Summary
Background
Hydrogen was proven to have anti-oxidative and anti-inflammation effects to various diseases.
Aim
We wish to investigate the acute effects of inhaled hydrogen on airway inflammation in patients with asthma and chronic obstructive pulmonary disease (COPD).
Design
Prospective study.
Methods
In total, 2.4% hydrogen containing steam mixed gas (XEN) was inhaled once for 45 min in 10 patients with asthma and 10 patients with COPD. The levels of granulocyte-macrophage colony stimulating factor, interferon-γ, interleukin-1β (IL-1β), IL-2, IL-4, IL-6 and so on in peripheral blood and exhaled breath condensate (EBC) before and after ‘XEN’ inhalation were measured.
Results
45 minutes ‘XEN’ inhalation once decreased monocyte chemotactic protein 1 level in both COPD (564.70–451.51 pg/mL, P = 0.019) and asthma (386.39–332.76 pg/mL, P = 0.033) group, while decreased IL-8 level only in asthma group (5.25–4.49 pg/mL, P = 0.023). The level of EBC soluble cluster of differentiation-40 ligand in COPD group increased after inhalation (1.07–1.16 pg/mL, P = 0.031), while IL-4 and IL-6 levels in EBC were significantly lower after inhalation in the COPD (0.80–0.64 pg/mL, P = 0.025) and asthma (0.06–0.05 pg/mL, P = 0.007) group, respectively.
Conclusions
A single inhalation of hydrogen for 45 min attenuated inflammatory status in airways in patients with asthma and COPD.
Introduction
Hydrogen, identified as antioxidants, in particular have been shown to have distinct characteristics, including its effect on specific reactive oxygen species (ROS) and excellent diffusion capacity.1 It has been demonstrated that hydrogen could provide protection against various diseases, including sepsis, stroke and ischemia-reperfusion injury.2,3 The major feature of chronic obstructive pulmonary disease (COPD) and other airway diseases is generally regarded as abnormal response to injury, chronic inflammation, excessive activation of macrophages, neutrophils, T lymphocytes and fibroblasts in the lung and oxidative stress is widely proposed as a pathogenic mechanism while ROS plays a pivotal role in the incidence and exacerbation of diseases.4 It is still unknown, however, whether hydrogen with low concentration inhalation has a therapeutic role on human diseases with airflow limitation. The purpose of this study was to investigate the effect of inhaled hydrogen gas on airway inflammation in patients with COPD and asthma.
Methods
Subjects
From March 2019 to June 2019, 10 COPD and 10 asthma patients (aged 20–65 years old) were recruited to participate in this study in Peking Union Medical College Hospital. COPD patients were restricted to those with spirometrically confirmed airflow obstruction (postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 0.7).5 Asthma patients met the diagnostic criteria of asthma.6 Oxygen saturation on resting-state using pulse oximetry and Medical Research Council (MRC) scores for dyspnea were recorded.7 Exclusion criteria included pregnancy, breast feeding, symptoms of acute airway infections or exacerbation 4 weeks prior to the test, and past history of malignancy, myocardial infarction, liver cirrhosis, renal failure and mental or intellectual disorders who were judged to not be able to provide informed consent.
The study protocol was reviewed and approved by the ethical committee of the Peking Union Medical College Hospital (HS-1948), and the protocol was carried out in accordance with relevant ethical guidelines and regulations. The written consents were obtained from all subjects.
Pulmonary function
Pulmonary function was measured by FEV1, FVC, FEV1/FVC and bronchial provocation test used a MasterScreen spirometer (CareFusion, Hoechberg, Germany). All measurements were performed according to the standards established by the American Thoracic Society.8
Hydrogen gas administration
A machine developed by Earth Engineering Co. (Suisonia, FRJ-003, Kitakyushu, Japan) was used to decompose superheated steam to produce a mixed gas containing hydrogen (H2) gas. The stream produced by heating sterile water for inhalation, and it decomposes into H2 and oxygen (O2) at a decomposition ratio of 67% vs. 33%. As air is present inside the machine, the concentration of H2 gas is ∼2.4%, according to the Manufacture’s instruction confirmed by a portable type hydrogen detector. After transfer through nasal cannula the H2 concentration is ∼0.1–0.3% when inhaled, while O2 is adsorbed with a cartridge. This steam mixed gas is designated as ‘XEN’ in preliminary study. All subjects performed the hydrogen gas inhalation for 45 min under close observation of researchers to ensure the compliance and to find any adverse reactions.
Exhaled breath condensate (EBC) from all participants was obtained according to the American Thoracic Society/European Respiratory Society guidelines using RTube™ (Respiratory Research, Inc, Austin, TX, USA) by breathing tidally into the device precooled to −20°C. Before and after 45 min of ‘XEN’ inhalation, peripheral blood and EBC were collected and storage in −80°C.
Reagents and measurement
MILLIPLEX MAP assay beads are comprised of polystyrene microspheres that have been impregnated with ferrite particles as well as a mixture of two colored dyes. The level of granulocyte-macrophage colony stimulating factor, interferon-γ, interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-17A, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, tumor necrosis factor alpha (TNF-α) were detected by multiplex analysis using T Cell Magnetic Bead Panel (HSTCMAG-28SK-14) and macrophage-derived chemokine, soluble cluster of differentiation-40 ligand, monocyte chemotactic protein 1 (MCP-1), vascular endothelial growth factor A using Human Cytokine/Chemokine Magnetic Bead Panel (HCYTOMAG-60K-04) (EMD Millipore Corp, Billerica, MA, USA). Luminex® xMAP® technology was used for detection and analysis concentrations of multiple target cytokine in a single sample, as recommended by the manufacturer. Sandwich enzyme-linked immunosorbent assay was used to measure the level of human superoxide dismutase 3 (SOD3) using LF-EK0107 kit (AbFrontier, Seoul, South Korea).
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (SPSS Inc, Chicago, IL, USA). Values were presented as the mean ± the standard deviation and the Shapiro–Wilk Test was used to assess the normal distribution of data. To compare the mean of a single group before and after ‘XEN’ inhalation, paired samples t test after taking natural logarithms were performed and statistical significance was established at P < 0.05.
Results
The characteristics of the subjects
The characteristics of the 20 subjects are listed in Table 1. Patients with COPD were older, with more male participates than asthma group. Pulmonary function showed COPD patients had lower levels in FEV1, FVC and FEV1/FVC compared with asthma patients (P < 0.05).
Table 1.
Characteristics of the study population
| COPD (N = 10) | Asthma (N = 10) | P value | |
|---|---|---|---|
| Age, years (range) | 61.9±5.9 (52–70) | 46.8±13.3 (21–64) | 0.006* |
| Sex (M/F) | 10/0 | 3/7 | 0.001* |
| BMI (kg/m2) | 25.30±3.02 | 24.32±3.01 | 0.499 |
| Pack-years | 36.9±12.9 | 8.2±7.2 | 0.001* |
| Oxygen saturation (%) | 95.0±1.9 | 96.3 ±1.1 | 0.098 |
| mMRC of dyspnea | 3.3±1.0 | 1.7±0.8 | 0.001* |
| FEV1 (%pred) | 46.08±16.21 | 74.32±27.34 | 0.016* |
| FVC (%pred) | 79.50±13.45 | 96.36±12.87 | 0.014* |
| FEV1/FVC, % | 45.55±13.58 | 74.06±15.10 | 0.001* |
Sex ratio were compared using Pearson Chi-square test. Quantitative data are expressed as mean ± SD, and P values were obtained by the independent sample t test.
BMI: body mass index; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; mMRC: modified Medical Research Council.
Values indicate significant differences (P < 0.05).
Measurement of inflammatory factors
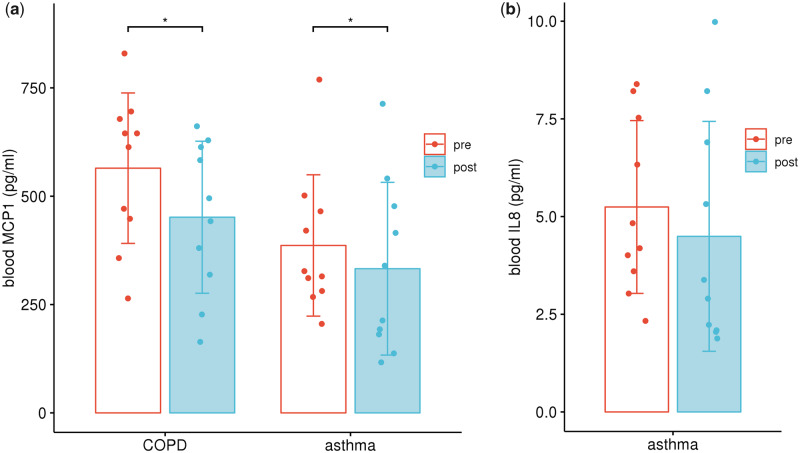
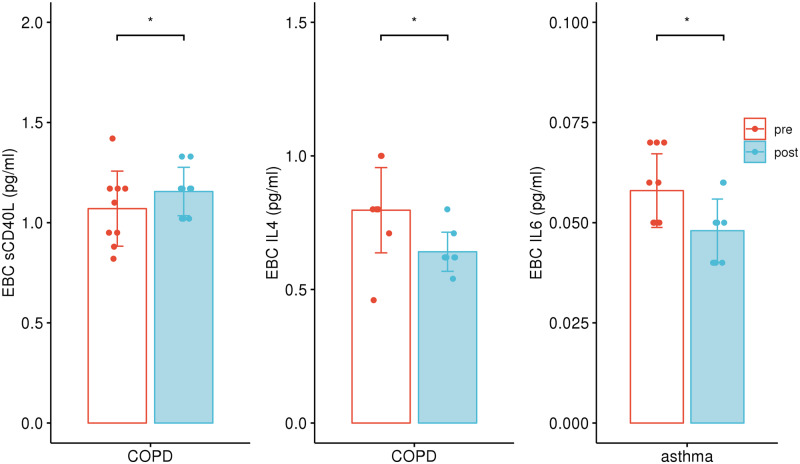
Regarding the proinflammatory mediators in peripheral blood, MCP-1 level in both COPD (564.70–451.51 pg/mL, P = 0.019) and asthma (386.39–332.76 pg/mL, P = 0.033) group decreased significantly after inhalation (Figure 1a), while IL-8 level decreased only in asthma group (5.25–4.49 pg/mL, P = 0.023, Figure 1b). In the case of CD40L in COPD group, the level in EBC increased after inhalation (1.07–1.16 pg/mL, P = 0.031). However, IL-4 and IL-6 level in EBC was significantly lower after inhalation in the COPD (0.80–0.64 pg/mL, P = 0.025) and asthma (0.06–0.05 pg/mL, P = 0.007) group, respectively, compared with the data before inhalation (Figure 2). There was no significant difference in SOD3 level before and after inhalation (Table 2).
Figure 1.
Blood cytokine levels before and after hydrogen inhalation. (a) MCP-1 level decreased after hydrogen inhalation in both COPD and asthma group; (b) IL-8 level decreased significantly after hydrogen inhalation in asthma group. *P < 0.05, paired t test.
Figure 2.
Cytokine levels in EBC before and after hydrogen inhalation. Soluble cluster of differentiation-40 ligand (sCD40L) level increased and IL-4 level decreased after hydrogen inhalation in COPD group. IL-6 level decreased after hydrogen inhalation in asthma group. *P < 0.05, paired t test.
Table 2.
Proinflammatory mediators before and after hydrogen inhalation
| Before inhalation | After inhalation | P value | ||
|---|---|---|---|---|
| COPD | ||||
| Peripheral blood (pg/mL) | MCP1 | 564.70 + 164.67 | 451.51 + 166.39 | 0.019* |
| Exhaled breath condensate (pg/mL) | sCD40L | 1.07 + 0.18 | 1.16 + 0.11 | 0.031* |
| IL-4 | 0.80 + 0.15 | 0.64 + 0.07 | 0.025* | |
| Asthma | ||||
| Peripheral blood (pg/mL) | MCP1 | 386.39 + 154.72 | 332.76 + 189.07 | 0.033* |
| IL-8 | 5.25 + 2.10 | 4.49 + 2.79 | 0.023* | |
| Exhaled breath condensate (pg/mL) | IL-6 | 0.06 + 0.01 | 0.05 + 0.01 | 0.007* |
P values were obtained by paired samples t test after taking natural logarithms.
MCP1: monocyte chemoattractant protein-1; sCD40L: soluble cluster of differentiation-40 ligand; IL-4: interleukin-4; IL-8: interleukin-8; IL-6: interleukin-6.
Values indicate significant differences (P < 0.05).
Discussion
Hydrogen, which exhibits anti-oxidative and anti-inflammation effects, was proved to be relatively safe for inhalation in diving.9,10 The concentration of hydrogen used in our study is quite lower (∼2.4%). Due to its small molecular weight, hydrogen can easily penetrate bio-membranes and diffuse into cytosol and organelles, and the tissue compatibility of hydrogen is stronger than many other oxidant scavengers. In this study, we demonstrated that a single inhalation of 45 min hydrogen gas could reduce airway inflammatory mediators in patients with asthma or COPD.
Antioxidants may be effective in the protection against the damage of oxidative stress from trauma or infection. Molecular hydrogen specifically quenches detrimental ROS (⋅OH), while maintaining the metabolic oxidation–reduction reaction and other less potent ROS, such as hydrogen peroxide (H2O2), and nitric oxide(NO⋅). Ohsawa et al.1 demonstrated that 2% hydrogen inhalation can alleviate oxidative stress by selectively neutralizing hydroxyl radicals (⋅OH) and antagonizing peroxynitrite (ONOO−), and proved to be effective for other cytotoxic ROS-related diseases.2,11 SOD is an important antioxidant enzyme in vivo, which can scavenge superoxide radical and decompose them into low-activity H2O2. Hydrogen was proved to increase the expression of antioxidative enzymes such as NF-E2-related factor 2 (Nrf2) and SOD, subsequently ameliorating oxidative stress and inflammatory responses.12,13 Huang et al.14 found that 42% hydrogen inhalation enhanced alveolar macrophage phagocytosis in ovalbumin-induced asthmatic mice, which may be associated with the antioxidant effects of hydrogen and the activation of the Nrf2 pathway, that significantly alleviated airway hyperresponsiveness, inflammation and goblet cell hyperplasia, diminished type-2 helper T-cell (TH2) response, malondialdehyde (MDA) production, decreased IL-4 and immunoglobulin E levels and increased SOD activity.
In our study, we used SOD3 level to evaluate the antioxidative effect and found the level did not change significantly after ‘XEN’ inhalation, that there were some limitations. First, early study measured SOD activity, which calls for higher requirements for sample storage and measurement, not the content of SOD. Second, we did not investigate anti-oxidative properties of hydrogen, and previous studies evaluate oxidative damage by measuring the content of MDA and 8-hydroxydeoxyguanosine.15,16
As oxidants can promote inflammation by activating nuclear factor kappa-B (NF-kB) and other pathways, and oxidative stress can lead to a protease/antiprotease imbalance.4 TNF-α and IL-1β are considered as initiator cytokines that initiate subsequent activations of other mediators, release of prostaglandin, and induction of chemotaxis of leukocytes due to inflammation.17 The expression levels of TNF-α and IL-6 was proved to increase in the lungs with COPD,18–20 and IL-6 may also contribute to acute lung injury as an inflammatory mediator,21 and elevates in patients with acute respiratory distress syndrome (ARDS) or at risk of ARDS due to infection, injury or inflammatory diseases, which is associated with higher mortality.
Recent studies proved that 2% hydrogen can inhibit the expression of inflammatory cytokines, including IL-6 and mitigate lung injury through an antiapoptotic effect.22,23 Qiu et al.24 found 2% hydrogen could alleviate acute lung injury by reducing IL-6 and TNF-α expression. Liu et al.25 proposed that hydrogen treatment might become a new and effective method for COPD and reported that 2% hydrogen inhalation significantly reduced the number of inflammatory cells in the bronchoalveolar lavage fluid (BALF) on a rat COPD model, and the mRNA and protein expression levels of TNF-α, IL-6, IL-17, IL-23, matrix metalloproteinase-12, caspase-3 and caspase-8, but increased the tissue inhibitor of metalloproteinase-1 expression. Furthermore, hydrogen inhalation ameliorated lung pathology, lung function and cardiovascular function and reduced the right ventricular hypertrophy index.26 Terasaki et al.27 found that hydrogen-rich water reduced inflammatory cell infiltration of the lung, the expression of IL-6 in BALF and the accumulation of cells expressing MCP-1 and IL-6 in the airway with lung injury.
MCP-1, an activating factor of both monocytes and T lymphocytes, can act as a chemoattractant. Capelli et al.28 reported increased concentrations of MCP-1 in BAL fluid from smokers with or without chronic bronchitis compared with healthy non-smoking subjects, and Traves et al.29 found MCP-1 levels were significantly increased in sputum from COPD patients compared with non-smokers and healthy smokers, suggesting that this chemokine might play a role in the inflammatory cell recruitment associated with cigarette smoking. Fang et al.30 found hydrogen inhalation inhibited the overexpression of MCP-1 in oxidant-induced endothelia and reduced inflammatory cells infiltration and proinflammatory cytokines (TNF-α, IL-6 and IL-8) production in cutaneous ischemia/reperfusion injury in a mouse model of pressure ulcer. Our study also found the MCP-1 level in peripheral blood significantly decreased after hydrogen inhalation, that hydrogen inhalation may produce protection against smoking.
Inflammatory cells, such as activated eosinophils and neutrophils identified in sputum and bronchial lavages in severe acute asthma are associated with increased levels of IL-5, IL-831 and IL-1β, IL-6 and TNF-α are detected in BAL from patients with symptomatic asthma and there is an increase in TNF-α production by macrophages after the late-phase response consecutive to allergen challenge,32 while the immune inflammatory changes associated with COPD are linked to a tissue-repair and -remodeling process, that generates a broad spectrum of cytokines including TNF-α, TGF-β, IL-8 and so on.33 To compare the effects of this hydrogen gas on COPD and asthma airway inflammation, previous studies based on COPD model animals showed hydrogen could alleviate the network of inflammatory factors like TNF-α, IL-6, IL-17 and IL-23, restore the balance of protease/antiprotease, and reduce apoptosis and alveolar structure damage26; while in asthmatic mice, the effect of hydrogen mainly focused on inhibition of NF-κB activation and activation of Nrf2 pathway, as well as to diminish TH2 response.14 In our study, IL-4 and IL-6 level in EBC decreased after inhalation in the COPD and asthma group, respectively; however, the values measured were near lower limit of detection and the results require more investigation.
Through the antioxidant pathway and the following anti-inflammatory effect, hydrogen may attenuate lung injury. Studies demonstrated hydrogen had a protective effect on hyperoxia-induced alveolar type II epithelial cell damage34 and hydrogen inhalation could attenuate seawater instillation-induced acute lung injury in rabbits, that markedly improved lung endothelial permeability and decreased both MDA content and MPO activity in lung tissue. Hydrogen gas also alleviated histopathological changes and cell apoptosis, while Nrf2 and heme oxygenase 1 (HO-1) expressions were significantly activated and caspase-3 expression was inhibited.11 Hydrogen-rich solution may also work that Terasaki et al.35 found hydrogen therapy could attenuate irradiation-induced lung damage. Wang et al.36 demonstrated hydrogen-rich saline alleviated lipopolysaccharide-induced acute lung injury by inhibiting excessive autophagy activation via the ROS/AMPK/mTOR pathway in mice with lung histopathological changes.
As previous studies on hydrogen inhalation were based on model animals or healthy human groups, this study provides important findings that even single inhalation of hydrogen was effective in modulating airway inflammation in patients with asthma and COPD. There are some limitations in this study. The first is the small sample size as this is a pilot study to evaluate the effect of hydrogen gas (XEN) inhalation on COPD and asthma, further studies are required to determine optimal dosing method for long-term treatment for patients. Other limitations include that we artificially set the time of hydrogen gas (XEN) inhalation to 45 min according to previous studies, that whether longer inhalation time may produce better effects remains unknown.
Conclusions
This study suggested that inhaled hydrogen (XEN) once with low concentration for 45 min had a positive therapeutic effect on airway inflammation in patients with asthma and COPD. Further studies are required in a larger sample size and longer duration of XEN treatments in asthma and COPD.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFC1313600) and the National Key Basic Research Program of China (973 Program) (2015CB553402).
Conflict of interest. None declared.
References
- 1. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007; 13:688–94. [DOI] [PubMed] [Google Scholar]
- 2. Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 2008; 373:30–5. [DOI] [PubMed] [Google Scholar]
- 3. Zhang J, Zhou H, Liu J, Meng C, Deng L, Li W.. Protective effects of hydrogen inhalation during the warm ischemia phase against lung ischemia-reperfusion injury in rat donors after cardiac death. Microvasc Res 2019; 125:103885. [DOI] [PubMed] [Google Scholar]
- 4. Park HS, Kim SR, Lee YC.. Impact of oxidative stress on lung diseases. Respirology 2009; 14:27–38. [DOI] [PubMed] [Google Scholar]
- 5.Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. http://goldcopdorg (4 October 2018, date last accessed).
- 6.Global Strategy for Asthma Management and Prevention. Workshop Report. Global Initiative for Asthma. 2018. https://ginasthmaorg (4 October 2018, date last accessed).
- 7. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA.. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991; 144:1202–18. [DOI] [PubMed] [Google Scholar]
- 9. Friess SL, Hudak WV, Boyer RD.. Toxicology of hydrogen-containing diving environments. I. Antagonism of acute CO2 effects in the rat by elevated partial pressures of H2 gas. Toxicol Appl Pharmacol 1978; 46:717–25. [DOI] [PubMed] [Google Scholar]
- 10. Abraini JH, Gardette-Chauffour MC, Martinez E, Rostain JC, Lemaire C.. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J Appl Physiol 1994; 76:1113–8. [DOI] [PubMed] [Google Scholar]
- 11. Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S.. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun 2007; 361:670–4. [DOI] [PubMed] [Google Scholar]
- 12. Diao M, Zhang S, Wu L, Huan L, Huang F, Cui Y, et al. Hydrogen gas inhalation attenuates seawater instillation-induced acute lung injury via the Nrf2 pathway in rabbits. Inflammation 2016; 39:2029–39. [DOI] [PubMed] [Google Scholar]
- 13. Yu Y, Yang Y, Yang M, Wang C, Xie K, Yu Y.. Hydrogen gas reduces HMGB1 release in lung tissues of septic mice in an Nrf2/HO-1-dependent pathway. Int Immunopharmacol 2019; 69:11–8. [DOI] [PubMed] [Google Scholar]
- 14. Huang P, Wei S, Huang W, Wu P, Chen S, Tao A, et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int Immunopharmacol 2019; 74:105646. [DOI] [PubMed] [Google Scholar]
- 15. Abe T, Li XK, Yazawa K, Hatayama N, Xie L, Sato B, et al. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation 2012; 94:14–21. [DOI] [PubMed] [Google Scholar]
- 16. Shigeta T, Sakamoto S, Li XK, Cai S, Liu C, Kurokawa R, et al. Luminal injection of hydrogen-rich solution attenuates intestinal ischemia-reperfusion injury in rats. Transplantation 2015; 99:500–7. [DOI] [PubMed] [Google Scholar]
- 17. Sato H, Kasai K, Tanaka T, Kita T, Tanaka N.. Role of tumor necrosis factor-alpha and interleukin-1beta on lung dysfunction following hemorrhagic shock in rats. Med Sci Monit 2008; 14:BR79–87. [PubMed] [Google Scholar]
- 18. Amrani Y, Panettieri RA Jr, Frossard N, Bronner C.. Activation of the TNF alpha-p55 receptor induces myocyte proliferation and modulates agonist-evoked calcium transients in cultured human tracheal smooth muscle cells. Am J Respir Cell Mol Biol 1996; 15:55–63. [DOI] [PubMed] [Google Scholar]
- 19. Barczyk A, Pierzchala W, Kon OM, Cosio B, Adcock IM, Barnes PJ.. Cytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2006; 117:1484–92. [DOI] [PubMed] [Google Scholar]
- 20. Hackett TL, Holloway R, Holgate ST, Warner JA.. Dynamics of pro-inflammatory and anti-inflammatory cytokine release during acute inflammation in chronic obstructive pulmonary disease: an ex vivo study. Respir Res 2008; 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaper F, Rose-John S.. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 2015; 26:475–87. [DOI] [PubMed] [Google Scholar]
- 22. Huang CS, Kawamura T, Peng X, Tochigi N, Shigemura N, Billiar TR, et al. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem Biophys Res Commun 2011; 408:253–8. [DOI] [PubMed] [Google Scholar]
- 23. Kohama K, Yamashita H, Aoyama-Ishikawa M, Takahashi T, Billiar TR, Nishimura T, et al. Hydrogen inhalation protects against acute lung injury induced by hemorrhagic shock and resuscitation. Surgery 2015; 158:399–407. [DOI] [PubMed] [Google Scholar]
- 24. Qiu X, Li H, Tang H, Jin Y, Li W, Yu S, et al. Hydrogen inhalation ameliorates lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol 2011; 11:2130–7. [DOI] [PubMed] [Google Scholar]
- 25. Liu SL, Liu K, Sun Q, Liu WW, Tao HY, Sun XJ.. Hydrogen therapy may be a novel and effective treatment for COPD. Front Pharmacol 2011; 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu X, Ma C, Wang X, Wang W, Li Z, Wang X, et al. Hydrogen coadministration slows the development of COPD-like lung disease in a cigarette smoke-induced rat model. Int J Chron Obstruct Pulmon Dis 2017; 12:1309–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terasaki Y, Suzuki T, Tonaki K, Terasaki M, Kuwahara N, Ohsiro J, et al. Molecular hydrogen attenuates gefitinib-induced exacerbation of naphthalene-evoked acute lung injury through a reduction in oxidative stress and inflammation. Lab Invest 2019; 99:793–806. [DOI] [PubMed] [Google Scholar]
- 28. Capelli A, Di Stefano A, Gnemmi I, Balbo P, Cerutti CG, Balbi B, et al. Increased MCP-1 and MIP-1beta in bronchoalveolar lavage fluid of chronic bronchitics. Eur Respir J 1999; 14:160–5. [DOI] [PubMed] [Google Scholar]
- 29. Traves SL, Culpitt SV, Russell RE, Barnes PJ, Donnelly LE.. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002; 57:590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang W, Wang G, Tang L, Su H, Chen H, Liao W, et al. Hydrogen gas inhalation protects against cutaneous ischaemia/reperfusion injury in a mouse model of pressure ulcer. J Cell Mol Med 2018; 22:4243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tillie-Leblond I, Hammad H, Desurmont S, Pugin J, Wallaert B, Tonnel AB, et al. Chemokines and interleukin-5 in bronchial lavage fluid from patients with status asthmaticus. Potential implication in eosinophil recruitment. Am J Respir Crit Care Med 2000; 162:586–92. [DOI] [PubMed] [Google Scholar]
- 32. Gosset P, Tillie-Leblond I, Oudin S, Parmentier O, Wallaert B, Joseph M, et al. Production of chemokines and proinflammatory and anti-inflammatory cytokines by human alveolar macrophages activated by IgE receptors. J Allergy Clin Immunol 1999; 103:289–97. [DOI] [PubMed] [Google Scholar]
- 33. James CH, Wim T.. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol Mech Dis 2009; 4:435–59. [DOI] [PubMed] [Google Scholar]
- 34. Yao L, Xu F, Luo C, Yu P, Dong X, Sun X, et al. Protective effect of hydrogen against hyperoxia-induced type II alveolar epithelial cell injury. Nan Fang Yi Ke Da Xue Bao 2013; 33:193–6. [PubMed] [Google Scholar]
- 35. Terasaki Y, Ohsawa I, Terasaki M, Takahashi M, Kunugi S, Dedong K, et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol 2011; 301:L415–26. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Zhang J, Bo J, Wang X, Zhu J.. Hydrogen-rich saline ameliorated LPS-induced acute lung injury via autophagy inhibition through the ROS/AMPK/mTOR pathway in mice. Exp Biol Med (Maywood) 2019; 244:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]