Abstract
Ehlers-Danlos syndrome (EDS) is a multifaceted debilitating disease. Affected patients are at risk for complications such as joint hypermobility and cardiac disease, but the prevalence, course, and management of these conditions are not well understood. The objective of this retrospective cohort study was to investigate the demographic characteristics and systemic manifestations in EDS. We performed a retrospective analysis of 98 EDS patients seen in a physical medicine and rehabilitation clinic between January 2015 and April 2019. Charts were reviewed for demographic information, subtype of EDS, characteristics of musculoskeletal pain, and presence of certain systemic comorbid diagnoses: autonomic dysfunction, headaches/migraines, gastrointestinal conditions, cardiovascular anomalies, mast cell activation syndrome, and temporomandibular joint dysfunction. Of 98 patients, 75 were diagnosed with EDS-hypermobile type (EDS-HT); 94 patients were women, and the mean age was 36.7 years. On average, each patient reported involvement of 5.4 joints, with the shoulder, knee, and lumbar spine as the most common. The average number of comorbid systemic conditions was 2.8, of which autonomic dysfunction was the most common. This study aims to provide a better understanding of this disease to promote earlier and more accurate diagnoses to guide treatment and prevent complications.
Keywords: Arthralgia, Ehlers-Danlos syndrome, joint instability
Ehlers-Danlos syndrome (EDS) is a connective tissue disease that predominantly affects women and has a prevalence of approximately 1 in 2500.1 There are several different subtypes, with the classical and hypermobility type (EDS-HT) encompassing 90% of cases.2 EDS-HT, the most common but least severe, has no genetic marker and shares characteristics with juvenile hypermobility syndrome.2–4 Therefore, the only way to confirm a diagnosis of EDS-HT is with a thorough clinical exam and clinical tools such as the Villefranche, Brighton, or Beighton criteria.2,5 Despite recent advances in research, studies have shown that up to 56% of patients receive a misdiagnosis and 70% undergo inappropriate treatments, with time between symptom onset and diagnosis as high as 28 years.6 This limited understanding may hinder the detection and treatment of otherwise preventable complications.6 The aim of this study was to describe and analyze the common syndromes that afflict patients with EDS and provide a review of the literature to better understand this condition, facilitate earlier and more accurate diagnosis, and provide appropriate treatment.
METHODS
This research was approved by the institutional research ethics committee. Patients provided written informed consent to be included in the study, and all patient information was deidentified. A retrospective chart review of all patients seen in a physical medicine and rehabilitation clinic between January 2015 and April 2019 was conducted to identify patients with a documented diagnosis of EDS. From the medical records of each included patient, we determined the reasons for each visit and assessed the medical history to identify relevant information. Demographic data such as age, gender, subtype of EDS, and number of clinic visits were extracted. Further detailed review of subspecialty records was performed to elicit information pertaining to musculoskeletal manifestations and presence of systemic conditions such as migraines, autonomic dysfunction, cardiovascular conditions, mast cell activation syndrome, gastrointestinal dysfunction, and temporomandibular joint (TMJ) dysfunction. Associated conditions were included only if formally diagnosed by a physician. Duplicate data were excluded.
RESULTS
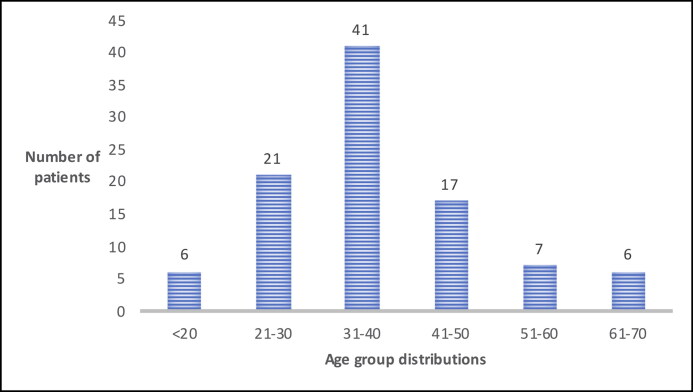
A total of 98 patients with a diagnosis of EDS were included in this study. Patient ages ranged from 18 to 67 years old (mean 37.5 ± 11.8), with 42% of patients in their 30s (Figure 1). Most (96%) were women. Seventy-eight percent of the patients carried a diagnosis of EDS-HT, 1% had classic type, 1% had cardiac valvular type, and 20% were uncharacterized. The mean number of physical medicine and rehabilitation clinic visits per patient was 4.2 (ranges 1–34), with 24% of patients seen once, 32% seen two to four times, and 44% seen five or more times. All patients had established care with genetics. Other frequent subspecialty clinic referrals were neurology (54%), cardiology (38%), gastroenterology (34%), and psychiatry (26%).
Figure 1.
Ehlers-Danlos syndrome patients clustered by age.
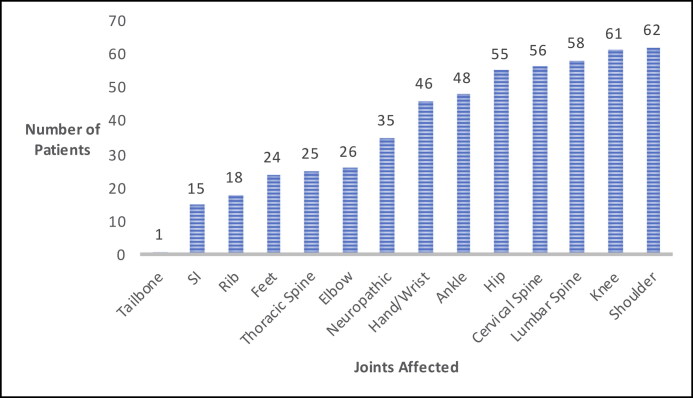
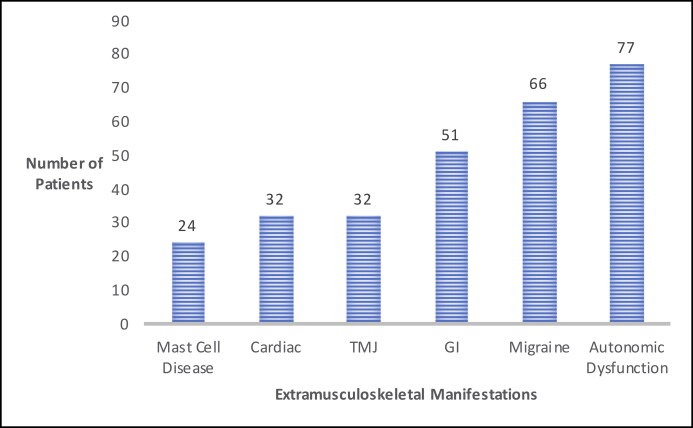
The mean number of joints affected was 5.4. Figure 2 depicts the most commonly affected joints. On average, each patient was diagnosed with 2.8 nonmusculoskeletal systemic conditions. Figure 3 depicts the number of patients with the systemic manifestations of autonomic dysfunction, headaches/migraines, gastrointestinal conditions, TMJ dysfunction, cardiac conditions, and mast cell activation syndrome. Certain symptoms seemed to coincide with others. Of the 66 patients diagnosed with headaches/migraines, 47 patients were also diagnosed with autonomic dysfunction (71%). Further, 27 of the 32 patients (84%) diagnosed with cardiac conditions also were diagnosed with autonomic dysfunction; 23 of the 24 patients (95%) with mast cell activation syndrome had headaches/migraines; 22 patients (91%) of the same population also had autonomic dysfunction; and 36 patients (70%) with gastrointestinal disease also had autonomic dysfunction.
Figure 2.
Most commonly affected joints in Ehlers-Danlos syndrome. SI indicates sacroiliac.
Figure 3.
Extramusculoskeletal manifestations in Ehlers-Danlos syndrome. GI indicates gastrointestinal; TMJ, temporomandibular joint.
DISCUSSION
In this study, most patients were women, with the greatest proportion between 31 and 40 years old (41%). Other studies have similarly reported a high proportion of young women in the EDS population. The cause is still unclear, with some suggesting a female sex hormone influence.1 In addition, many chronic pain syndromes also tend to preferentially affect women.1 Another explanation for the large variability of symptoms among different patients is the inheritance pattern of autosomal dominant with incomplete penetrance and variable expressivity.7 Age-related reduction in joint laxity may also partially explain why hypermobility symptoms are more pronounced in younger adults.8
All patients in this study reported musculoskeletal pain with more than one joint affected, consistent with a study by Rombaut et al that found joint pain in 100% of patients.9 Interestingly, the most commonly affected joints in our study besides the spine were the shoulder, knee, and hip, suggesting that the larger, higher activity-bearing joints with greater ranges of motion may be more susceptible to injury and pain. The lower prevalence of pain in smaller, less mobile joints such as the ribs, sacroiliac joint, and coccyx are consistent with this. Stern et al described similar findings in a study of 209 patients, reporting the knee, back, shoulder, ankle, and hips as the most common sites of pain.8
It is believed that central sensitization, nerve subluxations, and proprioceptive deficits may cause neuropathic symptoms.8–11 The prevalence in the literature varies with respect to neuropathic pain, with some studies reporting up to 68% as opposed to 36% in this study.10 This discrepancy highlights the difficulty in accurately identifying and treating pain syndromes in EDS. It is plausible that both the nociceptive and neuropathic pain generators are multifactorial, with contributors from both neuropathic and mechanical stressors. Thus, a treatment strategy optimizing both neuropathic and nociceptive pain management may be superior to treating each symptom individually. Specifically, Scheper et al and Bathen et al found reasonable success when utilizing physical and psychological treatments.12,13 While many patients opt for medication and procedural remedies, evidence of benefit is variable.8
Autonomic dysfunction, or dysautonomia, often presents with palpitations, dizziness, and syncope.4 The prevalence in the literature varies from 31% to 94%.4,14–16 One explanation for this variability is the use of separate methods of autonomic testing, ranging from questionnaires to validated measurements.4,14–16 In addition, studies have used different criteria for dysautonomia; some measured subjective presyncopal symptoms while others used specialized testing and exam maneuvers.4,14–16 Even when utilizing formal diagnostic criteria, current literature is also limited by factors such as age, duration/type of challenge, or medication use during testing.17 Initial treatment is conservative, focusing on optimizing fluid and electrolyte intake.1 Physical therapy consisting of graded, low resistance programs and activity modifications to improve muscle and vascular tone is also recommended.1 As cardiac manifestations can present similarly, referral to a cardiologist is recommended to rule out conditions such as mitral valve prolapse.18
Sacheti et al estimated that 30% to 40% of EDS patients suffer from chronic headaches and migraines.19 These symptoms in the EDS population exhibit an earlier symptom onset and a higher frequency of occurrence.7 Headaches may also be warning signs of dysautonomia, cerebrospinal fluid leak, or intracranial hypertension.14 Other causes include cervical spine hypermobility, atlantoaxial junction instability, meningeal fragility, TMJ dysfunction, and abnormal cerebral vasculature.7,20 Because the root causes are multifactorial and difficult to elucidate, measures of prevalence vary between studies. Surprisingly, some studies have reported a decreased consumption of analgesic medications for headache in this population, but this could be explained by patients’ acceptance of the headaches as part of their chronic living condition and thus not treatable.7
About 50% of patients in this study had been evaluated for at least one gastrointestinal-related symptom: 69% of patients had unspecified chronic abdominal pain, 54% had frequent nausea and vomiting, 40% had gastroesophageal reflux disorder, 29% had chronic constipation or diarrhea, and 7% had hernias. The prevalence varied from 37% to 87%, with one study in Italy describing patients with similar manifestations of dyspepsia (67%), gastroesophageal reflux disorder (57%), recurrent abdominal pain (62%), alternating constipation and diarrhea (33%), and abdominal hernias (5%).21 The high prevalence of gastrointestinal symptoms in our study is consistent with the literature, and variability in individual categories could be influenced by the subjective nature of symptom reporting, provider differences, and bias. Symptoms can be exacerbated by frequent use of opiates, which can worsen constipation and gastrointestinal motility.22 Unfortunately, typical diagnostic tests such as esophageal motility testing and endoscopic procedures are not as reliable.1
About 30% of patients in this study had been diagnosed with at least one cardiac condition, the most common being arrhythmia (23%), aortic root dilatation (15%), and valvular anomalies (9%). Similarly, Antani et al reported 34% of patients with diagnosed cardiac anomalies.23 While cardiac conditions are known to be highly associated with disorders of hypermobility due to collagen abnormalities in the vasculature, there is a lack of studies reporting prevalence. Of note, EDS patients are also at risk for other complications including aortic regurgitation, aortic root dilation, valvular anomalies, and Raynaud phenomenon.4 Insidious symptoms such as chest discomfort, presyncope, and palpitations should be thoroughly worked up, as they may be life-threatening indications of mitral valve prolapse.4
TMJ dysfunction is often present in conjunction with other findings such as friable/sensitive oral mucosa, absent frenulum, periodontitis, or abnormally shaped teeth.1 The prevalence of TMJ dysfunction in the literature is unclear, varying from 40% to 100%.24 De Coster et al found unilateral and bilateral TMJ dysfunction in 28% and 51% of patients, respectively.25 The current study did not differentiate between unilateral and bilateral TMJ but found relatively consistent prevalence at 33%. Initial treatment is preventive and combined with physical therapy to improve neck and upper back posture.24 Other treatments include splinting, prolotherapy, botulinum toxin, or surgery.24 Dental procedures can be challenging due to the decreased responsiveness to anesthetics in this population.1
Mast cell activation syndrome can present as flushing, pruritus, hypotension, asthma, diarrhea, bloating, and cramping.1 Patients may also exhibit food insensitivity and intolerance, and laboratory testing can reveal increased blood levels of mast cells and mast cell mediators such as histamine and tryptase.1 Cheung and Vadas reported a prevalence of 66% with mast cell syndrome as opposed to 24% in our study.26 This disparity could be due to several reasons. For one, mast cell activation syndrome can present similarly to common conditions such as seasonal allergies or the common cold.1 In addition, accurate diagnosis of mast cell activation syndrome often requires an allergist and utilization of advanced laboratory or tissue sampling measures.1 As no cure yet exists for mast cell activation syndrome, patients are treated symptomatically.27 Desensitization therapy can be used initially, and medications such as antihistamines, omalizumab, or leukotriene antagonists are alternatives.27 However, steroids should be avoided.2 Caution should also be exercised with using taping treatments for musculoskeletal pain as the skin can be hypersensitive to adhesives.28
In addition to establishing baseline data on the prevalence of comorbidities seen in EDS, this study was also able to establish certain trends. Hakim et al found that 12% of patients had concurrent autonomic, cardiac, and gastrointestinal symptoms.18 Our study demonstrated similar findings, with 19 of 98 (19%) patients diagnosed with the same spectrum of symptoms. De Wandele et al reported an average of four musculoskeletal and three nonmusculoskeletal symptoms per patient, which is also consistent with our data, which showed that patients had on average 5.4 and 2.8, respectively.22 The pathophysiological basis of these conditions is likely interrelated. For example, autonomic dysfunction can present with headaches related to hypotension, worsen gastrointestinal and secretomotor/thermoregulatory regulation, and mimic other symptoms such as reflux, bloating, or diarrhea; however, the exact mechanism is unknown.21,22 Medication side effects such as worsened orthostasis and constipation in opiates may also play a role.22
A major strength of this study is that it describes a large population of patients and provides a detailed overview of the manifestations seen in EDS. While the gender discrepancy favoring young women is consistent with the literature, there could be potential differences in symptom reporting between genders or different age brackets. Further investigation into gender differences in EDS should be considered. Most patients were diagnosed with EDS-HT, which limited the study’s ability to identify associations applicable to other subtypes. Future studies could investigate whether the prevalence and presentation of these manifestations are similar in other EDS subtypes. This study took place in a single academic clinic setting, which was well equipped with an established protocol for diagnosing and treating EDS patients. All patients suspected of having EDS were sent to genetics for confirmatory diagnosis. These data may not be applicable to other settings without the same resources.
In conclusion, EDS is a complex multidisciplinary condition. While the most common symptoms are musculoskeletal, many serious clinical features are systemic. Often, this disease is suspected only when evaluating the pattern of symptoms across multiple body systems. These symptoms may be insidious and misdiagnosed. Delay in diagnosis makes management of this condition difficult; therefore, early diagnosis is particularly important.
References
- 1.Tinkle B, Castori M, Berglund B, et al. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet C: Semin Med Genet. 2017;175(1):48–69. doi: 10.1002/ajmg.c.31538. [DOI] [PubMed] [Google Scholar]
- 2.Pennetti A. A multimodal physical therapy approach utilizing the Maitland concept in the management of a patient with cervical and lumbar radiculitis and Ehlers-Danlos syndrome-hypermobility type: a case report. Physiother Theory Pract. 2018;34(7):559–568. doi: 10.1080/09593985.2017.1422207. [DOI] [PubMed] [Google Scholar]
- 3.Nourissat G, Vigan M, Hamonet C, Doursounian L, Deranlot J.. Diagnosis of Ehlers-Danlos syndrome after a first shoulder dislocation. J Shoulder Elbow Surg. 2018;27(1):65–69. doi: 10.1016/j.jse.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Gazit Y, Jacob G, Grahame R.. Ehlers-Danlos syndrome—hypermobility type: a much neglected multisystemic disorder. Rambam Maimonides Med J. 2016;7(4):e0034. doi: 10.5041/RMMJ.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheper M, de Vries JE, Verbunt J, Engelbert RHH.. Chronic pain in hypermobility syndrome and Ehlers-Danlos syndrome (hypermobility type): it is a challenge. J Pain Res. 2015;8:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demmler JC, Atkinson MD, Reinhold EJ, Choy E, Lyons RA, Brophy ST.. Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: a national electronic cohort study and case-control comparison. BMJ Open. 2019;9(11):e031365. doi: 10.1136/bmjopen-2019-031365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puledda F, Viganò A, Celletti C, et al. A study of migraine characteristics in joint hypermobility syndrome a.k.a. Ehlers–Danlos syndrome, hypermobility type. Neurol Sci. 2015;36(8):1417–1424. doi: 10.1007/s10072-015-2173-6. [DOI] [PubMed] [Google Scholar]
- 8.Stern CM, Pepin MJ, Stoler JM, Kramer DE, Spencer SA, Stein CJ.. Musculoskeletal conditions in a pediatric population with Ehlers-Danlos syndrome. J Pediatr. 2017;181:261–266. doi: 10.1016/j.jpeds.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 9.Rombaut L, Malfait F, De Wandele I, et al. Muscle mass, muscle strength, functional performance, and physical impairment in women with the hypermobility type of Ehlers-Danlos syndrome. Arthritis Care Res (Hoboken). 2012;64(10):1584–1592. doi: 10.1002/acr.21726. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Rewari A, Shanthanna H.. Management of chronic pain in Ehlers-Danlos syndrome: two case reports and a review of literature. Medicine (Baltimore). 2018;97(45):e13115. doi: 10.1097/MD.0000000000013115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ericson WB Jr, Wolman R.. Orthopaedic management of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):188–194. doi: 10.1002/ajmg.c.31551. [DOI] [PubMed] [Google Scholar]
- 12.Scheper MC, Juul-Kristensen B, Rombaut L, et al. Disability in adolescents and adults diagnosed with hypermobility-related disorders: a meta-analysis. Arch Phys Med Rehabil. 2016;97(12):2174–2187. doi: 10.1016/j.apmr.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Bathen T, Hangmann AB, Hoff M, Andersen LO, Rand-Hendriksen S.. Multidisciplinary treatment of disability in Ehlers-Danlos syndrome hypermobility type/hypermobility syndrome: a pilot study using a combination of physical and cognitive-behavioral therapy on 12 women. Am J Med Genet A. 2013;161A(12):3005–3011. doi: 10.1002/ajmg.a.36060. [DOI] [PubMed] [Google Scholar]
- 14.Celletti C, Camerota F, Castori M, et al. Orthostatic intolerance and postural orthostatic tachycardia syndrome in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type: neurovegetative dysregulation or autonomic failure? Biomed Res Int. 2017;2017:9161865–9161867. doi: 10.1155/2017/9161865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakim AJ, Grahame R.. Non-musculoskeletal symptoms in joint hypermobility syndrome. Indirect evidence for autonomic dysfunction? Rheumatology (Oxford). 2004;43(9):1194–1195. doi: 10.1093/rheumatology/keh279. [DOI] [PubMed] [Google Scholar]
- 16.De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum. 2014;44(3):353–361. doi: 10.1016/j.semarthrit.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Roma M, Marden CL, De Wandele I, Francomano CA, Rowe PC.. Postural tachycardia syndrome and other forms of orthostatic intolerance in Ehlers-Danlos syndrome. Auton Neurosci. 2018;215:89–96. doi: 10.1016/j.autneu.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Hakim A, O'Callaghan C, De Wandele I, Stiles L, Pocinki A, Rowe P.. Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome-hypermobile type. Am J Med Genet C Semin Med Genet. 2017;175(1):168–174. doi: 10.1002/ajmg.c.31543. [DOI] [PubMed] [Google Scholar]
- 19.Sacheti A, Szemere J, Bernstein B, Tafas T, Schechter N, Tsipouras P.. Chronic pain is a manifestation of the Ehlers-Danlos syndrome. J Pain Symptom Manage. 1997;14(2):88–93. doi: 10.1016/S0885-3924(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 20.Chopra P, Tinkle B, Hamonet C, et al. Pain management in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):212–219. doi: 10.1002/ajmg.c.31554. [DOI] [PubMed] [Google Scholar]
- 21.Fikree A, Chelimsky G, Collins H, Kovacic K, Aziz Q.. Gastrointestinal involvement in the Ehlers-Danlos syndromes. Am J Med Genet C: Semin Med Genet. 2017;175(1):181–187. doi: 10.1002/ajmg.c.31546. [DOI] [PubMed] [Google Scholar]
- 22.De Wandele I, Rombaut L, Malfait F, De Backer T, De Paepe A, Calders P.. Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos syndrome. Res Dev Disabil. 2013;34(3):873–881. doi: 10.1016/j.ridd.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Antani J, Srinivas HV.. Ehlers-Danlos syndrome and cardiovascular abnormalities. Chest. 1973;63(2):214–217. doi: 10.1378/chest.63.2.214. [DOI] [PubMed] [Google Scholar]
- 24.Mitakides J, Tinkle BT.. Oral and mandibular manifestations in the Ehlers-Danlos syndromes. Am J Med Genet C: Semin Med Genet. 2017;175(1):220–225. doi: 10.1002/ajmg.c.31541. [DOI] [PubMed] [Google Scholar]
- 25.De Coster PJ, Van den Berghe LI, Martens LC.. Generalized joint hypermobility and temporomandibular disorders: inherited connective tissue disease as a model with maximum expression. J Orofac Pain. 2005;19:47–57. [PubMed] [Google Scholar]
- 26.Cheung I, Vadas P.. A new disease cluster: Mast cell activation syndrome, postural orthostatic tachycardia syndrome, and Ehlers-Danlos syndrome. J Allergy Clinical Immunol. 2015;135(2):AB65. doi: 10.1016/j.jaci.2014.12.1146. [DOI] [Google Scholar]
- 27.Seneviratne SL, Maitland A, Afrin L.. Mast cell disorders in Ehlers-Danlos syndrome. Am J Med Genet C: Semin Med Genet. 2017;175(1):226–236. doi: 10.1002/ajmg.c.31555. [DOI] [PubMed] [Google Scholar]
- 28.Russek LN, Stott P, Simmonds J.. Recognizing and effectively managing hypermobility-related conditions. Phys Ther. 2019;99(9):1189–1200. doi: 10.1093/ptj/pzz078. [DOI] [PubMed] [Google Scholar]