Abstract
Purpose of review:
To provide updates on terminology, epidemiology, diagnosis, and treatment of combined hepatocellular-cholangiocarcinoma (cHCC-CCA).
Recent findings:
cHCC-CCAs are tumors that in the same nodule contain a variable degree of HCC and CCA components with a transition zone. cHCC-CCAs develop in cirrhotic and non-cirrhotic livers like and is associated with poor outcomes. Mutations in TP53, TERT promoter, and ARID1A are the most common genetic aberrations in cHCC-CCA. Fusion gene PTMS-AP1G1 is unique for cHCC-CCA. A biopsy is required for diagnosis. Surgical resection remains treatment of choice, while liver transplantation for early cHCC-CCA is associated with favorable outcomes. Gemcitabine-based therapy shows benefits for advanced cHCC-CCA.
Summary:
cHCC-CCAs are a heterogeneous group of primary liver cancers with unique biological behavior. Multicenter studies are required for a molecular analysis to inform novel therapeutic approaches, and understand epidemiology and benefits of liver transplantation, liver-directed and targeted therapies for this rare aggressive cancer.
Keywords: primary liver cancer, mixed liver cancer, genetic aberrations, liver transplantation, loco-regional therapy
Introduction
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary liver cancer (PLC) which has been increasingly recognized as a neoplasm with a unique biological behavior. Its epidemiology, radiological and histological characteristics, and clinical behavior are distinct from classical HCC and intrahepatic CCA (iCCA). While cHCC-CCA was first described more than 100 years ago [1] due to lack of use of unified terminology and diagnostic criteria, its true incidence and prevalence are not well known and were likely underestimated in the past. It had been reported to account for 0.4–14.2% of all PLCs. Lately, increased awareness of the unique biological behavior of cHCC-CCA has resulted in increased attention and number of studies dedicated to this cancer.
To improve cHCC-CCA diagnosis, treatment and research approaches, an international group of pathologists, clinicians, and radiologists offered a new consensus document on cHCC-CCA [2]. Additionally, the World Health Organization (WHO) updated the definition of cHCC-CCA in their 5th, most recent edition [3]. According to the international consensus and WHO, cHCC-CCA is now defined as a cancer with simultaneous presence of both hepatocytic and cholangiocytic cytologies and architecture within the same tumor [2, 3]. These tumors often have transitional features and various degree of cellular differentiation and proportion of each cell subtype making this group very heterogenous. Routine histopathology with hematoxylin and eosin staining (Figure 1) is sufficient to make the cHCC-CCA diagnosis. Specialized immunostains are no longer required for the cHCC-CCA diagnosis. While it has been recommended that pathologists document presence of stem cell phenotype [2, 4], cHCC-HCCs are no longer subclassified on classical and stem cell-like subtypes. Further subclassification on typical, intermediate and cholangiocellular types also is no longer recommended. Moreover, the latter is currently termed cholangiolocarcinoma and is considered a subtype of intrahepatic CCA (iCCA) [4–6]. Finally, cHCC-CCA should be distinguished from collision tumors, when HCC and iCCA develop separately in the same liver but meet to form one nodule; and from multifocal HCC and iCCA. This review will focus specifically on cHCC-CCA.
Figure 1.
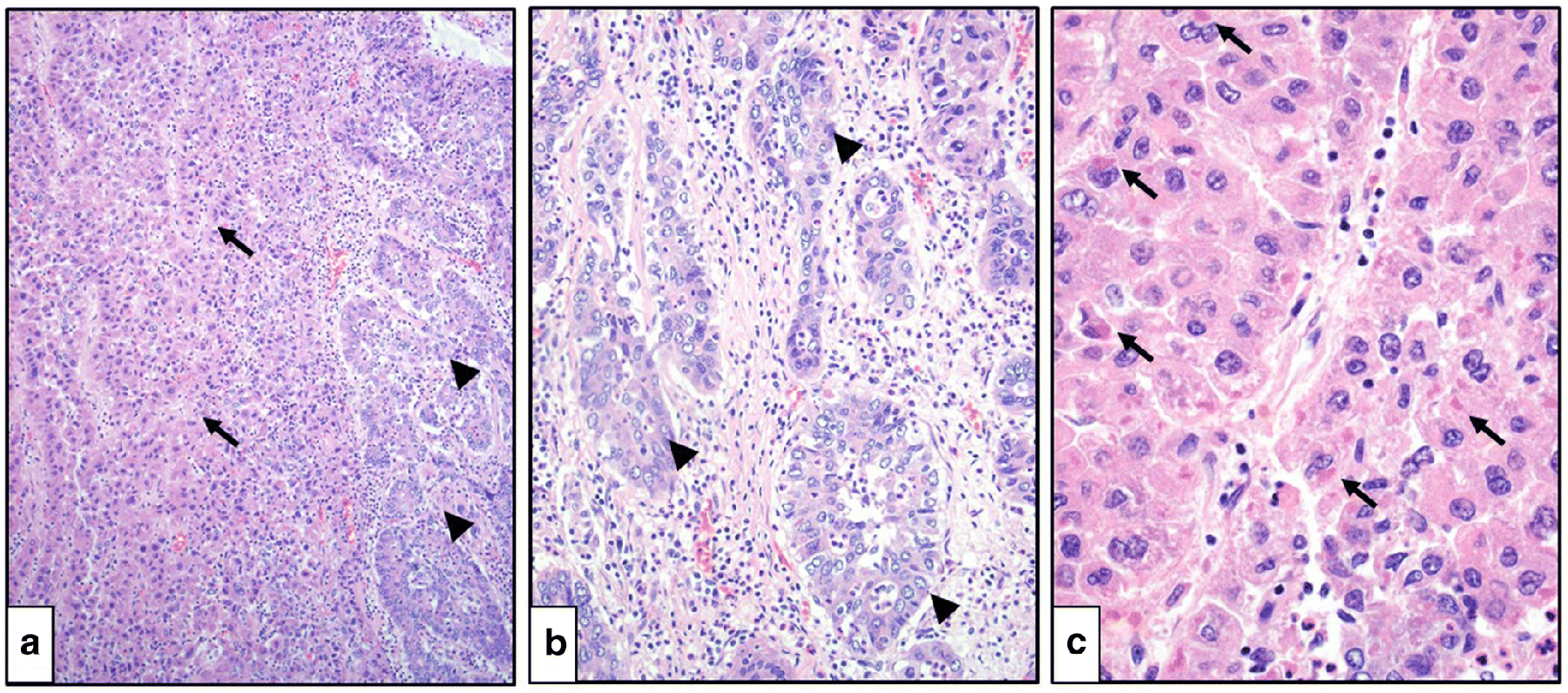
Combined hepatocellular and cholangiocellular carcinoma (hematoxylin and eosin staining). A. Hepatocellular (arrows) and cholangiocellular (arrowheads) components are present in the same tumor (100x magnification). B. A cholangiocellular tumor component demonstrates distinct tubular structures with lumens (arrowheads; 200x magnification). C. A hepatocellular tumor component demonstrates Mallory-Denk bodies (arrows; 400x magnification).
Epidemiology
The incidence of cHCC-CCA is increasing worldwide [7–9]. Its prevalence varies depending on the geographic region, risk factors within a population, and means of diagnosis [2, 10, 11]. In fact, most combined tumors are diagnosed post-operatively [6], and are thought to represent 1–4.7% of all resected liver cancers [12, 13]. In Western countries, cHCC-CC is more often diagnosed before 60 years of age [7, 8]. In Asia, it accounts for 0.25–0.70% of PLCs [14, 15] with the mean age at diagnosis of 67 years old [14, 15]. Association of cHCC-CCA with sex has not been well established. Some reports from Asia indicate male predominance [7, 8, 14–16]. While reports from Western countries do not support this sex difference [2, 16].
cHCC-CCA can be diagnosed in both cirrhotic and non-cirrhotic livers. This is unlike HCC, which is more often associated with cirrhosis [17], and iCCA, which is most often diagnosed de Novo [18]. In Western countries, only 15% of patients diagnosed with cHCC-CCA have history of viral Hepatitis B or C, and do not have cirrhosis. In some Asian studies, etiological factors were reported to be similar between HCC and cHCC-CCA [19, 20]. While in other studies from Asia, a history of cirrhosis and viral hepatitis among patients with cHCC-CCA reported to be less common than in patients with HCC, but more frequent than in iCCA patients [11, 21]. Prevalence of cHCC-CCA in patients with non-alcoholic fatty liver- and alcohol-related liver diseases is not well defined but warrants investigation. Overall, the epidemiological profile of cHCC-CCA is likely intermediate between HCC and iCCA (Figure 2) [22].
Figure 2.

Characteristics of cHCC-CCA.
Diagnosis
Clinical presentation
A mass lesion in patients undergoing surveillance for liver cancer or in symptomatic patients with or without known liver disease are most common clinical presentations of cHCC-CCA [10, 19, 23, 24]. Fatigue, weight loss, abdominal pain, new jaundice, night sweats, or worsening of portal hypertension can be observed. Owing to their transitional nature, combined tumors can cause symptoms secondary to portal vein tumor invasion [25] or with biliary obstruction. Enlarged due to metastases lymph nodes (LN) had been reported in 27%−73% of cHCC-CCs [15]. The wide range of reported frequency of metastases can be due to differences in diagnostic criteria for combined cancers between studies leading to misclassification. History of risk factors for liver disease, including obesity, alcohol misuse, intravenous drug use or immigration from areas with high prevalence of viral hepatitis can prompt abdominal imaging. The average tumor size at diagnosis is 5.3 cm [26]. However, patients who undergo cHCC-CCA resection and have cirrhosis, tend to have smaller, asymptomatic cancers as compared to patients without prior history of liver disease likely due to earlier diagnosis during active cancer surveillance [27].
Imaging studies
Presence of both hepatocellular and cholangiocellular components makes diagnosis of combined cancer based on imaging studies challenging as these components have a different degree of vascularization and fibrosis among other factors influencing radiological appearance (Figure 2) [28]. Not surprisingly, the majority of patients undergoing surgery based on radiological appearance alone are often misdiagnosed with either HCC or iCCA preoperatively [24]. Abdominal ultrasound is commonly used for cancer surveillance and can reveal a hypoechoic mass with a central hyperechoic focus or a heterogeneous hypoechoic mass, which requires further characterization with dynamic contrast-enhanced abdominal imaging such as computed tomography or magnetic resonance imaging (MRI).
Radiological characteristics of cHCC-CCA depend on the predominant histological features of HCC or CCA. Classical HCC demonstrates a homogenous diffuse enhancement during the arterial phase, a washout on the delayed venous phase, and pseudocapsule. However, owing to the cholangiocellular cancer component, cHCC-CCAs can also exhibit a peripheral ring-like enhancement with progressive centripetal enhancement due to presence of the fibrotic stroma [6, 15, 29–31]. Biliary dilatation and capsular retraction can be observed similar with iCCA [32]. The retrospective analysis of resection or post liver transplantation samples of patients with cHCC-CCA had shown, that tumors with >50% HCC histology are more radiologically similar to HCC with arterial enhancement and washout, while lesions with >50% iCCA histology demonstrate delayed enhancement like iCCA [33]. Another analysis has reported that masses showing a mixed pattern with progressive enhancement; arterial enhancement with or without washout; and hypovascular areas all in one lesion have a 48% sensitivity and 81% specificity for diagnosis of cHCC-CCA [34]. The MRI can additionally demonstrate intralesional fat, hypointensity on T1- weighted and hyperintensity on T2-weighted images due to hepatocellular cancer part [35].
The Liver Imaging Reporting and Data System (LI-RADS) was developed to standardize radiological approach to liver lesion classification and facilitate HCC diagnosis in patients with cirrhosis [36]. It grades lesions from LR-1 (definitely benign) to LR-5 (definitely HCC). cHCC-CCA lesion is reported as LR-malignant (LR-M) as it does not meet HCC criteria but appears as a malignant mass [37, 38]. Accurate preoperative diagnosis of cHCC-CCA is imperative because the treatment approach and outcomes differ from that of other PLCs. Thus, the frequency of lymph node metastases in cHCC‐CCA is high which mandates LN dissection during surgery as in iCCA [28]. Serological markers, and ultimately an imaging-guided mass biopsy should aid the correct diagnosis.
Histopathological diagnosis
When a suspicious liver mass is associated with elevation of CA 19–9 characteristic for CCA and AFP typical for HCC, cHCC-CCA should be ruled out and warrants a mass biopsy. At a cutoff value of 20 ng/mL, AFP can be lower in cHCC-CC as compared to HCC [19, 24]. Histologically, cHCC-CCA is confirmed by unequivocal histological areas of HCC and CCA in the same tumor nodule [39, 40]. The hepatocellular component can demonstrate Mallory-Denk hyaline bodies, alpha-1 antitrypsin granules, albumin, pseudoglandular trabecular arrangement, presence of bilirubin in neoplastic canaliculi lined by the well differentiated liver cells (Figure 1). Nodule boundaries may contain cells with eosinophilic cytoplasm [41]. A cholangiocellular cancer component is often characterized by gland-like structures composed of mucin producing cells positive for biliary differentiation markers CK7 and CK19, and by a prominent desmoplastic stroma. A periductal infiltration or intraductal growth can also be observed [6].
There is no minimum amount of HCC and CCA components that qualify patients for combined tumor diagnosis and each component can be independently well or poorly differentiated [16, 23]. As PLCs often arise in the cirrhotic livers, histopathological features of underlying liver disorder such as alcoholic and non-alcoholic fatty liver disease, or viral hepatitis-related liver changes can be present [16, 42].
Differentiation of cHCC-CCA from other rare types of PLCs for prognostication. Thus, recently re-classified as a subtype of iCCA intermediate cell PLC is more common in females and associated with larger tumor size and less fibrosis compared to cHCC-CCA. The latter can have less inflammation in surrounding tissue in comparison to cholangiolocarcinoma [43]. In turn, the cholangiolocarcinomas are often smaller tumors with a lower differentiation grade than cHCC-CCA making them more aggressive [25, 39].
Molecular pathogenesis
Genetic profile of cHCC-CCA appears to be distinct from this of HCC and CCA. Thus, a recent study of 133 patients from Asia demonstrated a higher frequency of TP53 mutations (49%) in cHCC-CCA as compared to HCC and CCA [44], which was supported by other studies [5, 16, 45]. Mutations in CTNNB1 and KRAS, which are frequently observed in HCC and CCA, respectively, are rare in cHCC-CCA (6% and 0%, respectively) [44]. A unique for cHCC-CCA fusion gene PTMS-AP1G1 had been reported in 11.7% of cases and might be clinically relevant. Overexpression of NESTIN in cHCC-CCA had been reported in an association with poor prognosis [44]. Study of 53 cHCC-CCA patients from Japan had demonstrated that mutations in the TERT promoter (31.3% of cases) are mainly observed in females, in previously treated tumors, and are associated with Hepatitis B and poorer outcomes [4]. In the same study, mutations in ARID1A (13.2% of cases) were more frequently observed in alcoholic liver disease-associated cHCC-CCA and in smaller tumors [4]. Mutations in IDH1/2, which are frequent in iCCAs, were reported in 11.8% of patients with cHCC-CCA. However, there were not predictive of clinical outcomes [4]. Highly targetable FGFR aberrations, which are also common in iCCA, are infrequent in combined cancers [4]. An analysis of combined tumors in 18 U.S. patients showed that tumors with high prevalence of stem cells and SALL4 positivity are enriched for progenitor-like signatures, demonstrate activated MYC and IGF pathways, and are associated with poorer outcomes [5].
Staging and Outcome Predictors
Several staging systems have been developed for HCC and CCA [46–49]. According to the 8th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual, cHCC-CCA is staged the same as iCCA [50] However, these staging systems cannot be directly applied to combined cancers due to their transitional nature. A risk prediction preoperative model and a prognostic estimation of cHCC-CCA after resection (PECAR) systems have been proposed for outcome prediction [44, 51]. Risk predictors in former model include age; presence of anti-HBc and tumor in vein; levels of AFP, CEA, blood urea nitrogen; and red blood cell count [44, 52]. Predictors of recurrence in the PECAR system include male sex, elevated GGT, presence of the macrovascular invasion and hilar LN metastases. These models were not validated externally.
Treatment
Surgical Approach
Surgical resection with dissection of hilar LNs is a treatment of choice for patients with compensated liver function and without distant metastases. Underlying liver function, degree of portal hypertension, volume of a potential liver remnant, and patient age influence patient surgical candidacy. Portal vein embolization for pre-operative enhancement of the future liver remnant can be considered. Despite curative intent of the resection, the 5-year survival rate is still suboptimal at approximately 30% [20] with a 5-year post-surgical resection recurrence rate is 78%. A resection margin >10 mm (R0 resection) is associated with a longer disease-free survival [44, 53]. One- and 3-year survival rates in cHCC-CCA have been reported at 81.9% and 47.0%, respectively [11]. cHCC-CCA patients more often have poorly differentiated tumor (29.2%) as compared with HCC and iCCA (10.3% and 17.2%, respectively, P<0.001), which might explain a shorter median overall survival of 7.9 months versus 10.8 months in HCC and 8.2 months in iCCA (P<0.001) [12]. The post-resection stage-specific survival for cHCC-CCA patients has been reported to be similar with those with HCC [12].
Role for Liver Transplantation
Liver transplantation is a well-established treatment approach associated with improved survival in patients with HCC, who meet Milan criteria [44, 54]. iCCA is not a widely accepted indication for liver transplantation, while there is an accumulating body of evidence for favorable outcomes in patients with early (<2 cm) iCCA [55, 56], and some patients with more advanced iCCA after neoadjuvant chemotherapy [57]. Notably, the majority of small iCCAs and cHCC-CCAs are diagnosed post-transplantation incidentally, with 0.48–2.7% of cHCC-CCA found in the explanted livers [39, 44, 52].
Some studies, including those using adjuvant chemotherapy [28, 44] have reported the 1-, 3-, and 5-year cHCC-CCA survival rates post-transplantation of 79%, 66%, and 16%, respectively [44]. Another study directly comparing outcomes for liver transplantation for cHCC-CCA, iCCA, and HCC had reported the 5-year survival rates of 40%, 47%, and 62%, respectively [44]. Tumors >2 cm, presence of concurrent HCC, LN metastases, and portal vein invasion were associated with poorer prognosis after liver transplantation [44].
Data from transplant centers show comparable outcomes of the surgical resection and liver transplantation for cHCC-CCA with the 1-, 3-, and 5-year survival rates after resection at 71%, 46%, and 42.1%, respectively, and after transplantation at 89%, 48%, and 50%, respectively [44]. Taking into a consideration a scarce pool of donor organs, resection should be considered as a first line treatment for localized to the liver cHCC-CCA in acceptable surgical candidates. The role of neoadjuvant or adjuvant therapy is not known.
Loco-regional Therapies
Extrapolating from experience with HCC and iCCA [13, 25], inoperable relatively small cHCC-CCs (<4 cm) can potentially be treated with image-guided ablation. Radiation therapy can be used when a tumor location precludes safe ablation, the tumor cannot be visualized on an imaging study, or in patients with suboptimal liver function. An unpublished experience with stereotactic radiation therapy (SBRT) for cHCC-CC from our center is highly favorable. A paucity of data on outcomes of ablation and radiation therapy in cHCC-CC is likely due to rarity of these tumors and difficulties with diagnosis.
Degree of cHCC-CCA arterialization is determined by HCC component and can predict a potential response to transarterial catheter-based therapies. cHCC-CCAs treated with transarterial therapies versus resection were reported to be larger (mean size of 8.9 cm versus 5.8 cm, respectively) and more often were associated with LN metastases (33% versus 8%, respectively) [8, 16, 35]. The partial response rate to transarterial chemoembolization, radioembolization, and hepatic arterial infusional chemotherapy has been reported at 20%, 50%, and 66%, respectively [35]. The median progression-free and overall survival after catheter-based therapies has been reported at 8.3 and 16 months, respectively [8, 16]. Thus, catheter-based transarterial therapies might have utility for well-vascularized large or multifocal inoperable cHCC-CCAs in patients with acceptable liver function. Overall, more data are needed to demonstrate effectiveness of loco-regional therapies for cHCC-CCA.
Systemic therapy
There are no standardized recommendations on systemic agents for cHCC-CCA. Most centers use a combination of gemcitabine with platinum-based regimens [58], which can be justified by the data from several recent retrospective studies. One had compared outcomes in 36 patients treated with gemcitabine/cisplatin, gemcitabine/5-fluorouracil, sorafenib, and other regimens, including S-1 alone or in a combination with gemcitabine, or gemcitabine alone [44, 59]. The median overall survival with gemcitabine/cisplatin, gemcitabine/5-fluorouracil, sorafenib, and “other regimens” was 11.9, 10.2, 3.5, and 8.1 months, respectively, and there was no difference in a progression-free survival between groups [44]. Thus, sorafenib use was associated with an inferior overall survival (hazard ratio 15.83, P=0.006) as compared with gemcitabine-based regimens. Sorafenib inferiority for treatment of cHCC-CCA also was demonstrated in a study of 123 patients treated with either gemcitabine alone or in combination with 5-fluorouracil or platinum-based agents, sorafenib alone, or “other drugs” [8, 44]. The overall survival after treatment with gemcitabine combinations versus sorafenib were 11.5 and 9.6 months, respectively. The progression-free survival was also lower in the sorafenib group (4.8 months) as compared with the gemcitabine combination with platinum-based agents (8 months) or the gemcitabine combination with 5-fluorouracil (6.6 months) groups [44].
Based on clinical trials, molecular targeted therapies are effective for HCC [60] and iCCA [61]. The data on targeted therapies use in cHCC-CCA are very limited. One case review has shown that bevacizumab, which had been recently approved by the Federal Drug Administration as a first line agent in a combination with atezulizumab for advanced HCC [60, 62], is associated with improved outcomes in cHCC-CCA patients when used in a combination with gemcitabine-based therapy [59]. Immunotherapy alone or in combination with other systemic agents and liver-directed modalities had been shown to improve outcomes in HCC [63]. Its utility for cHCC CCA is not known.
Conclusions
cHCC-CCAs are a distinct group of PLCs with unique genetic landscape and aggressive biological behavior. Due to its rarity and non-uniform use of cHCC-CCA terminology and diagnostic criteria, its epidemiology and best therapeutic approaches to treat it are not well established. Histological tumor evaluation is required for diagnosis of combined cancers and can be based on a routine hematoxylin and eosin staining. Patients outcomes with cHCC-CCA appear to be worse than with HCC but better when compared with iCCA. Surgical resection is the treatment of choice for early stage cHCC-CCA confined to the liver. The role of liver transplantation for cHCC-CCA is emerging, while the role of liver-directed therapies and adjuvant and neoadjuvant therapies is unknown. Catheter-based therapy can be beneficial for patients with highly vascularized tumors. Limited retrospective studies support use of a combination of gemcitabine with platinum-based chemotherapeutic agents until data for other regimens are available. Studies investigating the role of molecular targeted therapies in cHCC-CCA are highly desirable but might be difficult to conduct due to rarity of this cancer. Consistent use of proper cHCC-CCA terminology and multicenter collaborations will promote better understanding of this rare cancer epidemiology and genetics to inform potentially actionable targets and facilitate clinical trials.
Acknowledgments
This work was supported by NIDDK K08 (NR); Gilead Research Scholar Award (NR).
List of Abbreviations:
- cHCC-CCA
combined hepatocellular-cholangiocarcinoma
- iCCA
intrahepatic cholangiocarcinoma
- WHO
World Health Organization
- MRI
magnetic resonance imaging
- PECAR
prognostic estimation of cHCC-CCA after resection
- LN
lymph node
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Olga Raevskaya, Henry Appelman, and Natalita Razumilava declare that they have no conflict of interest
REFERENCES
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.H.G W, Primary carcinoma of the liver. Am.J.M.Sc, 1903. 26(126): p. 403–417. [Google Scholar]
- 2. ••.Brunt E, et al. , cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology, 2018. 68(1): p. 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]; A latest consensus document on a uniform cHCC-CCA terminology.
- 3. ••.Nagtegaal ID, et al. , The 2019 WHO classification of tumours of the digestive system. Histopathology, 2020. 76(2): p. 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]; A manuscript summarizing the World Helath Organization recommendations on cHCC-CCA classification and diagnosis.
- 4. •.Sasaki M, Sato Y, and Nakanuma Y, Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology, 2017. 70(3): p. 423–434. [DOI] [PubMed] [Google Scholar]; An important work providing information on genetic abbeartions in cHCC-CCAs.
- 5.Moeini A, et al. , Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol, 2017. 66(5): p. 952–961. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, et al. , Clinicopathological, radiologic, and molecular study of 23 combined hepatocellular-cholangiocarcinomas with stem cell features, cholangiolocellular type Hum Pathol, 2017. 64: p. 118–127. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB and Kanwal F, Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology, 2014. 60(5): p. 1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]; *(epidemiology of liver cancers in US population)
- 8.Rahib L, et al. , Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res, 2014. 74(11): p. 2913–21. [DOI] [PubMed] [Google Scholar]
- 9.Shaib YH, et al. , Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol, 2004. 40(3): p. 472–7. [DOI] [PubMed] [Google Scholar]
- 10.Jarnagin WR, et al. , Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer, 2002. 94(7): p. 2040–6. [DOI] [PubMed] [Google Scholar]
- 11.Koh KC, et al. , Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg, 2005. 189(1): p. 120–5. [DOI] [PubMed] [Google Scholar]
- 12.Bergquist JR, et al. , Mixed hepatocellular and cholangiocarcinoma: a rare tumor with a mix of parent phenotypic characteristics. HPB (Oxford), 2016. 18(11): p. 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon YI, et al. , Postresection Outcomes of Combined Hepatocellular Carcinoma-Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Gastrointest Surg, 2016. 20(2): p. 411–20. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, et al. , Synchronous development of HCC and CCC in the same subsegment of the liver in a patient with type C liver cirrhosis. World J Hepatol, 2009. 1(1): p. 103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao J, et al. , Double primary hepatic cancer (hepatocellular carcinoma and intrahepatic cholangiocarcinoma) in a single patient: a clinicopathologic study of 35 resected cases. J Gastroenterol Hepatol, 2013. 28(6): p. 1025–31. [DOI] [PubMed] [Google Scholar]
- 16. •.Stavraka C, Rush H, and Ross P, Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma, 2019. 6: p. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review on pathophysiology, treatment approaches, and prognostic factors in cHCC-CCA.
- 17.Llovet JM, Burroughs A, and Bruix J, Hepatocellular carcinoma. Lancet, 2003. 362(9399): p. 1907–17. [DOI] [PubMed] [Google Scholar]
- 18.Razumilava N and Gores GJ, Liver transplantation for intrahepatic cholangiocarcinoma—Authors’ reply. Lancet, 2014. 384(9949): p. 1182–3. [DOI] [PubMed] [Google Scholar]
- 19.Chantajitr S, et al. , Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg, 2006. 13(6): p. 537–42. [DOI] [PubMed] [Google Scholar]
- 20.Chu KJ, et al. , Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol, 2014. 26(2): p. 192–9. [DOI] [PubMed] [Google Scholar]
- 21.Murakami Y, et al. , Comprehensive analysis of transcriptome and metabolome analysis in Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Sci Rep, 2015. 5: p. 16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Serag HB and Davila JA, Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol, 2011. 4(1): p. 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda T, et al. , Combined hepatocellular and cholangiocarcinoma: proposed criteria according to cytokeratin expression and analysis of clinicopathologic features. Hum Pathol, 1995. 26(9): p. 956–64. [DOI] [PubMed] [Google Scholar]
- 24.Tang D, et al. , Clinical and pathological features of Allen’s type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg, 2006. 10(7): p. 987–98. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, et al. , Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology, 2010. 255(1): p. 270–7. [DOI] [PubMed] [Google Scholar]
- 26.Ono Y, et al. , Claudins-4 and −7 might be valuable markers to distinguish hepatocellular carcinoma from cholangiocarcinoma. Virchows Arch, 2016. 469(4): p. 417–26. [DOI] [PubMed] [Google Scholar]
- 27.Portolani N, et al. , Intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma: a Western experience. Ann Surg Oncol, 2008. 15(7): p. 1880–90. [DOI] [PubMed] [Google Scholar]
- 28.Kassahun WT and Hauss J, Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract, 2008. 62(8): p. 1271–8. [DOI] [PubMed] [Google Scholar]
- 29. •.Ni T, et al. , Different MR features for differentiation of intrahepatic mass-forming cholangiocarcinoma from hepatocellular carcinoma according to tumor size. Br J Radiol, 2018. 91(1088): p. 20180017. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important review on radiological features of PLCs.
- 30.Connell LC, et al. , Combined intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Chin Clin Oncol, 2016. 5(5): p. 66. [DOI] [PubMed] [Google Scholar]
- 31.Li R, et al. , Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer, 2016. 16: p. 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maximin S, et al. , Current update on combined hepatocellular-cholangiocarcinoma. Eur J Radiol Open, 2014. 1: p. 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu CH, et al. , Combined Hepatocellular Carcinoma and Cholangiocarcinoma: Diagnosis and Prognosis After Resection or Transplantation. Transplant Proc, 2016. 48(4): p. 1100–4. [DOI] [PubMed] [Google Scholar]
- 34.Gigante E, et al. , Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int, 2019. 39(12): p. 2386–2396. [DOI] [PubMed] [Google Scholar]
- 35.Fowler K, et al. , Biphenotypic Primary Liver Carcinomas: Assessing Outcomes of Hepatic Directed Therapy. Ann Surg Oncol, 2015. 22(13): p. 4130–7. [DOI] [PubMed] [Google Scholar]
- 36. ••.Abdel Razek AAK, et al. , Liver Imaging Reporting and Data System Version 2018: What Radiologists Need to Know. J Comput Assist Tomogr, 2020. 44(2): p. 168–177. [DOI] [PubMed] [Google Scholar]; The summary document on radiological classification of liver lesions.
- 37.Jeon SK, et al. , Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol, 2019. 29(1): p. 373–382. [DOI] [PubMed] [Google Scholar]; * (modern MRI diagnostic toll with improving diagnostic outcomes)
- 38.Potretzke TA, et al. , Imaging Features of Biphenotypic Primary Liver Carcinoma (Hepatocholangiocarcinoma) and the Potential to Mimic Hepatocellular Carcinoma: LI-RADS Analysis of CT and MRI Features in 61 Cases. AJR Am J Roentgenol, 2016. 207(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 39.Gera S, et al. , Clinical features, histology, and histogenesis of combined hepatocellular-cholangiocarcinoma. World J Hepatol, 2017. 9(6): p. 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim R, et al. , CD44 expression in patients with combined hepatocellular cholangiocarcinoma. Ann Surg Treat Res, 2015. 89(1): p. 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, et al. , [Combined hepatocellular-cholangiocarcinoma (cholangiolocellular type) with stem-cell features: a clinicopathologic analysis of 26 cases]. Zhonghua Bing Li Xue Za Zhi, 2016. 45(3): p. 175–9. [DOI] [PubMed] [Google Scholar]
- 42.Akiba J, et al. , Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol, 2013. 37(4): p. 496–505. [DOI] [PubMed] [Google Scholar]
- 43.Kim GJ, Kim H, and Park YN, Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLoS One, 2013. 8(9): p. e75449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. •.Leoni S, et al. , Treatment of Combined Hepatocellular and Cholangiocarcinoma. Cancers (Basel), 2020. 12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]; An important review on approaches to treatment and prognostication of cHCC-CCA.
- 45.Llovet JM and Bruix J, Molecular targeted therapies in hepatocellular carcinoma. Hepatology, 2008. 48(4): p. 1312–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llovet JM, Bru C, and Bruix J, Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis, 1999. 19(3): p. 329–38. [DOI] [PubMed] [Google Scholar]
- 47.You HL, et al. , Copy number aberrations in combined hepatocellular carcinoma and cholangiocarcinoma. Exp Mol Pathol, 2012. 92(3): p. 281–6. [DOI] [PubMed] [Google Scholar]
- 48.Nathan H, et al. , A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol, 2009. 16(1): p. 14–22. [DOI] [PubMed] [Google Scholar]
- 49.Tian MX, et al. , A Novel Risk prediction Model for Patients with Combined Hepatocellular-Cholangiocarcinoma. J Cancer, 2018. 9(6): p. 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. ••.Amin MB, et al. , The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin, 2017. 67(2): p. 93–99. [DOI] [PubMed] [Google Scholar]; The formative document of the American Joint Committee on Cancer on cancer staging.
- 51.Herraez E, et al. , Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology, 2013. 58(3): p. 1065–73. [DOI] [PubMed] [Google Scholar]
- 52.Berretta M, et al. , Serum and tissue markers in hepatocellular carcinoma and cholangiocarcinoma: clinical and prognostic implications. Oncotarget, 2017. 8(8): p. 14192–14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong S, et al. , Nervous system and primary liver cancer. Biochim Biophys Acta Rev Cancer, 2018. 1869(2): p. 286–292. [DOI] [PubMed] [Google Scholar]
- 54.Sasaki M, et al. , Clinicopathological significance of ‘subtypes with stem-cell feature’ in combined hepatocellular-cholangiocarcinoma. Liver Int, 2015. 35(3): p. 1024–35. [DOI] [PubMed] [Google Scholar]
- 55. •.Song P, et al. , Patients’ prognosis of intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma after resection. Cancer Med, 2019. 8(13): p. 5862–5871. [DOI] [PMC free article] [PubMed] [Google Scholar]; A retrospective study on outcomes of surgical resection for cHCC-CCA.
- 56. •.De Martin E, et al. , Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl, 2020. 26(6): p. 785–798. [DOI] [PubMed] [Google Scholar]; A study of outcomes in cHCC-CCA patients after resection and liver transplantation.
- 57.Lunsford KE, et al. , Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol, 2018. 3(5): p. 337–348. [DOI] [PubMed] [Google Scholar]
- 58.Valle J, et al. , Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med, 2010. 362(14): p. 1273–81. [DOI] [PubMed] [Google Scholar]
- 59. •.Rogers JE, et al. , Systemic therapy for unresectable, mixed hepatocellular-cholangiocarcinoma: treatment of a rare malignancy. J Gastrointest Oncol, 2017. 8(2): p. 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]; An analysis of effectiveness of different systemic therapy regimens in cHCC-CCA.
- 60.Faivre S, Rimassa L, and Finn RS, Molecular therapies for HCC: Looking outside the box. J Hepatol, 2020. 72(2): p. 342–352. [DOI] [PubMed] [Google Scholar]
- 61.Harris WP, et al. , Biomarker-Driven and Molecular Targeted Therapies for Hepatobiliary Cancers. Semin Oncol, 2018. 45(3): p. 116–123. [DOI] [PubMed] [Google Scholar]
- 62.Finn RS, et al. , Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med, 2020. 382: p. 1894–1905. [DOI] [PubMed] [Google Scholar]
- 63.Pinato DJ, et al. , Immune-based therapies for hepatocellular carcinoma. Oncogene, 2020. 39(18): p. 3620–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]


