Abstract
We report the case of an HIV-1-infected patient, treated with anti-CD20 monoclonal antibody for a B-cell lymphoma previously treated by autologous stem cell transplant. He suffered from chronic COVID19 and we monitored by plasma SARS-CoV-2 RNA by highly sensitive droplet-based digital PCR technology (ddPCR). Under tocilizumab therapy and despite a first clinical improvement biologically associated with decreasing inflammatory markers, a slight increase of SARS-CoV-2 RNAaemia quantified by ddPCR was highlighted, confirming the absence of viral efficacy of this treatment and predicting the subsequent observed deterioration. As expected, his complete recovery, finally achieved after COVID-19 convalescent plasmatherapy, strictly paralleled plasma SARS-CoV-2 RNA clearance. With these results, we confirmed the interest of SARS-CoV-2 RNAaemia monitoring by ddPCR in COVID-19 patients, particularly during treatment, and firstly showed that this new and specific biomarker could be helpful to select eligible patient for anti-IL6 receptors therapy considering the variable levels of efficacy recently observed with such therapy.
Case Report
We herein report the case of an HIV-1 infected and treated patient with stable disease and undetectable HIV viral load. The patient received first line chemotherapy treatment followed by autologous stem cell transplant for a B-cell lymphoma diagnosed in June 2017. After an early relapse, he received 2 lines of immunochemotherapy regimen with rituximab (last infusion received in December 2018). Due to disease progression, he was treated with obinutuzumab associated with ibrutinib from January 2019 to February 2020, with very good partial response but with a deep lymphopenia (CD4 T cell count < 50/mm3) and hypogammaglobulinemia.
On April 1st, 2020, he was referred to Cochin Hospital (Paris, France), for severe Coronavirus Disease 2019 (COVID-19) with positive SARS-CoV-2 nasopharyngeal RT-PCR (Cycle threshold (Ct) at 23). He presented as dry cough, diarrhea, fever, dyspnea and biological worsening as peripheral thrombocytopenia, increased acute phase reactants with upper values of ferritin at 5150 µg/L and CRP at 28.5 mg/dL, hepatic cytolysis and cholestasis, and hypertriglyceridemia. Oxygenotherapy at 3 L/min was lasted for more than 6 weeks. In the meantime, bronchoalveolar lavage fluid remained SARS-CoV-2 positive by RT-PCR (Ct at 20) 5 weeks after his admission, confirming a chronic COVID-19.
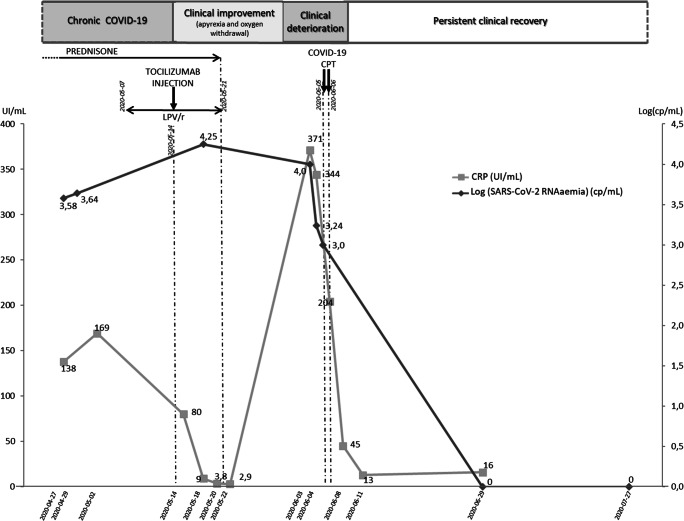
Intravenous dexamethasone (20 mg/day for the first three days then 10 mg/day for three more days) was initiated on April 14th, 2020, and relayed by oral prednisone (60 mg daily) until May 21th, 2020, without any significant clinical improvement. Next, he received lopinavir/ritonavir (LPV/r) (400 mg x 2 /day) for one week before the initiation of tocilizumab (480 mg in one infusion) on May 14th, 2020. After two weeks of clinical and biological improvement with apyrexia, oxygen withdrawal and decreased CRP (Fig. 1), the patient is getting clinically worse again.
Fig. 1.
Biological monitoring of CRP (UI/mL) and SARS-CoV-2 RNAaemia expressed in Log(cp/mL) during clinical and therapeutic history. CPT: Convalescent plasma therapy
Considering the recurrence of severe clinical symptoms on May 30th with persistent positive SARS-CoV-2 RT-PCR in respiratory samples and his absence of seroconversion (negative anti-SARS-CoV-2 IgG on June 5th ), COVID-19 convalescent plasma transfusion (CPT) was started considering his profound B-cell depletion (hypogammaglobulinemia at 2.9 g/L on April 24th, 2020). Indeed, anti-CD20 monoclonal antibody constitutes a therapeutic option for most patients with B-cell lymphoproliferative disorders. However, the induced profound B cell depletion is lasting for several months and treated-patients are unable to produce neutralizing antibodies. During SARS-CoV-2 pandemic, such patients were at major risk of developing extended form of COVID-19 [1]. Thus, containing specific SARS-CoV-2 neutralizing antibodies, COVID-19 CPT appears to be an interesting therapeutic approach in such patients [2].
The patient received one dose (2 units) daily of COVID-19 CPT on June 5th and 6th which induced a rapid and complete clinical remission, persistent at last follow-up three months later.
With patient consent, SARS-CoV-2 RNAaemia was retrospectively quantified by droplet-based digital PCR (ddPCR) on 8 plasma samples collected for medical care during his hospitalization (Fig. 1).
By optimizing sensitivity and allowing absolute quantification compared with classical q(RT)PCR, ddPCR technology was primarily developed for cancer research to quantify mutated circulating tumoral DNA among cell-free DNA in liquid biopsies. The increased tolerance of ddPCR to PCR inhibitory molecules makes it an attractive alternative to qPCR for medical applications including viral diagnostics. We recently adapted this innovative and ultrasensitive technology for SARS-CoV-2 RNAaemia monitoring in COVID-19 patients and recently showed its great interest for the first time in the world [3].
Despite a first clinical response to tocilizumab correlating with CRP decrease (Fig. 1), a slight increase of SARS-CoV-2 RNAaemia (from 3.58 log cp/mL to 4.25 log cp/mL) was noticed by ddPCR and preceded the observed clinical deterioration. Considering the variable levels of efficacy recently observed with anti-interleukin-6 receptor treatments in COVID-19 patients [4], our observation could be of particular importance in the medical use of such treatment. Indeed, SARS-CoV-2 RNAaemia could represent an helpful biomarker to select eligible patient for anti-IL6 receptors therapy by reserving it for SARS-CoV-2 RNAaemia negative patients if our data are confirmed on largest cohort of patients.
Moreover, after COVID-19 CPT, a clear negativation of plasma SARS-CoV-2 RNAaemia was rapidly observed and correlated with complete clinical recovery. In contrast, none of acute phase reactants like CRP or other classical virologic marker such as SARS-CoV-2 detection by conventional RT-PCR in respiratory samples still positive after clinical recovery were correlated with prolonged clinical recovery confirming that SARS-CoV-2 RNAaemia remained the only useful biomarker to monitor for confirming treatment success.
As circulating SARS-CoV-2 RNAaemia becomes negative under COVID-19 CPT treatment composed by specific SARS-CoV-2 antibodies, we can hypothesize that this detected viral RNA comes from complete SARS-CoV-2 viral particles and not only represents circulating released RNA.
This case illustrates the interest of SARS-CoV-2 RNAaemia monitoring by ddPCR particularly during treatment, as the only quantitative biomarker strictly correlated with the patient disease history and predictive of subsequent prolonged clinical recovery in chronic COVID-19 patient with haematological malignancy.
Finally, with the development of recent therapies against COVID-19 such as immunomodulators like anti-IL-6R, mesenchymal stem cells therapy or convalescent COVID-19 plasmatherapy which have recently showed their efficacy to prevent clinical deterioration [4] or to cure [2,5] COVID-19, we particularly need specific biomarkers to monitor such treatments and SARS-CoV-2 RNAaemia by ddPCR seems to present such qualities in the global care of COVID-19.
Acknowledgements
All authors have read the journal’s policy on disclosure of potential conflicts of interest and a statement that all authors have disclosed any financial or personal relationship with organizations that could potentially be perceived as influencing the described research. We also would like to acknowledge and thank the patient and Stilla Technologies for their prompt support with this project.
Funding
This work was supported by the Assistance Publique Hôpitaux de Paris (AP-HP), Ministère de l’Enseignement Supérieur et de la Recherche, the Université de Paris, the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Collecte Crise COVID/APHP Centre-Université de Paris.
A patent EP20305571.0 filed June 1, 2020, is pending, for “QUANTIFICATION OF CORONAVIRUS RNAemia”.
Footnotes
This article belongs to the Topical Collection: Special issue on COVID19 Pandemic and Stem Cells
Guest Editor: Mariusz Z. Ratajczak
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tali-Anne Szwebel and David Veyer contributed equally to this manuscript.
References
- 1.Avouac J, Airó P, Carlier N, Matucci-Cerinic M, Allanore Y. Severe COVID-19-associated pneumonia in 3 patients with systemic sclerosis treated with rituximab. Annals of the Rheumatic Diseases. 2020 doi: 10.1136/annrheumdis-2020-217864. [DOI] [PubMed] [Google Scholar]
- 2.Hueso T, Pouderoux C, Péré H, Beaumont A-L, Raillon L-A, Ader F, Lacombe K. Convalescent plasma therapy for B-cell depleted patients with protracted COVID-19 disease. Blood. 2020 doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veyer D, Kernéis S, Poulet G, Wack M, Robillard N, Taly V, Péré H. Highly sensitive quantification of plasma SARS-CoV-2 RNA shelds light on its potential clinical value. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parr JB. Time to Reassess Tocilizumab’s Role in COVID-19 Pneumonia. JAMA Internal Medicine. 2020 doi: 10.1001/jamainternmed.2020.6557. [DOI] [PubMed] [Google Scholar]
- 5.Debuc B, Smadja DM. Is COVID-19 a New Hematologic Disease? Stem Cell Reviews and Reports. 2020 doi: 10.1007/s12015-020-09987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]