Abstract
The aim of this cross-sectional study was to evaluate the use and application of the atherogenic index of plasma (AIP) in the prediction of cardiovascular risk factors including mixed hyperlipidemia, hypertension, hyperuricemia, and metabolic syndrome in a population of young Mexican adults. Values were obtained for metabolic parameters, such as glucose, triglycerides, cholesterol (total, high-density, low-density, and very low density), systolic and diastolic blood pressure, and uric acid. Through univariate and multivariate analysis, parametric comparisons were applied and receiver operating characteristic curves were plotted. Logistic regression analysis was used to assess the risk of hyperuricemia, hypertension, mixed hyperlipidemia, and metabolic syndrome from a high AIP. Metabolic parameters and AIP had a significant correlation, with higher rates observed with increased AIP. As a set, metabolic parameters increased with an AIP >0.21 (λ Wilks = 0.58, F(14,344) = 7.7, P < 0.0001). The area under the curve was statistically significant for prediction of hyperuricemia (0.6), mixed hyperlipidemia (0.9), hypertension (0.8), and metabolic syndrome (0.95). In conclusion, in a sample of young Mexican adults, AIP was strongly associated with cardiovascular risk factors and could serve as a useful marker for the prediction of metabolic alterations related to cardiovascular disease.
Keywords: Atherogenic index of plasma, cardiovascular risk factors, young adults
The atherogenic index of plasma (AIP) is the logarithmically transformed ratio of triglycerides and high-density lipoprotein cholesterol (HDL-C). This index has been studied as a biomarker of the smaller and denser low-density lipoprotein (LDL) molecule1 and cardiovascular risk.2 AIP corrected the normative distribution that showed a correlation with smaller LDL particles and an increase in fractional esterification rate.1 It was later shown to be consistent with the phenotype of LDL-C and HDL-C particles. Hypoalphalipoproteinemia and hypertriglyceridemia induce an increase in the percentage of small HDL-C particles, as well as small and dense LDL-C particles, which indicates that the simultaneous use of triglycerides and HDL-C in the AIP reflects the complex interactions of the lipoprotein metabolism as a whole and may be useful in the prediction of plasma atherogenicity.3,4 In this study, we evaluated the utility of AIP as a predictor of cardiovascular risk factors such as mixed hyperlipidemia, hypertension, hyperuricemia, and metabolic syndrome in a population of young Mexican adults.
METHODS
A cross-sectional study was carried out in the department of biochemistry of the School of Medicine of the Benemérita Universidad Autónoma de Puebla in Mexico. Apparently healthy young Mexican adults were enrolled from July 2011 to May 2018. The inclusion criteria were half-blood Hispanic-Americans by birth; an age of 18 to 22 years; no family history of chronic and metabolic diseases for first-generation relatives; no previous history of hospitalization for the previous year or diagnosis of chronic diseases; and no present or recent (6-month) use of pharmacological treatments. Exclusion criteria included having fasting times <12 h, a diagnosis of a metabolic disorder within the previous year, smoking, and hemolyzed or lipemic blood samples. Enrollment in the study was voluntary.
Anthropometric measures and body composition parameters (weight, height, waist circumference) were taken by a certified anthropometrist in compliance with International Society for the Advancement of Kinanthropometry standards of measurement. Clinical assessment was done by a certified physician, who also measured blood pressure and obtained a peripheral blood sample by venipuncture. Glucose, triglycerides, cholesterol (total, HDL, LDL, and very low-density lipoprotein [VLDL]), and uric acid were measured by a VITROS DT60 II analyzer (Ortho-Clinical Diagnostics, Raritan, NJ).
Hyperuricemia was defined as uric acid levels >6 mg/dL for women and >7 mg/dL for men. An elevated blood pressure level was defined as a systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mm Hg. Mixed hyperlipidemia was characterized as the presence of triglycerides ≥150 mg/dL and hypercholesterolemia (total cholesterol ≥200 mg/dL) in the same subject. Based on Adult Treatment Panel III criteria, metabolic syndrome was diagnosed if at least three of five criteria were met: waist circumference (modified parameter according to standards for Hispanic people) ≥85 cm in women and ≥90 cm in men; serum fasting glucose ≥100 mg/dL; triglycerides ≥150 mg/dL; HDL-C <40 mg/dL for men or <50 mg/dL for women; blood pressure >130/85 mm Hg.
AIP was calculated as log10 (triglycerides/HDL-C) and, according to previous studies, classified into three groups: low risk (<0.11), intermediate risk (0.11–0.21), and increased risk (>0.21).
This study was approved and renewed every year by the ethics and research committee of the School of Medicine of the Benemérita Universidad Autónoma de Puebla, with the registration number 279, since 2011.
Variables were presented as mean ± standard deviation. A normality test was carried out using the Kolmogorov-Smirnov statistic. One-way analysis of variance determined parametric comparisons for univariate changes of metabolic parameters, and multivariate analysis of variance was used to observe multivariate changes by the same parameters. A Pearson correlation coefficient was obtained for bivariate correlations. Nonparametric comparisons were performed by χ2 test for independent categorical variables. Receiver operating characteristic curves were plotted, and the area under the curves and their confidence intervals were obtained for prediction of different cardiovascular risk factors with AIP; sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratio were also calculated. Multivariate logistic regression analysis was performed to evaluate the risk of hyperuricemia, hypertension, mixed hyperlipidemia, and metabolic syndrome due to elevated AIP. Different regression models were created in which AIP >0.21 was used as the independent variable and hyperuricemia, hypertension, mixed hyperlipidemia, and metabolic syndrome were used as dependent variables. All models were adjusted for age, body mass index, and sex. Statistical significance was set at P < 0.05. All analyses were performed with SPSS version 21 for Windows (IBM Corp., Armonk, NY), and figures were plotted with GraphPad Prism scientific graphing software, version 6.01 for Windows (GraphPad Software, La Jolla, CA).
RESULTS
The total number of enrolled participants was 1004. Men comprised 37% of the population, and the mean age was 18.9 ± 2 years. Participants had average values of standard body composition (waist circumference, hip circumference, body mass index, and fat mass percentage). Averages for metabolic parameters were also within the normal range.
Body composition parameters differed for sex, being higher in men (all P < 0.01), with the exception of fat mass percentage, which was higher in women (27.1% vs. 17.1%, P < 0.0001). Metabolic parameters did not differ by sex, except for VLDL-C, which was higher in men (24.9 ± 11.9 vs. 21.9 ± 9.8, P < 0.0001), and HDL-C and uric acid. The AIP value was 0.02 ± 0.01 for men and −0.03 ± 0.01 for women (P < 0.0001).
Individuals were categorized according to cardiovascular and atherogenic risk assessed by AIP into low risk (70%), intermediate risk (13%), and increased risk (17%). Clinical variables and metabolic parameters were compared for each risk group by one-way analysis of variance (Table 1). There were no significant differences for age between groups, whereas somatometric variables and metabolic parameters increased with higher values of AIP.
Table 1.
Age, body composition, blood pressure, and metabolic parameters of Mexicans aged 18 to 22 years divided according to the AIPa
| Variable | Total (n = 1004) | Low risk (n = 700) | Intermediate risk (n = 137) | Increased risk (n = 167) | P |
|---|---|---|---|---|---|
| Women, n (%) | 639 (63%) | 471 (67%) | 86 (63%) | 82 (49%) | |
| Men, n (%) | 385 (37%) | 229 (33%) | 51 (37%) | 85 (51%) | |
| Age (years) | 18.9 ± 2 | 18.8 ± 1.9 | 19.1 ± 3.7 | 19 ± 1.7 | 0.4 |
| Weight (kg) | 64.3 ± 13.6 | 61.6 ± 12.2b | 67.1 ± 13.8b | 72.3 ± 15.8 | <0.0001 |
| Height (m) | 1.64 ± 0.08 | 1.63 ± 0.08b | 1.64 ± 0.07b | 1.66 ± 0.09 | <0.0001 |
| Waist circumference (cm) | 81.4 ± 11.2 | 80.3 ± 10.1b | 86 ± 10.5 | 86.9 ± 11.7 | <0.0001 |
| Hip circumference (cm) | 98 ± 8.6 | 96.7 ± 8.1b | 101 ± 8.7 | 101 ± 9.4 | <0.0001 |
| Body mass index (kg/m2) | 23.7 ± 4.1 | 22.9 ± 3.7b | 24.8 ± 4.4 | 25.8 ± 4.5 | <0.0001 |
| Fat mass (%) | 23.2 ± 7.1 | 23 ± 7.1 | 24.8 ± 4.4 | 25.8 ± 4.4 | 0.007 |
| Glucose (mg/dL) | 89.3 ± 16.6 | 88.3 ± 17.4b | 90.8 ± 14.3 | 92 ± 14.1 | 0.009 |
| Total cholesterol (mg/dL) | 164.3 ± 38.8 | 163.5 ± 32.2b | 169.3 ± 28.7b | 182.6 ± 42.4 | <0.0001 |
| Triglycerides (mg/dL) | 117.9 ± 55.9 | 92.3 ± 28.6b | 140.6 ± 23.9b | 206.7 ± 62 | <0.0001 |
| HDL-C (mg/dL) | 50.1 ± 13.2 | 54.5 ± 12.8b | 42.6 ± 6.8b | 37.7 ± 6.9 | <0.0001 |
| LDL-C (mg/dL) | 92.9 ± 24.7 | 89.7 ± 23.8b | 97.3 ± 20.7 | 102.3 ± 28.1 | <0.0001 |
| VLDL-C (mg/dL) | 23.1 ± 10.8 | 18.02 ± 5.5b | 28 ± 5b | 39.9 ± 11.3 | <0.0001 |
| Ureic acid (mg/dL) | 5.5 ± 1.4 | 5.3 ± 1.2b | 5.8 ± 1.4b | 6.1 ± 1.5 | <0.0001 |
| Systolic blood pressure (mm Hg) | 115.7 ± 13.5 | 112.3 ± 11.7b | 119.1 ± 12.6b | 125.2 ± 15.5 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 70.9 ± 9.4 | 69.5 ± 9.1b | 73.3 ± 10.6 | 74.3 ± 8.7 | 0.01 |
| Total cholesterol/HDL-C | 3.5 ± 1.2 | 3 ± 0.6b | 4 ± 0.6b | 5 ± 1.9 | <0.0001 |
| Triglycerides/HDL-C | 2.6 ± 1.7 | 1.7 ± 0.5b | 3.2 ± 0.2b | 5.6 ± 2.1 | <0.0001 |
| LDL-C/HDL-C | 2 ± 0.7 | 1.7 ± 0.5b | 2.3 ± 0.5b | 2.8 ± 0.9 | <0.0001 |
Mean ± standard deviation. Comparison was made by one-way analysis of variance and post hoc Tukey test for independent samples in the continuous quantitative variables.
Statistical differences (P < 0.05) vs. increased risk group.
HDL-C indicates high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
The prevalence of obesity was higher in patients with AIP >0.21 (16.8%) and lower in those with an AIP of 0.11 to 0.21 (12.4%) and <0.11 (4.4%) (P < 0.0001), Similarly, a trend of a higher prevalence of increased waist circumference (≥85 cm in women and ≥90 cm in men), or abdominal adiposity, was observed in subjects with AIP >0.21 (21.8%) compared with those with an AIP of 0.11 to 0.21 (19.5%) and <0.11 (16.1%), although there was no statistically significant difference (P = 0.2)
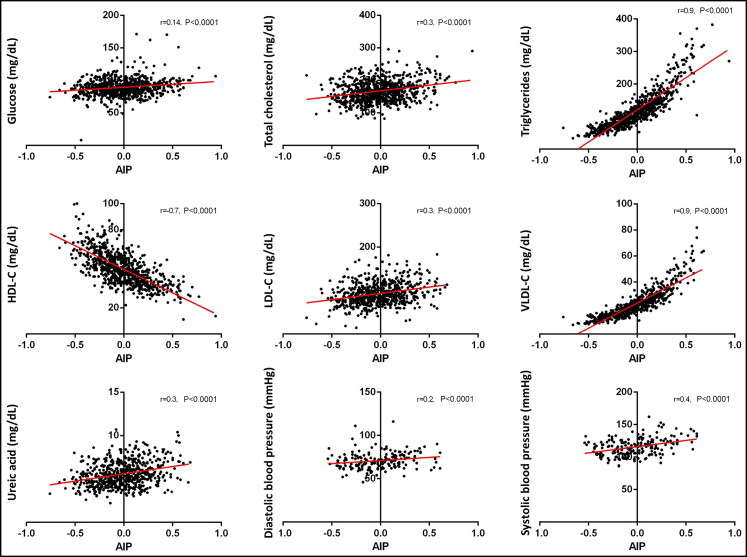
Total cholesterol, LDL-C, uric acid, and systolic blood pressure values had the most significant differences between low and increased risk groups and also had the greatest correlation with AIP; the rest of the parameters also had significant correlations (Figure 1). Triglycerides, HDL-C, and VLDL-C were increased in all three groups. Since triglycerides and HDL-C are used to calculate AIP and VLDL-C is calculated from triglycerides, the correlation was deemed invalid.
Figure 1.
Correlation of atherogenic index of plasma (AIP) with metabolic parameters in young Mexican adults. Row 1: Glucose, total cholesterol, triglycerides. Row 2: High-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and very low-density lipoprotein cholesterol. Row 3: Uric acid, diastolic blood pressure, systolic blood pressure.
A subanalysis was performed for sex in each risk group. In the low risk group (AIP <0.11), values of uric acid (6.1 ± 1.1 vs. 4.9 ± 1.1, P < 0.0001), systolic blood pressure (120.5 ± 8 vs. 109.4 ± 11.6, P < 0.0001), and diastolic blood pressure (72.5 ± 9.5 vs. 68.5 ± 9.7, P = 0.03) were higher in men than in women. In the intermediate risk group (AIP 0.11–0.21), glucose (95.7 ± 16.1 vs. 87.9 ± 12.3, P = 0.002), uric acid (6.8 ± 1.3 vs. 5.1 ± 1.14, P < 0.0001), and systolic blood pressure (127 ± 13 vs. 112.4 ± 7.3, P < 0.0001) were higher in men than in women. In the increased risk group (AIP >0.21), uric acid (6.8 ± 1.5 vs. 5.4 ± 1.3, P < 0.0001), systolic blood pressure (131.5 ± 12.4 vs. 113.9 ± 13.2, P = 0.001), and diastolic blood pressure (76.5 ± 8.2 vs. 70.1 ± 8.3, P = 0.04) were higher in men than in women. Further subanalyses of correlation between AIP, uric acid, and blood pressure in men and women showed that AIP correlated with values of uric acid (men: r = 0.3, P < 0.0001; women: r = 0.3, P < 0.0001) and systolic blood pressure (men: r = 0.4, P = 0.001; women: r = 0.2, P = 0.3) in both sexes.
The effect of increasing values of AIP in metabolic parameters was analyzed by multivariate analysis of variance. All metabolic parameters increased with an AIP >0.21 (ƛ of Wilks = 0.58, F(14,344) = 7.7, P < 0.0001), but not when obesity was present (ƛ of Wilks = 0.95, F(7,172) = 1.4, P = 0.2). Glucose, total cholesterol, uric acid, and LDL-C were the parameters in which increases were not due to obesity.
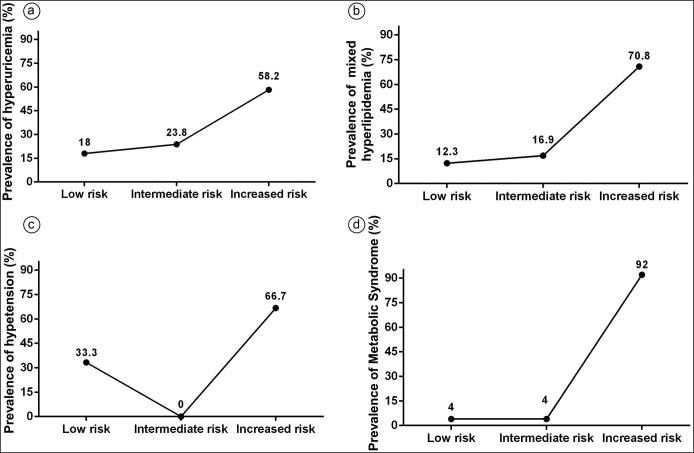
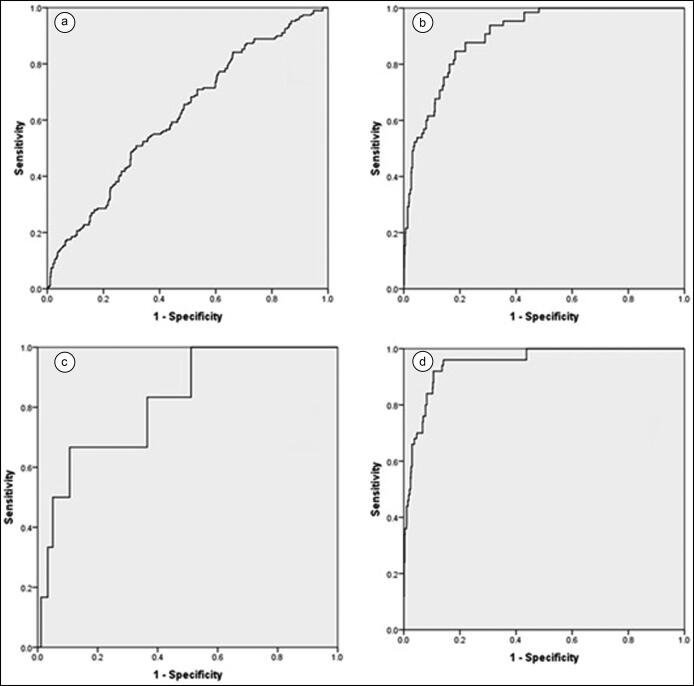
The prevalence of hyperuricemia (58.2%), hypercholesterolemia (29.3%), mixed hyperlipidemia (70.5%), hypertension (66.7%), and metabolic syndrome (92%) were statistically higher (P < 0.0001) in individuals with high AIP (>0.21) (Figure 2). The area under the curve with 95% confidence intervals was 0.62 (0.6–0.7, P < 0.0001) for prediction of hyperuricemia, 0.62 (0.6–0.66, P < 0.0001) for hypercholesterolemia, 0.9 (0.87–0.93, P < 0.0001) for mixed hyperlipidemia, 0.8 (0.7–0.97, P = 0.008) for hypertension, and 0.95 (0.92–0.98, P < 0.0001) for metabolic syndrome (Figure 3). The sensitivity of AIP was 0.23 (0.2–0.31) for hyperuricemia, 0.66 (0.2–0.95) for hypertension, 0.32 (0.25–0.40) for hypercholesterolemia, 0.7 (0.58–0.81) for mixed hyperlipidemia, and 0.92 (0.81–0.98) for metabolic syndrome. Specificity was 0.85 (0.82–0.87) for hyperuricemia, 0.83 (0.77–0.88) for hypertension, 0.86 (0.84–0.88) for hypercholesterolemia, 0.87 (0.84–0.89) for mixed hyperlipidemia, and 0.81 (0.85–0.89) for metabolic syndrome. The positive predictive value was 0.34 (0.26–0.42) for hyperuricemia, 0.2 (0.1–0.3) for hypertension, 0.29 (0.23–0.36) for hypercholesterolemia, 0.3 (0.2–0.3) for mixed hyperlipidemia, and 0.27 (0.21–0.34) for metabolic syndrome. The negative predictive value was 0.77 (0.73–0.80) for hyperuricemia, 0.99 (0.95–0.99) for hypertension, 0.88 (0.85–0.89) for hypercholesterolemia, 0.97 (0.96–0.98) for mixed hyperlipidemia, and 0.99 (0.98–0.99) for metabolic syndrome. The likelihood ratio was 1.6 for hyperuricemia, 3.9 for hypertension, 2.3 for hypercholesterolemia, 5.4 for mixed hyperlipidemia, and 7.2 for metabolic syndrome. The multivariate logistic regression analysis is shown in Table 2. The results suggest that an AIP >0.21 is an independent risk factor for the presence of hyperuricemia, mixed hyperlipidemia, and metabolic syndrome. Likewise, an AIP >0.21 was associated with an increased risk for hypercholesterolemia (odds ratio = 2.9, 95% confidence interval 1.9–4.5, P < 0.0001).
Figure 2.
Prevalence of (a) hyperuricemia, (b) mixed hyperlipidemia, (c) hypertension, and (d) metabolic syndrome in the three AIP risk groups.
Figure 3.
Receiver operating characteristic curve for prediction of (a) hyperuricemia, (b) mixed hyperlipidemia, (c) hypertension, and (d) metabolic syndrome by AIP.
Table 2.
Adjusteda odds ratios for hyperuricemia, dyslipidemia, hypertension, and metabolic syndrome
| b coefficient | Standard error | Odds ratio (95% CI) | P | |
|---|---|---|---|---|
| Model for hyperuricemia | 0.2 | 0.110 | 1.24 (1.1–1.5) | 0.04 |
| Model for mixed hyperlipidemia | 1.7 | 0.187 | 5.2 (3.6–7.5) | <0.0001 |
| Model for hypertension | 0.83 | 0.564 | 2.3 (0.76–6.9) | 0.14 |
| Model for metabolic syndrome | 2.5 | 0.37 | 11.9 (5.7–24.5) | <0.0001 |
Adjusted for age, body mass index, and sex.
DISCUSSION
In this study, we found that higher values of AIP were associated with a higher prevalence of obesity and abdominal adiposity. Similar results were found by Zhu et al who reported that higher AIP was positively and strongly associated with obesity.5 However, in our study, the prevalence of obesity or abdominal adiposity was low, which suggests that alterations in AIP may even precede obesity and abdominal adiposity. In addition, multivariate analysis indicated that variations in metabolic parameters were due to increased AIP and not due to increased body mass index; all the values of the metabolic parameters were significantly higher with an AIP >0.21. The presence of obesity only explains the increase in triglycerides, HDL-C, and VLDL-C. Since the studied population consisted of young adults, AIP could be a good predictor of cardiovascular risk even before the appearance of significant clinical manifestations of cardiovascular diseases or other metabolic disorders, which could lead to greater opportunities to deliver early preventive interventions to groups at risk.6–8
The smallest LDL particles, which have a role in the development of atherosclerosis, are not routinely measured.9,10 LDL-C is one of the main laboratory determinations which if abnormally elevated confers a high risk of cardiovascular disease; these molecules have also been the main target for pharmacological treatment.11 Current international guidelines for the management of cardiovascular risk and mixed hyperlipidemia rely on lipoprotein indexes, mainly triglycerides/HDL-C, for stratification of risk and to establish pharmacological treatment objectives.12,13 Atherogenic indexes, including AIP, have been shown to have a higher predictive value than independent parameters. The predictive ability of atherogenic indexes has been attributed to evidence that increased HDL-C is associated with regression of the atheromatous plaques, while decreases in LDL-C cause slowdown of plaque progression. Increased blood pressure levels have been correlated with AIP in other studies. The relationship between high blood pressure and atherogenesis is common in the metabolic syndrome.14
We have shown that an AIP >0.21 was significantly associated with hyperuricemia, hypercholesterolemia, mixed hyperlipidemia, and metabolic syndrome, which are all risk factors for cardiovascular diseases. These results in conjunction with the calculated area under the curve allowed us to validate the use of AIP with a cutoff point of >0.21 as a predictor of cardiovascular disease risk in young Mexican adults. This cutoff point has been validated, both in men and women, in different populations.1,15,16
The AIP has also been shown to be useful in a clinical setting, since it was found to be a superior marker for cardiovascular events (i.e., death, atherosclerosis, and stroke) than other biomarkers such as LDL-C, triglycerides, and non-HDL cholesterol.17–19 Similarly, AIP has been found to be increased in patients with acute myocardial infarction, making it a lower-cost alternative biomarker.20
The AIP correlates with the most frequently employed clinical anthropometric and metabolic parameters and has the ability of predicting their alterations in young Mexican adults. This index was also strongly associated with cardiovascular risk factors, being a useful predictor of metabolic alterations conferring risk for cardiovascular disease.
ACKNOWLEDGMENTS
The authors thank Dr. Javier Mancilla-Galindo from Unidad de Investigación UNAM-INC, Instituto Nacional de Cardiología Ignacio Chávez, México, for his advice and corrections regarding English grammar and style.
References
- 1.Dobiásová M, Frohlich J.. The plasma parameter log (TG/HDLC) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apo B-lipoprotein-depleted plasma (FER(HDL). Clin Biochem. 2001;34((7):583–588. doi: 10.1016/S0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 2.Dobiásová M. AIP-atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek. 2006;52(1):64–71. [PubMed] [Google Scholar]
- 3.Dobiásová M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004;50(7):1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G, Montagnana M, Luca Salvagno G, Targher G, Cesare Guidi G.. Epidemiological association between uric acid concentration in plasma, lipoprotein(a), and the traditional lipid profile. Clin Cardiol. 2010;33(2):E76–E80. doi: 10.1002/clc.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Yu L, Zhou H, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. 2018;17(1):37. doi: 10.1186/s12944-018-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezeukwu AO, Agwubike EO.. Anthropometric measures of adiposity as correlates of atherogenic index of plasma in non-obese sedentary Nigerian males. Libyan J Med. 2014;9(1):23798. doi: 10.3402/ljm.v9.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliarsingh S, Sharma N, Mukherjee R.. Serum uric acid: Marker for atherosclerosis as it is positively associated with “atherogenic index of plasma”. Arch Physiol Biochem. 2013;119(1):27–31. doi: 10.3109/13813455.2012.732580. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y, Li Y, Guo X, et al. Atherogenic index of plasma predicts hyperuricemia in rural population: a cross-sectional study from Northeast China. IJERPH. 2016;13(9):879. doi: 10.3390/ijerph13090879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björnheden T, Babyi A, Bondjers G, Wiklund O.. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis. 1996;123(1–2):43–56. doi: 10.1016/0021-9150(95)05770-6. [DOI] [PubMed] [Google Scholar]
- 10.Notarnicola M, DE Nunzio V, Tutino V, et al. ; MICOL Group . Integrated small dense low-density lipoprotein profile in cardiovascular disease and cancer: a longitudinal study. Anticancer Res. 2019;39(11):6035–6039. doi: 10.21873/anticanres.13809. [DOI] [PubMed] [Google Scholar]
- 11.Superko HR, King S, III.. Lipid management to reduce cardiovascular risk: a new strategy is required. Circulation. 2008;117(4):560–568. doi: 10.1161/CIRCULATIONAHA.106.667428. [DOI] [PubMed] [Google Scholar]
- 12.Frohlich J, Fodor G, McPherson R, et al. Rationale for an outline of the recommendations of the Working Group on Hypercholesterolemia and Other Dyslipidemias. Interim Report Can J Cardiol. 1998;14(A):17–21. [PubMed] [Google Scholar]
- 13.International Lipid Information Bureau. The ILIB Lipid Handbook for Clinical Practice: Blood Lipids and Coronary Heart Disease. New York, NY: ILIB; 2000. [Google Scholar]
- 14.Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 15.Raslova K, Dobiasova M, Hubacek JA, et al. Association of metabolic and genetic factors with cholesterol esterification rate in HDL plasma and atherogenic index of plasma in a 40 years old Slovak population. Physiol Res. 2011;60:785–795. doi: 10.33549/physiolres.932069. [DOI] [PubMed] [Google Scholar]
- 16.Akbas EM, Timuroglu A, Ozcicek A, et al. Association of uric acid, atherogenic index of plasma and albuminuria in diabetes mellitus. Int J Clin Exp Med. 2014;7(12):5737–5743. [PMC free article] [PubMed] [Google Scholar]
- 17.Bendzala M, Sabaka P, Caprnda M, et al. Atherogenic index of plasma is positively associated with the risk of all-cause death in elderly women: A 10-year follow-up. Wien Klin Wochenschr. 2017;129(21–22):793–798. doi: 10.1007/s00508-017-1264-1. [DOI] [PubMed] [Google Scholar]
- 18.Njajou OT, Kanaya AM, Holvoet P, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the health, aging and body composition study. Diabetes Metab Res Rev. 2009;25(8):733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobiásová M, Urbanova Z, Samanek M.. Relations between particle size of HDL and LDL lipoproteins and cholesterol esterification rate. Physiol Res. 2005;54:159–165. [PubMed] [Google Scholar]
- 20.Sujatha R, Subramanian K.. Atherogenic indices in stroke patients: A retrospective study. Iran J Neurol. 2017;16(2):78–82. [PMC free article] [PubMed] [Google Scholar]