Abstract
The COVID-19 pandemic has profoundly influenced public health and contributed to global economic divergences of unprecedented dimensions. Due to the high prevalence and mortality rates, it is then expected that the consequence and public health challenges will last for long periods. The rapid global spread of COVID-19 and lack of enough data regarding the virus pathogenicity multiplies the complexity and forced governments to react quickly against this pandemic. Stem cells represent a small fraction of cells located in different tissues. These cells play a critical role in the regeneration and restoration of injured sites. Because of their specific niche and a limited number of stem cells, the key question is whether there are different anti-viral mechanisms against viral infection notably COVID-19. Here, we aimed to highlight the intrinsic antiviral resistance in different stem cells against viral infection. These data could help us to understand the possible viral infections in different stem cells and the activation of specific molecular mechanisms upon viral entrance.
Graphical Abstract
Keywords: COVID-19, Stem cells, Mature cells, Anti-viral defense system
Background
The global emergence of human coronavirus disease, namely COVID-19, with severe bronchopneumonia and respiratory symptoms raised concerns about public health at the beginning of 2020 [1–3]. According to released statistics, SARS-CoV-2 is easily spread from person-to-person with deep socioeconomic influences on healthcare systems [4, 5]. The rapid worldwide spread of COVID-19 between human societies has led to enormous pressure on the health care system and changed the auspices toward therapeutic priorities [6]. Transplant patients are a certain population that needs unique and special health care systems [7]. Considering the urgent need for potent regenerating cells and biological products, this raises the question of whether stem cells within different tissues, especially bone marrow stem cells, could transmit the SARS-CoV-2 virus from person-to-person. In other words, are there any differences or similarities in the susceptibility of differentiated and undifferentiated cells to viral infections such as COVID-19? Of course, here is assumed all hygienic principles are toughly respected during cell transplantation to minimize the transmission of COVI-19 among individuals.
In this regard, the present review article aimed to address the possible differences in antiviral defense system between differentiated and undifferentiated cells. The logical answer to this question can acknowledge us to exploit policies against this COVID-19 in healthy donors and transplant recipients.
COVID-19 Pathophysiology and Mechanism of Action
SARS-CoV-2 belongs to the B lineage of the β-coronaviruses [8]. The virus is enveloped in a spherical nucleocapsid. The genomic pool of SARS-CoV-2 consists of + ssRNA in association with nucleoprotein inside a capsid shell [9]. At the external surface of the envelope, numerous projections (Spike proteins) and glycoproteins (hemagglutinin) are seen. Using spike proteins, the SARS-CoV-2 could attach to surface receptor ACE2 on the target cells (Figs. 1 and 2) [10]. In general, infection with SARS-CoV-2 contributes to the onset of inflammation in the human upper and lower respiratory tract [3]. Nearly a few months after the onset of COVID-19 disease, people with acute atypical respiratory disease and pneumonia refer to the hospitals and clinical settings. According to clinical observations and the susceptibility of patients, mild, moderate, severe, and critical forms of the COVID-19 disease have been recorded [8]. In the mild form, minor respiratory and gastrointestinal symptoms are common. The patients with moderate form are diagnosed with a lack of prominent hypoxemia while CT imaging exhibits some lesion inside the pulmonary tissue. In the severe forms of COVID-19 disease, patients suffer from severe hypoxemia and acute pneumonitis. With the progression of symptoms and the occurrence of critical form, an acute respiratory distress syndrome was initiated followed by renal and myocardial injury, encephalopathy, and coagulation disorders [8]. The surface ACE2 receptors account for the cellular entry of SARS-CoV-2. This virus uses the serine protease termed TMPRSS2 for priming S protein [11]. Like other cells, the ACE2 receptor is present on the surface of respiratory epithelial cells which makes these cells more susceptible to SARS-CoV-2 infection rather than cells with low levels of ACE2 receptor [12]. This coronavirus harbors a nucleocapsid with 30 kb + ssRNA [13, 14]. The whole-genome sequence of SARS-CoV-2 is greatly identical and unique [15, 16]. It has been thought that the main pathological effects of the coronaviruses correlate with + ssRNA with a 5′-cap structure and 3′poly-A tail [17–19]. After entry into the target cells, RNA plays as a template sequence to translate the non-structural polyproteins like replicase polyprotein 1a and 1ab [20]. The complex of polyprotein 1a/1ab and sgRNAs sequences acts as a replicon-transcription machinery system that is surrounded by a primary double-membrane vesicle [21]. The transcription regulatory sequence, namely ORF, controls the production of subgenomic mRNAs [22, 23]. Among different ORFs identified in the whole genome segments, the sequence located between the ORF1a and ORF1b plays a critical role in the transcription and translation of polypeptides 1a and 1ab. These proteins are further modified by viral chymotrypsin-like protease, Mpro, and two papain-like proteases [10, 14]. Along with ORF1a and 1b, other ORFs participate in the synthesis of different structural polypeptides such as envelope, external spike, viral membrane peptides, and nucleocapsid [24]. The previous experiments have shown that the variety of protein products is closely associated with the activity of sgRNAs [25–27]. Once a cell is infected with coronaviruses, the cytopathic effects are detectable in the host cells due to the activity of both viral structural and functional proteins. In support of this notion, detailed investigations of viral pathogenesis revealed that the non-structural proteins could prohibit the innate immune system activity [28]. It has been established that the activity of spike glycoproteins is essential to make a physical connection between virions and the host cell receptor. Spike proteins are classified into main subunits namely S1 and S2 [29]. The subunit S2 possesses a transmembrane domain and a cytoplasmic tail with a fusion-like activity and is highly conserved among all members of the family Coronaviridae [30]. On this basis, many pharmaceutical interventions target S2 as the main anti-viral therapeutic medication [31]. The estimated mutation rate is high within the genomic pool of SARS-CoV-2 and other RNA viruses which supports rapid expansion and transmission to human and other species [32]. The proliferation and site-direct activity of SARS-CoV2 in the host cells are set actually by absolute mutations. In most COVID-19 confirmed cases, severe pulmonary inflammation has been reported to coincide with excessive activity of immune cells [33]. Polyclonal recruitment of immune cells with disturbed activation thresholds and cytokine release contribute to irreversible tissue injury (Fig. 2) [34, 35]. Cytokine array analysis has revealed that IL-6 is the ringmaster of this story. B lymphocytes and numerous cells within the tissues are capable of IL-6 production and release. This cytokine accelerates the differentiation of B lymphocytes into inflammatory cells and increases a fraction of cell populations required for acute phase reactions [36, 37]. Elevated local levels of IL-6 in the target tissues after infection with SARS-CoV-2, mainly lungs, trigger the synthesis and release of acute-phase proteins and intensify pathological changes [38].
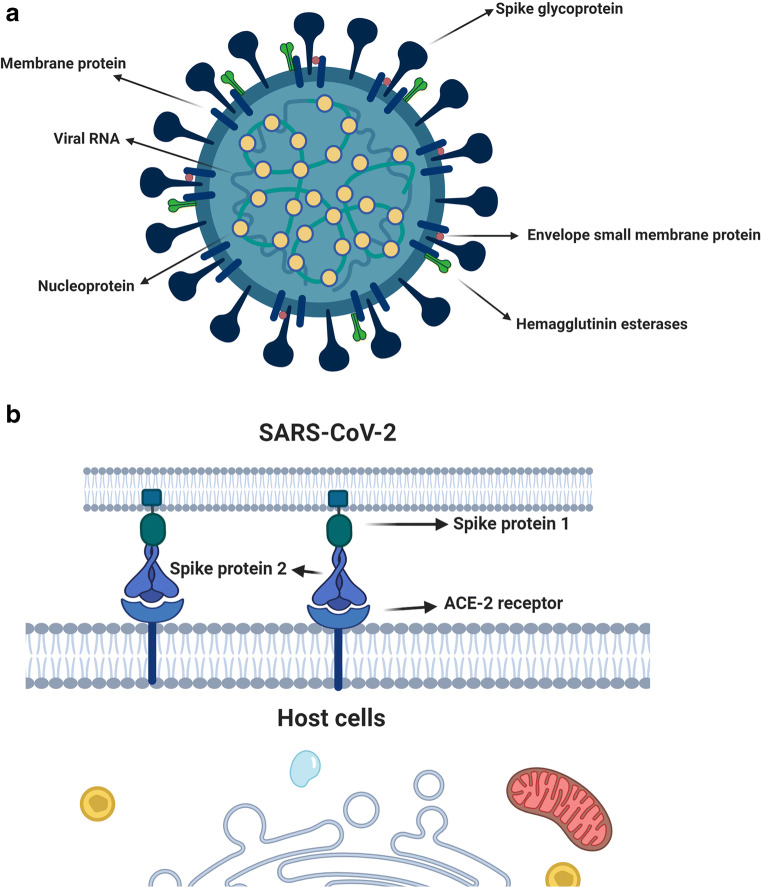
Fig. 1.
SARS-CoV-2 is encased within a fatty membrane (envelop) and has a very large genomic pool with nucleotides around 3 × 104. The viral structure is composed of membrane protein, nucleoprotein, envelope small membrane protein, hemagglutinin, single-strand positive sensel RNA, and spike glycoprotein (a). Two types of cup-shaped spike glycoproteins subunits S1 and S2 are present on the viral surface which attach the viral body to the host cellular receptor ACE-2 (b). Membrane protein = M; Nucleoprotein = N; Envelope small membrane protein = E; and Hemagglutinin = HE. The illustration was created with BioRender.com
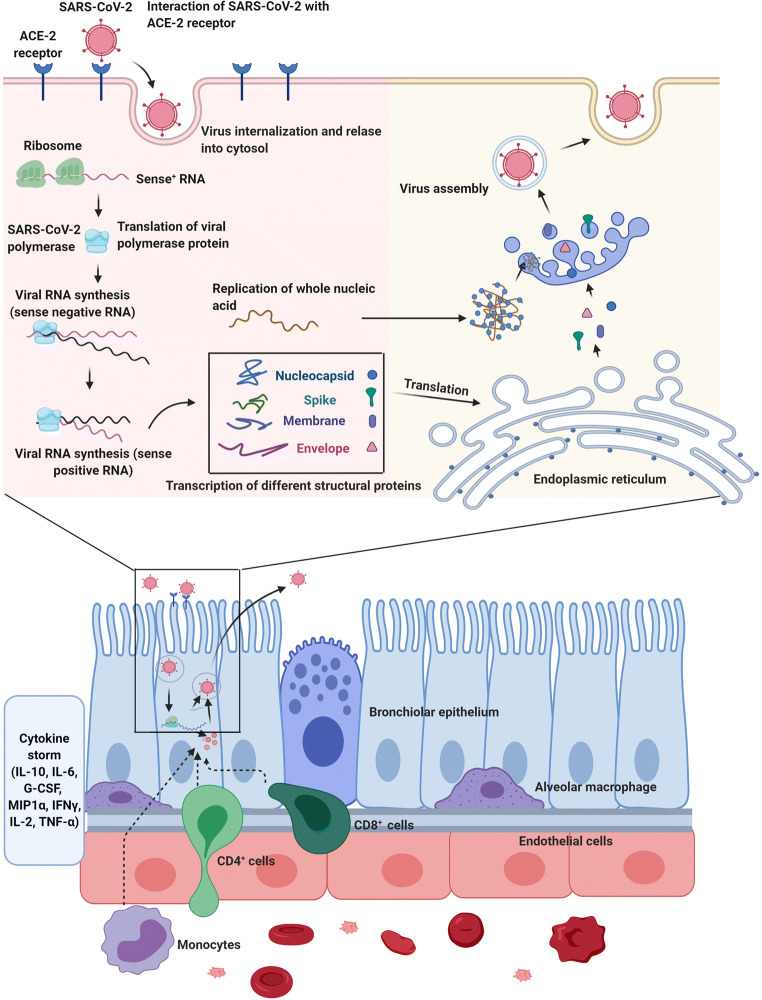
Fig. 2.
The proliferation of SARS-CoV-2 within the host cells initiates soon after attachment of S protein (S1 and S2) to the cell membrane-bound ACE-2 receptor. Allosteric changes in S protein promote viral envelope fusion with the cell membrane through endosomal signaling. Inside the cells, the genomic pool is released, transcribed, and translated to synthesize various components of viral structure. Finally, viral proteins and genome RNA are assembled into virions in the endoplasmic reticulum and Golgi apparatuses and transported into microvacuoles. In the next, step, the microvesicles containing virions are released. After infection of host cells with COVID-19 nanoparticles, the release of virions promotes pyroptosis and massive cellular damage. The neighboring cells such as endothelial cells, dust cells (alveolar macrophages) start to release an array of cytokines and chemokines. With the progression of cellular damage, blood lymphocytes (either T and B), as well as macrophages, are recruited to the site of infection. Accumulation of immune cells exacerbates the inflammatory responses by the continuous production of inflammatory cytokines. Interleukine 10: IL-10; Interleukine 6: IL-6; granulocyte colony-stimulating factor: G-CSF; Macrophage inflammatory protein 1α: MIP1α; Interferon-gamma: IFNγ; Interleukine 2: IL-2; Tumour necrosis factor-α: TNF-α. The illustration was created with BioRender.com
Antiviral Mechanisms of Stem Cells
As a common belief, stem cells are undifferentiated multipotential cells with the potential to commit toward multiple cell types. This capacity is referred to as trans-differentiation [39]. These cells commonly enter the asymmetrical division to produce a large number of the same cell phenotype to self-renew and maintain tissue homeostasis [40, 41]. The main question is whether the sensitivity of stem cells and other cell types varies against certain viral infections and there are distinct sets of antiviral responses and innate immunity in stem cells enabling them to reduce the risk of viral infection. If we hypothesize an equal probability between differentiated and undifferentiated cells of being infected by a certain virus, thus stem cell sources will be eliminated in the not too distant future The potential reasons why the prominent antiviral responses are integral to stem cells supported by evidence has been gathered from previously published studies [42]. Given the inability of viruses like myxoma virus, West Nile virus, and cytomegalovirus, to infect stem cells, one could propose that these cells are armed with rapid anti-viral clearance systems [42, 43]. Such defense shields allow stem cells to be in a constant state of health after exposure to different external stimuli (Fig. 3; Table 1) [44–46]. This notion also derives from the fact that stem cells can produce functional cells and are capable of repopulating injured cells under pathological conditions [47]. Of course, stem cells are variably permissive and susceptible to different viruses and favor diverse responses after infection. For instance, it has been shown that human hematopoietic stem cells could be infected with retroviruses and herpesviruses [48]. This process could lead to the selective loss of certain stem cell types in a distinct niche, showing relative resistance of different stem cells to certain viral infections. Preliminary evidence of human NSCs infected with cytomegalovirus was presented previously. Although NSCs were permissive for cytomegalovirus, the replication of the virus was prohibited at the levels of the immediate-early gene expression [43]. Monitoring the number of viral genomes in the infected NSCs using sensitive gene-analysis methods showed that the average viral genome copy number was diminished over time but was not eliminate, suggesting viral genome integration with the DNA [43]. Regarding the existence of a complete machinery system for viral replication in mature cells, this phenomenon can lead to the propagation of the virus from stem cells to the neighbor mature cells, showing that these cells might act as a reservoir for the cytomegalovirus. If we propose the lack of highly resistant mechanisms in stem cells to the viral infections, the reduction of stem cell pool per se decreases the regeneration potential of tissues and triggers subsequent aging and functional defects [49]. In this line, several intricate resistance mechanisms with prompt activity are mandatory to support host stem cells at the time of viral infections thus favors the stem cell resistance hypothesis [50, 51]. Yet, this capacity, which without a doubt is of interest in regenerative medicine should be determined by further investigations. Perhaps it should be noted that the existence of a unique genomic profile and proteomic machinery in stem cells potentiates these cells to maintain and restore normal physiological activity after viral infections [52]. In particular, it confirmed that the stem cells do not express specific receptors or limited areas of the cell membrane are coved with target receptors. These features limit direct contact and crosstalk between the viruses and stem cells [53, 54]. Mutual crosstalk between the different cellular constituents of tissues (stem cells and differentiated cells) has been shown in the local microenvironment namely niche [55]. Stem cells are in close contact, either paracrine or juxtacrine manner, with the neighboring cells to sense the external clues, exchange the biological information, and respond appropriately to the injuries [56, 57]. Even though, if we consider the existence of unique anti-viral resistance responses in stem cells, the infection of differentiated neighboring cells can, to a lesser extent, affect the normal function of stem cells [58]. Yet, the detrimental effects of viral infections on differentiated cells and the dynamic interaction of these cells with stem cells have not been elucidated completely. Commensurate with these descriptions, it would not be nonsense to say that antiviral resistance is vital to each cell type and is distinctively regulated between stem cells and differentiated cells [42, 59, 60]. Progress in our data about stem cell biology has highlighted the crucial impact of several anti-viral defense mechanisms in these cells once exposed to the viruses. In the below sections, we highlighted the putative anti-viral mechanisms used by the stem cells.
Fig. 3.
different intracellular mechanisms used by stem cells to inhibit the proliferation and expansion inside these cells. The illustration was created with BioRender.com
Table 1.
List of antiviral mechanisms in different stem cell types
| Stem Cell type | Effector | Mechanism of action | Ref |
|---|---|---|---|
| Mouse ESCs, PSCs, hiPSCs, TSCs, MSCs, NSCs, and PnSCs | RNA interference (RNAi) pathway | Viral RNA synthesis ↓ | [42, 61, 62] |
| Mouse ESCs, iPSCs, PSCs | Dicer-1 and Dicer-2 | miRNA biogenesis and siRNA biogenesis↑ | [63–65] |
| Human iPSCs, TSCs, mouse ESCs, iPSCs, | Argonaute (Ago) |
Formation of RNA-induced silencing complex (RISC) ↑ |
[42, 66] |
| Somatic stem cells, ESCs, TSCs, Skeletal stem cells, iPSCs, MSCs, NSCs, and PnSCs | Component 3 Promoter of RISC |
Activation of RISC ↑, Argonaute2 (Ago2)-associated RNAi↑ |
[62, 67] |
| iPSCs, MSCs, NSCs, and PnSCs | Ars2 and heat shock proteins | siRNA biogenesis↑, RNA-protein complexes ↑, Conformational changes during RISC loading↑ | [68–70] |
| SoSCs | piRNA | Antiviral defense↑ | [69, 71] |
| ESCs | RNase-III enzyme Dicer-2 | Recognition of cytoplasmic dsRNA↑ | [72, 73] |
| ESCs and respiratory epithelial cells | miRNA | miRNA-induced silencing complex (miRISC) attachment to target sites in the 3’ untranslated regions (UTR) of mRNAs↑, translational repression↑, de-adenylation↑, and mRNA decay↑ | [74–76] |
| NSCs | interferon-α/β receptor (IFNAR) | JAK-STAT pathway↑, ISGs↑ | [77–79] |
| Primary stem cells, NSC, Human ESCs | Interferon stimulated genes (ISGs) | Viral replication↓ Adaptive immune response↑, transcription of Mx1, and RIM5↓, translation of PKR, IFIT family members, OASL↓, RNA degradation and apoptosis (RNase L)↑ | [80, 81] |
| NSCs, MSCs, mouse ESCs | Type I IFNs | Chemokine release↑, Antigen presentation by innate immune cells↑, antibody production↑, and T cell responses↑ | [82, 83] |
| NSCs, MSCs, mouse ESCs | TLR3, RIG-I, and MDA5 | Recognition of viral dsRNA↑, IRF3↑, IRF7↑, and NF-kB ↑, IFN ↑ | [78, 83, 84] |
| HSCs, ESCs, iPSCs, germ layer cells | ISG12 | Cell death ↑, Cytochrome C release↑, Caspase activation↑ | [46, 85] |
| Mouse ESCs, HSCs, ESCs, iPSCs | OAS1 | Innate immune response to viral infection↑, RNase L activity ↑, Viral RNA degradation↑ | [46, 86–88] |
| iPSCs, ESCs, MSCs, NSCs derived from iPSCs | DNA sensors absent in melanoma 2 (AIM2) | Activation of the NLRP3 inflammasome↑, production of IL-1β↑, | [89–91] |
| ESCs, iPSCs | Protein kinase R (PKR) |
Virus translation↓, Protein phosphorylation↓, Innate immune responses↑ |
[92, 93] |
| Human ESCs, HLCs, multipotent germ layer cells, human hiPSCs, TSCs, HSCs, NSCs, MSCs | IFITM1, IFITM3, EIF3L, and BST2 | Replication of viruses↓ Cytosolic entry↓ | [94] |
| Human ESCs, HLCs, multipotent germ layer cells, human iPSCs, TSCs, HSCs, NSCs, MSCs | IFN Response and IFN pathway | Phosphorylation and nuclear import of IRF-3↑, Post-transcriptional processing of cellular antiviral pre-mRNAs↓ dsRNA binding properties↓, RNA processing↓, trafficking ↓, translational ↓ | [94, 95] |
| Bone marrow MSCs, HSCs, ESCs, iPSCs | Mitochondrial antiviral-signaling protein (MAVS) | Activation of NF-kB, IRF3 and IRF7 and ISGs↑ | [64, 96, 97] |
| HSCs, ESCs, iPSCs, germ layer cells. | IFIT family | Recognition of 5ʹ triphosphate ↑, Viral protein translation↓ | [46, 98–100] |
| Mouse ESCs, and human ESCs | Ribonuclease L (RNase L) |
Single-stranded RNA degradation in U-rich sequences↑, Antiviral innate Immunity↑ |
[101–103] |
| Human ESCs, HLCs, Multipotent germ layer cells, human iPSCs, TSCs, HSCs, NSCs, MSCs | Interferon regulatory factor 3 (IRF3) | Glial cytokine expression↑, pro-inflammatory cytokines ↓, Anti-inflammatory or immunoregulatory cytokines↑ | [46, 104] |
| Glioma stem cells | Interferon Regulatory Factor (IRF-7) | Antiviral responses ↑ and NF-κB expression↓ | [105, 106] |
| CySCs, Germline stem cells | JAK/STAT pathway | Upregulation of ISGs↑ | [107, 108] |
| HSCs, ESCs, iPSCs, germ layer cells. | Interferon-inducible transmembrane proteins (IFITMs) | Cytosolic entry↓ | [46, 109–111] |
| ESCs, HSC, multipotent adult stem cells, BMSCs, Skeletal stem cells, SoSCs | Bone marrow stromal antigen 2 (BST-2) | Inoculation site viral load↓, Viremia ↓, and lymphoid tissues tropism↑ | [112, 113] |
| Human ESCs, human hiPSCs, mouse ESCs | Suppressor of cytokine signaling 1 (SOCS1) | IFN signaling↓, phosphorylation of type I IFN receptor ↓ JAK kinase activity↓ phosphorylation of STAT1↓ | [114, 115] |
| NSCs, MSCs, ESCs | IFN-β | Virus entry↓, Transcription↓, Translation↓, Genome Replication↓, Assembly↓, and egress ↓ | [83, 116] |
| NSCs, MSCs, mouse ESCs | IFN-λ1 and -λ2 | Replication of virus ↓, Cytotoxic activity ↓, CC chemokine expression ↑, Viral entrance ↓ | [83, 117, 118] |
| ESCs, iPSCs, NSCs | NF-κB | NF-κB-LTRs attachment↑, Replication early during the viral life cycle↑ | [119–121] |
| Human ESCs, HLCs, multipotent germ layer cells, human iPSCs, TSCs, HSCs, NSCs, MSCs | RNA helicase MOV10 |
Retro-transposition and Interferon-stimulated genes ↓, Repression of ERVs beyond antiviral proteins↑ |
[46, 122, 123] |
| HSCs, CD34+ stem cells, ESCs, iPSCs | small interfering RNAs (siRNAs) | Sequence-specific defense against viruses and transposons, Bind to Argonaut protein↑ | [124–128] |
Bone marrow stromal cells: BMSCs; Hematopoietic stem cells; Hepatocyte-like cells: HLCs; Induced pluripotent stem cells: iPSCs; Mesenchymal stem cells; MSCs; Neural Stem Cells; NSCs; Pancreatic stem cells: PnSCs; Somatic cyst stem cells: CySCs; Somatic stem cells: SoSCs; Tissue stem cells: TSCs
Interferon Associated Signaling Pathways and Antiviral Activity in Stem Cells
Most of the cells use different mechanisms to prohibit the infection with various intracellular agents. IFN signaling cascade is the cellular defense in the frontline which is commonly engaged by most cell types [129, 130]. IFN signaling is activated against the dsRNA of different viral masses except for retroviruses. Immediately after attachment of the virions to cell membrane-bound receptors, such as TLR-3, MDA5, and RIG-I, the expression of downstream effectors, mainly IRF-3, -7, and NF-κB increased, leading to bulk production of IFNs [84]. In the next step, IFN is released from the target cells and alert cells in autocrine and paracrine manners [131]. Upon IFN binding to receptors, activation of the JAK/STAT pathway can lead to the expression of multiple genes commonly termed ISGs [78]. ISGs initiate diverse intracellular anti-viral mechanisms inhibiting the cellular machinery that regulate virus proliferation. Importantly, the stimulation of target cells with IFN activates specific proteins which in turn limit horizontal transmission of viruses through the cell membrane [132]. For instance, the IFITM protein, as innate effector proteins, restrict cell entry of enveloped viruses. Along with IFITM activity, another peptide so-called BST2 prohibits the evasion of virions from infected host cells to other cells [133]. At the genetic levels, IFN ignites the production of non-coding RNAs consisted of long non-coding RNAs, microRNAs, and circular RNAs [134].
Despite the occurrence of these molecular pathways inside most mature cells, it is thought that stem cells are exceptional to some extent concerning viral infection. Evidence points out the intensity and durability of IFN-related responses are different in stem cells than that of most mature cells. The data suggested the absence of IFN synthesis in stem cells after the exposure to active viral particles or incubation with poly I: C (a mimic of dsRNA) [135]. This apparent discrepancy could be explained by different factors. It should be suggested that strong tolerance and higher thresholds are seen in stem cells for viral infections and these cells could lower the concentration of cellular effectors which are critical for viral replication [43].
Based on a recent study, it has been shown that the absence of effective IFN-related responses in multipotent cells is linked to a lack of diverse signaling effectors [86, 136]. Unlike immortalized cells such as HeLa cells, human ESCs harbor lower contents of dsRNA biological sensors such as TLR3, MDA5, OAS1, and PKR [86]. Attempts to show the potential importance of stemness in restricting viral infection did reveal that stem cell commitment toward hepatic and neural lineages induces the production of dsRNA sensors such as MDA5 and OAS1, which provides essential elements for viral replication [115]. Of course, the potency of negative regulators in viral replication in different stem cells should not be neglected [115]. As described by Hong et al., the basal level of SOCS1, an inhibitor of the JAK/STAT pathway, is more in ESCs compared to most of the differentiated cells [115]. Unlike most cells, canonical IFN-associated responses have been determined in stem cells against viral infections. Although some controversial studies showed that ESCs and embryonic carcinoma cells could not produce type I IFN following exposure to viruses and displayed a very faint reaction against exogenous IFN [115, 135, 137]. These data show that the antiviral activity of stem cells is not completely dependent on canonical IFN signaling [115, 135, 137]. This apparent discrepancy may relate to the fact that tissue-dependent activity and the activation of quiescent stem cells could affect IFN-based anti-viral mechanisms [115, 135, 137]. Further investigations are highly demanded to address the ambiguity. Lin and co-workers confirmed the potency of human NSCs to synthesize IFN-β and -λ1 after incubation with dsRNA mimic and activation of ISGs [83]. All effectors like RIG-I, MDA5, and TLR3 are induced in human NSCs in the presence of dsRNA mimic and exogenous IFN-β [83]. In NSCs co-cultured with Zika, Japanese encephalitis viruses, RIG-I-associated IFN-β expression has been detected [83]. Hong and co-workers declared mouse and human ESCs, and human iPSCs exhibit interferon stimulation resistance [115]. However, the critical role of ISGs and their association with the cellular innate immune system remains unidentified [138, 139]. Calling attention, a limited subset of ISGs is produced in human, chimpanzee, and mouse ESCs, iPSCs, and adult stem cells [46, 140]. The expression profiling of ISGs varies between the different stem cell types. However, the basal levels of the ISCs are diminished during commitment toward mature cells and maintain at the minimum levels. It was postulated that some ISG products have a close association with stem cell fate [141, 142]. Hence, the mature and differentiated cells respond differently to the IFN [143]. Similar to other stem cells, MSCs could respond to viral infections by the alteration of ISGs in two ways. Upon exposure of MSCs to virions, the levels of ISGs increase which can lead to exogenous IFN in other cells. Therefore, MSCs display both intrinsic and inducible ISG-associated antiviral activities [144]. These data likely demonstrate that local and systemic application of MSCs contributes to therapeutic outcomes in COVID-19 patients through the alteration of anti-viral mechanisms in other cells. Compared to mature cells, IFITM family members are abundantly seen on the stem cell surface [145, 146]. Of note, the suppression of IFITM1, 2, and 3 in ESCs sensitizes these cells to viral infection, suggesting the anti-viral activity of IFITMs [145, 146]. The activation of ISGs could provoke other anti-viral mechanisms, such as BST2, MOV10, and IDO1, in the human and mouse stem cells. These elements bind directly to retroviruses. Similarly, enhanced ISGs activity with the increase of BST2, MOV10, and IDO1 factors could suppress the activity of endogenous retroviruses [122, 123].
Antiviral Activity of RNA Interference in Stem Cells
RNAi is a biological phenomenon by which specific genes are silenced by using nucleic acids consisted of 20–30 nucleotides [62, 127, 147]. It has been shown that RNAi has a unique antiviral activity [127]. Upon viral infection, a cytoplasmic RNase, namely Dicer, hydrolyzes dsRNA, and produce a large set of siRNAs [127]. These siRNAs are not free inside the cytosol thus captured by Ago. The combination of siRNAs-Ago with other proteins forms a multi-protein complex namely RISC. After maturation of the RISC structure, siRNAs are degraded according to their sequences [74]. It was postulated that Ago and RNAi need each other for maximum activities. For example, Schuster and colleagues discovered a less potent anti-viral activity of RNAi against + sRNA viruses like yellow fever virus, and encephalomyocarditis virus in Argonaute-2−/− human cells [62]. Certain viruses circumvent these innate antiviral responses in different ways. For instance, Coxsackievirus B3 harbors a viral 3A protein complex with a virus-encoded suppressor of RNAi to inhibit the activity of eukaryotes RNAi [148]. Even, transfection of cells with mature siRNAs to stimulate RNAi does not contribute to the synthesis of long dsRNA [149]. Compared to differentiated cells, progenitors and ESCs have the potential to produce long dsRNAs [150, 151]. On this basis, ESCs have distinct siRNAs reservoirs that are not detectable in most cells. It is thought that these siRNAs are originated from dsRNAs produced by endogenous retrotransposons activity [42, 152, 153]. This capacity enables stem cells to acquire unique anti-viral defense mechanisms which are largely due to the modulation of RNAi. Transfection of murine ESCs with EMCV produced intracellular EMCV-derived siRNAs with a certain size and 3’ overhangs nucleotides [66, 95]. It seems that the simultaneous degradation of RNAi and the promotion of IFN-related responses could overlap once anti-viral immunity is initiated [154, 155]. The suppression of MAVS in mouse embryonic fibroblasts aborted the functionality and collaboration of dsRNA sensors with IFN products [96]. Unlike mature cells, stem cells commonly use different anti-viral pathways once infected with viruses to alert other cells and to regulate their activity. For instance, the initiation of IFN response in HepSCs, activates cytotoxic T cells, NK cells, and dendritic cells and prevents viral infections in most cells [156]. The existence of endogenous retroviral infection in stem cells is another mechanism by which these cells could decrease the viral entry [140]. This pattern suggests that stem cells limit the integration of exogenous viral genome with DNA by the occupation of common integration sites [140]. Studies have shown that the activation of HERVK endogenous retroviruses in ESCs produce Rec, an RNA transport factor, and activates IFITM1, leading to restricted viral replication [140]. This strategy will work when common integration sites are pre-occupied by the endogenous viral genome or the same replication machinery systems exploited.
TLRs Have Antiviral Activity in Stem Cells
The TLR signaling pathway plays a fundamental role in most cells during inflammation. TLRs belong to the type 1 integral proteins and are stimulated by different PAMPs, for example, proteins, lipids, nucleic acids, lipoproteins, etc. [157]. Different subsets of TLRs including TLR1, 2, 4, 5, 6, and 11 are located at cell membranes while other family members such as TLR3, 7, 8, 9 are associated with organelles like lysosomes, endosomes, and reticular endothelium (Fig. 4) [158]. Like mature cells, stem cells could express different classes of TLRs [159]. The activation of TLRs could increase the synthesis of different pro-inflammatory cytokines and regulate paracrine activity in stem cells via the modulation of the NF-ƙB/IKB/TRIF/MYD88 pathway, leading to the inhibition of apoptotic changes [160, 161]. Among TLRs, endosomal TRLs such as TLR3, 7, and 8 are recognized with the potential to detect and bind to viral ssRNA and dsRNA (Fig. 4) [162]. Control of inflammatory responses is an efficient strategy that allows the stem cells to escape from apoptosis [163]. As described previously, the apoptosis signaling pathway is commonly induced in the host cells to limit the spread of the virus to juxtaposed cells [164]. Concerning certain antiviral properties and crucial importance for stem cell survival, it appears that apoptosis is differently regulated in these cells [165]. Unlike differentiated cells, stem cells are highly resistant to apoptosis [165]. In other words, a greater degree of resistance to apoptotic changes makes stem cells efficiently eliminate viral particles by using inflammatory mechanisms that are not well-tolerated by differentiated cells. Whether apoptosis is involved in viral clearance in stem cells has been the subject of debate.
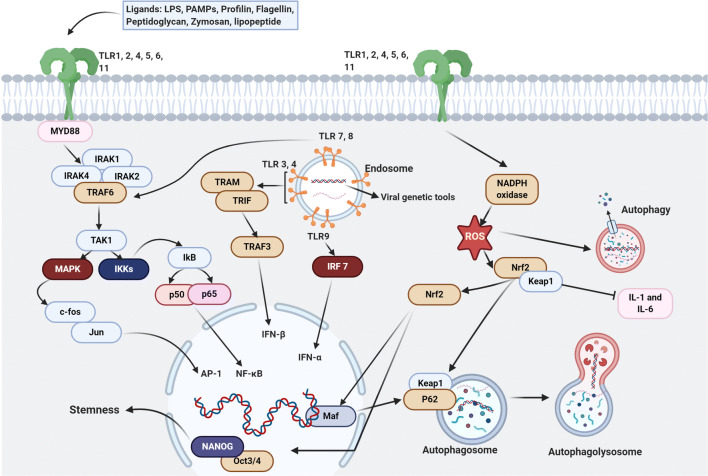
Fig. 4.
Cross-talk between TLRs and NrF2 signaling pathways in the viral infection. Endosomal TLRs including TLR 3,4,7,8,9 recognize the viral ssRNA and dsRNA. Stimulation of TLR7 by viral RNAs causes the production of NADPH oxidase which is an imperative factor in the connection of two signaling pathways and in results activation of NrF2 downstream pathways. Additionally, activation of TLR3 leads to the production of other anti-oxidant elements related to NrF2 pathways such as HO-1, which participates in the activation process of stress response transcription factors including NrF2, NF-KB, and AP-1. TLR 3 and 7 also appreciate the initiation of autophagy which can deliver the nucleic acid fragments of viruses to the endosomal TLRs and leads them to degradation by recruiting autolysosomes. On the other hand, NrF2 signaling pathways are in a relationship with the stemness of stem cells by inhibiting the activation of OCT4 and NANOG proteins by using ubiquitin/proteasome. The illustration was created with BioRender.com
Among different factors triggered after stimulation of TLRs, the Nrf2 signaling pathway plays a pivotal role in the regulation of inflammatory [166]. Upon activation of TLRs in stem cells, both NF-ƙB and Nrf2 factors are recruited [167, 168]. When activated, Nrf2 regulates the activity of antioxidant enzymes, cytoprotective properties against endogenous and exogenous stresses, and autophagolysosome formation (Fig. 4) [169, 170]. In normal conditions, Nrf2 is bounded to actin-associated Keap1 protein inside the cytosol to keep Nrf2 away from proteasomal degradation following ubiquitination [171]. Under conditions like oxidative stress, the NrF2-keap1 complex is degraded coincides with markedly decreased E3 ligase activity of Keap1. Downstream signaling events lead to NrF2 phosphorylation by protein kinase C activity and translocation into the nucleus [172, 173].
Pluripotency factors such as OCT4 and NANOG are highly ubiquitinated in stem cells and co-localized with Nrf2 to maintain stemness feature [172, 173]. The heterodimerization of NrF2 with Maf proteins in the nucleus produces anti-oxidant elements [174]. Besides, the activation of endosomal TLRs such as TLR3, 7, 8, and 9 promotes the formation of Nrf2-keap1 and prominent anti-oxidant capacity in stem cells [174, 175]. It has been shown that the stimulation of TLR7 by Resiquimod leads to NADPH oxidase activation and promotion of the Nrf2 signaling pathway [176]. Similar to these changes, the up-regulation of TLR3 could also promote Nrf2 anti-oxidant activity via the regulation of HO-1 [177]. HO-1 is touted as key transcriptional factors of stress responses including Nrf2, NF-ƙB, and plasminogen activator inhibitor-1 [178]. Owing to the distinct activity of the Nrf2 in stem cells and its association with TLRs [179], it seems that the promotion of inflammatory response and Nrf2 activity is integral to antiviral defense in these cells.
Like a close association of the Nrf2 signaling pathway with diverse cellular activities, Nrf2 could also activate autophagy and proteasomal activity during pathological conditions [179, 180]. To this end, Nrf2 controls the biogenesis of the 20S proteasome via the modulation of chaperones activity [179, 180]. The was recently shown that Nrf2 regulates proteasome activity in ESCs via the action of the proteasome maturation protein [172]. Upon viral entry, the close crosstalk between autophagy and TLR signaling pathway potentiates the cells to deliver the genomic fragments of viruses to endosomal TLRs and activates antiviral innate immune response (Fig. 4) [181]. Interestingly, the inhibition of MYD88 and TRIF, belonging to the TLR signaling pathway, prevents autophagic response in stem cells [182]. Besides, autophagy machinery digest several viral components by the formation of autolysosomes [183]. The activation of TLR3 and 7 promotes autophagic responses once ssRNA and dsRNA are released inside the host cells [182]. Upon activation of TLR3 and 7, the formation of the Beclin-1-Vsp34 complex initiates consecutive autophagic reactions [182]. The recruitment of selective autophagy receptors such as NBR, NDP52, p62 helps cells to kill intracellular virions [184]. These autophagy receptors possess capture domains like LC3-interacting regions which direct viral components to autophagosomes [185]. For example, the capsid of Sindbis, as RNA positive virus, is captured by a p62-dependent manner without using the ubiquitin system [185]. The exact mechanisms of the autophagy machinery system have been remained unclear. Overall, TLRs could have an axis role in the antiviral mechanisms of stem cells. These properties of stem cells might be a shed of light to use them in the field of stem cell therapy for the rest of human life especially for COVID-19 which has been spread globally.
The Antiviral Activity of Stem Cell Via EVs
The shedding of viral particles using EVs such as exosomes is an alternative defensive strategy in stem cells to eliminate virions [186]. Exosomes are nanosize vesicles ranging from 40 to 200 nm with the ability to carry the arrays of genomic and proteomic elements out of the cells. These particles maintain reciprocal paracrine crosstalk between the different cells [187]. It has been shown that the activation of exocytosis mechanisms is a compensatory mechanism in virus-infected cells [186]. EVs have some structural similarities to viruses. Due to the existence of inherent similarities between the EVs and viruses, in particular, small size dimensions, it is logical to propose that viruses and EVs use common mechanisms for intracellular trafficking, cell entry, and exocytosis (Fig. 5) [188, 189]. Upon the replication of viral particles inside the host cells, EVs act as delivery vectors. EVs collaborate with virus assembly machinery to pack viral-derived nucleic acids, lipids, proteins, and lipids, and take them to out of the cells [190]. Concerning similarities in the mechanism of EVs and viruses biogenesis, sophisticated manipulations could lead to the control of virus replication or vice versa. Notably, exosomes isolated from virus-infected cells harbor genetic elements which further inhibit the propagation of the virus in neighboring cells. For instance, studies have shown that up-regulation of let-7f, miR-145, miR-199a, and miR-221 in exosomes from umbilical MSCs can inhibit the replication of the Hepatitis C virus in the other cells. These genetic tools enter the acceptor cells and inhibit the propagation of virions after direct binding to viral genomes via targeting host factor insulin-like growth factor 2 mRNA-binding protein 1 [191, 192]. Of course, the shedding of viral particles from stem cells could decrease the intracellular accumulation of viral bodies meanwhile increases the risk of viral infection in neighboring cells. Based on recent studies, it has been shown that some exosomal microRNAs could regulate the specific genes correlated with SARS-CoV-2 RNA replication [193, 194]. The existence of miR-23b in bone marrow MSCs exosomes could inhibit ORF8 protein and limit the interspecies transmission of the SARS-CoV-2 virus [193, 194]. Besides, exosomal miR-1246 has the potential to regulates angiogenesis by the activation of the Smad 1/5/8 signaling cascade. This miRNA also controls ORF3a, a sodium or calcium ion channel protein, and thus the replication of the virus is diminished [195, 196].
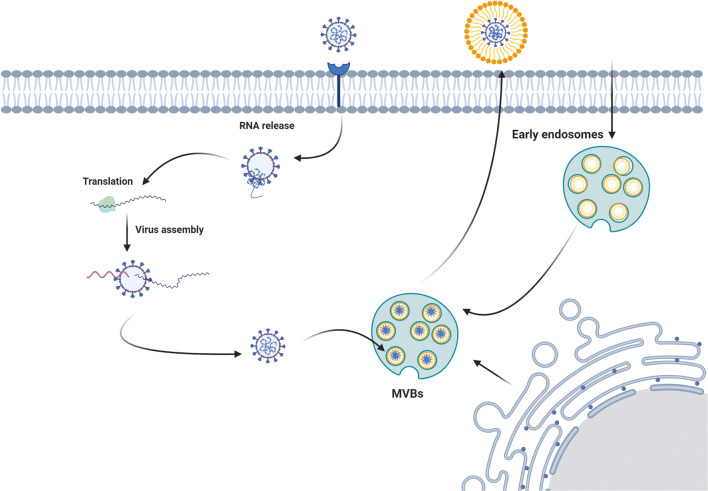
Fig. 5.
The similarity in exosome biogenesis and virus assembly system makes virus to use exosome biogenesis pathways for delivery and cell exit. The illustration was created with BioRender.com
The hemagglutinin-esterase, a glycoprotein, is located on a viral envelope that facilitates reversible attachment to O-acetylated sialic acids via lectin-like activity [197]. This enzyme exists in both Influenza and coronaviruses like the SARS-CoV-2 virus. It was suggested that MSCs exosomes could inhibit the hemagglutination activity of influenza viruses and decrease viral entry to most cells [198]. Taken together, the existence of exocytosis in stem cells helps these cells to decrease the load of virions inside cytosol and to use exocytosis-related mechanisms in neighboring cells to inhibit the transfection rate.
Conclusions
Commensurate with the above-mentioned comments, it is logical to mention that stem cells are less sensitive to coronaviruses such as SARS-CoV-2, unlike differentiated cells. This concept is not absolute and caution must be considered when we talk about the SARS-CoV-2 resistance of stem cells. There is close crosstalk between the stem cells and different cells within different organs to maintain basal stem cell function. Therefore, it seems that the occurrence and duration of COVID-19 and injury of mature cells could interrupt reciprocal crosstalk between undifferentiated and differentiated cells which further affects the supportive role of other cells on stem cells. Concerning the existence of various escaping ways in stem cells to adopt a virus-resistant state, it is less likely these cells become infected with the coronaviruses in the early stages. If we hypothesize that they are eventually will be infected, they are not a front-line cell target.
Acknowledgements
All authors would thank the Stem Cell Research Center staff for guidance and help. This study was supported by a grant from Tabriz University of Medical Sciences.
Abbreviations
- OAS1
2’-5’-oligoadenylate synthetase 1
- ACE2
angiotensin-converting enzyme 2
- Ago
Argonaute
- BST2
Bone marrow stromal antigen 2
- dsRNA
Double-stranded RNA
- EMCV
Encephalomyocarditis virus
- EVs
Extracellular vesicles
- HepSCs
Hepatic stem cells
- HO-1
Heme oxygenase-1
- ESCs
Human embryonic stem cells
- iPSCs
Induced pluripotent stem cells
- IFN
Interferon
- IRF-3, -7
interferon regulatory transcription factor-3, 7
- IFITM
Interferon-induced transmembrane
- ISGs
Interferon-stimulated genes
- IL-6
Interleukin-6
- Mpro
Main protease
- MDA5
Melanoma differentiation-associated protein 5
- MAVS
Mitochondrial antiviral-signaling components
- NK cells
Natural killer cells
- NBR
NBR Neighbor of BRCA1
- NDP52
Nuclear dot protein
- NSCs
Neural stem cells
- Nrf2
Nuclear factor erythroid 2-related factor 2
- MSCs
Mesenchymal stem cells
- NF-κB
Nuclear factor kappa B
- ORF
Open reading frame
- PAMP
Pathogen-associated molecular patterns
- PKR
Protein kinase R
- RIG-I
Retinoic acid-inducible gene I
- RNAi
RNA interference
- RISC
RNA-induced silencing complex
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus-2
- +ssRNA
Single-strand positive-sense RNA
- siRNAs
Small interfering RNAs
- sgRNAs
Subgenomic RNAs
- SOCS1
Suppressor of cytokine signaling 1
- TLR
Toll-like receptor
Author Contributions
H.S.B., M.K., M.H. and H. R. collected data, prepared draft and wrote the manuscript. E.S., and R.R. edited and supervised the study.
Data Availability
Not applicable.
Compliance with Ethical Standards
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
All authors declare there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Emel Sokullu and Reza Rahbarghazi contributed equally to this work.
Contributor Information
Emel Sokullu, Email: esokullu@ku.edu.tr, Email: emelsu@gmail.com.
Reza Rahbarghazi, Email: rezarahbardvm@gmail.com, Email: rahbarghazir@tbzmed.ac.ir.
References
- 1.Li H, Liu S-M, Yu X-H, Tang S-L, Tang C-K. Coronavirus disease 2019 (COVID-19): current status and future perspective. International Journal of Antimicrobial Agents. 2020;55:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singhal, T. (2020). A review of coronavirus disease-2019 (COVID-19). The Indian Journal of Pediatrics, 87, 1–6. [DOI] [PMC free article] [PubMed]
- 3.Rezabakhsh A, Ala A, Khodaei SH. Novel Coronavirus (COVID-19): A new emerging pandemic threat. Journal of Research in Clinical Medicine. 2020;8(1):5. [Google Scholar]
- 4.Team, E. E. (2020). Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Eurosurveillance, 25(10). [DOI] [PMC free article] [PubMed]
- 5.McIntosh, K. (2020). Coronavirus disease 2019 (COVID-19). Disponível em: https://www.uptodatecom/contents/coronavirus-disease-2019-covid-19-epidemiology-virology-clinical-features-diagnosis-and-prevention. Accessed 30 Dec 2020.
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Schaffhausen CR, Bruin MJ, McKinney WT, Snyder JJ, Matas AJ, Kasiske BL, Israni AK. How patients choose kidney transplant centers: A qualitative study of patient experiences. Clinical Transplantation. 2019;33(5):e13523. doi: 10.1111/ctr.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clinical Immunology. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esquivel D, Mishra R, Soni P, Seetharaman R, Mahmood A, Srivastava A. Stem cells therapy as a possible therapeutic option in treating COVID-19 patients. Stem Cell Reviews and Reports. 2020 doi: 10.1007/s12015-020-10017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mousavizadeh, L., & Ghasemi, S. (2020). Genotype and phenotype of COVID-19: Their roles in pathogenesis. Immunology: Journal of Microbiology. 10.1016/j.jmii.2020.03.022 . [DOI] [PMC free article] [PubMed]
- 11.Hoffmann, M., Kleine-Weber, H., Krüger, N., Mueller, M. A., Drosten, C., & Pöhlmann, S. (2020). The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 10.1101/2020.01.31.929042.
- 12.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science. 2020;12(1):1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomar S, Mahajan S, Kumar R. Genomics and biotechnological advances in veterinary, poultry, and fisheries. Amsterdam: Elsevier; 2020. Advances in structure-assisted antiviral discovery for animal viral diseases; pp. 435–468. [Google Scholar]
- 14.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. Journal of Medical Virology. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sah R, Rodriguez-Morales AJ, Jha R, Chu DK, Gu H, Peiris M, Bastola A, Lal BK, Ojha HC, Rabaan AA. Complete genome sequence of a 2019 novel coronavirus (SARS-CoV-2) strain isolated in Nepal. Microbiology Resource Announcements. 2020;9(11):e00169–20. doi: 10.1128/MRA.00169-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Current Biology. 2020;30(8):1578. doi: 10.1016/j.cub.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Organization, W. H. (2020) Coronavirus disease 2019 (COVID-19): situation report, 72.
- 18.Nelemans T, Kikkert M. Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses. 2019;11(10):961. doi: 10.3390/v11100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehr AR, Perlman S. Coronaviruses. Berlin: Springer; 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa, K., Lokugamage, K., & Makino, S. (2016). Viral and cellular mRNA translation in coronavirus-infected cells (Vol. 96, pp. 165–192). Amsterdam: Elsevier. [DOI] [PMC free article] [PubMed]
- 21.Grdzelishvili VZ, Garcia-Ruiz H, Watanabe T, Ahlquist P. Mutual interference between genomic RNA replication and subgenomic mRNA transcription in brome mosaic virus. Journal of Virology. 2005;79(3):1438–1451. doi: 10.1128/JVI.79.3.1438-1451.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual, M. R. (2020). Coronavirus SARS-CoV-2: Analysis of subgenomic mRNA transcription, 3CLpro and PL2pro protease cleavage sites and protein synthesis. arXiv preprint arXiv:200400746.
- 23.Sawicki SG, Sawicki DL, Siddell SG. A contemporary view of coronavirus transcription. Journal of Virology. 2007;81(1):20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nature Reviews Microbiology. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng F, Cui T, Gao Q, Guo L, Zhou Q, Li W. Artificial sgRNAs engineered for genome editing with new Cas12b orthologs. Cell Discovery. 2019;5(1):1–4. doi: 10.1038/s41421-019-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yunyan F, Jie Y, Fangquan W, Fangjun F, Wenqi L, Jun W, Yang X, Jinyan Z, Weigong Z. Production of two elite glutinous rice varieties by editing wx gene. Rice Science. 2019;26(2):118–124. [Google Scholar]
- 27.Mu W, Zhang Y, Xue X, Liu L, Wei X, Wang H. 5′ capped and 3′ polyA-tailed sgRNAs enhance the efficiency of CRISPR-Cas9 system. Protein & Cell. 2019;10(3):223–228. doi: 10.1007/s13238-018-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral Research. 2018;149:58–74. doi: 10.1016/j.antiviral.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathogens. 2018;14(8):e1007236. doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Yang C, Xu X, Xu W, Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil C, Ginex T, Maestro I, Nozal V, Barrado-Gil L, Cuesta-Geijo M, Urquiza J, Ramírez D, Alonso C, Campillo NE, Martinez A. COVID-19: drug targets and potential treatments. Journal of Medicinal Chemistry. 2020;63(21):12359–12386. doi: 10.1021/acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 32.Biswas A, Bhattacharjee U, Chakrabarti AK, Tewari DN, Banu H, Dutta S. Emergence of Novel Coronavirus and COVID-19: whether to stay or die out? Critical Reviews in Microbiology. 2020;46(2):182–193. doi: 10.1080/1040841X.2020.1739001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Zhou Y, Yang Z. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cellular & Molecular Immunology. 2016;13(1):3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xi-zhi, J. G., & Thomas P. G. (2017). New fronts emerge in the influenza cytokine storm. In: Seminars in immunopathology (Vol. 5, pp. 541–550). Berlin: Springer. [DOI] [PMC free article] [PubMed]
- 36.Wang J, Duan Y, Sluijter JP, Xiao J. Lymphocytic subsets play distinct roles in heart diseases. Theranostics. 2019;9(14):4030. doi: 10.7150/thno.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda, K., Malykhin, A., Teague-Weber, B. N., Sun, X.-H., Farris, A. D., & Coggeshall, K. M. (2009). Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus–prone B6. Sle1. Yaa animalsBlood, The Journal of the American Society of Hematology,113(19), 4534–4540. [DOI] [PMC free article] [PubMed]
- 38.Mukaino M, Nakamura M, Yamada O, Okada S, Morikawa S, Renault-Mihara F, Iwanami A, Ikegami T, Ohsugi Y, Tsuji O. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Experimental Neurology. 2010;224(2):403–414. doi: 10.1016/j.expneurol.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. Journal of Cellular and Molecular Medicine. 2004;8(3):301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita YM. Regulation of asymmetric stem cell division: spindle orientation and the centrosome. Frontiers in Bioscience: A Journal and Virtual Library. 2009;14:3003. doi: 10.2741/3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoltz J-F, De Isla N, Li Y, Bensoussan D, Zhang L, Huselstein C, Chen Y, Decot V, Magdalou J, Li N. Stem cells and regenerative medicine: myth or reality of the 21th century. Stem Cells International. 2015;2015:734731. doi: 10.1155/2015/734731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X, Kwong AC, Rice CM. Antiviral resistance of stem cells. Current Opinion in Immunology. 2019;56:50–59. doi: 10.1016/j.coi.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belzile J-P, Stark TJ, Yeo GW, Spector DH. Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. Journal of Virology. 2014;88(8):4021–4039. doi: 10.1128/jvi.03492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015;29(4):776–782. doi: 10.1038/leu.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Research & Therapy. 2019;10(1):1–22. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X, Thi VLD, Huang Y, Billerbeck E, Saha D, Hoffmann H-H, Wang Y, Silva LAV, Sarbanes S, Sun T. Intrinsic immunity shapes viral resistance of stem cells. Cell. 2018;172(3):423–438. doi: 10.1016/j.cell.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnsdorf EJ, Jones LM, Carter DR, Jacobs CR. The periosteum as a cellular source for functional tissue engineering. Tissue Engineering. Part A. 2009;15(9):2637–2642. doi: 10.1089/ten.tea.2008.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell J, Bixby D, Savona MR, Collins KL. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nature Medicine. 2010;16(4):446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yun MH. Changes in regenerative capacity through lifespan. International Journal of Molecular Sciences. 2015;16(10):25392–25432. doi: 10.3390/ijms161025392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yilmaz NK, Swanstrom R, Schiffer CA. Improving viral protease inhibitors to counter drug resistance. Trends in Microbiology. 2016;24(7):547–557. doi: 10.1016/j.tim.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Koo B-K, Yoon K-J. Modeling host-virus interactions in viral infectious diseases using stem-cell-derived systems and CRISPR/Cas9 technology. Viruses. 2019;11(2):124. doi: 10.3390/v11020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedli M, Turelli P, Kapopoulou A, Rauwel B, Castro-Díaz N, Rowe HM, Ecco G, Unzu C, Planet E, Lombardo A, Mangeat B, Wildhaber BE, Naldini L, Trono D. Loss of transcriptional control over endogenous retroelements during reprogramming to pluripotency. Genome Research. 2014;24(8):1251–1259. doi: 10.1101/gr.172809.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung S-L, Lee P-L, Chen H-W, Chen L-K, Kao C-L, King C-C. Analysis of the steps involved in dengue virus entry into host cells. Virology. 1999;257(1):156–167. doi: 10.1006/viro.1999.9633. [DOI] [PubMed] [Google Scholar]
- 54.Llorente-García, I., & Marsh, M. (2019) A biophysical perspective on receptor-mediated virus entry with a focus on HIV. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1862(6), 183158. [DOI] [PMC free article] [PubMed]
- 55.Zhao R, Xi R. Stem cell competition for niche occupancy: emerging themes and mechanisms. Stem Cell Reviews and Reports. 2010;6(3):345–350. doi: 10.1007/s12015-010-9128-3. [DOI] [PubMed] [Google Scholar]
- 56.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochimica et Biophysica Acta (BBA)-General Subjects. 2014;1840(8):2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nature Reviews Molecular Cell Biology. 2017;18(12):728. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Wu Q, Sun X, Shen J, Chen H. Organoids as a powerful model for respiratory diseases. Stem Cells International. 2020;2020:5847876. doi: 10.1155/2020/5847876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komarova, N. L., Weiss, L. D., Van Den Driessche, P., Lowengrub, J. S., & Wodarz, D. (2019) Determinants of stem cell enrichment in healthy tissues and tumors: implications for non-genetic drug resistance. BioRxiv, 663146.
- 60.Larribère L, Utikal J. Stem cell-derived models of neural crest are essential to understand melanoma progression and therapy resistance. Frontiers in Molecular Neuroscience. 2019;12:111. doi: 10.3389/fnmol.2019.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes & Development. 2005;19(4):489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schuster S, Miesen P, van Rij RP. Antiviral RNAi in insects and mammals: parallels and differences. Viruses. 2019;11(5):448. doi: 10.3390/v11050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witteveldt J, Knol LI, Macias S. MicroRNA-deficient mouse embryonic stem cells acquire a functional interferon response. Elife. 2019;8:e44171. doi: 10.7554/eLife.44171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurung C, Sapkota K, Huang F, Guo Y-L. Dicer as a repressor of antiviral responses in mouse embryonic stem cells. The FASEB Journal. 2020;34(S1):1–1. [Google Scholar]
- 66.Maillard P, Ciaudo C, Marchais A, Li Y, Jay F, Ding S, Voinnet O. Antiviral RNA interference in mammalian cells. Science. 2013;342(6155):235–238. doi: 10.1126/science.1241930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Tan H, Tian H, Liang C, Chen S, Liu Q. Autoantigen La promotes efficient RNAi, antiviral response, and transposon silencing by facilitating multiple-turnover RISC catalysis. Molecular Cell. 2011;44(3):502–508. doi: 10.1016/j.molcel.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marques JT, Kim K, Wu P-H, Alleyne TM, Jafari N, Carthew RW. Loqs and R2D2 act sequentially in the siRNA pathway in Drosophila. Nature Structural & Molecular Biology. 2010;17(1):24. doi: 10.1038/nsmb.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyoshi T, Takeuchi A, Siomi H, Siomi MC. A direct role for Hsp90 in pre-RISC formation in Drosophila. Nature Structural & Molecular Biology. 2010;17(8):1024–1026. doi: 10.1038/nsmb.1875. [DOI] [PubMed] [Google Scholar]
- 70.Fan, G.-C. (2012) Role of heat shock proteins in stem cell behavior. In: Progress in molecular biology and translational science (Vol. 111, pp. 305–322). Amsterdam: Elsevier. [DOI] [PMC free article] [PubMed]
- 71.Miesen P, Joosten J, van Rij RP. PIWIs go viral: arbovirus-derived piRNAs in vector mosquitoes. PLoS Pathogens. 2016;12(12):e1006017. doi: 10.1371/journal.ppat.1006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 73.Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118(1):57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 74.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nature Reviews Molecular Cell Biology. 2013;14(8):475–488. doi: 10.1038/nrm3611. [DOI] [PubMed] [Google Scholar]
- 76.Peng S, Wang J, Wei S, Li C, Zhou K, Hu J, Ye X, Yan J, Liu W, Gao GF. Endogenous cellular microRNAs mediate antiviral defense against influenza A virus. Molecular Therapy-Nucleic Acids. 2018;10:361–375. doi: 10.1016/j.omtn.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goubau D, Deddouche S, e Sousa CR. Cytosolic sensing of viruses. Immunity. 2013;38(5):855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annual Review of Immunology. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reder AT, Feng X. How type I interferons work in multiple sclerosis and other diseases: some unexpected mechanisms. Journal of Interferon & Cytokine Research. 2014;34(8):589–599. doi: 10.1089/jir.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Current Opinion in Virology. 2011;1(6):519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li G, Xiang Y, Sabapathy K, Silverman RH. An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and c-Jun NH2-terminal kinase. Journal of Biological Chemistry. 2004;279(2):1123–1131. doi: 10.1074/jbc.M305893200. [DOI] [PubMed] [Google Scholar]
- 82.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nature Reviews Immunology. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin J-Y, Kuo R-L, Huang H-I. Activation of type I interferon antiviral response in human neural stem cells. Stem Cell Research & Therapy. 2019;10(1):1–17. doi: 10.1186/s13287-019-1521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annual Review of Immunology. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 85.Chawla-Sarkar M, Lindner DJ, Liu Y-F, Williams B, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8(3):237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 86.Chen L-L, Yang L, Carmichael G. Molecular basis for an attenuated cytoplasmic dsRNA response in human embryonic stem cells. Cell Cycle. 2010;9(17):3552–3564. doi: 10.4161/cc.9.17.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clemens MJ, Williams BR. Inhibition of cell-free protein synthesis by pppA2′ p5′ A2′ p5′ A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- 88.Hoenen A, Liu W, Kochs G, Khromykh AA, Mackenzie JM. West Nile virus-induced cytoplasmic membrane structures provide partial protection against the interferon-induced antiviral MxA protein. Journal of General Virology. 2007;88(11):3013–3017. doi: 10.1099/vir.0.83125-0. [DOI] [PubMed] [Google Scholar]
- 89.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 90.Mehle A, Doudna JA. A host of factors regulating influenza virus replication. Viruses. 2010;2(2):566–573. doi: 10.3390/v2020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen G-Y, Hwang S-M, Su H-J, Kuo C-Y, Luo W-Y, Lo K-W, Huang C-C, Chen C-L, Yu S-H, Hu Y-C. Defective antiviral responses of induced pluripotent stem cells to baculoviral vector transduction. Journal of Virology. 2012;86(15):8041–8049. doi: 10.1128/JVI.00808-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pindel A, Sadler A. The role of protein kinase R in the interferon response. Journal of Interferon & Cytokine Research. 2011;31(1):59–70. doi: 10.1089/jir.2010.0099. [DOI] [PubMed] [Google Scholar]
- 93.Guo YL. Utilization of different anti-viral mechanisms by mammalian embryonic stem cells and differentiated cells. Immunology and Cell Biology. 2017;95(1):17–23. doi: 10.1038/icb.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strick-Marchand H, Durantel D. Who defends the stem cell’s citadel? Cell Stem Cell. 2018;22(3):287–289. doi: 10.1016/j.stem.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 95.Qiu Y, Xu Y, Zhang Y, Zhou H, Deng Y-Q, Li X-F, Miao M, Zhang Q, Zhong B, Hu Y. Human virus-derived small RNAs can confer antiviral immunity in mammals. Immunity. 2017;46(6):992–1004. doi: 10.1016/j.immuni.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Maillard PV, Van der Veen AG, Deddouche-Grass S, Rogers NC, Merits A, e Sousa CR. Inactivation of the type I interferon pathway reveals long double‐stranded RNA‐mediated RNA interference in mammalian cells. The EMBO Journal. 2016;35(23):2505–2518. doi: 10.15252/embj.201695086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao L, Bird AK, Meednu N, Dauenhauer K, Liesveld J, Anolik J, Looney RJ. Human SLE bone marrow mesenchymal stem cells (BMSCs) have a senescence-associated secretory phenotype (SASP) mediated by a MAVS-IFNβ feedback loop. Arthritis & Rheumatology (Hoboken, NJ) 2017;69(8):1623. doi: 10.1002/art.40142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin T-Y, Schneller S, Zust R, Dong H. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468(7322):452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Züst R, Cervantes-Barragan L, Habjan M, Maier R, Neuman BW, Ziebuhr J, Szretter KJ, Baker SC, Barchet W, Diamond MS. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nature Immunology. 2011;12(2):137. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pichlmair A, Lassnig C, Eberle C-A, Górna MW, Baumann CL, Burkard TR, Bürckstümmer T, Stefanovic A, Krieger S, Bennett KL. IFIT1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nature Immunology. 2011;12(7):624. doi: 10.1038/ni.2048. [DOI] [PubMed] [Google Scholar]
- 101.Chakrabarti A, Jha BK, Silverman RH. New insights into the role of RNase L in innate immunity. Journal of Interferon & Cytokine Research. 2011;31(1):49–57. doi: 10.1089/jir.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malathi K, Dong B, Gale M, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448(7155):816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang R, Wang J, Acharya D, Paul AM, Bai F, Huang F, Guo Y-L. Antiviral Responses in Mouse Embryonic Stem Cells differential development of cellular mechanisms in type i interferon production and response. Journal of Biological Chemistry. 2014;289(36):25186–25198. doi: 10.1074/jbc.M113.537746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tarassishin L, Bauman A, Suh H-S, Lee SC. Anti-viral and anti-inflammatory mechanisms of the innate immune transcription factor interferon regulatory factor 3: relevance to human CNS diseases. Journal of Neuroimmune Pharmacology. 2013;8(1):132–144. doi: 10.1007/s11481-012-9360-5. [DOI] [PubMed] [Google Scholar]
- 105.Rollenhagen C, Macura SL, Lathrop MJ, Mackenzie TA, Doncel GF, Asin SN. Enhancing interferon regulatory factor 7 mediated antiviral responses and decreasing nuclear factor Kappa B expression limit HIV-1 replication in cervical tissues. PLoS One. 2015;10(6):e0131919–e0131919. doi: 10.1371/journal.pone.0131919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin X, Kim S-H, Jeon H-M, Beck S, Sohn Y-W, Yin J, Kim J-K, Lim YC, Lee J-H, Kim S-H. Interferon regulatory factor 7 regulates glioma stem cells via interleukin-6 and Notch signalling. Brain. 2012;135(4):1055–1069. doi: 10.1093/brain/aws028. [DOI] [PubMed] [Google Scholar]
- 107.Fleming SB. Viral inhibition of the IFN-induced JAK/STAT signalling pathway: Development of live attenuated vaccines by mutation of viral-encoded IFN-antagonists. Vaccines. 2016;4(3):23. doi: 10.3390/vaccines4030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herrera SC, Bach EA. JAK/STAT signaling in stem cells and regeneration: from Drosophila to vertebrates. Development. 2019;146(2):dev167643. doi: 10.1242/dev.167643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen J-L, Liao Y, Goraya MU, Yuan X, Zhang B, Chiu S-H. Functional involvement of interferon-inducible transmembrane proteins in antiviral immunity. Frontiers in Microbiology. 2019;10:1097. doi: 10.3389/fmicb.2019.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JPY. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feeley EM, Sims JS, John SP, Chin CR, Pertel T, Chen L-M, Gaiha GD, Ryan BJ, Donis RO, Elledge SJ. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathogens. 2011;7(10):e1002337. doi: 10.1371/journal.ppat.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mahauad-Fernandez WD, Jones PH, Okeoma CM. Critical role for bone marrow stromal antigen 2 in acute Chikungunya virus infection. The Journal of General Virology. 2014;95(Pt 11):2450. doi: 10.1099/vir.0.068643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sicco CL, Reverberi D, Villa F, Pfeffer U, Quarto R, Cancedda R, Tasso R. Circulating healing (CH) cells expressing BST2 are functionally activated by the injury-regulated systemic factor HGFA. Stem Cell Research & Therapy. 2018;9(1):1–13. doi: 10.1186/s13287-018-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fenner JE, Starr R, Cornish AL, Zhang J-G, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nature Immunology. 2006;7(1):33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 115.Hong X-X, Carmichael GG. Innate immunity in pluripotent human cells ATTENUATED RESPONSE TO INTERFERON-β. Journal of Biological Chemistry. 2013;288(22):16196–16205. doi: 10.1074/jbc.M112.435461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Subramanian G, Kuzmanovic T, Zhang Y, Peter CB, Veleeparambil M, Chakravarti R, Sen GC, Chattopadhyay S. A new mechanism of interferon’s antiviral action: Induction of autophagy, essential for paramyxovirus replication, is inhibited by the interferon stimulated gene, TDRD7. PLoS Pathogens. 2018;14(1):e1006877. doi: 10.1371/journal.ppat.1006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. Journal of Virology. 2006;80(9):4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hou W, Wang X, Ye L, Zhou L, Yang Z-Q, Riedel E, Ho W-Z. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. Journal of Virology. 2009;83(8):3834–3842. doi: 10.1128/JVI.01773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sauter D, Hotter D, Van Driessche B, Stürzel CM, Kluge SF, Wildum S, Yu H, Baumann B, Wirth T, Plantier J-C. Differential regulation of NF-κB-mediated proviral and antiviral host gene expression by primate lentiviral Nef and Vpu proteins. Cell Reports. 2015;10(4):586–599. doi: 10.1016/j.celrep.2014.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.D’Angelo W, Acharya D, Wang R, Wang J, Gurung C, Chen B, Bai F, Guo Y-L. Development of antiviral innate immunity during in vitro differentiation of mouse embryonic stem cells. Stem Cells and Development. 2016;25(8):648–659. doi: 10.1089/scd.2015.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.FitzPatrick LM, Hawkins KE, Delhove JM, Fernandez E, Soldati C, Bullen LF, Nohturfft A, Waddington SN, Medina DL, Bolaños JP. NF-κB activity initiates human ESC-derived neural progenitor cell differentiation by inducing a metabolic maturation program. Stem Cell Reports. 2018;10(6):1766–1781. doi: 10.1016/j.stemcr.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang X, Han Y, Dang Y, Fu W, Zhou T, Ptak RG, Zheng Y-H. Moloney leukemia virus 10 (MOV10) protein inhibits retrovirus replication. Journal of Biological Chemistry. 2010;285(19):14346–14355. doi: 10.1074/jbc.M110.109314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goodier JL, Cheung LE, Kazazian HH., Jr MOV10 RNA helicase is a potent inhibitor of retrotransposition in cells. PLoS Genetics. 2012;8(10):e1002941. doi: 10.1371/journal.pgen.1002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakanishi K, Weinberg DE, Bartel DP, Patel DJ. Structure of yeast Argonaute with guide RNA. Nature. 2012;486(7403):368–374. doi: 10.1038/nature11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nowotny M, Gaidamakov SA, Crouch RJ, Yang W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell. 2005;121(7):1005–1016. doi: 10.1016/j.cell.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 126.Baulcombe D. RNA silencing in plants. Nature. 2004;431(7006):356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 127.Levanova A, Poranen MM. RNA interference as a prospective tool for the control of human viral infections. Frontiers in Microbiology. 2018;9:2151. doi: 10.3389/fmicb.2018.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bodak M, Cirera-Salinas D, Luitz J, Ciaudo C. The role of RNA interference in stem cell biology: beyond the mutant phenotypes. Journal of Molecular Biology. 2017;429(10):1532–1543. doi: 10.1016/j.jmb.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 129.Isaacs, A., & Lindenmann, J. (1957) Virus interference. I. The interferon. Proceedings of the Royal Society of London Series B-Biological Sciences 147(927), 258–267. [DOI] [PubMed]
- 130.Isaacs, A., Lindenmann, J., & Valentine, R. C. (1957) Virus interference. II. Some properties of interferon. Proceedings of the Royal Society of London Series B-Biological Sciences, 147(927), 268–273. [DOI] [PubMed]
- 131.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25(3):373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 132.Teijaro JR. Type I interferons in viral control and immune regulation. Current Opinion in Virology. 2016;16:31–40. doi: 10.1016/j.coviro.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host & Microbe. 2008;3(4):245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, Chapman R, Hertzog PJ. Interferome v2. 0: an updated database of annotated interferon-regulated genes. Nucleic Acids Research. 2012;41(D1):D1040–D1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burke DC, Graham CF, Lehman JM. Appearance of interferon inducibility and sensitivity during differentiation of murine teratocarcinoma cells in vitro. Cell. 1978;13(2):243–248. doi: 10.1016/0092-8674(78)90193-9. [DOI] [PubMed] [Google Scholar]
- 136.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nature Reviews Molecular Cell Biology. 2019;20(5):303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prompetchara E, Ketloy C, Palaga TJAPJAI. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific Journal of Allergy and Immunology. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 138.Crosse KM, Monson EA, Beard MR, Helbig KJ. Interferon-stimulated genes as enhancers of antiviral innate immune signaling. Journal of Innate Immunity. 2018;10(2):85–93. doi: 10.1159/000484258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ashley CL, Abendroth A, McSharry BP, Slobedman B. Interferon-independent innate responses to cytomegalovirus. Frontiers in Immunology. 2019;10:2751. doi: 10.3389/fimmu.2019.02751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grow EJ, Flynn RA, Chavez SL, Bayless NL, Wossidlo M, Wesche DJ, Martin L, Ware CB, Blish CA, Chang HY. Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature. 2015;522(7555):221–225. doi: 10.1038/nature14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, Zaretsky I, Jaitin DA, David E, Keren-Shaul H, Mildner A, Winter D, Jung S. Chromatin state dynamics during blood formation. Science. 2014;345(6199):943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Langlais D, Barreiro LB, Gros P. The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation. Journal of Experimental Medicine. 2016;213(4):585–603. doi: 10.1084/jem.20151764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Winkler CW, Woods TA, Groveman BR, Carmody AB, Speranza EE, Martens CA, Best SM, Haigh CL, Peterson KE. Neuronal maturation reduces the type I IFN response to orthobunyavirus infection and leads to increased apoptosis of human neurons. Journal of Neuroinflammation. 2019;16(1):1–16. doi: 10.1186/s12974-019-1614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PR, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. European Respiratory Journal. 2020;55(6):2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailey CC, Zhong G, Huang I-C, Farzan M. IFITM-family proteins: the cell’s first line of antiviral defense. Annual Review of Virology. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao X, Li J, Winkler CA, An P, Guo J-T. IFITM genes, variants, and their roles in the control and pathogenesis of viral infections. Frontiers in Microbiology. 2019;9:3228. doi: 10.3389/fmicb.2018.03228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Berkhout B. RNAi-mediated antiviral immunity in mammals. Current Opinion in Virology. 2018;32:9–14. doi: 10.1016/j.coviro.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 148.Schuster S, Overheul GJ, Bauer L, van Kuppeveld FJ, van Rij RP. No evidence for viral small RNA production and antiviral function of Argonaute 2 in human cells. Scientific Reports. 2019;9(1):1–11. doi: 10.1038/s41598-019-50287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paddison PJ, Caudy AA, Hannon GJ. Stable suppression of gene expression by RNAi in mammalian cells. Proceedings of the National Academy of Sciences. 2002;99(3):1443–1448. doi: 10.1073/pnas.032652399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes & Development. 2008;22(20):2773–2785. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453(7194):534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang S, Tutton S, Pierce E, Yoon K. Specific double-stranded RNA interference in undifferentiated mouse embryonic stem cells. Molecular and Cellular Biology. 2001;21(22):7807–7816. doi: 10.1128/MCB.21.22.7807-7816.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soifer, H. S. (2006) Do small RNAs interfere with LINE-1? BioMed Research International, 2006(1), 29049. [DOI] [PMC free article] [PubMed]
- 154.Takahashi T, Ui-Tei K. Mutual regulation of RNA silencing and the IFN response as an antiviral defense system in mammalian cells. International Journal of Molecular Sciences. 2020;21(4):1348. doi: 10.3390/ijms21041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wong M-T, Chen SS. Emerging roles of interferon-stimulated genes in the innate immune response to hepatitis C virus infection. Cellular & Molecular Immunology. 2016;13(1):11–35. doi: 10.1038/cmi.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gujar S, Bell J, Diallo J-S. SnapShot: cancer immunotherapy with oncolytic viruses. Cell. 2019;176(5):1240–1240. doi: 10.1016/j.cell.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 157.Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. International Reviews of Immunology. 2018;37(1):20–36. doi: 10.1080/08830185.2017.1380200. [DOI] [PubMed] [Google Scholar]
- 158.Leifer CA, Medvedev AE. Molecular mechanisms of regulation of Toll-like receptor signaling. Journal of Leukocyte Biology. 2016;100(5):927–941. doi: 10.1189/jlb.2MR0316-117RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heidarzadeh M, Roodbari F, Hassanpour M, Ahmadi M, Saberianpour S, Rahbarghazi R. Toll-like receptor bioactivity in endothelial progenitor cells. Cell and Tissue Research. 2020;379(2):223–230. doi: 10.1007/s00441-019-03119-2. [DOI] [PubMed] [Google Scholar]
- 160.Fawzy El-Sayed KM, Elahmady M, Adawi Z, Aboushadi N, Elnaggar A, Eid M, Hamdy N, Sanaa D, Dörfer CE. The periodontal stem/progenitor cell inflammatory‐regenerative cross talk: A new perspective. Journal of Periodontal Research. 2019;54(2):81–94. doi: 10.1111/jre.12616. [DOI] [PubMed] [Google Scholar]
- 161.Sánchez-Tarjuelo, R., Cortegano, I., Manosalva, J., Rodríguez, M., Ruíz, C., Alía, M., Prado, M. C., Cano, E. M., Ferrándiz, M. J., de la Campa, A. G., Gaspar, M. L., & de Andrés, B. (2020). The TLR4-MyD88 signaling axis regulates lung monocyte differentiation pathways in response to streptococcus pneumoniae. Frontiers in Immunology, 11, 2120. 10.3389/fimmu.2020.02120. [DOI] [PMC free article] [PubMed]
- 162.Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. Journal of Biological Regulators and Homeostatic Agents. 2020;34(2):71. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 163.Gutierrez-Martinez P, Hogdal L, Nagai M, Kruta M, Singh R, Sarosiek K, Nussenzweig A, Beerman I, Letai A, Rossi DJ. Diminished apoptotic priming and ATM signalling confer a survival advantage onto aged haematopoietic stem cells in response to DNA damage. Nature Cell Biology. 2018;20(4):413–421. doi: 10.1038/s41556-018-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaminskyy V, Zhivotovsky B. To kill or be killed: how viruses interact with the cell death machinery. Journal of Internal Medicine. 2010;267(5):473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 165.Watanabe J, Yamada M, Niibe K, Zhang M, Kondo T, Ishibashi M, Egusa H. Preconditioning of bone marrow-derived mesenchymal stem cells with N-acetyl-L-cysteine enhances bone regeneration via reinforced resistance to oxidative stress. Biomaterials. 2018;185:25–38. doi: 10.1016/j.biomaterials.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 166.Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Molecular and Cellular Biology. 2002;22(15):5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu HL, Yang C, Liu HF. p62 links the autophagy pathway and the ubiqutin–proteasome system upon ubiquitinated protein degradation. Cellular & Molecular Biology Letters. 2016;21(1):29. doi: 10.1186/s11658-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ishii T, Warabi E, Siow RC, Mann GE. Sequestosome1/p62: a regulator of redox-sensitive voltage-activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radical Biology and Medicine. 2013;65:102–116. doi: 10.1016/j.freeradbiomed.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 169.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. Journal of Biological Chemistry. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Levonen A-L, Patel RP, Brookes P, Go Y-M, Jo H, Parthasarathy S, Anderson PG, Darley-Usmar VM. Mechanisms of cell signaling by nitric oxide and peroxynitrite: from mitochondria to MAP kinases. Antioxidants and Redox Signaling. 2001;3(2):215–229. doi: 10.1089/152308601300185188. [DOI] [PubMed] [Google Scholar]
- 171.Wasik U, Milkiewicz M, Kempinska-Podhorodecka A, Milkiewicz P. Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in Primary Biliary Cholangitis. Scientific Reports. 2017;7:44769. doi: 10.1038/srep44769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zieba BA, Henry L, Lacroix M, Jemaà M, Lavabre-Bertrand T, Meunier L, Coux O, Stoebner P-E. The proteasome maturation protein POMP increases proteasome assembly and activity in psoriatic lesional skin. Journal of Dermatological Science. 2017;88(1):10–19. doi: 10.1016/j.jdermsci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 173.van der Stoop P, Boutsma EA, Hulsman D, Noback S, Heimerikx M, Kerkhoven RM, Voncken JW, Wessels LF, van Lohuizen M. Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. PLoS One. 2008;3(5):e2235. doi: 10.1371/journal.pone.0002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jin L, Ni J, Tao Y, Weng X, Zhu Y, Yan J, Hu B. N-acetylcysteine attenuates PM2. 5-induced apoptosis by ROS-mediated Nrf2 pathway in human embryonic stem cells. Science of The Total Environment. 2019;666:713–720. doi: 10.1016/j.scitotenv.2019.02.307. [DOI] [PubMed] [Google Scholar]
- 175.Mohan S, Gupta D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomedicine & Pharmacotherapy. 2018;108:1866–1878. doi: 10.1016/j.biopha.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 176.Nadeem A, Siddiqui N, Al-Harbi NO, Al-Harbi MM, Ahmad SF. TLR-7 agonist attenuates airway reactivity and inflammation through Nrf2-mediated antioxidant protection in a murine model of allergic asthma. The International Journal of Biochemistry & Cell Biology. 2016;73:53–62. doi: 10.1016/j.biocel.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 177.Yin S, Cao W. Toll-like receptor signaling induces Nrf2 pathway activation through p62-triggered Keap1 degradation. Molecular and Cellular Biology. 2015;35(15):2673–2683. doi: 10.1128/MCB.00105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rushworth SA, Chen X-L, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. The Journal of Immunology. 2005;175(7):4408–4415. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- 179.Jang J, Wang Y, Kim HS, Lalli MA, Kosik KS. Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2014;32(10):2616–2625. doi: 10.1002/stem.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ramezani A, Nahad MP, Faghihloo E. The role of Nrf2 transcription factor in viral infection. Journal of Cellular Biochemistry. 2018;119(8):6366–6382. doi: 10.1002/jcb.26897. [DOI] [PubMed] [Google Scholar]
- 181.Delgado M, Deretic V. Toll-like receptors in control of immunological autophagy. Cell Death & Differentiation. 2009;16(7):976–983. doi: 10.1038/cdd.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nabar NR, Shi C-S, Kehrl JH. Immunology. Amsterdam: Elsevier; 2018. Signaling by the toll-like receptors induces autophagy through modification of Beclin 1: Molecular mechanism; pp. 75–84. [Google Scholar]
- 183.Park Y-E, Hayashi YK, Bonne G, Arimura T, Noguchi S, Nonaka I, Nishino I. Autophagic degradation of nuclear components in mammalian cells. Autophagy. 2009;5(6):795–804. doi: 10.4161/auto.8901. [DOI] [PubMed] [Google Scholar]
- 184.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nature Reviews Immunology. 2013;13(10):722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chiramel AI, Brady NR, Bartenschlager R. Divergent roles of autophagy in virus infection. Cells. 2013;2(1):83–104. doi: 10.3390/cells2010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Akbari A, Rezaie J. Potential therapeutic application of mesenchymal stem cell-derived exosomes in SARS-CoV-2 pneumonia. Stem Cell Research & Therapy. 2020;11(1):356. doi: 10.1186/s13287-020-01866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hassanpour M, Cheraghi O, Brazvan B, Hiradfar A, Aghamohammadzadeh N, Rahbarghazi R, Nouri M. Chronic exposure of human endothelial progenitor cells to diabetic condition abolished the regulated kinetics activity of exosomes. Iranian Journal of Pharmaceutical Research. 2018;17(3):1068. [PMC free article] [PubMed] [Google Scholar]
- 188.Agostini, L., Martinon, F., Burns, K., McDermott, M. F., Hawkins, P. N., & Tschopp, J. J. I. (2004) NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity, 20(3), 319–325. [DOI] [PubMed]
- 189.Van Dongen HM, Masoumi N, Witwer KW, Pegtel DM. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiology and Molecular Biology Reviews. 2016;80(2):369–386. doi: 10.1128/MMBR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proceedings of the National Academy of Sciences. 2003;100(19):10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Qian X, Xu C, Fang S, Zhao P, Wang Y, Liu H, Yuan W, Qi Z. Exosomal microRNAs derived from umbilical mesenchymal stem cells inhibit hepatitis C virus infection. Stem Cells Translational Medicine. 2016;5(9):1190–1203. doi: 10.5966/sctm.2015-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cheng M, Si Y, Niu Y, Liu X, Li X, Zhao J, Jin Q, Yang W. High-throughput profiling of alpha interferon-and interleukin-28B-regulated microRNAs and identification of let-7 s with anti-hepatitis C virus activity by targeting IGF2BP1. Journal of Virology. 2013;87(17):9707–9718. doi: 10.1128/JVI.00802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling. 2014;7(332):ra63–ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 194.Lau SK, Feng Y, Chen H, Luk HK, Yang W-H, Li KS, Zhang Y-Z, Huang Y, Song Z-Z, Chow W-N. Severe acute respiratory syndrome (SARS) coronavirus ORF8 protein is acquired from SARS-related coronavirus from greater horseshoe bats through recombination. Journal of Virology. 2015;89(20):10532–10547. doi: 10.1128/JVI.01048-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Scientific Reports. 2018;8(1):1–12. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Castaño-Rodriguez C, Honrubia JM, Gutiérrez-Álvarez J, DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, Regla-Nava JA, Fernandez-Delgado R, Verdia-Báguena C, Queralt-Martín M. Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis. MBio. 2018;9(3):e02325–17. doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zeng Q, Langereis MA, Van Vliet AL, Huizinga EG, De Groot RJ. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proceedings of the National Academy of Sciences. 2008;105(26):9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Research & Therapy. 2018;9(1):1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.