Abstract
The association of the coronavirus disease 2019 (COVID-19) with significant neurological and neuropsychiatric complications has been increasingly reported, both during the acute illness and in its aftermath. However, due to the short duration of patient follow up until now, it is not clear whether this infection will be associated with longer-term neurological and/or neuropsychiatric sequelae. In particular, the question of whether COVID-19 will be associated with an increased risk and rate of future dementia remains open and subject to speculation. During the course of the COVID-19 pandemic, an increasing number of patients have reported sudden anosmia or other olfactory dysfunction as concurrent symptoms. The possibility that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may reach the brain via the olfactory nerve or an upper nasal trancribrial route is an interesting working hypothesis. Among the identified genetic risk factors for Late-onset Alzheimer’s disease (LOAD), Apo E4 is one of the strongest and most frequent. People carrying one or two copies of the e4 allele of Apo E4 have significant odor recognition deficits in comparison to those not carrying this haplotype.
The hypothesis invoked in this paper is that anosmia/olfactory dysfunctions induced by SARS-CoV-2 may cause an increased a risk of future neurodegenerative dementia in ApoE4 carriers, and that this risk would be higher than in Apo E4 carriers affected by anosmia not induced by SARS-CoV-2. This would be associated with virus-induced chronic modifications in the central nervous system. It is proposed that COVID-19 patients with anosmia and no other serious symptoms should be followed up as part of specifically designed and approved studies in order to identify the early stages of dementia (especially LOAD and Dementia with Lewy Bodies), thereby improving our knowledge of the mechanisms involved in pre-cognitive stages of neurodegenerative dementia and making best use of any available therapies. This latter opportunity is unique and should not be lost.
Keywords: COVID-19, SARS-CoV-2, Anosmia, Alzheimer's disease, Dementia, Apolipoprotein E
Background to hypothesis
A number of studies so far have highlighted the association of the coronavirus disease 2019 (COVID-19) with significant neurological and neuropsychiatric complications, both during the acute illness and in its aftermaths (referred to as long COVID-19) [1], [2], [3], [4], [5]. However, due to the short duration of patient follow up until now, it is not clear whether this viral infection will be associated with longer-term neurological and/or neuropsychiatric sequelae. In particular, the question of whether COVID-19 will be associated with an increased risk and rate of future major cognitive disorders such as dementia remains open and subject to speculation.
During the course of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, an increasing number of patients reported sudden anosmia or other olfactory dysfunction as concurrent symptoms [6], [7]. As a multicenter European study reported, infected patients may just present with olfactory dysfunctions without other significant complaints [8]. The Angiotensin‐converting enzyme‐2 (ACE2) has been proven to be the functional host receptor for SARS-CoV-2. This receptor is also expressed on the surface of the basal layer epithelial cells of nasal mucosa and nasopharynx. Hence, the possibility that SARS-CoV-2 may reach the brain via the olfactory nerve or an upper nasal trancribrial route is an interesting working hypothesis, already suggested for other coronaviruses [9]. It is worth noticing, in this respect, that the olfactory bulb is the only part of the central nervous system not protected by duramater.
Statement of hypothesis
The role of olfactory dysfunction
Olfaction is affected by normal aging. However, olfactive functions are affected differently by Alzheimer’s disease (AD). In studies in healthy elderly population, discrimination (olfactory thresholds) is the most affected olfactory function. A possible explanation of these differences could be found in the association of executive and memory cognitive domains associated with olfaction, related in part to performance on olfactory tests that involve identification and recognition, both closely linked to semantic memory. The association between olfactory dysfunction and clinical AD is largely due to the accumulation of neurofibrillary tangles in central olfactory regions, especially the entorhinal cortex and hippocampus. The involvement of these sites is very important because they are considered to be among the first areas affected by the pathologic changes of classical AD [10]. In addition, a possible change in components of γ-secretase enzymes has been reported to be related to olfactory alterations in patients with recent-onset AD, as well as an influence of tau protein deposits in the olfactory bulb affecting the limbic system directly – a finding very frequently found in AD and infrequently in healthy individuals [11], [12]. Moreover, as seen, in patients enrolled in the Rush Memory and Aging Project, the difficulty in identifying familiar odors predicted the subsequent development of mild cognitive impairment (MCI, a precursor of dementia), robustly correlating with the presence and the level of AD pathology on postmortem examination [13], [14]. A study highlighted that the sensitivity of predicting the conversion of MCI to AD was enhanced combining olfactory function assays to neuropsychological tests and magnetic resonance imaging (MRI) of hippocampus and entorhinal cortex volume [15].
On the other hand, another study first reported that anosmia was significantly frequent in patients with dementia with Lewy bodies (DLB) confirmed at post mortem, but not among patients affected with pure Alzheimer’s disease pathology [16]. It has been observed that anosmia is one of the most common clinical signs of DLB with superimposed Alzheimer pathology (LB-variant AD), and that is should be seen as an independent predictor in the patient group with minimal extra pyramidal signs early in the course of the disease [17].
The role of apo E alleles
A 2004 study demonstrated that people carrying one or two copies of the e4 allele of apolipoprotein E (Apo E4) have significant odor recognition deficits in comparison to those not carrying this haplotype. Notably, the authors reported that this effect was specific to olfactory, but not visual stimuli [18]. Apo E carries triglycerides and cholesterol in multiple tissues by interacting with lipoprotein receptors on target cells. The three common human isoforms (ApoE2, ApoE3 and ApoE4) differ from each other at amino acid positions 112 and 158. In particular, ApoE4 has arginine in both positions. The level of Apo E expression varies by genotype, with E2 typically having the greatest and E4 the lowest expression. ApoE3 is the most frequent isoform being present in 60–80% of general population whilst Apo E4 is present in 14%. [19], [20], [21]. The Apo E4 allele is a key associated genetic risk factor for late onset AD (LOAD), whilst the Apo E2 allele is considered protective [22]. In particular, Apo E protein levels in human cerebrospinal fluid proved to be reduced up to 30% in E4 compared to E2 carriers [23]. Astrocytes are the predominant producers of Apo E in the brain, but also other microglial cells and neurons can synthesize it. Once synthesized, the apolipoprotein is secreted to the extracellular space where it serves as the primary cholesterol carrier. Other cholesterol transporters such as ApoA1 and Apo B are virtually absent in the brain [24]. A recent study reported that Apo E4 could lead to blood–brain barrier breakdown, especially because of degeneration of brain capillary pericytes that maintain the integrity of this barrier [25]. To date, however, the working-hypothesis on whether Apo E4 cerebrovascular effects contribute to cognitive impairment remains little more than speculative.
The time factor
Among the identified genetic risk factors for LOAD, Apo E4 is one of the strongest and most frequent. Indeed, the odds ratio is above 10 for Apo E4 homozygotes, and apo E4 homozygosis is found in about 40% of LOAD patients [26], [27].
How long after the onset of olfactory dysfunctions does cognitive impairment appear? To the best of our knowledge, there are scant studies on the relationship between anosmia and clinical symptoms of LOAD, DLB and LB-variant AD, including cognitive impairment, and they are all retrospective studies [28], [29]. It is widely accepted that pathophysiological changes in these patients start many years prior to onset of cognitive impairment, in a so-called pre-clinical or pre-symptomatic stage. On the other hand, even if anosmia is not a recognized diagnostic or classification criterion for LOAD, DLB and LB-variant AD, much of the available published data seems to suggest that when anosmia occurs, the pathological process of dementia has already started, at least in some patients [30].
The pre-cognitive stage of AD, DLB and LB-variant AD
As already highlighted, AD, DLB and LB-variant AD tends to develop slowly and gradually worsens over several years. In the pre-clinical stage of AD, no cognitive impairment is present by definition. In the natural course of AD, MCI is a watershed period, even if not all patients with MCI progress towards AD.
Neurological involvement in COVID-19, different from olfactory dysfunction
A recent systematic review and meta-analysis of more than 41 studies involving approximately 4700 patients suggested that neurological manifestations are usually underestimated in patients affected with SARS-CoV-2. In this study, no case of dementia were reported, while several specific neurological manifestations and diseases were diagnosed, encephalitis and ischemic stroke among these. However, the authors admitted that, since their literature search was conducted during an ongoing outbreak, it was possible that many related studies had not yet been published, which could influence their results [31].
Acute neurological involvement is strongly linked to the severity of the disease, as first highlighted by the Wuhan series in which neurological and neuropsychiatric manifestations were more common in patients with severe disease [32].
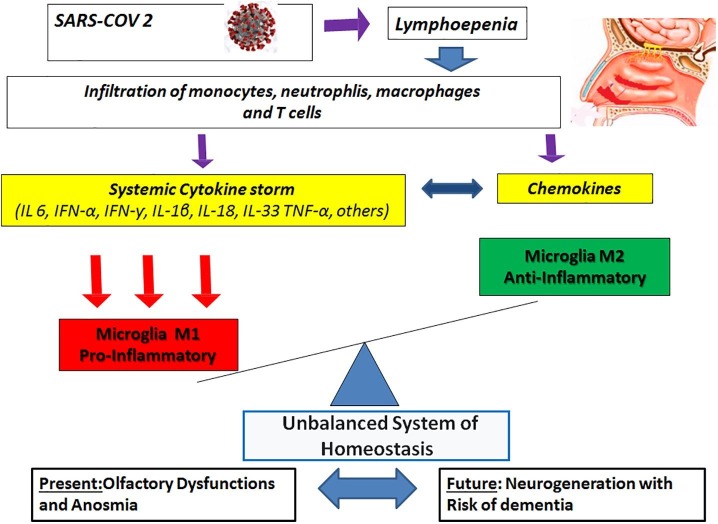
It is a common knowledge that neurodegenerative disorders can be accompanied by inflammation, which could be triggered by systemic infections or injuries [33]. In this case, the innate immune system recruits different cells in order to respond to inflammation. Such cells include many circulating lymphocytes and monocytes that can develop into both dendritic cells and macrophages. Microglia cells are usually found in microglia homeostasis balanced system M1-M2 expressed as M1 (pro-inflammatory) and M2 (anti-inflammatory). Disturbances in M1-M2 homeostasis involves many pro-inflammatory and anti-inflammatory cascading pathways depending on brain mediating environment [34]. The sharp increase of cytokine levels induced by SARS-CoV-2 can damage the blood–brain barrier and activate microglia M1 phenotype (pro-inflammatory). This may lead to the rapid accumulation of insoluble toxic aggregates in different brain regions where these neuropathological changes can be related to olfaction brain modifications following COVID-19 olfactory bulb penetration [35], [36], [37] ( Fig. 1 ).
Fig. 1.
Unbalanced system of microglial homeostasis induced by SARS-.CoV-2.
Furthermore, the upregulation of these cytokines can cause a significant activation of the hypothalamic pituitary adrenal (HPA) axis, in turn causing raised glucocorticoid (GC) levels in hippocampus. In the human brain, hippocampal CA1 neurons have the highest level of expression of GC receptors. Feedback regulation of the hypothalamus–pituitaryadrenal axis by glucocorticoids is mediated through two distinct intracellular receptor subtypes referred to as the mineralocorticoid receptor and the glucocorticoid receptor. The glucocorticoid receptor is therefore believed to be more important in the regulation of the response to stress when endogenous levels of glucocorticoids are high. These receptors are well expressed even in the elderly [38], [39]. This raised levels would make the limbic areas of the medial temporal lobe even more vulnerable to the insult caused by SARS-CoV-2 [40].
Effect of SARS-CoV-2 on the risk associated with Apo E4 phenotype
It is widely recognized that Apoe4 affects the severity of clinical manifestations and prognosis of COVID-19. However, how this happens is still debated. For instance, the UK Biobank Community cohort study found that ApoE4 homozygotes had 2.2-fold higher risk for COVID-19 positivity and 4.3-fold more case-fatality after COVID-19 than ApoE3 homozygotes. These strong associations were not diminished by excluding dementia, hypertension, coronary heart disease, or type II diabetes. Based on these reports, the investigators hypothesized that the negative effects of ApoE4 could be independent of these common age-related diseases. Interestingly, Apo E ε3ε4 COVID-19 positive subjects had a slight increase in mortality, with no statistically significant difference, compared to ApoE ε3ε3 positive subjects [41]. On the other hands, other investigators suggested that pleiotropies of ApoE4 may mediate multiple morbidities that increase vulnerability, involving genes in the so-called “ApoE cluster” on chromosome 19, that may contribute to SARS-CoV-2 susceptibility [42]. The role of Apo E in modulating immune responses, especially through pro-inflammatory cytokines, could be the common denominator [43].
A working-hypothesis
Our hypothesis is that anosmia/olfactory dysfunctions induced by SARS-CoV-2 may confer in ApoE4 carriers a risk of future dementia (especially LOAD, DLB and LB-variant AD) higher than in Apo E4 carriers affected by anosmia not induced by SARS-CoV-2. Indeed, this virus can damage the central nervous system (CNS) through several hypothesized mechanisms [40]. This involvement is the basis of our hypothesis.
Given that olfactory deficits develop at high frequency in cognitively intact Apo E4 carriers, olfaction may be a particularly useful early, noninvasive outcome measure. Data reported in the aforementioned European study showed a high prevalence (86%) of olfactory dysfunction in patients affected with mild-to-moderate forms of the COVID-19 [8]. We acknowledge that this high prevalence should be confirmed in non-European cohorts.
Specific acute neurological manifestations such as encephalitis or ischemic stroke have been reported during COVID-19 pandemic. However, whether these acute conditions increase the risk of future dementia is not the object of our hypothesis. Indeed, this subset of COVID-19 patients should be excluded from the proposed follow-up in order to avoid additional predisposing factors.
To date, only retrospective data are available from published literature about the relationship between anosmia, Apo E4 and risk of dementia.
In order to test our hypothesis, we propose to prospectively follow all the Apo E4 patients with the COVID-19 induced anosmia. Apo E4 is not normally tested in anosmic COVID-19 patients, and we suggest that it should be in specifically designed and approved research.
Conclusions
A high prevalence of anosmia and other olfactory dysfunctions has been reported in patients affected with mild-to-moderate forms of the COVID-19. The association of Apo E4 with anosmia is a recognized risk factor for future dementia.
Our hypothesis is that SARS-CoV-2 may be an increased risk factor a risk of future dementia in patients with Apo E4 affected with anosmia higher than in Apo E4 patients with anosmia not induced by this virus. This would be associated with virus-induced chronic modifications in the central nervous system.
Furthermore, the follow-ups of these patients would allow for the identification of the early stages of dementia (specifically LOAD, DLB and LB-variant AD), thereby improving our knowledge about the mechanism involved in pre-cognitive stages and making best use of any available therapies. This latter opportunity is unique and should not be lost.
Funding
No specific funding was received from any bodies in the public, commercial or not-for- profit sectors to carry out the work described in this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020;12:69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varatharaj A., Thomas N., Ellul M.A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kacem I, Gharbi A, Harizi C, et al. Characteristics, onset, and evolution of neurological symptoms in patients with COVID-19. . 2020 Nov 17 : 1–8. doi: [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 5.Jasti M, Nalleballe K, Dandu V, Onteddu S. A review of pathophysiology and neuropsychiatric manifestations of COVID-19. J Neurol. 2020 Jun 3: 1–6. doi: 10.1007/s00415-020-09950-w [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 6.Hornuss D., Lange B., Schroter N., et al. Anosmia in COVID-19 patients. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.05.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speth M.M., Singer-Cornelius T., Obere M., Gengler I., Brockmeier S.J., Sedaghat A.R. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163:114–120. doi: 10.1177/0194599820929185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277: :2251-2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed]
- 9.Dubè M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 2018; 92:e00404-18. . [DOI] [PMC free article] [PubMed]
- 10.Price J.L., Davies P.B., Morris J.C., White D.L. The distribution of tangles, plaques and related histochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 11.Attems J., Lintner F., Jellinger K.A. Olfactory involvement in aging and Alzheimer’s disease: an autopsy study. J Alzheimers Dis. 2005;7:149–157. doi: 10.3233/jad-2005-7208. [DOI] [PubMed] [Google Scholar]
- 12.Wilson R.S., Arnold S.E., Schneider J.A., Tang Y., Bennett D.A. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett D.A., Schneider J.A., Buchman A.S., Mendes de Leon C.F., Bienias J.L., Wilson R.S. The rush memory and aging project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Arnold SE, Julie A. Schneider JA, Patricia A. Boyle PA, Aron S. Buchman AS, and David A. Bennett DA. Olfactory Impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009; 1170: 730–735. [DOI] [PMC free article] [PubMed]
- 15.Devanand D.P., Liu X., Tabert M.H., et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer's disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McShane R.H., Nagy Z., Esiri M.M., et al. Anosmia in dementia is associated with Lewy bodies rather than Alzheimer’s pathology. J Neurol Neurosurg Psychiatry. 2001;70:739–743. doi: 10.1136/jnnp.70.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olichney J.M., Murphy C., Hofstette C.R., et al. Anosmia is very common in the Lewy body variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1342–1347. doi: 10.1136/jnnp.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert P.E., Murphy C. The effect of the ApoE ε4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer's disease, probableAlzheimer's disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26:779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- 19.Hanlon C.S., Rubinsztein D.C. Arginine residues at codons 112 and 158 in the apolipoprotein E gene correspond to the ancestral state in humans. Atherosclerosis. 1995;112:85–90. doi: 10.1016/0021-9150(94)05402-5. [DOI] [PubMed] [Google Scholar]
- 20.Cruchaga C., Kauwe J.S., Nowotny P., et al. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease. Hum Mol Genet. 2012;21:4558–4571. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease:pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C., Najm R., Xu Q., et al. Gain of toxic apolipoprotein e4 effects in human ipsc-derived neurons is ameliorated by a small-molecule structure corrector. Nat Med. 2018;24:647–6657. doi: 10.1038/s41591-018-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corder E.H., Saunders A.M., Strittmatter W.J., et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 24.Jeong W., Lee H., Cho S., Soo J. ApoE4-induced cholesterol dysregulation and its brain cell type-specific implications in the pathogenesis of Alzheimer’s disease. Mol Cells. 2019;42:739–746. doi: 10.14348/molcells.2019.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagne A., Nation D.A., Sagare A.P., et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Q., Guo P., Li D., et al. Olfactory Dysfunction and Its Relationship with Clinical Symptoms of Alzheimer Disease. Aging Dis. 2018;9:1084–1095. doi: 10.14336/AD.2018.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djordjevic J., Jones-Gotman M., De Sousa K., Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Swan G.E., Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21:58–67. doi: 10.1159/000048618. [DOI] [PubMed] [Google Scholar]
- 29.Sperling R.A., Aisen P.S., Beckett L.A., et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford R.J., Jr, Holtzman D.M., Reisa Sperling R. Dementia is not synonymous with Alzheimer's disease. Sci Transl Med. 2019;11(522) doi: 10.1126/scitranslmed.aav0511. eaav0511. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Shen Y., Li M., Chuang H., Ye Y., ZhaoH Wang H., et al. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARSCoV2: a systematic review and metaanalysis. J Neurol. 2019;2020(267):2777–2789. doi: 10.1007/s00415-020-09974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao I., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. Cina JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anwar MM, , Badawi AM, Nadia A, Eltablawy Can the coronavirus infection penetrates the brain resulting in sudden anosmia followed by severe neurological disorders? eNeurologicalSci 21 (2020) 100290, https://doi.org/10.1016/j.ensci.2020.100290. [DOI] [PMC free article] [PubMed]
- 35.Cuffaro L., Di Lorenzo F., Bonavita S., Tedeschi G., Leocani L., Lavorgna L. Dementia care and COVID-19 pandemic: a necessary digital revolution. Neurol Sci. 2020;41:1977–1979. doi: 10.1007/s10072-020-04512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippi R., Domingues C., Setz T., Outeiro F. SARS-CoV-2: at the crossroad between aging and. Neurodegeneration. 2020;35:716–720. doi: 10.1002/mds.28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naughton S.X., Raval U., Pasinetti G.M. Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J Alzheimers Dis. 2020;76:21–25. doi: 10.3233/JAD-200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q., Van Heerikhuize J., Aronica E., Kawata M., Seress L., Joels M., et al. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol Aging. 2013;34:1662–1673. doi: 10.1016/j.neurobiolaging.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Manzo C., Serra-Mestres J., Castagna A., Isetta M. Behavioral, psychiatric, and cognitive adverse events in older persons treated with glucocorticoids. Medicines (Basel) 2018;5(3):82. doi: 10.3390/medicines5030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;1(183):16–27.e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo C.L., Pilling L.C., Atkins J.L., et al. APOE e4 genotype predicts severe COVID-19 in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2231–2232. doi: 10.1093/gerona/glaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finch EC, Kulminski AM. The ApoE Locus and COVID-19: Are We Going Where We Have Been? J Gerontol A Biol Sci Med Sci, 2020, Vol. XX, No. XX, 1–3 doi:10.1093/gerona/glaa200. [DOI] [PMC free article] [PubMed]
- 43.Gale S.C., Gao L., Mikacenic C., et al. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol. 2014;134:127–134. doi: 10.1016/j.jaci.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]