Abstract
Statins are the most widely used class of drug in the United States. They lower blood cholesterol levels by inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase. Common side effects include myalgias and a mild increase in liver function tests. Statin-induced necrotizing autoimmune myopathy (SINAM) is a very rare side effect that is independent of the type and duration of statin use. Treatment involves high-dose steroids and immunosuppressants such as azathioprine, methotrexate, or mycophenolate mofetil. Nonresponders and patients with severe weakness can be treated with intravenous immunoglobulin or rituximab. We present a case of SINAM that was successfully treated with intravenous immunoglobulin.
Keywords: Immunosuppressants, rhabdomyolysis, SINAM, statin-induced necrotizing autoimmune myopathy, statins
Since their approval by the Food and Drug Administration in 1987, statins have become the most commonly prescribed class of drug in the United States.1 Statins cause a decrease in blood cholesterol levels by selective competitive inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, an enzyme involved in the rate-limiting step of cholesterol synthesis. Statins have the potential to cause many side effects, with the most common being myalgias, rhabdomyolysis, and transaminitis. Less common side effects include proteinuria, kidney dysfunction, and increased risk of developing diabetes mellitus.2 Statin-induced necrotizing autoimmune myopathy (SINAM) is very rare and occurs due to the formation of autoantibodies directed toward HMG-CoA reductase. Here we present a case of SINAM.
CASE DESCRIPTION
A 62-year-old black man with a past medical history of cerebrovascular accident, hypertension, and hyperlipidemia presented to the hospital with a 3-month history of diffuse myalgias, progressive proximal muscle weakness, and intermittent dark brown urine. He also had an unintentional 10-pound weight loss during this period. On presentation, his vitals were stable. Physical examination revealed normal heart sounds and lungs that were clear to auscultation. He had no focal neurological deficit or sensory loss, but strength was decreased (4/5) in upper extremities bilaterally. Lower-extremity strength was normal. Pertinent laboratory findings included a serum creatinine of 0.8 mg/dL (reference range 0.7–1.4), aspartate transaminase of 791 U/L (6–32), alanine aminotransferase of 686 U/L (10–55), alkaline phosphate of 125 U/L (50–136), creatine kinase of 35,817 U/L (21–232), lactate dehydrogenase of 1233 U/L (105–235), aldolase of 320.4 U/L (<8.1), erythrocyte sedimentation rate of 49 mm/h (0–30), and thyroid-stimulating hormone of 1.40 µIU/mL (0.4–4.0). The anti-HMG-CoA reductase antibody IgG level was >200 units (reference range <20). Our hospital did not have the capacity to test anti-HMG-CoA reductase IgG antibody, so the patient underwent an extensive workup while the final result was pending. Results were normal for antinuclear antibodies, rheumatoid factor, anti-cyclic citrullinated peptide IgG, extractable nuclear antigen antibody, viral hepatitis panel, alpha-1-antitrypsin antibodies, tissue transglutaminase, serum ceruloplasmin, liver/kidney microsomal antibodies, mitochondrial M2 antibodies, anti-smooth muscle antibodies, and myositis antibody panel.
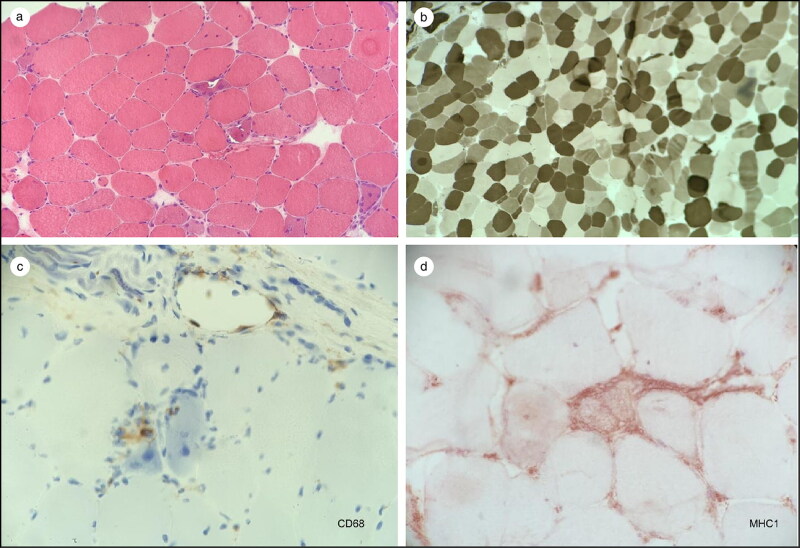
A nerve conduction study of the right upper and lower extremity was normal. However, needle electromyography of the right upper and lower extremity revealed a myopathic process associated with acute denervation that was concerning for inflammatory or toxic myopathy. A left quadricep muscle biopsy (Figure 1) showed immune myopathy with frequent necrotic fibers and scattered vacuoles. Immunochemistry for major histocompatibility class–1 (HLA-ABC) revealed mildly increased staining in occasional fascicles. CD3 and CD20 staining demonstrated scattered endomysial lymphocytes with more frequent B cells than T cells. CD68 staining highlighted frequent macrophages most prominently in the necrotic myofibers.
Figure 1.
(a) Hematoxylin and eosin stain showing necrotic fibers with mononuclear cell inflammatory infiltrate. (b) ATPase 4.6 stain showing no selective fiber atrophy. (c) CD68 stain showing darkly staining macrophages within the necrotic fibers. (d) Major histocompatibility complex increased in occasional fascicles, a feature consistent with inflammatory myopathies.
The patient was discharged on a high-dose prednisone taper. When seen in the clinic 10 days later with no improvement in symptoms or laboratory results, he was started on monthly intravenous immunoglobulin (100 g on day 1 and 100 g on day 2) while prednisone was continued. He received a second 2-day course of intravenous immunoglobulin at 1 month. At that time, he reported improvement in his symptoms, with serum creatine kinase trending down to 5174 U/L. He continued to feel better and received two more cycles of intravenous immunoglobulin monthly, with his serum creatine kinase levels decreasing to 3100 and 2100, respectively. His liver function normalized at the time of the fourth cycle. The plan is to continue intravenous immunoglobulin for six cycles and then switch to methotrexate.
DISCUSSION
SINAM is rare, with an estimated incidence of 2 to 3 per 100,000 people who use statins.3 There is no definitive association with any particular statin.4 There is great variation in statin use duration before symptoms develop,5 and the diagnosis usually lags disease onset, as it is almost exclusively diagnosed in tertiary care centers.6 It is characterized by significant loss of muscle power, pronounced myonecrosis on muscle biopsy, and irritable pattern on electromyography with elevated serum creatine kinase. The exact pathogenesis is not well understood. Statin exposure causes up-regulation of HMG-CoA reductase in statin-exposed muscles. The development of autoantibodies against HMG-CoA reductase suggests a direct role of statin exposure in development of this pathology.6
Due to SINAM’s rarity, it is essential to rule out other common medical entities. The identification of HMG-CoA reductase antibodies aids in the diagnosis, but given the low positive predictive value, it is not sufficient for diagnosis. Serum creatine kinase is markedly elevated, with electromyography revealing an irritable myopathy pattern similar to that seen with other inflammatory myopathies.7,8 Muscle biopsy is characterized by considerable myonecrosis without significant inflammation and lymphocytic infiltration.
Treatment involves starting oral prednisone with a dose of 1 mg/kg of body weight per day. Immunosuppressants such as azathioprine, methotrexate, or mycophenolate mofetil should be used as adjunct therapy. Nonresponders and patients with severe weakness can be treated with intravenous immunoglobulin or rituximab.9
References
- 1.Weintraub WS. Perspective on trends in statin use. JAMA Cardiol. 2017;2(1):11–12. doi: 10.1001/jamacardio.2016.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F, Ray KK, Wiklund O, et al; European Atherosclerosis Society Consensus Panel . Adverse effects of statin therapy: perception vs. the evidence—focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39(27):2526–2539. doi: 10.1093/eurheartj/ehy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixit A, Abrudescu A.. A case of atorvastatin-associated necrotizing autoimmune myopathy, mimicking idiopathic polymyositis. Case Rep Rheumatol. 2018;2018:5931046. doi: 10.1155/2018/5931046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molokhia M, McKeigue P, Curcin V, Majeed A.. Statin induced myopathy and myalgia: time trend analysis and comparison of risk associated with statin class from 1991-2006. PloS One. 2008;3(6):e2522. doi: 10.1371/journal.pone.0002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troyanov Y, Landon-Cardinal O, Fritzler MJ, et al. Atorvastatin-induced necrotizing autoimmune myositis: an emerging dominant entity in patients with autoimmune myositis presenting with a pure polymyositis phenotype. Medicine (Baltimore). 2017;96(3):e5694. doi: 10.1097/MD.0000000000005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamann PD, Cooper RG, McHugh NJ, Chinoy H.. Statin-induced necrotizing myositis—a discrete autoimmune entity within the “statin-induced myopathy spectrum.” Autoimmun Rev. 2013;12(12):1177–1181. doi: 10.1016/j.autrev.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selva-O'Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215–224. doi: 10.1080/1744666X.2018.1440206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casciola-Rosen L, Mammen AL.. Myositis autoantibodies. Cur Opin Rheumatol. 2012;24(6):602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374(7):664–669. doi: 10.1056/NEJMra1515161. [DOI] [PubMed] [Google Scholar]