Abstract
Graph theoretical analyses of nervous systems usually omit the aspect of connection polarity, due to data insufficiency. The chemical synapse network of Caenorhabditis elegans is a well-reconstructed directed network, but the signs of its connections are yet to be elucidated. Here, we present the gene expression-based sign prediction of the ionotropic chemical synapse connectome of C. elegans (3,638 connections and 20,589 synapses total), incorporating available presynaptic neurotransmitter and postsynaptic receptor gene expression data for three major neurotransmitter systems. We made predictions for more than two-thirds of these chemical synapses and observed an excitatory-inhibitory (E:I) ratio close to 4:1 which was found similar to that observed in many real-world networks. Our open source tool (http://EleganSign.linkgroup.hu) is simple but efficient in predicting polarities by integrating neuronal connectome and gene expression data.
Author summary
The fundamental way neurons communicate is by activating or inhibiting each other via synapses. The balance between the two is crucial for the optimal functioning of a nervous system. However, whole-brain synaptic polarity information is unavailable for any species and experimental validation is challenging. The roundworm Caenorhabditis elegans possesses a fully mapped connectome with an emerging gene expression profile of its 302 neurons. Based on the consideration that the polarity of a synapse can be determined by the neurotransmitter(s) expressed in the presynaptic neuron and the receptors expressed in the postsynaptic neuron, we conceptualized and created a tool that predicts synaptic polarities based on connectivity and gene expression information. Using currently available datasets we propose for the first time that the ratio of excitatory and inhibitory synapses in a partial connectome of C. elegans is around 4 to 1 which is in line with the balance observed in many natural systems. Our method opens a way to include spatial and temporal dynamics of synaptic polarity that would add a new dimension of plasticity in the excitatory:inhibitory balance. Our tool is freely available to be used on any network accompanied by any expression atlas.
Introduction
Chemical synapses of a neuronal network are both directed and signed, since a neuron is able to excite or inhibit another neuron. The nervous system of the nematode Caenorhabditis elegans has been fully mapped and reconstructed [1–3]. However, except for a few connections there is no comprehensive chemical synapse polarity data available [1]. While the direction of a synaptic connection can be inferred from its structure, the experimental determination of its polarity requires delicate electrophysiological methods with limited system-level use (e.g. patch-clamping) or more recent calcium-imaging or optogenetic techniques. Instead, in silico approaches using reverse engineering and genetic algorithms have efficiently predicted synaptic signs for different subnetworks of the C. elegans connectome [4–8].
Many synaptic sign prediction models have relied on the widely accepted assumption that the polarity of a chemical synapse is solely determined by the type of neurotransmitter released by the presynaptic neuron [4,5]. Therefore, in C. elegans excitatory glutamatergic and cholinergic, as well as inhibitory ɣ-aminobutyric acid (GABA)-ergic ionotropic chemical connections have been modeled. However, with this approach, approximately 6% of the connections turned to be inhibitory [9,10]. A low proportion of inhibitory connections can result in an unbalanced, over-excited network, as has been shown by previous publications [11–13]. Moreover, there is evidence of unconventional postsynaptic effects of neurotransmitters, such as cholinergic inhibition [14,15] or glutamatergic inhibition [16–18], meaning that a neuron can simultaneously excite and inhibit its postsynaptic partners with the same neurotransmitter due to variable neurotransmitter receptor expression on the postsynaptic neuron membrane. For example, the cholinergic AIY interneuron can activate RIB neurons and inhibit AIZ neurons in an acetylcholine-mediated fashion [15].
We aimed to predict synaptic polarities in the C. elegans ionotropic chemical synapse connectome (297 neurons and 20,589 synapses) relying on presynaptic neurotransmitter and postsynaptic receptor gene expression data for the three main neurotransmitters glutamate, acetylcholine and GABA. In this study of the C. elegans nervous system, we predicted the polarity of more than 70% of ionotropic chemical synapses and predicted a sign-balance of excitatory:inhibitory connections close to 4:1 that has been observed as functionally stable in many real-world circumstances. Presenting a new dataset, we show that the concept of gene expression-based polarity prediction can efficiently be applied to demonstrate a balanced E:I ratio in a nervous system.
Results
Creating a prediction tool of the synaptic polarities of the C. elegans connectome
Our primary goal was to infer synaptic polarity from combining connectivity and gene expression data. We created a simple, yet powerful algorithmic database (S1 Data) that takes as input connectome and gene expression data to predict synaptic polarity of ionotropic glutamatergic, cholinergic and GABA-ergic connections. We used the C. elegans WormWiring connectome data primarily in the form of a weighted edge list representing 20,589 chemical synapses in 3,638 connections. Our prediction tool is available here: http://EleganSign.linkgroup.hu.
Update of the previous neurotransmitter expression tables
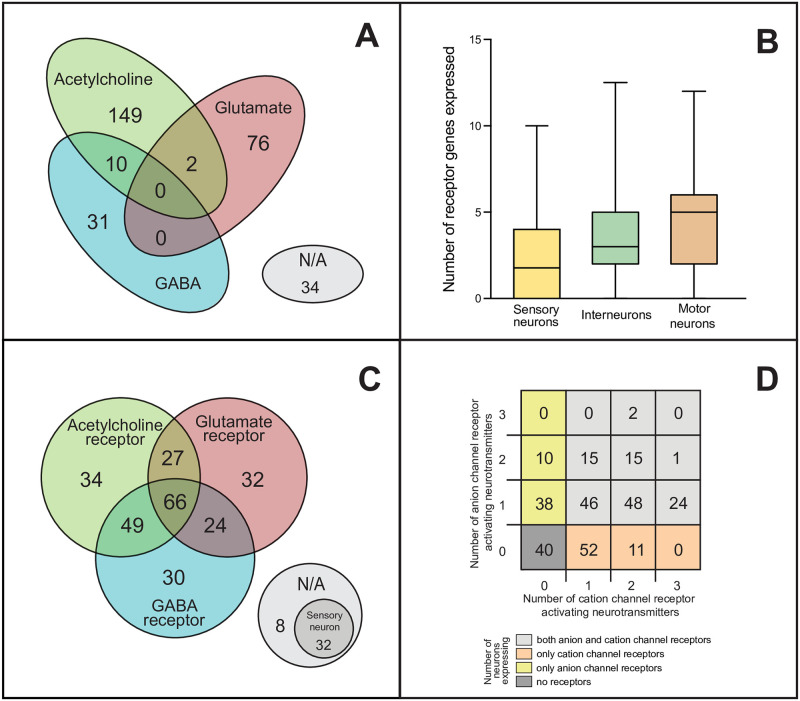
We updated the C. elegans neuronal neurotransmitter tables previously published [19–22] with recent evidence [10,23] (Methods). After this update, 256 neurons had a single neurotransmitter expressed, while 12 neurons had double neurotransmitter expression (Fig 1A). There were 34 neurons which did not express any of the three neurotransmitters investigated.
Fig 1. Neurotransmitter and receptor expression patterns of C. elegans neurons.
Expression data of the three major synaptic neurotransmitters and their receptors of C. elegans were collected from multiple datasets and were manually curated (see Methods). (A) Distribution of neurons according to their neurotransmitter expression: glutamate (red), acetylcholine (green), GABA (blue) or none (grey). (B) Number of receptor genes expressed by neurons, grouped by neuron modality. (C) Distribution of neurons based on their neurotransmitter receptor gene expression (colors are the same as in panel A). (D) Distribution of neurons according to the number of neurotransmitters for which anion and/or cation channel receptor genes are expressed.
Extraction of gene expression data
In parallel, we extracted gene expression data from Wormatlas [24], Wormbase (www.wormbase.org), and a recent RNA-sequencing dataset [23] which we curated manually to assign ionotropic receptor expression pattern to each neuron (see Methods). To do this we first sorted the previously identified 62 ionotropic receptor genes into six functional classes based on their suggested neurotransmitter ligand (glutamate, acetylcholine or GABA) and putative ion channel type (cationic or anionic, i.e. excitatory or inhibitory), as shown in Table 1. We found evidence for postsynaptic neuronal expression of 42 out of the 62 receptor genes in the C. elegans nervous system (genes marked bold in Table 1; also S1 Data). We also found an increasing average number of receptor genes in sensory, inter- and motor neurons, respectively (Fig 1B).
Table 1. Neurotransmitter receptor genes.
| Glutamate | Acetylcholine | GABA | |
|---|---|---|---|
| Cation channel receptor gene |
glr-1 glr-2 glr-3 glr-4 glr-5 glr-6 glr-7 glr-8 nmr-1 nmr-2 |
acr-1 acr-16 acr-2 acr-17 acr-3 acr-18 acr-4 acr-19 acr-5 acr-20 acr-6 acr-21 acr-7 acr-23 acr-8 acr-25 acr-9 deg-3 acr-10 des-2 acr-11 eat-2 acr-12 lev-8 acr-13 unc-29 acr-14 unc-38 acr-15 unc-63 |
exp-1 lgc-35 |
| Anion channel receptor gene |
glc-1 glc-2 glc-3 glc-4 avr-14 avr-15 |
acc-1 acc-2 acc-3 lgc-47 lgc-48 lgc-49 |
gab-1 ggr-1 ggr-2 ggr-3 lgc-36 lgc-37 lgc-38 unc-49 |
The Caenorhabditis elegans genome contains 62 ionotropic postsynaptic receptor genes for glutamate, acetylcholine, and GABA. acc-4 and lgc-46 genes were excluded from our database due to suggested presynaptic expression (S1 Table). In this table genes are grouped according to their neurotransmitter ligand and whether forming cationic (+) or anionic (−) ion channels (based on [24] and other references listed in S1 Table). In C. elegans "unconventional signaling", namely, glutamate-mediated inhibition, cholinergic inhibition and GABA-ergic excitation, is facilitated by 6, 6, and 2 receptor genes, respectively. In the gene expression database used in this work, expression in at least one neuron was found in the case of 42 genes (marked bold), while for 20 genes no neuronal expression was found.
Next, for all the 302 neurons of the C. elegans connectome we determined which receptor classes were expressed. 166 neurons had an overlapping expression of receptors for two or three different neurotransmitters (Fig 1C). The distribution of neurons according to their expression of cationic and/or anionic glutamate, acetylcholine and/or GABA receptors suggested functional diversity due to the high number of neurons expressing both excitatory and inhibitory receptors (Fig 1D). Surprisingly, 85 neurons expressed both excitatory and inhibitory receptor genes for the same neurotransmitter (S1 Data). Forty out of 302 neurons showed no receptor expression, out of which 32 neurons were primarily sensory neurons (S1 Data). The average number of receptor genes expressed was 3.7 per neuron (S2 Table).
Neurotransmitter and receptor gene expression-based polarity prediction
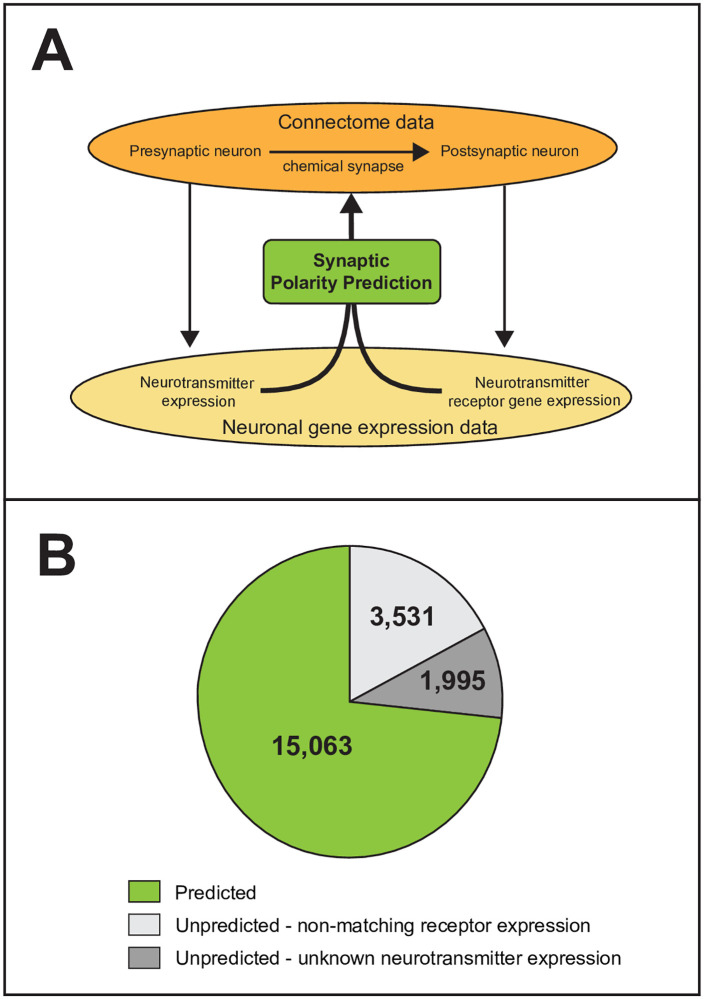
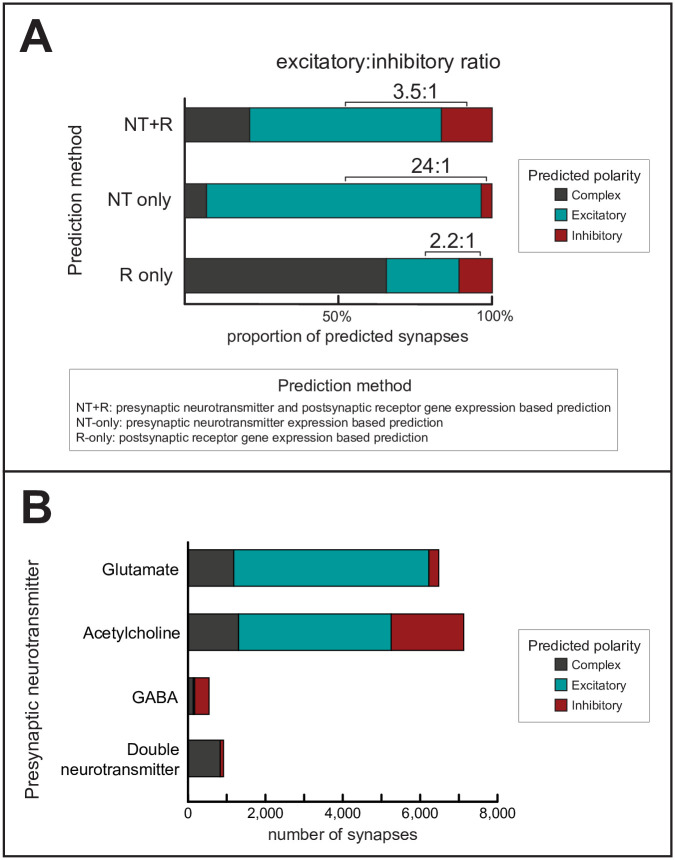
After assigning neurotransmitter and receptor expression patterns to each neuron, we predicted synaptic polarities by looking for matches between the neurotransmitter expression of the presynaptic neuron and the receptor gene expression of the postsynaptic neuron (Fig 2A). This way, we labeled synapses as one of the following: excitatory, inhibitory, complex, or unpredicted (see Methods). “Excitatory” or “inhibitory” label were given when the neurotransmitter-matched postsynaptic receptor genes were only cation or anion channel related, respectively. A synapse was labeled as “complex” if data suggested both excitatory and inhibitory function. With this approach, we predicted synaptic polarity for 73% of chemical synapses of the C. elegans connectome (Fig 2B). We could not predict polarity for the remaining synapses due to missing neurotransmitter data or mismatch in neurotransmitter/receptor expression (Fig 2B). We predicted that 9,070 of the synapses are excitatory and 2,580 are inhibitory, while 3,413 synapses have complex function (Fig 3A and S1 Data). These findings suggest that the overall ratio of excitatory and inhibitory synapses (E:I ratio) in the C. elegans ionotropic chemical synapse network is close to 4:1 (Fig 3A, NT+R method).
Fig 2. Prediction of synaptic polarities of the C. elegans ionotropic chemical synapse connectome.
(A) Prediction method. Connectome and gene expression data were manually curated (see Methods). Polarities of chemical synapses were predicted based on the neurotransmitter expression of presynaptic neurons and the matching receptor gene expression of the postsynaptic neurons. (B) Distribution of predicted and unpredicted synapses. We were able to predict polarity for 73% of chemical synapses (green). The polarities of the rest of synapses were unpredicted due to unknown neurotransmitter expression of the presynaptic neurons (dark grey) or non-matching receptor gene expression of the postsynaptic neurons (light grey).
Fig 3. Predicted synaptic polarities.
(A) Distributions of predicted polarities, using the method developed in this paper (NT+R) and two alternative methods as comparison (NT-only and R-only). Polarities were predicted by considering the neurotransmitter expression of the presynaptic neuron and/or the receptor gene expression of the postsynaptic neuron (see Methods). (B) Distributions of predicted synaptic polarities (using the NT+R method) according to the presynaptic neurotransmitter. Colors are the same as in panel A. Unpredicted synapses are not shown.
Alternative polarity prediction methods
To put our results in context, we applied two alternative prediction methods for comparison (NT-only and R-only; see Methods). The NT-only method yielded a much higher E:I ratio (Fig 3A, NT-only method; S2 Data), which is in line with the dominance of purely glutamatergic or cholinergic (traditionally excitatory) neurons over GABA-ergic (traditionally inhibitory) neurons (Fig 1A). To explain the difference further, the NT+R method predicted that 30% of cholinergic and 5% of glutamatergic synapses were inhibitory (Fig 3B) which is a significant fraction of synapses otherwise predicted excitatory with the NT-only method. A pairwise comparison of polarities predicted with the NT+R and NT-only methods is presented in S2 Data. The other, R-only method yielded a markedly smaller E:I ratio, however predicted an excessive number of complex synapses (Fig 3A, R-only method, S3 Data). This is due to the fact that many neurons express both cation and anion channel receptor genes (Fig 1D).
Feedback inhibition between neuron groups
Notably, in subsets of connections which connect neurons of different modalities of sensory neurons, motor neurons, interneurons and polymodal neurons, the E:I ratios varied between 1:10 (motor–> sensory) and 14:1 (inter–> motor). Importantly, we observed dominant inhibition in the motor–> sensory, motor–> inter, and inter–> sensory directions (Fig 4A), exhibiting inhibitory backward signaling as discussed in the literature previously [25,26], as opposed to a forward (sensory -> motor) excitatory excess. Besides, a significant presence of inhibitory and complex connections was found in the locomotion circuit as well (Fig 4B and S2 Fig).
Fig 4. Excitatory:inhibitory balance of different neuron groups.
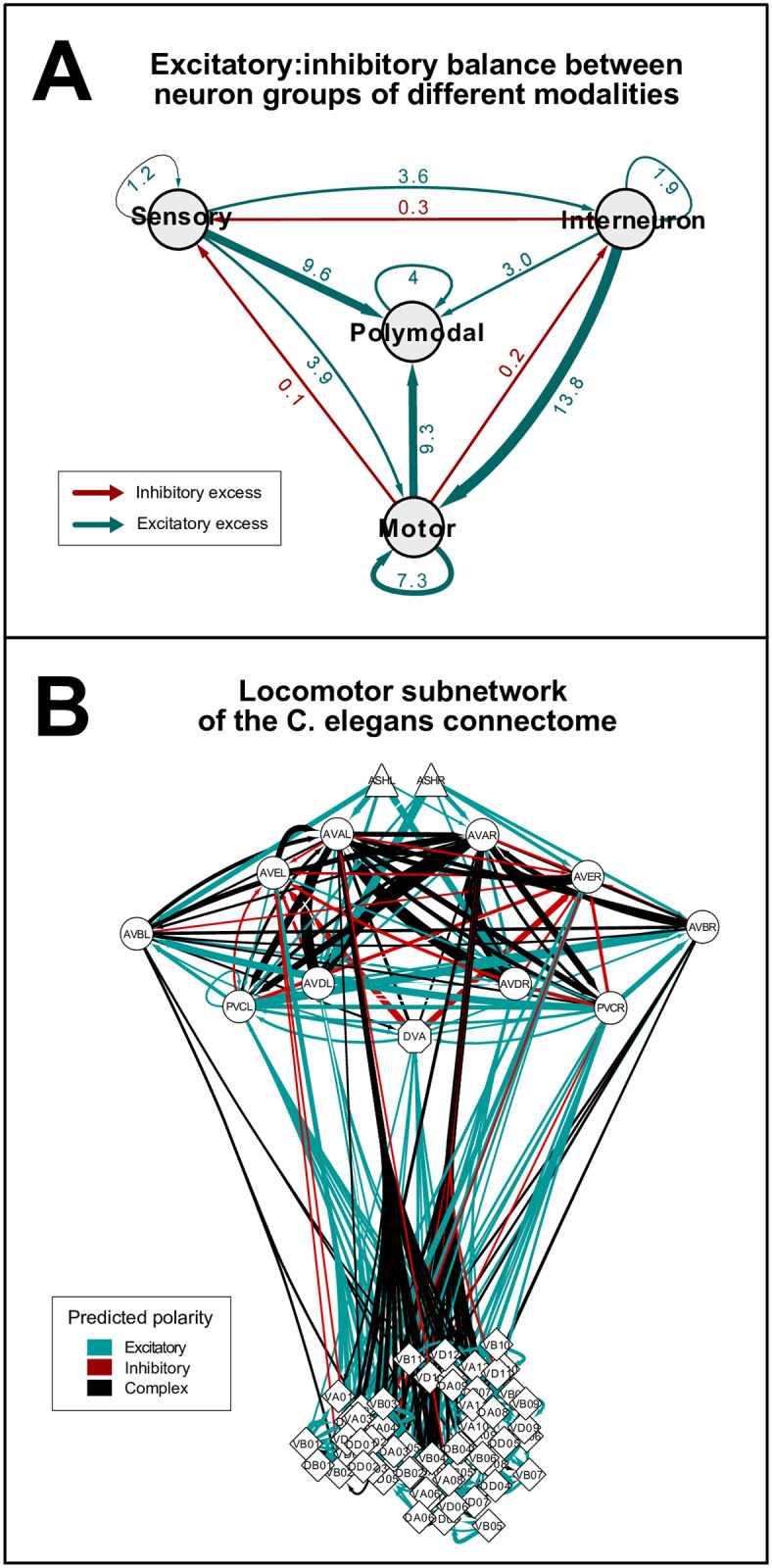
(A) Excitatory:inhibitory balance between neuron groups of different modalities. Nodes represent groups of neurons by modality. Edges are weighted according to the excitatory:inhibitory (E:I) ratios (see numbers). Green and red colors represent excitatory (E:I>1) and inhibitory (E:I<1) excess in sign-balances, respectively. (B) Network representation of the locomotion subnetwork. Edges represent excitatory (blue), inhibitory (red), or complex (black) chemical connections. Edges are weighted according to synapse number. The shape of vertices (Δ,○,◇) represent the modality (sensory, inter, motor, respectively) of neurons. Separate representations of the head circuit and the ventral nerve cord motor neurons are in S2 Fig.
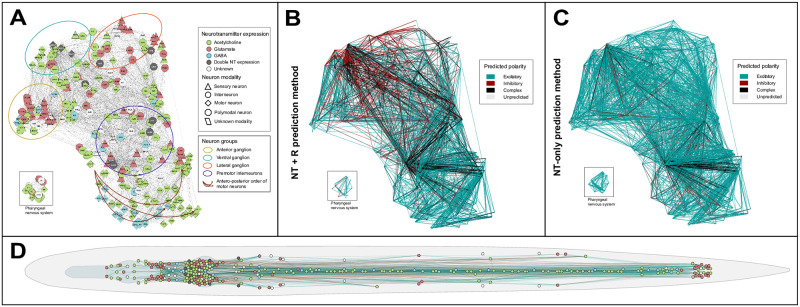
Network representations of the signed C. elegans connectome
Network representations of synaptic polarities in the C. elegans ionotropic chemical synapse connectome using the EntOptLayout plugin of Cytoscape [27] are in Fig 5. Fig 5A shows that the modular structure of the C. elegans connectome visualized by the EntOptLayout method nicely captures the anatomical locations of the anterior, ventral and lateral ganglions, as well as the premotor interneurons of the worm. While the anterior and lateral ganglions show a large glutamate expression, this is much less characteristic to the ventral ganglion (Fig 5A). Fig 5B shows that the ventral ganglion has predominantly inhibitory connections, while connections in the other locations are predominantly excitatory if predicted by our NT + R method. Fig 5C demonstrates that the prediction of polarities by neurotransmitters only (NT-only method) results in a large excitatory excess, mainly because the connections predicted as inhibitory or complex with the NT+R method turn into excitatory. This difference can be observed amongst head neurons and premotor interneurons, but less amongst motor neurons. Many connections of the polymodal neurons are predicted as complex only with the NT+R method. The anatomical locations of neurons expressing various neurotransmitters (Fig 5D) correspond well to the network representation shown in Fig 5A. Links between inhibitory function and anatomical structures have been shown in the human brain [28,29], but have not been demonstrated previously in the nematode.
Fig 5. Network representations of the C. elegans chemical synapse network.
(A-C) Network representations using the EntOpt layout plugin in Cytoscape [27]. (A) Color and shape of vertices represent neurotransmitter expression and modality of neurons, respectively (see inset for definitions). (B) Edges represent excitatory (blue), inhibitory (red), or complex (black) chemical connections predicted by the NT+R method (see Methods), weighted according to synapse numbers. (C) Colors of edges (see panel B) represent the polarities of chemical synapses predicted by the NT-only method. (D) Layout of vertices is representing the anatomic position of neurons. Node and edge colors are as in panels A and B, respectively. High-resolution representation is available in S1 File.
Validation of our predictions
To validate our results, we contrasted our predictions to previously published in silico and experimental work as well. Comparison to the computational findings of Rakowski & Karbowski [30] in the C. elegans locomotion circuit of 7 neuron groups and 652 synapses showed a 70% consistency in predicted synaptic polarities (53% on the level of connections) (S3 Table), albeit using a completely different concept. When testing our predictions against experimental evidence based on the literature we found that the majority of predicted polarities using our method were consistent with earlier findings in C. elegans: only 1 out of 12 interneuronal connections (29 / 501 synapses) reviewed was predicted an opposing polarity to what has experimentally been confirmed (S4 Table). This ratio is 6 /12 when validating the NT-only prediction method, supporting the importance of receptor expression.
To test the robustness of our NT+R prediction method to predict E:I balance, we applied the same rules to predict polarities of connections regardless of synapse number data, and after perturbations in the network like deletion of the 20 pharyngeal nervous system neurons or deletion of potentially variable (i.e. single-synapse) connections (S4 Data). Furthermore, we repeated our analyses in other published connectomes [2,3] of different sizes as well (S5 Table and S5 Data). In all five cases, the excitatory:inhibitory ratios were in the range of 3.1 to 4.1 (S1 Fig). When predicting not using the yet preprint-published RNA-seq expression dataset [23], this range was 3.7–4.1 (S6 Data). All together, these findings suggest that the observed sign-balance is a remarkably robust property of the C. elegans ionotropic chemical synapse network.
Discussion
Nervous systems are not only directed but signed networks as well since neurons either activate or inhibit other neurons [12]. The balance of excitatory and inhibitory connections (i.e. the sign-balance) is a fundamental feature of brain networks, clearly marked by the variety of disorders associated with its impairment [12,31,32]. However, direct evidence of single synapse polarity is rather sporadic even in simple species. In this work we predicted that the sign-balance in the C. elegans ligand-gated ionotropic chemical synapse network is approximately 4:1 (excitatory-inhibitory, E:I). This is consistent with previous in vitro and in vivo studies of nervous systems [33–36], and also with observations of different social networks [37,38], as shown in Table 2. However, this ratio can only be predicted if not only the neurotransmitter expression of the presynaptic neuron but also the receptor gene expression of the postsynaptic neuron is taken into consideration. Its significance is due to fourteen receptor genes that are presumed to encode inhibitory glutamatergic/cholinergic or excitatory GABA-ergic postsynaptic ion channel receptors. This concept of unconventional signaling is not new, but has already been described in C. elegans [14,39,40] and other primitive species [41–43], and also in mammals in the postnatal period [44,45]. This concept has already motivated the prediction of connection polarity instead of neuron polarity, yet on a subcircuit level [30]. Complementing recent work that used gene expression data for structural and functional modelling of the nervous system [46–48], our prediction model is a novel attempt to predict polarities of all ligand-gated ionotropic chemical synapses of the C. elegans connectome.
Table 2. Proportions of negative edges in signed networks.
| Network | Proportion of negative edges | Reference |
|---|---|---|
| C. elegans chemical synapse network | 20% | this work |
| Rat hippocampus (in vivo) | 5–30% | [35] |
| Rat excitatory neocortical neurons | 20% | [36] |
| Cerebral cortex (in vivo; GAD expression) | 10–20% | [17] |
| Cerebral cortex (in vivo; GABA neurons) | 20–25% | [17] |
| Optimal network for synchronized bursting activity (in silico) | 10–20% | [6] |
| Primary visual cortex (V1; in silico) | 25% | [49] |
| Neuronal network (ex vivo) and neuronal network model (in silico) | 20% | [18] |
| Wikipedia (social network) | 21% | [37] |
| Epinions (social network) | 15% | [37] |
| Slashdot (social network) | 23% | [37] |
| University freshman network (social) | 12–14% | [38] |
A surprisingly high proportion of synapses were predicted to have a complex, i.e. both excitatory and inhibitory polarity. This is due to parallel expression of cationic and anionic receptor genes–often for the same neurotransmitter–in half of the neurons. This suggests a highly complex functioning of neuronal connections that extends beyond the permanently exclusive concept of excitation-inhibition dichotomy. Since our work is mostly based on expression data of subunits instead of functional receptors, predictions made are derived from genetic permissibility rather than direct receptor complex presence. While ionotropic transmission in a single synapse is typically either excitatory or inhibitory, the predicted "complexity" can be resolved mainly in two physiologically well-established ways. One is that postsynaptic receptors are not homogenously distributed across the plasma membrane but their subcellular localization is regulated. This allows the receptors to act independently [50–55] and allows the same neurotransmitter to excite and inhibit at distinct postsynaptic sites. For example, such mechanisms have been identified in the AIA [56–58] and AIB neuron groups [59].
Another explanation of "complexity" is the dynamic change of gene expression in time which is observed all through the life-span of a worm e.g. during development, learning (synaptic plasticity), and aging [60–68]. Ultimately, changes in gene expression can lead to neurotransmitter-switching and consequential up- and downregulation of receptors of opposing polarity [69,70]. In its complexity, co-transmission by parallel expression of different neurotransmitters and receptors is one of the mechanisms of plasticity [71].
Currently, there is not enough data to address either the spatial or the temporal aspects of receptor expression regulation on the worm-scale. As expression-profiling methods will provide whole-brain and dynamic proteomics data of subcellular resolution, complex synapses might be further resolved.
There are several mechanisms of interneuronal communication acting in concert to transmit signals while maintaining a responsive but balanced system. The balance of excitation and inhibition—crucial for network stability—is reached via a number of mechanisms. Both synaptic and extrasynaptic, electrical and chemical, voltage-gated and ligand-gated, ion channel-mediated and G-protein coupled neurotransmission have diverse but intertwining roles in promoting and modulating excitation and inhibition. As there are significant mechanistic and functional differences (e.g. speed, modulatory role) between transmission modes, the neuronal connectome can be comprehended as a multiplex network of partially independent layers with each layer representing a distinct type of signaling [47,72,73]. Two dedicated cases were monoamine and metabotropic transmission which were excluded from our workflow. Since monoamine transmission via serotonin, dopamine and tyramine typically occurs extrasynaptically (i.e. 94% in case of tyramine-responsive neurons), expression data of even ionotropic channels (e.g. mod-1, lgc-55) would have been difficult to apply on the hard-wired connectome used in our study [47,74,75]. Additionally, in [47] the authors created a wireless (extrasynaptic) connectome of C. elegans based on matching monoamine/neuropeptide expression with receptor gene expression showing a network structurally different from the hard-wired connectome. Wireless networks possibly exist for ionotropic receptors as well via mechanism of spillover transmission [10,76]. Likewise, metabotropic neurotransmission typically acting via G-protein coupled receptors plays a rather modulatory than direct excitatory/inhibitory role in the nervous system by inducing broad, long-lasting, slow time-scale changes which is distinctive [71,73,77–80]. Our paper covers ligand-gated ionotropic synaptic connections, which account for the fast-acting system of neurotransmitter-mediated synaptic signaling. Additional layers of neural signaling can be targets of polarity prediction in subsequent studies and potentially overlayed on this signed network.
There are a number of limitations of our study which limit its generalizability at the current state: 1) although the most complete of any species, new connectivity data of the worm is still emerging [3,60] as well as 2) gene expression data [23,81]; 3) although our assumption that cation and anion channels are consistently excitatory and inhibitory, respectively, is generally valid based on their ion selectivity, the direction of a channel-mediated ion current is ultimately determined by a set of additional biophysical conditions e.g. ion gradients and the membrane potential [39,82,83] which were not considered in our work; 4) neurotransmission types other than ligand-gated ionotropic chemical signaling (e.g. G-protein coupled, monamine or neuropeptide) were excluded to avoid mixing different layers of neurotransmission (i.e. extrasynaptic, slow-scale, neuromodulatory transmission) [47,74,75]; 5) even in the case of ionotropic receptors, there are likely a number of additional ligand-gated ion channels that are still uncharacterized [84].
Although the strength of prediction of our work is generally acceptable (>70%), as new data of connectivity and gene expression emerge, our method can be used to provide more accurate predictions of synaptic polarity.
Within the scope of our aims and subject to the limitations discussed above we predicted synaptic polarities in the ionotropic chemical synapse network of C. elegans using expression data, for the first time. We developed and applied a novel method that combines connectivity with presynaptic and postsynaptic gene expression data and made its tool available for users at the website http://EleganSign.linkgroup.hu/. Amongst ionotropic chemical synaptic connections, balance of excitatory and inhibitory connections similar to other real-world networks can be well approximated only if one considers both pre- and postsynaptic gene expression, a concept that was lacking from previous work. Our method opens a way to include spatial and temporal dynamics of synaptic polarity that would add a new dimension of plasticity in the excitatory:inhibitory balance. When sufficient data is available, our polarity prediction method can be applied to any neuronal (and as a concept non-neuronal) network.
Methods
Description of C. elegans connectome data
Connectome reconstruction of the adult hermaphrodite worm published by WormWiring (http://wormwiring.org) consists of 3,638 chemical connections and 2,167 gap junctions, connecting 300 neurons (the two canal-associated neurons, CANL and CANR remained isolated in this reconstruction, and therefore were omitted from the connectome-related analyses). Each of the connections has 4 attributes: the presynaptic neuron, the postsynaptic neuron, the type of the connection (chemical or electrical), and the number of synapses. The chemical connections subset consisting of 20,589 synapses connecting 297 neurons was used in our work (the sensory neuron pair PLML and PLMR, and the pharyngeal neuron M5 is isolated in the chemical synapse network). Additionally, two other connectome reconstructions–both covering a smaller number of neurons and synapses–were used for validation (S5 Table).
Description of gene expression data and processing
Initially, neuronal binary gene expression data was obtained from a previous publication based on Wormbase [21]. This was extended with data of neuronal neurotransmitter [10,19,20,61] and receptor [24] expression, and with expression data from the recently published CenGen database [23] after transformation to binary information (S1 Text, S6 Table, and S7 Data). For receptor expression scoring, only the genes coding postsynaptic ionotropic receptor subunits were evaluated according to the six functional classes based on their suggested neurotransmitter ligand (glutamate, acetylcholine or GABA) and putative ion channel type. Cation and anion channel genes were categorized as excitatory and inhibitory, respectively. Expression of one or more genes in a functional class was considered positive.
Prediction of synaptic polarities
Polarities were predicted for connections based on presynaptic neurotransmitter and postsynaptic receptor expression data, using nested logical and conditional formulas. In case of the method referenced as the NT+R method throughout the paper, synapses were predicted as excitatory or inhibitory if only cation channel or only anion channel receptor genes matched the presynaptic neurotransmitter, respectively; complex if both types of receptor genes matched; and unpredicted if no receptor gene matched. Alternative prediction methods were used according to the following rules. NT-only method: synapses were predicted excitatory or inhibitory if the presynaptic neurotransmitter was acetylcholine and/or glutamate or GABA, respectively; complex if acetylcholine and/or glutamate and GABA; and unpredicted if the neurotransmitter was none of these. R-only method: synapses were predicted excitatory or inhibitory if the postsynaptic receptor genes expressed were only cation channel or anion channel coding, respectively; complex if both types of ion channel receptor genes were expressed; and unpredicted if no receptor gene was expressed. Exact formulas are available in Supplementary Data.
Software and data
Data were processed and predictions were made using Microsoft Excel (ver. 16.32) and R (RStudio 1.1.456) using standard packages.
Supporting information
Predictions were made based on the neurotransmitter and receptor gene expression patterns of the presynaptic and postsynaptic neurons, respectively (NT+R method, see Methods). Red, blue, and grey colors mark inhibitory, excitatory, and complex polarities, respectively. (A) Excitatory-inhibitory balances in alternative networks of the WormWiring connectome reconstruction. Bars from top to bottom: 1. synapse weighted network for comparative purpose (same as in Fig 3A); 2. weak links (defined by synapse number of 1) deleted [3]; 3. links connecting any of the pharyngeal nervous system neurons deleted. The rationale is that many previous work analyzed the connectome without the pharyngeal nervous system [2,85]; 4. unweighted network. (B) Predicted synaptic polarities for two connectome reconstructions other than Wormwiring, covering a variable number of neurons and synapses [2,3] (S5 Table). In summary, excitatory:inhibitory sign-balance ratios were similar in all cases, ranging between 3.1–4.1. Source data are provided in S1, S4 and S5 Data.
(TIF)
Figure is a split network representation of Fig 4B. Edges represent excitatory (blue), inhibitory (red), or complex (black) chemical connections. Edges are weighted according to synapse number. The shape of vertices (Δ,○,◇) represent the modality (sensory, inter, motor, respectively) of neurons. (A) Head circuit neurons. (B) Ventral nerve cord motor neurons. Colors as in Fig 4B.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(PDF)
The Caenorhabditis elegans genome contains 62 ionotropic postsynaptic receptor genes for glutamate, acetylcholine, and GABA. In this table genes are listed in alphabetic order, and the type of channel (+ for cation channel,–for anion channel) is presented with relevant reference. The 42 genes that were expressed postsynaptically in at least one neuron in our database (marked bold) have been validated for being cationic or anionic.
(DOCX)
Neuronal gene expression database was compiled from available datasets and manually curated (Methods). Genes encoding ionotropic receptors for glutamate, acetylcholine or GABA were grouped according to the type of ion channel (cation or anion), i.e. whether being excitatory or inhibitory. Bold numbers represent number of neurons expressing certain numbers of excitatory and/or inhibitory receptor genes. 63 neurons express only cation-channel receptor genes (green), while 48 neurons express only anion-channel receptor genes (red). 151 neurons express a mixture of cation- and anion-channel receptor genes (grey). Source data is in S1 Data.
(DOCX)
Predicted polarities from our results (S1 Data) were compared to the polarities predicted by Rakowski and Karbowski, 2017, for the locomotion circuit of the C. elegans connectome. Each cell represents a connection between the named source and target neuron. Green colored cell means that the predicted polarity was the same in both cases. Orange colored cell means that the predicted polarity was different with the two methods. 456 of the 652 synapses (70%) were predicted the same.
(DOCX)
Predicted polarities from our results (S1 Data) were individually compared to previously published experimental data. Each row represents a single connection. If validated (“Yes” in column “Validated?”), the NT+R predicted polarity equals the reference polarity. Partial validation means that the predicted and/or reference polarity was complex or uncertain.
(DOCX)
The three most complete connectome reconstructions of C. elegans, namely the WormWiring (http://wormwiring.org), as well as published by Varshney et al., 2011, and Cook et al., 2019, have fundamental differences in their coverage of chemical connections and synapse numbers.
(DOCX)
Bulk gene expression data was updated manually according to literature data. Green and red text shows receptor gene additions and deletions, respectively, to (from) specified neuron groups. Blue text shows neurotransmitter expression additions. acc-4 and lgc-46 genes were excluded to avoid false predictions because of literature evidence supporting a presynaptic localization rather than postsynaptic. All neurons of a neuron group were updated unless specified otherwise.
(DOCX)
(DOCX)
Acknowledgments
We thank members of the LINK network science group (http://linkgroup.hu) for their helpful comments.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This work was supported by the Hungarian National Research, Development and Innovation Office [Hungarian Scientific Research Fund, K131458 to P.C. and K116525 to C.S.](https://kormany.hu/emberi-eroforrasok-miniszteriuma), by the Higher Education Institutional Excellence Programme of the Ministry for Innovation and Technology in Hungary (https://nkfih.gov.hu) within the framework of the Molecular Biology thematic programme of the Semmelweis University [to P.C.] (https://semmelweis.hu), as well as by the Thematic Excellence Programme (Tématerületi Kiválósági Program, 2020-4.1.1.-TKP2020) of the Ministry for Innovation and Technology in Hungary (Nemzeti Kutatási, Fejlesztési és Innovációs Alap), within the framework of the artificial intelligence, biomarker thematic programme of the Semmelweis University [to P.C]. B.G.F was supported by the Human Capacities Grant Management Office in Hungary(Emberi Eroforrások Minisztériuma) [NTP-NFTÖ-18-B-0179 and NTP-NFTÖ-19-B-0264], also by the Semmelweis University (EFOP-3.6.3-VEKOP-16-2017-00009). C.S. is a Merit Prize recipient of the Semmelweis University (https://semmelweis.hu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc London B, Biol Sci. 1986;314: 1–340. 10.1098/rstb.1986.0056 [DOI] [PubMed] [Google Scholar]
- 2.Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput Biol. 2011;7: e1001066 10.1371/journal.pcbi.1001066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571: 63–71. 10.1038/s41586-019-1352-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wicks SR, Roehrig CJ, Rankin CH. A dynamic network simulation of the nematode tap withdrawal circuit: predictions concerning synaptic function using behavioral criteria. J Neurosci. 1996;16: 4017–4031. 10.1523/JNEUROSCI.16-12-04017.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rakowski F, Srinivasan J, Sternberg PW, Karbowski J. Synaptic polarity of the interneuron circuit controlling C. elegans locomotion. Front Comput Neurosci. 2013;7: 128 10.3389/fncom.2013.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong C-Y, Cho K-H. An optimally evolved connective ratio of neural networks that maximizes the occurrence of synchronized bursting behavior. BMC Syst Biol. 2012;6: 23 10.1186/1752-0509-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izquierdo EJ, Beer RD. Connecting a connectome to behavior: An ensemble of neuroanatomical models of C. elegans klinotaxis. Graham LJ, editor. PLoS Comput Biol. 2013;9: e1002890 10.1371/journal.pcbi.1002890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cangelosi A, Parisi D. A Neural network model of Caenorhabditis elegans: The circuit of touch sensitivity. Neural Process Lett. 1997;6: 91–98. 10.1023/A:1009615807222 [DOI] [Google Scholar]
- 9.McIntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364: 337–341. 10.1038/364337a0 [DOI] [PubMed] [Google Scholar]
- 10.Gendrel M, Atlas EG, Hobert O. A cellular and regulatory map of the GABAergic nervous system of C. elegans. Elife. 2016; e17686 10.7554/eLife.17686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin R, Abbott LF, Sompolinsky H. Balanced excitation and inhibition are required for high-capacity, noise-robust neuronal selectivity. Proc Natl Acad Sci. 2017;114: E9366–E9375. 10.1073/pnas.1705841114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatia A, Moza S, Bhalla US. Precise excitation-inhibition balance controls gain and timing in the hippocampus. Elife. 2019;8: e43415 10.7554/eLife.43415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker C, Ebsch C, Lampl I, Rosenbaum R. Correlated states in balanced neuronal networks. Phys Rev E. 2019;99: 52414 10.1103/PhysRevE.99.052414 [DOI] [PubMed] [Google Scholar]
- 14.Putrenko I, Zakikhani M, Dent JA. A family of acetylcholine-gated chloride channel subunits in Caenorhabditis elegans. J Biol Chem. 2005;280: 6392–6398. 10.1074/jbc.M412644200 [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Liu J, Zheng M, Xu XZS. Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell. 2014;159: 751–765. 10.1016/j.cell.2014.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450: 63–70. 10.1038/nature06292 [DOI] [PubMed] [Google Scholar]
- 17.Marom S, Shahaf G. Development, learning and memory in large random networks of cortical neurons: lessons beyond anatomy. Q Rev Biophys. 2002;35: 63–87. 10.1017/s0033583501003742 [DOI] [PubMed] [Google Scholar]
- 18.Pastore VP, Massobrio P, Godjoski A, Martinoia S. Identification of excitatory-inhibitory links and network topology in large-scale neuronal assemblies from multi-electrode recordings. PLOS Comput Biol. 2018;14: e1006381 10.1371/journal.pcbi.1006381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loer CM, Rand JB. The evidence for classical neurotransmitters in Caenorhabditis elegans. Altun ZF, Herndon LA, editors. WormAtlas. 2016. 10.3908/wormatlas.5.200 [DOI] [Google Scholar]
- 20.Pereira L, Kratsios P, Serrano-Saiz E, Sheftel H, Mayo AE, Hall DH, et al. A cellular and regulatory map of the cholinergic nervous system of C. elegans. Elife. 2015;4: e12432 10.7554/eLife.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobert O, Glenwinkel L, White J. Revisiting neuronal cell type classification in Caenorhabditis elegans. Curr Biol. 2016;26: R1197–R1203. 10.1016/j.cub.2016.10.027 [DOI] [PubMed] [Google Scholar]
- 22.Serrano-Saiz E, Poole RJ, Felton T, Zhang F, De La Cruz ED, Hobert O. Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell. 2013;155: 659–673. 10.1016/j.cell.2013.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SR, Santpere G, Reilly M, Glenwinkel L, Poff A, McWhirter R, et al. Expression profiling of the mature C. elegans nervous system by single-cell RNA-sequencing. bioRxiv. 2019; 737577 10.1101/737577 [DOI] [Google Scholar]
- 24.Altun ZF. Neurotransmitter receptors in Caenorhabditis elegans. WormAtlas. 2011. 10.3908/wormatlas.5.202 [DOI] [Google Scholar]
- 25.Martikainen MH, Kaneko KI, Hari R. Suppressed responses to self-triggered sounds in the human auditory cortex. Cereb Cortex. 2005;15: 299–302. 10.1093/cercor/bhh131 [DOI] [PubMed] [Google Scholar]
- 26.Dalenoort G, de Vries PH. The essential role of binding for cognition in living systems In: Schaub H, Detje F, Bruggemann U, editors. Logic af artificial life: Abstracting and synthesizing the principles of living systems. Berlin: Aka GmbH; 2004. pp. 32–39. [Google Scholar]
- 27.Ágg B, Császár A, Szalay-Bekő M, Veres DV., Mizsei R, Ferdinandy P, et al. The EntOptLayout Cytoscape plug-in for the efficient visualization of major protein complexes in protein–protein interaction and signalling networks. Bioinformatics. 2019;35: 4490–4492. 10.1093/bioinformatics/btz257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buzsáki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56: 771–783. 10.1016/j.neuron.2007.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5: 793–807. 10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- 30.Rakowski F, Karbowski J. Optimal synaptic signaling connectome for locomotory behavior in Caenorhabditis elegans: Design minimizing energy cost. PLOS Comput Biol. 2017;13: e1005834 10.1371/journal.pcbi.1005834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohal VS, Rubenstein JLR. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry. 2019/05/14. 2019;24: 1248–1257. 10.1038/s41380-019-0426-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Błaszczyk JW. Parkinson’s Disease and neurodegeneration: GABA-collapse hypothesis. Front Neurosci. 2016;10: 269 10.3389/fnins.2016.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci. 2004;7: 373–379. 10.1038/nn1206 [DOI] [PubMed] [Google Scholar]
- 34.Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, et al. Reconstruction and simulation of neocortical microcircuitry. Cell. 2015;163: 456–492. 10.1016/j.cell.2015.09.029 [DOI] [PubMed] [Google Scholar]
- 35.Gulyás AI, Megías M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19: 10082–10097. 10.1523/JNEUROSCI.19-22-10082.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi K, Kukita F. Intracellular [Cl−] modulates synchronous electrical activity in rat neocortical neurons in culture by way of GABAergic inputs. Brain Res. 2000;863: 192–204. 10.1016/s0006-8993(00)02152-1 [DOI] [PubMed] [Google Scholar]
- 37.Leskovec J, Huttenlocher D, Kleinberg J. Signed networks in social media. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. New York, NY, USA: ACM; 2010. pp. 1361–1370.
- 38.Kirkley A, Cantwell GT, Newman MEJ. Balance in signed networks. Phys Rev E. 2019;99: 012320 10.1103/PhysRevE.99.012320 [DOI] [PubMed] [Google Scholar]
- 39.Beg AA, Jorgensen EM. EXP-1 is an excitatory GABA-gated cation channel. Nat Neurosci. 2003;6: 1145–1152. 10.1038/nn1136 [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Torres A, Miledi R. Expression of Caenorhabditis elegans neurotransmitter receptors and ion channels in Xenopus oocytes. Proc Natl Acad Sci. 2006;103: 5120–5124. 10.1073/pnas.0600739103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kehoe J, McIntosh JM. Two distinct nicotinic receptors, one pharmacologically similar to the vertebrate α7-containing receptor, mediate Cl currents in Aplysia neurons. J Neurosci. 1998;18: 8198–8213. 10.1523/JNEUROSCI.18-20-08198.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cully DF, Paress PS, Liu KK, Schaeffer JM, Arena JP. Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J Biol Chem. 1996;271: 20187–20191. 10.1074/jbc.271.33.20187 [DOI] [PubMed] [Google Scholar]
- 43.Liu WW, Wilson RI. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc Natl Acad Sci U S A. 2013;110: 10294–10299. 10.1073/pnas.1220560110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kullmann PHM, Ene FA, Kandler K. Glycinergic and GABAergic calcium responses in the developing lateral superior olive. Eur J Neurosci. 2002;15: 1093–1104. 10.1046/j.1460-9568.2002.01946.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolstenholme AJ. Glutamate-gated chloride channels. J Biol Chem. 2012;287: 40232–40238. 10.1074/jbc.R112.406280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barabási DL, Barabási A-L. A genetic model of the connectome. Neuron. 2020;105: 435–445.e5. 10.1016/j.neuron.2019.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bentley B, Branicky R, Barnes CL, Chew YL, Yemini E, Bullmore ET, et al. The multilayer connectome of Caenorhabditis elegans. Jbabdi S, editor. PLOS Comput Biol. 2016;12: e1005283 10.1371/journal.pcbi.1005283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarma GP, Lee CW, Portegys T, Ghayoomie V, Jacobs T, Alicea B, et al. OpenWorm: Overview and recent advances in integrative biological simulation of Caenorhabditis elegans. Philos Trans R Soc B Biol Sci. 2018;373 10.1098/rstb.2017.0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou D, Rangan AV, McLaughlin DW, Cai D. Spatiotemporal dynamics of neuronal population response in the primary visual cortex. Proc Natl Acad Sci. 2013;110: 9517–9522. 10.1073/pnas.1308167110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao L, Porto D, Li Z, Fechner S, Lee SA, Goodman MB, et al. Parallel processing of two mechanosensory modalities by a single neuron in C. elegans. Dev Cell. 2019;51: 543–658. 10.1016/j.devcel.2019.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nusser Z. Subcellular distribution of neurotransmitter receptors and voltage-gated ion channels In: Stuart G, Spruston N, Hausser M, editors. Dendrites. Oxford University Press; 2012. pp. 154–188. 10.1093/acprof:oso/9780198566564.003.0007 [DOI] [Google Scholar]
- 52.Megías M, Emri Z, Freund TF, Gulyás AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102: 527–540. 10.1016/s0306-4522(00)00496-6 [DOI] [PubMed] [Google Scholar]
- 53.Zou W, Fu J, Zhang H, Du K, Huang W, Yu J, et al. Decoding the intensity of sensory input by two glutamate receptors in one C. elegans interneuron. Nat Commun. 2018;9: 4311 10.1038/s41467-018-06819-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arey RN, Kaletsky R, Murphy CT. Nervous system-wide profiling of presynaptic mRNAs reveals regulators of associative memory. Sci Rep. 2019;9: 20314 10.1038/s41598-019-56908-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stetak A, Hörndli F, Maricq AV, van den Heuvel S, Hajnal A. Neuron-specific regulation of associative learning and memory by MAGI-1 in C. elegans. PLoS One. 2009;4: e6019 10.1371/journal.pone.0006019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi S, Taylor KP, Chatzigeorgiou M, Hu Z, Schafer WR, Kaplan JM. Sensory neurons arouse C. elegans locomotion via both glutamate and neuropeptide release. PLOS Genet. 2015;11: e1005359 10.1371/journal.pgen.1005359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shinkai Y, Yamamoto Y, Fujiwara M, Tabata T, Murayama T, Hirotsu T, et al. Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J Neurosci. 2011;31: 3007–3015. 10.1523/JNEUROSCI.4691-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13: 615–621. 10.1038/nn.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuramochi M, Doi M. An excitatory/inhibitory switch from asymmetric sensory neurons defines postsynaptic tuning for a rapid response to NaCl in Caenorhabditis elegans. Front Mol Neurosci. 2019;11: 484 10.3389/fnmol.2018.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Witvliet D, Mulcahy B, Mitchell JK, Meirovitch Y, Berger DK, Wu Y, et al. Connectomes across development reveal principles of brain maturation in C. elegans. bioRxiv. 2020. 10.1101/2020.04.30.066209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serrano-Saiz E, Pereira L, Gendrel M, Aghayeva U, Battacharya A, Howell K, et al. A neurotransmitter atlas of the Caenorhabditis elegans male nervous system reveals sexually dimorphic neurotransmitter usage. Genetics. 2017;206: 1251–1269. 10.1534/genetics.117.202127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kunitomo H, Sato H, Iwata R, Satoh Y, Ohno H, Yamada K, et al. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat Commun. 2013;4: 2210 10.1038/ncomms3210 [DOI] [PubMed] [Google Scholar]
- 63.Ho VM, Lee J-A, Martin KC. The cell biology of synaptic plasticity. Science. 2011;334: 623–628. 10.1126/science.1209236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hadziselimovic N, Vukojevic V, Peter F, Milnik A, Fastenrath M, Fenyves BG, et al. Forgetting is regulated via Musashi-mediated translational control of the Arp2/3 complex. Cell. 2014;156: 1153–1166. 10.1016/j.cell.2014.01.054 [DOI] [PubMed] [Google Scholar]
- 65.Ingrosso A, Abbott LF. Training dynamically balanced excitatory-inhibitory networks. PLoS One. 2019;14: e0220547 10.1371/journal.pone.0220547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freytag V, Probst S, Hadziselimovic N, Boglari C, Hauser Y, Peter F, et al. Genome-wide temporal expression profiling in Caenorhabditis elegans identifies a core gene set related to long-term memory. J Neurosci. 2017;37: 6661–6672. 10.1523/JNEUROSCI.3298-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hangya B, Ranade SP, Lorenc M, Kepecs A. Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell. 2015;162: 1155–1168. 10.1016/j.cell.2015.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoerndli FJ, Walser M, Fröhli Hoier E, de Quervain D, Papassotiropoulos A, Hajnal A. A conserved function of C. elegans CASY-1 calsyntenin in associative learning. PLoS One. 2009;4: e4880 10.1371/journal.pone.0004880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hammond-Weinberger DR, Wang Y, Glavis-Bloom A, Spitzer NC. Mechanism for neurotransmitter-receptor matching. Proc Natl Acad Sci. 2020;117: 4368–4374. 10.1073/pnas.1916600117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spitzer NC. Neurotransmitter switching in the developing and adult brain. Annu Rev Neurosci. 2017;40: 1–19. 10.1146/annurev-neuro-072116-031204 [DOI] [PubMed] [Google Scholar]
- 71.Tritsch NX, Granger AJ, Sabatini BL. Mechanisms and functions of GABA co-release. Nat Rev Neurosci. 2016;17: 139–145. 10.1038/nrn.2015.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bianconi G. Multilayer Networks: Structure and Function. Oxford: Oxford University Press; 2018. 10.1093/oso/9780198753919.001.0001 [DOI] [Google Scholar]
- 73.Pournaki A, Merfort L, Ruiz J, Kouvaris NE, Hövel P, Hizanidis J. Synchronization patterns in modular neuronal n etworks: A case study of C. elegans. Front Appl Math Stat. 2019;5: 52 10.3389/fams.2019.00052 [DOI] [Google Scholar]
- 74.Chase D. Biogenic amine neurotransmitters in C. elegans. WormBook; 2007. 10.1895/wormbook.1.132.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bentley B. Connectomics of extrasynaptic signalling: applications to the nervous system of Caenorhabditis elegans [Doctoral thesis]. University of Cambridge. 2017.
- 76.Jobson MA, Valdez CM, Gardner J, Garcia LR, Jorgensen EM, Beg AA. Spillover transmission is mediated by the excitatory GABA receptor LGC-35 in C. elegans. J Neurosci. 2015;35: 2803–2816. 10.1523/JNEUROSCI.4557-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50: 295–322. 10.1146/annurev.pharmtox.011008.145533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crupi R, Impellizzeri D, Cuzzocrea S. Role of metabotropic glutamate receptors in neurological disorders. Front Mol Neurosci. 2019;12: 20 10.3389/fnmol.2019.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koelle MR. Neurotransmitter signaling through heterotrimeric G proteins: insights from studies in C. elegans. WormBook; 2018. 10.1895/wormbook.1.75.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reiner A, Levitz J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron. 2018;98: 1080–1098. 10.1016/j.neuron.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yemini E, Lin A, Nejatbakhsh A, Varol E, Sun R, Mena GE, et al. NeuroPAL: A neuronal polychromatic atlas of landmarks for whole-brain imaging in C. elegans. bioRxiv. 2019; 676312 10.1101/676312 [DOI] [Google Scholar]
- 82.Branicky R, Miyazaki H, Strange K, Schafer WR. The voltage-gated anion channels encoded by clh-3 regulate egg laying in C. elegans by modulating motor neuron excitability. J Neurosci. 2014;34: 764–775. 10.1523/JNEUROSCI.3112-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Prog Brain Res. 2007;160: 59–87. 10.1016/S0079-6123(06)60005-8 [DOI] [PubMed] [Google Scholar]
- 84.Jones A, Sattelle D. The cys-loop ligand-gated ion channel gene superfamily of the nematode, Caenorhabditis elegans. Invert Neurosci. 2008;8: 41–47. 10.1007/s10158-008-0068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science. 2002;298: 824–827. 10.1126/science.298.5594.824 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predictions were made based on the neurotransmitter and receptor gene expression patterns of the presynaptic and postsynaptic neurons, respectively (NT+R method, see Methods). Red, blue, and grey colors mark inhibitory, excitatory, and complex polarities, respectively. (A) Excitatory-inhibitory balances in alternative networks of the WormWiring connectome reconstruction. Bars from top to bottom: 1. synapse weighted network for comparative purpose (same as in Fig 3A); 2. weak links (defined by synapse number of 1) deleted [3]; 3. links connecting any of the pharyngeal nervous system neurons deleted. The rationale is that many previous work analyzed the connectome without the pharyngeal nervous system [2,85]; 4. unweighted network. (B) Predicted synaptic polarities for two connectome reconstructions other than Wormwiring, covering a variable number of neurons and synapses [2,3] (S5 Table). In summary, excitatory:inhibitory sign-balance ratios were similar in all cases, ranging between 3.1–4.1. Source data are provided in S1, S4 and S5 Data.
(TIF)
Figure is a split network representation of Fig 4B. Edges represent excitatory (blue), inhibitory (red), or complex (black) chemical connections. Edges are weighted according to synapse number. The shape of vertices (Δ,○,◇) represent the modality (sensory, inter, motor, respectively) of neurons. (A) Head circuit neurons. (B) Ventral nerve cord motor neurons. Colors as in Fig 4B.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(PDF)
The Caenorhabditis elegans genome contains 62 ionotropic postsynaptic receptor genes for glutamate, acetylcholine, and GABA. In this table genes are listed in alphabetic order, and the type of channel (+ for cation channel,–for anion channel) is presented with relevant reference. The 42 genes that were expressed postsynaptically in at least one neuron in our database (marked bold) have been validated for being cationic or anionic.
(DOCX)
Neuronal gene expression database was compiled from available datasets and manually curated (Methods). Genes encoding ionotropic receptors for glutamate, acetylcholine or GABA were grouped according to the type of ion channel (cation or anion), i.e. whether being excitatory or inhibitory. Bold numbers represent number of neurons expressing certain numbers of excitatory and/or inhibitory receptor genes. 63 neurons express only cation-channel receptor genes (green), while 48 neurons express only anion-channel receptor genes (red). 151 neurons express a mixture of cation- and anion-channel receptor genes (grey). Source data is in S1 Data.
(DOCX)
Predicted polarities from our results (S1 Data) were compared to the polarities predicted by Rakowski and Karbowski, 2017, for the locomotion circuit of the C. elegans connectome. Each cell represents a connection between the named source and target neuron. Green colored cell means that the predicted polarity was the same in both cases. Orange colored cell means that the predicted polarity was different with the two methods. 456 of the 652 synapses (70%) were predicted the same.
(DOCX)
Predicted polarities from our results (S1 Data) were individually compared to previously published experimental data. Each row represents a single connection. If validated (“Yes” in column “Validated?”), the NT+R predicted polarity equals the reference polarity. Partial validation means that the predicted and/or reference polarity was complex or uncertain.
(DOCX)
The three most complete connectome reconstructions of C. elegans, namely the WormWiring (http://wormwiring.org), as well as published by Varshney et al., 2011, and Cook et al., 2019, have fundamental differences in their coverage of chemical connections and synapse numbers.
(DOCX)
Bulk gene expression data was updated manually according to literature data. Green and red text shows receptor gene additions and deletions, respectively, to (from) specified neuron groups. Blue text shows neurotransmitter expression additions. acc-4 and lgc-46 genes were excluded to avoid false predictions because of literature evidence supporting a presynaptic localization rather than postsynaptic. All neurons of a neuron group were updated unless specified otherwise.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.