranged from −0.93 to 1.37.
We also conducted a heterogeneity tests to assess subgroup-specific effects and regarding sex, results were significantly (p=0.041) in favor of women: ES=0.36 95%, CI=0.15 to 0.56, I2=41.6%; and between exercise groups results were in in favor of strength type of exercise (p=0.0001): ES=0.34, 95% CI=0.23 to both sexes?0.44, I2=0.0%, see Appendix E. The results also favored moderate exercise (p=0.025) (ES=0.27 95% CI=0.16 to 0.38, I2=9.0%, see Appendix F), and combining two categories, strength, and moderate exercise, resulted in higher ES: 0.45 95% CI=0.27 to 0.64. Finally, the heterogeneity test between subgroups was in favor of strength exercise in women: ES=0.67 95% CI=0.34, 1.01, p=0.001 and results considering age subgroups were not significant. The rest of the subgroups were no significant (Appendix G)
3.3. Risk of bias assessment and evidence certainty
Most of the included articles (75%) had low risks of bias (higher than 16 points). The mean MINORS score was 18.17 (SD=3.31) However, most of them (60.53%) had deficit reporting of the sample size calculation and selecting an adequate control group for the experiment. See Table 1. We judged the certainty of the pooled estimate as very low. We started the evaluation from low certainty since we included non-randomized studies (quasi-experimental). We downgraded according to the risk of bias of the studies (60.53% had a methodological limitation due to sample size and control group selection). We did not downgrade the evidence due to heterogeneity (the calculated I2 was 7.5%), nor by imprecision (the calculated 95% CI was precise), neither by publication bias (Table 2).
Table 2.
Quality of the body of evidence: Summary of findings
| Exercise-induced pain threshold modulation in healthy subjects | ||||
|---|---|---|---|---|
| Outcomes | Anticipated absolute effects (95% CI) | № of participants (Studies) | Certainty of the evidence (GRADE) | |
| Effect size (Hedge’s) | CI 95% | |||
| Effects on pain threshold due to physical exercise in healthy subjects | 0.19 | 0.11 to 0.27 | 1326 (36 studies) |
⨁◯◯◯ VERY LOW a, b |
| CI: Confidence interval. | ||||
Explanations
The certainty rating started from low certainty since we included non-randomized studies.
We downgraded according to the risk of bias of the studies (60.53% had a methodological limitation due to sample size and control group selection)
3.4. Sensitivity and meta-regression analysis
The sensitivity analysis showed not significant differences by risk of bias categories and by study design (RCTs vs. quasi-experimental). Moreover, the meta-regression analysis confirms that type of exercise is the main source of between-study heterogeneity (p<0.001), age, sex, and exercise intensity was not significant in the meta-regression model (Appendix G).
3.5. Publication bias
We did not find publication bias in this meta-analysis as indexed by symmetrical funnel plots and non-significant Egger’s (p=0.43) and Begg’s (p=0.67) test analysis (Appendix H).
4. DISCUSSION
4.1. Summary of results
We included thirty-six studies that have evaluated the effects of exercise on pain threshold (PT) in healthy adults. These studies have no publication bias but were methodologically heterogeneous and presented a small sample size. The main sources of heterogeneity were especially regarding the type and intensity of exercise. Also, it was found that exercise had different effects on PT in healthy adults; however, the heterogeneity test for women was marginally significant. Men and women presented their PTs modulated by exercise in opposite ways, independently of age or the intensity of the exercise. In other words, women significantly benefited from exercise, while men did not. In terms of the type of exercise, stretching increased PT significantly. Interestingly, in this study, no positive effect of aerobic exercise on PT was found, which was different from previous studies. From the point of exercise intensity, moderate intensity exercise increases PT, but light and high intensity exercise has no similar effects. In general, regardless of sex, exercise has a positive role in the PT in a healthy population, especially when doing strength exercises at a moderate intensity.
4.2. Comparison with previous meta-analysis results
Naugle et al. (Naugle et al., 2012) conducted a comprehensive review of the published articles to examine the effects of exercise on pain perception on healthy adults (Ambrose & Golightly, 2015). They found that aerobic exercise reduced pain sensitivity in response to the application of all types of pain stimulation (pressure, heat/cold, and electric) among healthy participants. They found the largest effect in studies using pressure stimuli and the smallest effect in those using cold and heat stimuli. The authors concluded that among healthy adults, aerobic exercise is very effective in reducing pain perception following different pain-inducing techniques and that PT reduction works best when exercise is performed at moderate to high-intensity pace. In our study, however, we did not find a significant effect on aerobic exercise. The reason for such differences may be the heterogenous outcome definition of pain perception response (combining PT and pain rating measures) that they used, which could overestimate the effect; also, our study included an updated evidence body that could provide a more robust pooled effect estimate.
4.3. Sex effects
The mechanisms that explain why exercise leads to a higher threshold increase in women that in men have not been elucidated. Similar to our results, several studies(Girdler et al., 2005; K. Koltyn, M. Trine, A. Stegner, & D. Tobar, 2001; Rhud & Meagher, 2001; Sternberg, Boka, Kas, Alboyadjia, & Gracely, 2001) found that females exhibit more efficient pain inhibitory responses when compared to males. For instance, Koltyn et al. (2001)(K. Koltyn et al., 2001) found PT elevated responses to isometric exercise in females but not in males; they hypothesized that differences in blood pressure between men and women were related to different pain perception responses; however, such relationship is exploratory and needs further research. The potential biological mechanism of sex differences in pain perception includes the effects of gonadal hormones on the endogenous pain modulation system. Estrogens and androgens have been proved to affect nociceptive pathways both via receptor-based mechanisms and indirectly through the influence on neuropeptides action (such as the endogenous opioid system) in nociceptive tracts(Bartley & Fillingim, 2013; Fillingim, King, Ribeiro-Dasilva, Rahim-Williams, & Riley III, 2009; Maurer, Lissounov, Knezevic, Candido, & Knezevic, 2016; Vincent & Tracey, 2008). Also, previous literature reported that estrogen could increase pain perception (Bartley & Fillingim, 2013; Fillingim et al., 2009; Maurer et al., 2016; Vincent & Tracey, 2008). Thus, we hypothesize that the sex difference in exercise-induced pain threshold in women participants can be attributed to the decrease of female hormone levels caused by the exercise effects and consequently, it could enhance the endogenous inhibitory pain pathways.
4.4. Type of exercise effects
Regular exercise has been associated with numerous physical and mental health benefits, including lower blood pressure; improve lipoprotein profile, enhance insulin sensitivity, release of neural growth factors, improve cognition and play an important role in pain management (Mazzeo & Tanaka, 2001). According to studies substantiating the American College of Sports Medicine Position Stand exercise prescription is based upon the frequency, intensity, and duration of training (Pescatello et al., 2004).
Regarding the type of exercise, our results showed that the overall effect was higher for strength exercise ES of 0.37 (0.23, 0.34) when compared to aerobic on pain threshold. These results are supported by some other studies (Focht & Koltyn, 2009; K. F. Koltyn & Arbogast, 1998; K. F. Koltyn et al., 2001; K. F. Koltyn & M. Umeda, 2007), that compared, in similar designs, the effect of exercise induced hypoalgesia in subjects submitted to aerobic and strength exercise. Some authors suggest that high threshold motor units need to be recruited during strength exercises to elicit a significant hypoalgesic response (Garland, Enoka, Serrano, & Robinson, 1994), which may explain the differences between the two modalities of exercise.
Several studies showed the effect of isometric strength exercise increasing PT multisegmental and not isolated to the contracting muscle (Gajsar et al., 2016; Garland et al., 1994; Hoeger Bement et al., 2011; Kelli F. Koltyn & Masataka Umeda, 2007; Eva Kosek & Ekholm, 1995). According to Kosek and Lundenburg et al.(E. Kosek & Lundberg, 2003), the widespread hypoalgesic response of the exercise is driven by central mechanisms that may include increased secretion of β-endorphins, attention mechanisms, interaction of the cardiovascular and pain regulatory systems or an activation of diffuse noxious inhibitory controls. In addition to this activation, it was shown that nociceptive afferents originated in the muscle also undergo extensive endogenous modulation which acts to enhance or diminish the intensity perceived pain(O’Connor & Cook, 1999).
Pain perception is modulated by the activity of central endogenous modulatory processing, which involves antagonist mechanisms(Le Bars, Dickenson, & Besson, 1979). This system acts in the central nervous system at both spinal and supraspinal level. Also, this process involves descending neural pathways with inputs from cortical, subcortical and spinal regions(Flood, Waddington, Keegan, Thompson, & Cathcart, 2017).
It is known that many areas involved in control of motivation, anxiety, fear, and mood have a strong influence in pain perception. Therefore, prefrontal, anterior cingulate, and insula cortices, amygdala and hypothalamus, project to the brainstem pain modulatory network (Benarroch, 2008). However, still, future studies need to address specific neural effects comparing different types of exercise to understand potential differences in activated networks.
4.5. Intensity of exercise effects
In our analysis, the greatest increase in PT was among the moderate-intensity group, with ES of 0.2795% CI=0.16, 0.38, followed by high-intensity, with ES of 0.13 95% CI=0, 0.27. Low-intensity exercise decreased PT by an ES of −0.195% CI=−0.36, 0.17, but this effect was not statistically significant.
Light, moderate and high-intensity exercise were defined according to the Borg Scale of Perceived Exertion (Borg, 1998) as reported by the analyzed studies; if the study did not report such scale, we defined it based on the description of the exercise protocols: none, very, very light or very light exertions were classified as light (Borg ratings 6–10), fairly light and somewhat hard were classified as moderate (Borg ratings 11 to 14) and hard, very hard and very, very hard were classified as high-intensity (Borg ratings 15 to 20).
Our results are consistent with the observations from Brümmer et al.(Brümmer, Schneider, Abel, Vogt, & Strueder, 2011), in which brain cortical activation patterns present a dose-response relationship with exercise intensity among strength training modes but not for aerobic exercise modes. In addition, that study suggests that, for exercises that are familiar or preferable to the participant, an intensity of 50 to 80% of the individual capacity is necessary to evoke significant brain activation.
Potential undocumented differences in baseline levels of fitness among participants between studies could also be part of the neural mechanisms behind the heterogeneity in results, as suggested by the study from Petruzzello et al. (Petruzzello & Landers, 1994), in which brain activation in response to exercise was mediated by the level of aerobic fitness. Another explanation could be a ceiling effect for highly-trained individuals, in which the long-term effects of exercise would already be reflected in their baseline PT levels, so no significant differences would be observed after the training sessions.
4.6. Limitations
In light of the findings, it is acknowledged that the interpretation for this study requires consideration of some limitations. First, database searching was limited to English-language papers, which possibly decreased the number of eligible papers for analysis. Second, the interaction of several other variables such as exercise training sessions, prior exercise conditioned state, and the detailed exercise selection could have influenced the neurophysiological outcomes. Furthermore, the lack of standard in the PPT protocol from the different studies combined in this meta-analysis might have increased the heterogeneity of outcomes and affected the overall effect sizes.
5. CONCLUSION
This meta-analysis provides evidence of significant small to moderate effects of exercise on PT in healthy subjects. This effect was higher for moderate strength exercise and in women population. These results support the idea of modulation of the endogenous pain system due to exercise and highlight the need of clinical translation to chronic pain population. However, validation of PT as a biomarker for pain perception requires further investigation under strict methodological settings and combination with other quantitative measurements.
Supplementary Material
FUNDING/SUPPORT STATEMENT
This study was funded by NIH grant R01 AT009491-01A1.
Footnotes
CONFLICTS OF INTERESTS STATEMENT
The authors declare to have no compelling interests with this article.
4. REFERENCES
- Agnew JW, Hammer SB, Roy AL, & Rahmoune A (2018). Central and peripheral pain sensitization during an ultra-marathon competition. Scand J Pain, 18(4), 703–709. doi: 10.1515/sjpain-2018-0079 [DOI] [PubMed] [Google Scholar]
- Alsouhibani A, Vaegter HB, & Hoeger Bement M (2019). Systemic Exercise-Induced Hypoalgesia Following Isometric Exercise Reduces Conditioned Pain Modulation. Pain Med, 20(1), 180–190. doi: 10.1093/pm/pny057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose KR, & Golightly YM (2015). Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract Res Clin Rheumatol, 29(1), 120–130. doi: 10.1016/j.berh.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anshel MH, & Russell KG (1994). Effect of aerobic and strength training on pain tolerance, pain appraisal and mood of unfit males as a function of pain location. J Sports Sci, 12(6), 535–547. doi: 10.1080/02640419408732204 [DOI] [PubMed] [Google Scholar]
- Arroyo-Morales M, Rodriguez LD, Rubio-Ruiz B, & Olea N (2012). Influence of gender in the psychoneuroimmunological response to therapeutic interval exercise. Biol Res Nurs, 14(4), 357–363. doi: 10.1177/1099800412448120 [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, … Guyatt GH (2011). GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology, 64(4), 401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Bartholomew JB, Lewis BP, Linder DE, & Cook DB (1996). Post-exercise analgesia: replication and extension. J Sports Sci, 14(4), 329–334. doi: 10.1080/02640419608727718 [DOI] [PubMed] [Google Scholar]
- Bartley EJ, & Fillingim RB (2013). Sex differences in pain: a brief review of clinical and experimental findings. British journal of anaesthesia, 111(1), 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2008). Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology, 71(3), 217–221. doi: 10.1212/01.wnl.0000318225.51122.63 [DOI] [PubMed] [Google Scholar]
- Borg G (1998). Borg’s perceived exertion and pain scales: Human kinetics.
- Brümmer V, Schneider S, Abel T, Vogt T, & Strueder HK (2011). Brain cortical activity is influenced by exercise mode and intensity. Medicine Science in Sports Exercise, 43(10), 1863–1872. [DOI] [PubMed] [Google Scholar]
- Burrows NJ, Booth J, Sturnieks DL, & Barry BK (2014). Acute resistance exercise and pressure pain sensitivity in knee osteoarthritis: a randomised crossover trial. Osteoarthritis Cartilage, 22(3), 407–414. doi: 10.1016/j.joca.2013.12.023 [DOI] [PubMed] [Google Scholar]
- DerSimonian R Fau - Laird N, & Laird N Meta-analysis in clinical trials. (0197–2456 (Print)). [Google Scholar]
- Edmonds M, McGuire H, & Price J (2004). Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev(3), Cd003200. doi: 10.1002/14651858.CD003200.pub2 [DOI] [PubMed] [Google Scholar]
- Ellingson LD, Koltyn KF, Kim JS, & Cook DB (2014). Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology, 51(3), 267–276. doi: 10.1111/psyp.12168 [DOI] [PubMed] [Google Scholar]
- Falla D, Gizzi L, Tschapek M, Erlenwein J, & Petzke F (2014). Reduced task-induced variations in the distribution of activity across back muscle regions in individuals with low back pain. Pain, 155(5), 944–953. doi: 10.1016/j.pain.2014.01.027 [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, & Riley J. L. J. T. j. o. p. III (2009). Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain, 10(5), 447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingleton C, Smart KM, & Doody CM (2017). Exercise-induced Hypoalgesia in People With Knee Osteoarthritis With Normal and Abnormal Conditioned Pain Modulation. Clin J Pain, 33(5), 395–404. doi: 10.1097/ajp.0000000000000418 [DOI] [PubMed] [Google Scholar]
- Flood A, Waddington G, Keegan RJ, Thompson KG, & Cathcart S (2017). The effects of elevated pain inhibition on endurance exercise performance. PeerJ, 5, e3028. doi: 10.7717/peerj.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht BC, & Koltyn KF (2009). Alterations in pain perception after resistance exercise performed in the morning and evening. J Strength Cond Res, 23(3), 891–897. doi: 10.1519/JSC.0b013e3181a05564 [DOI] [PubMed] [Google Scholar]
- Gajsar H, Titze C, Hasenbring MI, & Vaegter HB (2016). Isometric Back Exercise Has Different Effect on Pressure Pain Thresholds in Healthy Men and Women. Pain Medicine, 18(5), 917–923. doi: 10.1093/pm/pnw176 [DOI] [PubMed] [Google Scholar]
- Garland SJ, Enoka RM, Serrano LP, & Robinson GA (1994). Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol (1985), 76(6), 2411–2419. doi: 10.1152/jappl.1994.76.6.2411 [DOI] [PubMed] [Google Scholar]
- Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, & Smith BH (2017). Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev, 4(4), CD011279–CD011279. doi: 10.1002/14651858.CD011279.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, & Light KC (2005). Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain, 114(3), 372–385. [DOI] [PubMed] [Google Scholar]
- Gomolka S, Vaegter HB, Nijs J, Meeus M, Gajsar H, Hasenbring MI, & Titze C (2019). Assessing Endogenous Pain Inhibition: Test-Retest Reliability of Exercise-Induced Hypoalgesia in Local and Remote Body Parts After Aerobic Cycling. Pain Med. doi: 10.1093/pm/pnz131 [DOI] [PubMed] [Google Scholar]
- Harris S, Sterling M, Farrell SF, Pedler A, & Smith AD (2018). The influence of isometric exercise on endogenous pain modulation: comparing exercise-induced hypoalgesia and offset analgesia in young, active adults. Scand J Pain, 18(3), 513–523. doi: 10.1515/sjpain-2017-0177 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, … Sterne JAC (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger Bement MK, Weyer A, Hartley S, Drewek B, Harkins AL, & Hunter SK (2011). Pain Perception After Isometric Exercise in Women With Fibromyalgia. Archives of Physical Medicine and Rehabilitation, 92(1), 89–95. doi: 10.1016/j.apmr.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Jones MD, Booth J, Taylor JL, & Barry BK (2014). Aerobic training increases pain tolerance in healthy individuals. Med Sci Sports Exerc, 46(8), 1640–1647. doi: 10.1249/mss.0000000000000273 [DOI] [PubMed] [Google Scholar]
- Jones MD, Taylor JL, Booth J, & Barry BK (2016). Exploring the Mechanisms of Exercise-Induced Hypoalgesia Using Somatosensory and Laser Evoked Potentials. Front Physiol, 7, 581. doi: 10.3389/fphys.2016.00581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn K, Trine M, Stegner A, & Tobar D (2001). Effect of isometric exercise on pain perception and blood pressure in men and women. Medicine Science in Sports Exercise, 33(2), 282–290. [DOI] [PubMed] [Google Scholar]
- Koltyn KF (2000). Analgesia following exercise: a review. Sports Med, 29(2), 85–98. doi: 10.2165/00007256-200029020-00002 [DOI] [PubMed] [Google Scholar]
- Koltyn KF, & Arbogast RW (1998). Perception of pain after resistance exercise. British journal of sports medicine, 32(1), 20–24. doi: 10.1136/bjsm.32.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, & Hillard C (2014). Mechanisms of Exercise-Induced Hypoalgesia. The Journal of Pain, 15(12), 1294–1304. doi: 10.1016/j.jpain.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn KF, Trine MR, Stegner AJ, & Tobar DA (2001). Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sports Exerc, 33(2), 282–290. doi: 10.1097/00005768-200102000-00018 [DOI] [PubMed] [Google Scholar]
- Koltyn KF, & Umeda M (2007). Contralateral attenuation of pain after short-duration submaximal isometric exercise. J Pain, 8(11), 887–892. doi: 10.1016/j.jpain.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Koltyn KF, & Umeda M (2007). Contralateral Attenuation of Pain After Short-Duration Submaximal Isometric Exercise. The Journal of Pain, 8(11), 887–892. doi: 10.1016/j.jpain.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Kosek E, & Ekholm J (1995). Modulation of pressure pain thresholds during and following isometric contraction. Pain, 61(3), 481–486. doi: 10.1016/0304-3959(94)00217-3 [DOI] [PubMed] [Google Scholar]
- Kosek E, Ekholm J, & Hansson P (1996). Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain, 64(3), 415–423. doi: 10.1016/0304-3959(95)00112-3 [DOI] [PubMed] [Google Scholar]
- Kosek E, & Lundberg L (2003). Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain, 7(3), 251–258. doi: 10.1016/s1090-3801(02)00124-6 [DOI] [PubMed] [Google Scholar]
- Krüger S, Khayat D, Hoffmeister M, & Hilberg T (2016). Pain thresholds following maximal endurance exercise. Eur J Appl Physiol, 116(3), 535–540. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, & Besson JM (1979). Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain, 6(3), 283–304. doi: 10.1016/0304-3959(79)90049-6 [DOI] [PubMed] [Google Scholar]
- Lee HS (2014). The effects of aerobic exercise and strengthening exercise on pain pressure thresholds. J Phys Ther Sci, 26(7), 1107–1111. doi: 10.1589/jpts.26.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemley KJ, Drewek B, Hunter SK, & Hoeger Bement MK (2014). Pain relief after isometric exercise is not task-dependent in older men and women. Med Sci Sports Exerc, 46(1), 185–191. doi: 10.1249/MSS.0b013e3182a05de8 [DOI] [PubMed] [Google Scholar]
- Lemley KJ, Hunter SK, & Bement MKH (2015). Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Medicine & Science in Sports & Exercise. [DOI] [PubMed] [Google Scholar]
- Lemley KJ, Senefeld J, Hunter SK, & Hoeger Bement M (2016). Only women report increase in pain threshold following fatiguing contractions of the upper extremity. Eur J Appl Physiol, 116(7), 1379–1385. doi: 10.1007/s00421-016-3389-8 [DOI] [PubMed] [Google Scholar]
- Lemming D, Borsbo B, Sjors A, Lind EB, Arendt-Nielsen L, Graven-Nielsen T, & Gerdle B (2015). Single-point but not tonic cuff pressure pain sensitivity is associated with level of physical fitness--a study of non-athletic healthy subjects. PLoS One, 10(5), e0125432. doi: 10.1371/journal.pone.0125432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Z, & Sullivan PJ (2018). The effect of group size and synchrony on pain threshold changes. Small Group Research, 49(6), 723–738. [Google Scholar]
- Löfgren M, Opava CH, Demmelmaier I, Fridén C, Lundberg IE, Nordgren B, & Kosek E (2018). Pain sensitivity at rest and during muscle contraction in persons with rheumatoid arthritis: a substudy within the Physical Activity in Rheumatoid Arthritis 2010 study. Arthritis Research & Therapy, 20(1), 48. doi: 10.1186/s13075-018-1513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AJ, Lissounov A, Knezevic I, Candido KD, & Knezevic NN (2016). Pain and sex hormones: a review of current understanding. Pain management, 6(3), 285–296. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, & Tanaka H (2001). Exercise prescription for the elderly. Sports medicine, 31(11), 809–818. [DOI] [PubMed] [Google Scholar]
- Meeus M, Roussel NA, Truijen S, & Nijs J (2010). Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med, 42(9), 884–890. doi: 10.2340/16501977-0595 [DOI] [PubMed] [Google Scholar]
- Micalos PS, & Arendt-Nielsen L (2016). Differential pain response at local and remote muscle sites following aerobic cycling exercise at mild and moderate intensity. Springerplus, 5, 91. doi: 10.1186/s40064-016-1721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & The PG (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle KM, Fillingim RB, & Riley JL 3rd. (2012). A meta-analytic review of the hypoalgesic effects of exercise. J Pain, 13(12), 1139–1150. doi: 10.1016/j.jpain.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle KM, Naugle KE, Fillingim RB, Samuels B, & Riley JL 3rd. (2014). Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc, 46(4), 817–825. doi: 10.1249/MSS.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle KM, Naugle KE, Fillingim RB, Samuels B, & Riley JL 3rd. (2014). Intensity thresholds for aerobic exercise-induced hypoalgesia. Med Sci Sports Exerc, 46(4), 817–825. doi: 10.1249/mss.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle KM, Naugle KE, & Riley JL 3rd. (2016). Reduced Modulation of Pain in Older Adults After Isometric and Aerobic Exercise. J Pain, 17(6), 719–728. doi: 10.1016/j.jpain.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle KM, & Riley JL 3rd. (2014). Self-reported physical activity predicts pain inhibitory and facilitatory function. Med Sci Sports Exerc, 46(3), 622–629. doi: 10.1249/MSS.0b013e3182a69cf1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien AT, Deitos A, Trinanes Pego Y, Fregni F, & Carrillo-de-la-Pena MT (2018). Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J Pain, 19(8), 819–836. doi: 10.1016/j.jpain.2018.01.010 [DOI] [PubMed] [Google Scholar]
- O’Brien AT, El-Hagrassy MM, Rafferty H, Sanchez P, Huerta R, Chaudhari S, … Fregni F (2019). Impact of Therapeutic Interventions on Pain Intensity and Endogenous Pain Modulation in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Pain Med, 20(5), 1000–1011. doi: 10.1093/pm/pny261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PJ, & Cook DB (1999). Exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exerc Sport Sci Rev, 27, 119–166. [PubMed] [Google Scholar]
- Ohlman T, Miller L, Naugle KE, & Naugle KM (2018). Physical Activity Levels Predict Exercise-induced Hypoalgesia in Older Adults. Med Sci Sports Exerc, 50(10), 2101–2109. doi: 10.1249/mss.0000000000001661 [DOI] [PubMed] [Google Scholar]
- Persson AL, Hansson GA, Kalliomaki A, Moritz U, & Sjolund BH (2000). Pressure pain thresholds and electromyographically defined muscular fatigue induced by a muscular endurance test in normal women. Clin J Pain, 16(2), 155–163. doi: 10.1097/00002508-200006000-00009 [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, & Ray CA (2004). American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc, 36(3), 533–553. doi: 10.1249/01.mss.0000115224.88514.3a [DOI] [PubMed] [Google Scholar]
- Petruzzello SJ, & Landers DM (1994). Varying the duration of acute exercise: Implications for changes in affect. Anxiety, Stress Coping, 6(4), 301–310. [Google Scholar]
- Rhud JL, & Meagher MW (2001). Noise stress and human pain thresholds: divergent effects in men and women. Journal of Pain, 2(1), 57–64. [DOI] [PubMed] [Google Scholar]
- Rice D, Nijs J, Kosek E, Wideman T, Hasenbring MI, Koltyn K, … Polli A (2019). Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J Pain. doi: 10.1016/j.jpain.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Rogatgi. (2011). WebPlotDigitizer. Retrieved from http://arohatgi.info/WebPlotDigitizer/app/. [Google Scholar]
- Schunemann H (2008). GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 http://www.cc-ims.net/gradepro. [Google Scholar]
- Slater H, Theriault E, Ronningen BO, Clark R, & Nosaka K (2010). Exercise-induced mechanical hypoalgesia in musculotendinous tissues of the lateral elbow. Man Ther, 15(1), 66–73. doi: 10.1016/j.math.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, & Chipponi J. J. A. j. o. s. (2003). Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. 73(9), 712–716. [DOI] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Weyl EE, & Price DD (2010). Pain variability in fibromyalgia is related to activity and rest: role of peripheral tissue impulse input. J Pain, 11(12), 1376–1383. doi: 10.1016/j.jpain.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg WF, Boka C, Kas L, Alboyadjia A, & Gracely RH (2001). Sex-dependent components of the analgesia produced by athletic competition. Journal of Pain, 2(1), 65–74. [DOI] [PubMed] [Google Scholar]
- Treseler C, Bixby WR, & Nepocatych S (2016). The Effect of Compression Stockings on Physiological and Psychological Responses after 5-km Performance in Recreationally Active Females. J Strength Cond Res, 30(7), 1985–1991. doi: 10.1519/jsc.0000000000001291 [DOI] [PubMed] [Google Scholar]
- Umeda M, Newcomb LW, & Koltyn KF (2009). Influence of blood pressure elevations by isometric exercise on pain perception in women. Int J Psychophysiol, 74(1), 45–52. doi: 10.1016/j.ijpsycho.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Vaegter HB, Handberg G, Jorgensen MN, Kinly A, & Graven-Nielsen T (2015). Aerobic exercise and cold pressor test induce hypoalgesia in active and inactive men and women. Pain Med, 16(5), 923–933. doi: 10.1111/pme.12641 [DOI] [PubMed] [Google Scholar]
- Vaegter HB, Hoeger Bement M, Madsen AB, Fridriksson J, Dasa M, & Graven-Nielsen T (2017). Exercise increases pressure pain tolerance but not pressure and heat pain thresholds in healthy young men. Eur J Pain, 21(1), 73–81. doi: 10.1002/ejp.901 [DOI] [PubMed] [Google Scholar]
- Vaegter HB, Lyng KD, Yttereng FW, Christensen MH, Sorensen MB, & Graven-Nielsen T (2019). Exercise-Induced Hypoalgesia After Isometric Wall Squat Exercise: A Test-Retest Reliabilty Study. Pain Med, 20(1), 129–137. doi: 10.1093/pm/pny087 [DOI] [PubMed] [Google Scholar]
- van Weerdenburg LJ, Brock C, Drewes AM, van Goor H, de Vries M, & Wilder-Smith OH (2017). Influence of exercise on visceral pain: an explorative study in healthy volunteers. J Pain Res, 10, 37–46. doi: 10.2147/jpr.s121315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K, & Tracey I (2008). Hormones and their interaction with the pain experience. Reviews in pain, 2(2), 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra-Tanaka JH, Pacheco-Barrios K, Tellez WA, & Taype-Rondan A (2019). Effects of dog-assisted therapy in adults with dementia: A systematic review and meta-analysis. BMC psychiatry, 19(1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


