Abstract
Cancer immunotherapy has impacted the treatment of numerous tumor types, including skin, lung, and colon cancers. Immune checkpoint inhibitors (ICI) activate the immune system to attack cancer cells, but this mechanism can also impact healthy cells. Dermatomyositis, an autoimmune syndrome affecting multiple organ systems, is often associated with cancer as a paraneoplastic syndrome, but this syndrome can also be induced by ICI. Here, we describe a case of dermatomyositis in a patient receiving pembrolizumab for treatment of squamous cell carcinoma of the lung and discuss the importance of recognizing complications of ICI.
Keywords: Adverse drug reaction, dermatomyositis, immune-related adverse event, immunotherapy, squamous cell carcinoma of the lung
Immune checkpoint inhibitors (ICI), monoclonal antibodies that target specific immune cell sites, have transformed the treatment approach for many cancers. Although ICI can aid in the clearance of tumor cells, this mechanism of action can also manifest in an autoimmune-like attack on healthy cells. These immune-related adverse effects (irAEs) of ICI commonly affect the skin, with more than a third of ICI patients experiencing a dermatologic irAE.1–3 Dermatomyositis is a rare autoimmune myopathy with cutaneous manifestations, such as heliotrope rash, poikiloderma, and Gottron papules, and severe systemic symptoms. It is often associated with malignancy as a paraneoplastic syndrome; however, dermatomyositis has also been reported as an irAE.4–6 Here, we report a case of new-onset pembrolizumab-associated dermatomyositis in a patient with squamous cell carcinoma of the lung.
CASE REPORT
A 74-year-old man with adenocarcinoma of the left lung treated with lobectomy and newly diagnosed squamous cell carcinoma of the lung, recently being treated with pembrolizumab and an interleukin-2 clinical trial, presented with a 3-month history of a rash, periorbital edema, and profound muscle weakness suspicious for a paraneoplastic syndrome. Prior to the unmasking of these symptoms before initiating immunotherapy, the patient reported a nonspecific asymptomatic skin discoloration. Immediately after his infusion with pembrolizumab, the cutaneous symptoms flared, accompanied by new systemic symptoms of severe fatigue, chills, wheezing, and muscle weakness. The violaceous eruption, which began on the abdomen, spread to the chest and then the elbows, medial thighs, and dorsal hands. Neither topical betamethasone nor oral methylprednisolone improved his symptoms after 3 days of use. Due to the severity of these symptoms, the patient’s lung cancer treatment was put on hold.
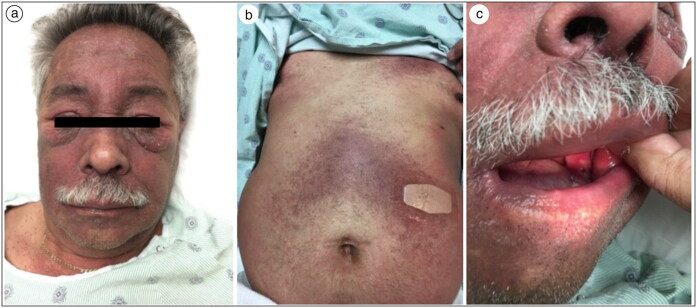
On exam, the patient’s scalp was diffusely erythematous with areas of scale. His face was violaceous with periorbital swelling (Figure 1). Erythematous plaques and violaceous macules covered the upper back, upper chest, abdomen, medial thighs, popliteal fossa, and left antecubital fossa. The right elbow had erythematous papules that converged into a plaque with overlying scale, and bilateral dorsal hands overlying the joints had erythematous patches. Oral mucosal ulceration was present. The new-onset muscle weakness made it difficult for the patient to raise his arms against gravity, and he required assistance to move from sitting to standing. His creatine kinase was 247 U/L and aldolase was within normal limits.
Figure 1.
(a) Violaceous facial rash and periorbital swelling, (b) abdomen with violaceous rash, and (c) oral cavity with buccal mucosal ulceration.
Punch biopsy revealed mild superficial perivascular dermatitis, with interstitial dermal mucin revealed via colloidal iron staining. Lymphocytes approximated the dermal-epidermal junction in association with necrotic basilar keratinocytes and pigment incontinence. Physical exam and laboratory results were deemed to be consistent with dermatomyositis. The patient was treated with a prednisone taper and intravenous immunoglobulin, which rapidly improved the symptoms of pain, muscle weakness, rash, and swelling around the eyes. All symptoms had virtually resolved within weeks, except for the rash, which steadily improved over the next 2 months. His immunotherapy was resumed and continued for 3 months, but the protocol was eventually discontinued after his fourth infusion due to cancer progression.
DISCUSSION
This report describes a case of dermatomyositis stimulated by pembrolizumab. It is key to appreciate multiple factors that can contribute to dermatomyositis in cancer patients treated with immunotherapy: tumor-associated paraneoplastic causes as well as drug-associated causes. Interestingly in this case, the patient previously developed a local, asymptomatic rash that transformed into a widespread, painful rash with ocular erythema and systemic symptoms only upon initiation of ICI therapy, which is suggestive of pembrolizumab inducing or unmasking this condition.7 However, despite the temporal relationship of the pembrolizumab infusion and the patient’s symptoms, it is possible that this dermatomyositis was due to a paraneoplastic syndrome. Further investigation into the intersection of irAEs and paraneoplastic disorders is warranted.7,8
Programmed cell death protein 1 (PD-1) inhibitors such as pembrolizumab commonly produce dermatologic and rheumatologic irAEs, such as psoriasis, lichenoid dermatitis, vitiligo, and lupus-like reactions.9 Although the precise mechanisms behind PD-1 inhibitors inducing autoimmunity has yet to be elucidated, these therapies may provoke the immune system to target tumor-associated antigens, which then cross-react with similar molecular patterns in normal tissues.7,8 Only a few cases of pembrolizumab-associated dermatomyositis have been documented, but these reports are likely to increase in number as PD-1 inhibitor use becomes more widespread.7–9
General management for dermatomyositis includes topical or systemic steroids, antihistamines, strict sun protection, and supportive treatment as required. Management of severe musculocutaneous irAEs may include immunotherapy treatment disruption or permanent discontinuation, which emphasizes the importance of prompt recognition and treatment of these toxicities.10 Here, our patient’s treatment regimen was disrupted due to the severity of his symptoms, and although his ICI treatment was resumed once this irAE was treated, unfortunately he died soon after due to the proliferation of his cancer.
Funding Statement
The study was funded by MD Anderson Cancer Center. The patient gave permission for this case to be published.
References
- 1.Rapoport BL, van Eeden R, Sibaud V, et al. . Supportive care for patients undergoing immunotherapy. Support Care Cancer. 2017;25(10):3017–3030. doi: 10.1007/s00520-017-3802-9. [DOI] [PubMed] [Google Scholar]
- 2.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP.. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28(4):254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 3.Curry JL, Tetzlaff MT, Nagarajan P, et al. . Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44(2):158–176., doi: 10.1111/cup.12858. [DOI] [PubMed] [Google Scholar]
- 4.Sheik Ali S, Goddard AL, Luke JJ, et al. . Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol. 2015;151(2):195–199. doi: 10.1001/jamadermatol.2014.2233. [DOI] [PubMed] [Google Scholar]
- 5.Kudo F, Watanabe Y, Iwai Y, et al. . Advanced lung adenocarcinoma with nivolumab-associated dermatomyositis. Intern Med. 2018;57(15):2217–2221. doi: 10.2169/internalmedicine.9381-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 7.Hinogami H, Yamashita C, Tanaka A, Shirai H, Nakano Y, Matsuura Y.. Case of dermatomyositis during treatment with pembrolizumab for lung cancer. J Dermatol. 2019;46(11):e430–e432. doi: 10.1111/1346-8138.14993. [DOI] [PubMed] [Google Scholar]
- 8.Liewluck T, Kao JC, Mauermann ML.. PD-1 Inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother. 2018;41(4):208–211. doi: 10.1097/CJI.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 9.Berger M, Legeay AL, Souci S, Streichenberger N, Thomas L, Dalle S.. Pembrolizumab-induced dermatomyositis in a patient with metastatic melanoma. Eur J Cancer. 2018;104:227–230. doi: 10.1016/j.ejca.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Plachouri K-M, Vryzaki E, Georgiou S.. Cutaneous adverse events of immune checkpoint inhibitors: a summarized overview. Curr Drug Saf. 2019;14(1):14–20. doi: 10.2174/1574886313666180730114309. [DOI] [PubMed] [Google Scholar]