Abstract
This study investigated the association between hematologic inflammatory markers derived from complete blood counts and obesity. We undertook a cross-sectional study that included self-reported healthy subjects above the age of 18 years from the 2011–2016 National Health and Nutrition Examination Survey, a US population database. Study parameters included mean corpuscular volume, red cell distribution width, mean platelet volume, total platelet count, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index. Body mass index was used as an index of obesity and was correlated with each hematologic inflammatory marker. Our analysis found a statistically significant association between each inflammatory parameter and higher body mass indices. We demonstrated an association between complete blood count–derived indices of inflammation and obesity, and these results provide the basis for future studies using complete blood count–derived variables and outcomes in patients with some chronic diseases.
Keywords: Complete blood count, inflammatory markers, neutrophil-to-lymphocyte ratio, NHANES, obesity, platelet-to-lymphocyte ratio, systemic immune-inflammation index
Obesity currently affects at least one-third of the US adult population, and its prevalence is increasing each year.1 Based on data from the 2015–2016 National Health and Nutrition Examination Survey (NHANES), over 39.8% of adults meet the criteria for obesity, defined as a body mass index (BMI) >30 kg/m2.2 Obesity has important health consequences and increases the frequency of cardiac disease, diabetes, and other diseases. In addition, several malignancies, including breast cancer and colon cancer, occur more frequently in obese individuals. Obesity causes low-grade inflammation, and this could contribute to the development of some of these complications. Simple hematological indices can reflect inflammation and have been associated with increases in BMI. However, the magnitude of these associations remains inconclusive and controversial, and the literature lacks a nationally representative study that analyzes them.3,4 Our study investigated the relation between hematologic inflammatory markers and BMI in a large number of healthy participants to establish background information for future clinical studies.
METHODS
Our study used data from NHANES (2011–2016), a nationally representative cross-sectional survey of the noninstitutionalized US adult population, administered every 2 years by the Centers for Disease Control and Prevention. It includes demographic and health questionnaires, a physical examination, and laboratory tests. White blood cell (WBC) counts, neutrophil counts, lymphocyte counts, total platelet counts, mean platelet volume (MPV), and red cell distribution width (RDW) are reported as a part of complete blood count (CBC) in the laboratory results.
Data from all subjects aged 18 and older (N = 17,969) were retrieved from the data set. From the adult sample, we defined a “healthy” subsample using questionnaire responses. Based on question HDS010, “What would you say your health in general is?” we retained individuals who self-reported their health as “good,” “very good,” or “excellent.” We excluded participants with self-reported physician diagnoses of diabetes, asthma, hypertension, high cholesterol, coronary heart disease, or congestive heart failure. Pregnant women were also excluded. The remaining healthy subpopulation included 3201 women and 3131 men.
NHANES 2011–2016 laboratory tests were analyzed on the Coulter HMX (Coulter Electronics Ltd, Bedfordshire, England) using the ethylenediaminetetraacetic acid–mixed blood sample from participants. The derived indices (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index) were calculated using CBC values. The neutrophil-to-lymphocyte ratio was calculated as the ratio between the neutrophil count and the lymphocyte count. The platelet-to-lymphocyte ratio was calculated as the ratio between the platelet count and the lymphocyte count. The systemic immune-inflammation index was calculated with the formula Platelet count × Neutrophil count/Lymphocyte count.5
Analysis used Stata 15.1 with weighting for the complex survey design of NHANES. Mean values for each CBC marker were calculated for BMI categories recommended by the World Health Organization6 and the National Institutes of Health.7 Wald tests were used to estimate differences between normal BMI (18.5 to <25 kg/m2) and other BMI categories. For clarity of presentation, we used ordinary least squares regression to predict each CBC marker with BMI as a continuous variable. Supplementary analysis (not shown) also ran ordinary least squares regression using BMI as a categorical independent variable. In some cases, this form of the BMI variable better predicted variation in some CBC markers, but model fit was relatively consistent between both forms. All analyses were split by gender, and ordinary least squares regression models were adjusted for age and race/ethnicity. Model sample sizes varied depending on completeness of values for each CBC marker. Scatterplots were used to display the relationship between BMI and systemic immune-inflammation index, with a small number of systemic immune-inflammation index outliers (value >3000; 1 man, 2 women) removed for clarity of presentation.
RESULTS
Table 1 reports sample characteristics for the healthy male and female subsamples. The mean age was 38.4 years for men and 40.2 years for women. The mean BMI was 27.4 kg/m2 for men and 27.2 kg/m2 for women.
Table 1.
Characteristics of the healthy male and female subsamples, NHANES 2011–2016
| Variable | Mean (SD) or n (%) |
|
|---|---|---|
| Men (n = 3131) | Women (n = 3201) | |
| Age (years) | 38.4 (13.6) | 40.2 (13.6) |
| Body mass index (kg/m2) | 27.4 (5.3) | 27.2 (6.1) |
| Non-Hispanic white | 1127 (62.0%) | 1169 (65.0%) |
| Non-Hispanic black | 669 (10.9%) | 630 (10.7%) |
| Hispanic | 712 (17.1%) | 743 (14.5%) |
| Other races | 623 (10.0%) | 659 (9.8%) |
NHANES indicates National Health and Nutrition Examination Survey.
Table 2 reports mean values for various inflammatory markers derived from the CBC in individual BMI categories based on the World Health Organization classification. Ordinary least squares regression showed that BMI significantly predicted variation in most CBC markers, but the modest r2 values suggested that the individual contribution of BMI was relatively small. NHANES values for all common CBC measures except for MPV were within typical reference ranges (Table 3).8,9 The lower MPV values from NHANES may be a product of different instrumentation; NHANES is a national sample that also publishes its own CBC reference ranges with each data release.10
Table 2.
Weighted mean and standard deviation of complete blood count values by body mass index category using data from NHANES 2011–2016
| Variable | Body mass index categories (kg/m2) |
||||||
|---|---|---|---|---|---|---|---|
| <18.5 | 18.5 to <25 | 25 to <30 | 30 to <35 | 35 to <40 | 40+ | Total | |
| Male | 1.8% | 35.5% | 35.6% | 18.7% | 5.4% | 3.1% | |
| Hb (g/dL) | 14.8 (1.2) | 15.0 (1.0) | 15.2 (1.0)* | 15.3 (0.9)* | 15.4 (0.9)* | 15.1 (1.2) | 15.2 (1.0) |
| Hct (%) | 43.4 (3.7) | 44.2 (3.0) | 44.5 (2.9) | 44.7 (2.7)* | 45.1 (2.3)* | 44.5 (3.1) | 44.5 (2.9) |
| WBC (103/μL) | 6.6 (2.2) | 6.8 (2.1) | 6.9 (1.7) | 7.2 (1.7)* | 7.4 (1.7)* | 9.0 (2.5)* | 7.0 (1.9) |
| NEUT (103/μL) | 4.0 (1.7) | 4.0 (1.7) | 3.9 (1.3) | 4.3 (1.3)* | 4.2 (1.3) | 5.6 (1.7)* | 4.1 (1.5) |
| LYMPH (103/μL) | 1.8 (0.6)* | 2.0 (0.7) | 2.1 (0.6)* | 2.1 (0.6)* | 2.3 (0.7)* | 2.5 (0.8)* | 2.1 (0.6) |
| MCV (fL) | 91.7 (4.8) | 90.4 (4.9) | 89.8 (4.3)* | 88.8 (4.1)* | 88.9 (4.8)* | 86.8 (5.3)* | 89.7 (4.6) |
| MCH (pg) | 31.2 (1.7)* | 30.7 (2.0) | 30.6 (1.7) | 30.4 (1.7)* | 30.3 (2.0)* | 29.5 (2.2)* | 30.6 (1.8) |
| MCHC (g/dL) | 34.3 (1.0) | 34.2 (0.9) | 34.3 (0.9) | 34.4 (0.8) | 34.4 (0.9) | 34.1 (1.1) | 34.3 (0.9) |
| RDW (%) | 13.0 (0.7) | 13.0 (0.8) | 13.0 (0.7) | 13.1 (0.7) | 13.1 (0.7) | 13.4 (0.9)* | 13.0 (0.8) |
| TPC (103/μL) | 210.0 (44.6)* | 223.1 (46.0) | 224.7 (47.0) | 228.7 (44.9) | 228.3 (53.3) | 254.5 (53.4)* | 225.9 (47.2) |
| MPV (fL) | 8.1 (0.7) | 8.3 (0.8) | 8.3 (0.8) | 8.4 (0.8)* | 8.5 (0.8)* | 8.4 (0.7) | 8.3 (0.8) |
| NLR | 2.3 (1.1) | 2.1 (1.1) | 2.0 (0.9) | 2.1 (0.8) | 2.0 (0.7) | 2.4 (0.8)* | 2.1 (0.9) |
| PLR | 127.0 (47.5) | 119.1 (39.9) | 115.7 (37.6) | 115.2 (35.9) | 106.8 (30.0)* | 109.8 (34.3) | 116.4 (38.0) |
| SII | 483.0 (241.2) | 473.2 (282.2) | 454.0 (223.8) | 492.5 (235.9) | 455.6 (199.7) | 608.5 (260.3)* | 474.6 (250.3) |
| Female | 2.3% | 42.7% | 28.1% | 14.6% | 6.9% | 5.4% | |
| Hb (g/dL) | 13.5 (1.0) | 13.3 (1.0) | 13.4 (1.0) | 13.4 (1.2) | 13.3 (1.0) | 13.1 (1.1)* | 13.3 (1.0) |
| Hct (%) | 40.0 (2.8) | 39.5 (2.7) | 39.6 (2.7) | 39.7 (3.2) | 39.6 (2.8) | 39.4 (3.0) | 39.6 (2.8) |
| WBC (103/μL) | 6.6 (2.1) | 6.7 (1.7) | 7.0 (1.7)* | 7.5 (2.1)* | 8.0 (2.1)* | 8.3 (2.1)* | 7.1 (1.9) |
| NEUT (103/μL) | 4.0 (1.7) | 3.9 (1.4) | 4.1 (1.3)* | 4.4 (1.7)* | 5.0 (1.6)* | 5.1 (1.7)* | 4.2 (1.5) |
| LYMPH (103/μL) | 2.0 (0.6) | 2.1 (0.6) | 2.1 (0.6) | 2.3 (0.6)* | 2.3 (0.6)* | 2.4 (0.7)* | 2.1 (0.6) |
| MCV (fL) | 92.0 (4.3)* | 90.2 (5.0) | 89.1 (5.3)* | 88.2 (6.1)* | 87.1 (4.9)* | 85.4 (5.4)* | 89.1 (5.5) |
| MCH (pg) | 31.1 (1.8)* | 30.4 (2.1) | 30.1 (2.2)* | 29.8 (2.6)* | 29.3 (2.1)* | 28.4 (2.2)* | 30.1 (2.3) |
| MCHC (g/dL) | 33.9 (0.9) | 34.0 (1.0) | 34.1 (1.0) | 34.0 (1.1) | 33.8 (1.0) | 33.6 (0.8)* | 34.0 (1.0) |
| RDW (%) | 12.9 (1.2) | 13.2 (1.3) | 13.3 (1.3) | 13.5 (1.5)* | 13.7 (1.2)* | 14.1 (1.3)* | 13.3 (1.3) |
| TPC (103/μL) | 243.1 (52.2) | 238.5 (48.3) | 246.1 (49.0)* | 259.2 (53.1)* | 270.4 (58.5)* | 273.8 (60.0)* | 248.0 (52.0) |
| MPV (fL) | 8.3 (0.9) | 8.4 (0.8) | 8.4 (0.8) | 8.4 (0.8) | 8.5 (0.9) | 8.5 (0.7) | 8.4 (0.8) |
| NLR | 2.2 (1.1) | 2.0 (0.9) | 2.0 (0.8) | 2.1 (0.8) | 2.2 (0.8)* | 2.2 (0.8)* | 2.1 (0.8) |
| PLR | 134.1 (52.2) | 125.1 (38.5) | 123.0 (37.2) | 123.7 (38.2) | 123.8 (38.2) | 122.6 (41.2) | 124.5 (39.0) |
| SII | 526.5 (260.0) | 488.5 (247.4) | 497.0 (207.0) | 541.4 (268.2)* | 606.4 (252.2)* | 615.9 (276.9)* | 515.3 (246.8) |
Hb indicates hemoglobin; Hct, hematocrit; LYMPH, lymphocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelet volume; NEUT, neutrophils; NHANES, National Health and Nutrition Examination Survey; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; TPC, total platelet count; WBC, white blood cell count.
Significantly different from body mass index 18.5 to <25 kg/m2 at P < 0.05 (Wald test).
Table 3.
Complete blood count reference ranges
| Parameters | Male | Female | Source |
|---|---|---|---|
| Hb (g/dL) | 13.2–16.6 | 11.6–15.0 | Mayo8 |
| Hct (%) | 28.2–48.6 | 35.5–44.9 | Mayo8 |
| WBC (103/μL) | 3.4–9.6 | 3.4–9.6 | Mayo8 |
| NEUT (103/μL) | 1.56–6.45 | 1.56–6.45 | Mayo8 |
| LYMPH (103/μL) | 0.95–3.07 | 0.95–3.07 | Mayo8 |
| MCV (fL) | 78.2–97.9 | 78.2–97.9 | Mayo8 |
| MCH (pg) | 26–33 | 26–33 | UChicago9 |
| MCHC (g/dL) | 32–35 | 32–35 | UChicago9 |
| RDW (%) | 11.8–14.5 | 12.2–16.1 | Mayo8 |
| TPC (103/μL) | 135–317 | 157–371 | Mayo8 |
| MPV (fL) | 9–12.4 | 9–12.4 | UChicago9 |
Hb indicates hemoglobin; Hct, hematocrit; LYMPH, lymphocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelet volume; NEUT, neutrophils; RDW, red blood cell distribution width; TPC, total platelet count; WBC, white blood cell count.
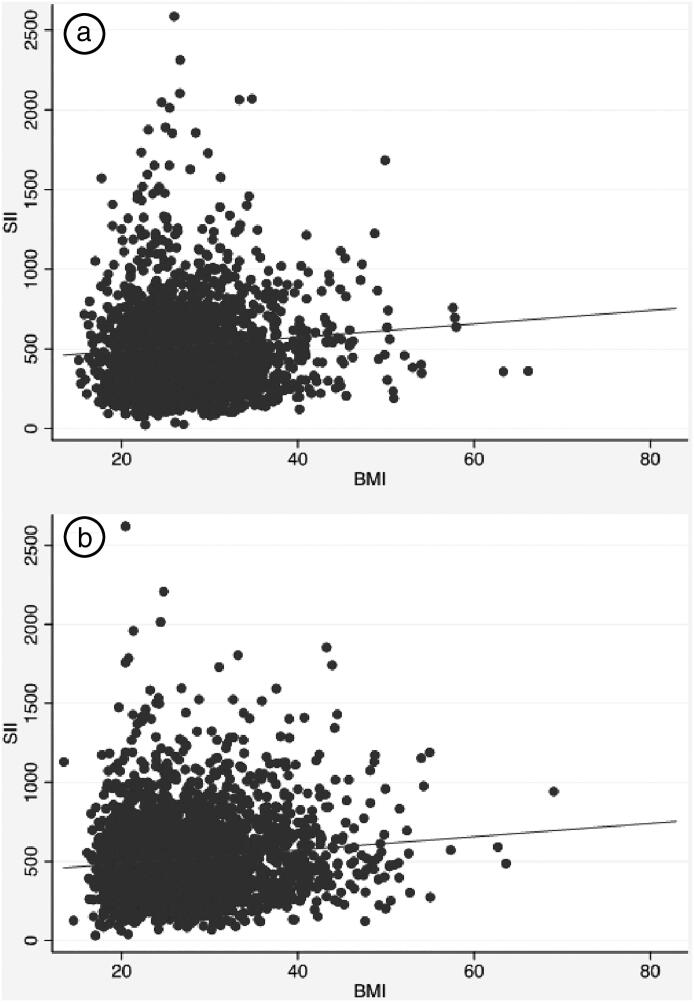
In the healthy adult male participants, RDW, total platelet count, MPV, and the systemic immune-inflammation index were significantly and positively correlated with BMI (P < 0.05) (Table 4, Figure 1a). Statistically significant inverse correlations with BMI were found for mean corpuscular volume (MCV) and platelet-to-lymphocyte ratio. Moreover, mean MCV in each obesity class was significantly lower than in participants with normal BMIs. Figure 1a shows the positive relationship between BMI and systemic immune-inflammation index for the health male sample.
Table 4.
Age- and race-adjusted OLS regression coefficients predicting complete blood count marker with body mass index as a continuous variable
| N | b | t | P value | r2 | |
|---|---|---|---|---|---|
| Male | |||||
| Hb (g/dL) | 2815 | 0.01 | 2.69 | 0.01 | 0.081 |
| Hct (%) | 2815 | 0.04 | 2.52 | 0.02 | 0.043 |
| WBC (103/μL) | 2815 | 0.06 | 6.39 | <0.001 | 0.052 |
| NEUT (103/μL) | 2814 | 0.04 | 5.28 | <0.001 | 0.042 |
| LYMPH (103/μL) | 2814 | 0.02 | 4.99 | <0.001 | 0.067 |
| MCV (fL) | 2815 | −0.16 | −10.02 | <0.001 | 0.107 |
| MCH (pg) | 2815 | −0.05 | −7.38 | <0.001 | 0.103 |
| MCHC (g/dL) | 1936 | 0.003 | 0.55 | 0.59 | 0.100 |
| RDW (%) | 2815 | 0.01 | 4.28 | <0.001 | 0.090 |
| TPC (103/μL) | 2815 | 0.94 | 4.36 | <0.001 | 0.030 |
| MPV (fL) | 2815 | 0.01 | 2.74 | 0.01 | 0.017 |
| NLR | 2814 | 0.003 | 0.64 | 0.52 | 0.043 |
| PLR | 2814 | −0.49 | −2.20 | 0.03 | 0.034 |
| SII | 2814 | 2.83 | 2.00 | 0.05 | 0.026 |
| Female | |||||
| Hb (g/dL) | 2878 | 0.001 | 0.29 | 0.78 | 0.102 |
| Hct (%) | 2878 | 0.01 | 1.31 | 0.20 | 0.067 |
| WBC (103/μL) | 2878 | 0.09 | 10.00 | <0.001 | 0.107 |
| NEUT (103/μL) | 2870 | 0.06 | 10.82 | <0.001 | 0.101 |
| LYMPH (103/μL) | 2870 | 0.02 | 5.46 | <0.001 | 0.058 |
| MCV (fL) | 2878 | −0.2 | −10.43 | <0.001 | 0.126 |
| MCH (pg) | 2878 | −0.07 | −11.03 | <0.001 | 0.125 |
| MCHC (g/dL) | 1903 | −0.01 | −1.68 | 0.10 | 0.070 |
| RDW (%) | 2878 | 0.03 | 6.60 | <0.001 | 0.078 |
| TPC (103/μL) | 2877 | 1.64 | 7.97 | <0.001 | 0.054 |
| MPV (fL) | 2877 | 0.002 | 0.61 | 0.55 | 0.014 |
| NLR | 2870 | 0.01 | 3.69 | 0.001 | 0.031 |
| PLR | 2870 | −0.24 | −1.63 | 0.11 | 0.014 |
| SII | 2870 | 6.22 | 8.57 | <0.001 | 0.037 |
Hb indicates hemoglobin; Hct, hematocrit; LYMPH, lymphocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; TPC, total platelet count; MPV, mean platelet volume; MPV, mean platelet volume; NEUT, neutrophils; NLR, neutrophil-to-lymphocyte ratio; OLS, ordinary least squares; PLR, platelet-to-lymphocyte ratio; RDW, red blood cell distribution width; SII, systemic immune-inflammation index; TPC, total platelet count; WBC, white blood cell count.
Figure 1.
Scatterplot and regression line between body mass index (BMI) and systemic immune-inflammation index (SII) for the sample of (a) 3131 healthy men and (b) 3201 healthy women from the 2011–2016 National Health and Nutrition Examination Survey.
In the healthy adult female participants, RDW, total platelet count, neutrophil-to-lymphocyte ratio, and the systemic immune-inflammation index were positively associated with BMI (P < 0.05) (Figure 1b). MCV was the only inflammatory parameter that significantly decreased with increasing BMI. BMI predicted CBC markers differently by gender but was not a clearly better predictor of variation for men or women across all CBC markers.
DISCUSSION
CBC is the easiest and simplest laboratory test that clinicians frequently order. Measuring the number of erythrocytes, leukocytes, and platelets can have important clinical implications. In particular, significant decreases or increases in cell counts usually represent an important clinical disease process. In addition, analyzing indices derived from the CBC potentially provides important information about disease states, including chronic low-grade inflammation. We found that hematologic inflammatory markers, including RDW, total platelet count, MPV, and the systemic immune-inflammation index, were positively correlated with BMI in both genders. MCV was the only marker that was negatively correlated with BMI. In addition, the neutrophil-to-lymphocyte ratio had a positive correlation with BMI only in women, and the platelet-to-lymphocyte ratio had a negative correlation only in men.
Although the correlations between most CBC parameters and BMI were statistically significant, the overall variance explained by BMI was not particularly high. BMI accounted for the highest variation in WBC, similar to what was found in a 2003–2006 NHANES study.4 The negative correlation with MCV was also noted in that same study, and the investigators concluded that the finding could be secondary to iron deficiency anemia. Prior studies have shown that obesity can lead to iron and other micronutrient deficiencies, which might explain this association.11 Several studies have investigated the potential association of RDW with waist circumference,4 metabolic syndrome,12 cardiovascular disease,13 and obesity.14 The underlying pathophysiology of these relationships is still unknown. Some investigators have suggested that inflammation affects the hepatic production of the iron regulatory peptide hormone (hepcidin), causing abnormal iron absorption, thereby affecting the MCV and RDW.15
Total platelet count and MPV reflect the platelet number and size, respectively. Platelets have roles in inflammation, thrombosis, and atherogenesis.16 Obesity is believed to be a prothrombotic state resulting from a combination of increased thrombin generation, platelet hyperactivity, and decreased fibrinolysis.17 Our study found results similar to previous studies because both total platelet counts and MPV were increased with higher BMIs.4 For other blood component ratios (i.e., neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index), there is no established relationship between these parameters and BMI. A previous small observational study showed a positive correlation.3 A possible explanation is that low-grade inflammation increases neutrophil and platelet counts. We found that the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio are gender specific regarding their relationship with BMI, but the systemic immune-inflammation index is positively correlated with BMI in both genders. These results need further study to find plausible explanations.
Our study had several limitations. First, we could not analyze individual details regarding diet, medication, iron level, and ferritin level, which might affect hematopoiesis. Second, the method of measurement, type of analyzer, and anticoagulant used for CBC studies might affect these parameters. This concern would require studies comparing results with the most common CBC analyzers.
In conclusion, BMI had a significant association with most common hematologic inflammatory markers. The most useful index is possibly the systemic immune-inflammation index because it involves three components of the CBC and might have more pronounced changes with small or early changes in clinical status.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Furuncuoglu Y, Tulgar S, Dogan AN, et al. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016;20:1300–1306. [PubMed] [Google Scholar]
- 4.Vuong J, Qiu Y, La M, et al. Reference intervals of complete blood count constituents are highly correlated to waist circumference: should obese patients have their own “normal values”? Am J Hematol. 2014;89:671–677. doi: 10.1002/ajh.23713. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33:e22964. doi: 10.1002/jcla.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 7.National Institutes of Health . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 8.Mayo Clinic Laboratories . Complete blood count (CBC) with differential, blood. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/9109. Accessed May 29, 2020.
- 9.University of Chicago Medical Laboratories. Blood count , complete. https://uchicagomedlabs.testcatalog.org/show/CBC. Accessed May 29, 2020.
- 10.National Center for Health Statistics. Laboratory Procedure Manual . https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/CBC_I_MET_Complete_Blood_Count.pdf. Accessed May 29, 2020.
- 11.Zhao L, Zhang X, Shen Y, et al. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. 2015;16:1081–1093. doi: 10.1111/obr.12323. [DOI] [PubMed] [Google Scholar]
- 12.Farah R, Khamisy-Farah R.. Significance of MPV, RDW with the presence and severity of metabolic syndrome. Exp Clin Endocrinol Diabetes. 2015;123:567–570. doi: 10.1055/s-0035-1564072. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Zhou H, Tang Q.. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. 2017;2017:7089493. doi: 10.1155/2017/7089493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaya A, Alis R, Hernandez-Mijares A, et al. Red blood cell distribution width is not related with inflammatory parameters in morbidly obese patients. Clin Biochem. 2014;47:464–466. doi: 10.1016/j.clinbiochem.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Wei S, Zhang W, Wang C, et al. Increased hepcidin expression in adipose tissue as a primary cause of obesity-related inhibition of iron absorption. J Biol Regul Homeost Agents. 2019;33:1135–1141. [PubMed] [Google Scholar]
- 16.Nording HM, Seizer P, Langer HF.. Platelets in inflammation and atherogenesis. Front Immunol. 2015;6:98. doi: 10.3389/fimmu.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens I, van Gaal LF.. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85–101. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]