Abstract
Objective
MTX remains the cornerstone for therapy for RA, yet research shows that non-adherence is significant and correlates with response to therapy. This study aimed to halve self-reported non-adherence to MTX at the Kellgren Centre for Rheumatology.
Methods
An anonymous self-report adherence questionnaire was developed and data collected for 3 months prior to the introduction of interventions, and then regularly for the subsequent 2.5 years. A series of interventions were implemented, including motivational interviewing training, consistent information about MTX and development of a summary bookmark. Information on clinic times was collected for consultations with and without motivational interviewing. Surveys were conducted to ascertain consistency of messages about MTX. A biochemical assay was used to test MTX serum levels in patients at two time points: before and 2.8 years following introduction of the changes. Remission rates at 6 and 12 months post-MTX initiation were retrieved from patient notes and cost savings estimated by comparing actual numbers of new biologic starters compared with expected numbers based on the numbers of consultants employed at the two time points.
Results
Between June and August 2016, self-reported non-adherence to MTX was 24.7%. Following introduction of the interventions, self-reported non-adherence rates reduced to an average of 7.4% between April 2018 and August 2019. Clinic times were not significantly increased when motivational interviewing was employed. Consistency of messages by staff across three key areas (benefits of MTX, alcohol guidance and importance of adherence) improved from 64% in September 2016 to 94% in January 2018. Biochemical non-adherence reduced from 56% (September 2016) to 17% (June 2019), whilst remission rates 6 months post-initiation of MTX improved from 13% in 2014/15 to 37% in 2017/18, resulting is estimated cost savings of £30 000 per year.
Conclusion
Non-adherence to MTX can be improved using simple measures including focussing on the adherence and the benefits of treatment, and providing consistent information across departments.
Keywords: methotrexate, adherence, quality improvement, motivational interviewing
Rheumatology key messages
MTX adherence can be improved in clinical practice to improve outcomes in inflammatory arthritis.
Motivational interviewing and consistent messaging by clinicians and health-care professionals are effective strategies.
Drug education that focuses on both benefits and harms of MTX improves adherence.
Introduction
Early intervention improves long-term outcomes in patients with RA, with data from inception longitudinal cohorts showing that effective treatment within the first 6 months of diagnosis is associated with better outcomes after 20 years of follow-up [1]. MTX remains the cornerstone for therapy for RA and is recommended as first-line treatment by national and international organizations [2, 3]. Clinical trials where MTX is used as the comparator arm consistently show response rates of 80% of patients achieving good or moderate EULAR response rates by 6 months in early RA; by contrast a recent real-world study reported a lower response rate of 57% [4–7]. A drug cannot be effective if it is not taken, and issues of non-adherence are recognized for all chronic conditions [8]. Non-adherence is defined by the World Health Organization as ‘the extent to which the patient’s behaviour – taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health-care provider.’ Previous research shows that non-adherence to MTX is considerable, and correlates with response to therapy (reviewed in [9]). Given that failure to respond to conventional synthetic DMARDs is part of the eligibility criteria for biologic therapies in the UK, and given the cost differential of MTX compared with biologic drugs, there is a health economic argument to optimize adherence to MTX in order to achieve individual health benefits as well as to reduce health costs. Furthermore, concomitant MTX use and higher dose MTX is associated with improved drug survival and lower frequency of anti-drug antibodies in biologic-treated patients [10], further supporting the need to optimize MTX adherence.
We aimed to use a quality improvement programme to halve self-reported non-adherence to MTX at a single, specialist rheumatology centre. We used both self-report and a biochemical assay of serum MTX levels to assess adherence over a 3-year period.
Methods
A multidisciplinary team comprising two consultant rheumatologists (A.B. and R.G.), an academic clinical lecturer in rheumatology (M.J.), a health psychologist (C.B.) and a senior specialist nurse (M.A.) underwent Health Foundation training in quality improvement methods over a 12-month period. Improvement Science for Leaders (IS4L) is a bespoke programme to support clinical academics and health-care professionals to close the gap between research and clinical practice by providing training in improvement science (www.haelo.org.uk).
An anonymous self-report questionnaire was developed to capture information on non-adherence specific to all patients taking MTX. This was adapted from a validated questionnaire and developed with patient involvement and subject to three Plan-Do-Study-Act (PDSA) cycles [10]. Patients reporting to the receptionist were asked if they were taking MTX and, if so, were asked to complete the form and return it anonymously to the care centre. Data were collected for 3 months prior to the introduction of interventions, then monthly for the subsequent 2 years and less frequently for the third year. Following discussions with medical, nursing, pharmacy and patient colleagues, a series of interventions were developed:
Process map: process mapping was undertaken to understand the pathway from the clinical decision to prescribe MTX to the patient being established on therapy. From this, points in the pathway that were potential barriers to adherence were identified.
Motivational interviewing (MI): training was organized for all permanent members of staff with refresher You-tube training commissioned (https://www.youtube.com/channel/UCoyHTp8AMW5-UjxfmqcVHiQ). MI is a patient-centred consultation technique designed to elicit the patient’s own goals and plans for behaviour change [11].
- Personalized approach to tackling MTX concerns: at the point of decision about starting MTX, clinicians could flag on the drug education referral form if they had initial concerns regarding non-adherence specific to the patient. This allowed addressing the appropriate raised issues in more detail during the 1:1 drug education consultation. Clinicians were given the following choices on the form:
- Patient seems anxious (not taking in information/conversation jumping around)
- Not convinced patient believes this is the right treatment
- Patient concerned about side effects
- I have no concerns and am sure that this patient will have no issues with non-adherence
A theory informed ‘agenda-setting’ tool was developed, following three PDSA cycles, in order to focus consultations and ensure that adherence was addressed directly.
‘What happens next’ sheet: a flowchart for patients was developed following three rounds of PDSA to provided clearer information about progression through the prescribing pathway once MTX was commenced. It included contact numbers in case of difficulty obtaining MTX prescriptions, as these are prescribed from the hospital until patients are established on a stable dose of MTX according to shared care protocols (http://gmmmg.nhs.uk).
Drug education: MTX patient education slides were extensively revised to include positive messages about the benefits of treatments, information on absolute risks of adverse events as well as strategies to improve adherence through identified barriers to taking MTX regularly. Six PDSA cycles were undertaken before a final slide set was agreed.
Points to remember for MTX: six education points were agreed within the core and wider clinical team, as being imperative for patients to remember about MTX. A summary bookmark containing the main points to remember about MTX was developed following three PDSA cycles to be handed to patients at the time they received their MTX drug education from the nursing team. These points were also included on laminated sheets in clinic rooms and in the junior doctors’ office to re-inforce the consistent messaging. Additionally, a banner was made with these points in the waiting area, to promote a culture for openly talking about adherence and any concerns about MTX.
Maintaining long-term momentum: a series of regular meetings with all consultant, nursing and pharmacy colleagues were held, during which consensus was reached on information to be provided to patients to ensure consistency of messages by all clinical team members. The core team met on a weekly basis to discuss monthly data, patient feedback, plan/revise strategies as required and keep up the momentum of introducing a culture of improving adherence to medicines in the department.
Outcomes
Our primary outcome was self-reported non-adherence, as measured by anonymous surveys. A classification of adherent was given if the MTX tablet or injection was administered by the patient on the day agreed with the health care professional. If the patient had paused treatment due to being on an antibiotic whilst experiencing an infection for instance, they were classed as adherent (as would be consistent with the recommendations from the health-care provider).
Information on length of clinic reviews was collected for consultations with and without MI as a balancing measure to determine whether using MI approach and techniques increased consultation times. Surveys were conducted for all medical and nursing staff at four points during the 3 year process asking about information provided when talking to patients about MTX, in order to determine whether message consistency was improved.
Prior to introducing interventions, blood samples from 20 consecutive patients receiving oral MTX were collected in October 2016 and samples from 21 patients collected in June 2019. Patients were asked verbally if they had taken their MTX, and the day and dose. The samples were tested for MTX levels using a mass spectrometry method developed by our group [12] to provide an objective measure of adherence behaviour; this test is not in routine clinical use and patients were not told that the purpose of the blood sampling was to measure MTX drug levels.
An audit of DAS28 scores by 6 and 12 months following MTX initiation was carried out for patients starting therapy up until 2016 and compared with patients initiating therapy between May 2017 and April 2018. The number of patients starting a biologic for the first time was retrieved from a biologics prescribing database for 2014 and 2015. The number of new starters was averaged according to the number of whole-time equivalent consultants. Assuming a constant prescribing rate per whole-time equivalent consultant, the expected number of new biologic starters was estimated for 2018 and 2019 and compared with actual numbers of new starters.
Manchester University National Health Service (NHS) Trust R&I Department approved the study as a Quality Improvement (QI) project, and no other ethical approval was necessary.
Results
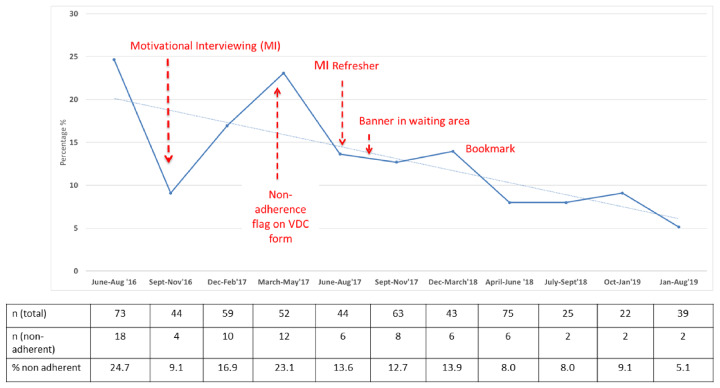
The process map for MTX prescribing is shown in supplementary Fig. S1, available at Rheumatology online. MI training was conducted in September 2016 with a face-to-face refresher organized in April 2017, along with reminder YouTube videos available from December 2017. The agenda setting tool developed was not found to be helpful by patients and so was discarded. Following meetings with clinical colleagues, the six main points to be conveyed to patients by all staff were agreed and were used to populate a bookmark reminder for patients (Fig. 1). The most common self-reported reasons for non-adherence were forgetting and stopping due to an infection such as a cold. Patient education slides were subsequently amended in two ways. Firstly, information was given on how to set Smartphone reminders with details of Smartphone apps to help manage medications for those on multiple drug treatment; and secondly, not needing to discontinue MTX treatment for coughs/colds/viral infections was reinforced in the slide set. Patients were recommended to stop MTX only if they were prescribed an antibiotic for an infection.
Fig. 1.

Bookmark handed to patients highlighting key points regarding MTX
Table 1 shows the number of forms collected in each 3 month period and the number reporting non-adherence. Between June and August 2016, mean self-reported non-adherence to MTX was 24.7%, consistent with previous reports [9]. Post-interventions, self-reported non-adherence rates had reduced to an average of 7.4% in the 17 months between April 2018 and August 2019 (Fig. 2). Clinic times were not significantly increased when MI was employed (data not shown). Consistency of messages by staff across three key areas (benefits of MTX, alcohol guidance and importance of adherence) improved from 64% in September 2016 to 94% in January 2018 (supplementary Fig. S2, available at Rheumatology online).
Table 1.
Number of non-adherent patients between June 2016 and August 2019
| Time frame | Number of forms | Non-adherent | Percentage non-adherence |
|---|---|---|---|
| June–August 2016 | 73 | 18 | 24.7 |
| September–November 2016 | 44 | 4 | 9.1 |
| December 2016–February 2017 | 59 | 10 | 16.9 |
| March–May 2017 | 52 | 12 | 23.1 |
| June–August 2017 | 44 | 6 | 13.6 |
| September–November 2017 | 63 | 8 | 12.7 |
| December 2017–March 2018 | 43 | 6 | 13.9 |
| April–June 2018 | 75 | 6 | 8 |
| July–September 2018 | 25 | 2 | 8 |
| October 2018–January 2019 | 22 | 2 | 9.1 |
| January–August 2019 | 39 | 2 | 5.1 |
| Total | 539 |
A total of 539 forms were collected between June 2016 and August 2016 representing a random sample of patients on MTX. The data are presented in 3-month intervals. However, sampling periods were longer towards the end of the study, reflecting when the forms were collected.
Fig. 2.
Self-reported non-adherence to MTX in rheumatology outpatients
Of the 20 consecutive participants taking MTX recruited for biochemical testing in October 2016, two reported non-adherence, equating to 10% self-reported non-adherence. The two patients self-reporting non-adherence were confirmed as biochemically non-adherent (negative predictive value = 100%). Ten out of the 18 who reported they were adherent were identified as biochemically non-adherent (56% non-adherence). In the second round of testing following introduction of the QI interventions in June 2019, 21 patients receiving oral MTX were tested. Of these, three reported non-adherence, equating to 14% self-reported non-adherence, and all three were all correctly identified as biochemically non-adherent (negative predictive value = 100%). Three out of the 18 who reported that they were adherent were identified as biochemically non-adherent (17% non-adherence).
For patients initiating MTX prior to 2016, 8/61 (13%) patients achieved remission (DAS28 <2.6) by 6 months and 12/58 (21%) by 12 months. This compared with 20/54 (37%) and 20/43 (46%), respectively, for patients initiating MTX between May 2017 and April 2018. There were 135 expected new starters for first biologic drugs in 2018/19 but only 105 actual new starters, meaning 10 fewer starters than expected each year. Using public data for drug costs and assuming all new starters would receive a biosimilar drug, estimated cost savings were >£30 000 per year.
Discussion
We have shown that non-adherence to MTX can be improved using a series of measures including asking about adherence, increasing the focus on the benefits of treatment and providing consistent information across a single department. We used a mixed-methods approach to quantify improvements in adherence in the absence of a standardized method to demonstrate improvement. By using a combination of personalized and population-level approaches and maintaining momentum through regular meetings, we were able to demonstrate long-term sustained improvements over a 3-year period.
Strengths of the study include the long follow-up post-introduction of interventions demonstrating maintenance over 2 years following the year-long implementation phase. Furthermore, using an objective biochemical assay as well as patient self-report has confirmed this sustained improvement. We measured consultation times with and without the use of MI and showed that it did not significantly increase the length of appointments. The interventions employed were informed by patient involvement, health psychology theory and previous research [13]. For example, anxiety has previously been reported to correlate with subsequent non-adherence to MTX, so we addressed this by adapting nurse education referral forms to highlight patients who seem anxious about starting the treatment [7].
Limitations include that, first, due to a lack of a prescribing database before the introduction of these interventions, we have not been able to show that improved adherence translates to increased persistence on MTX; however, we have identified fewer new starters on biologic drugs than would be expected. Furthermore, previous studies have consistently reported that non-adherence correlates with subsequent poorer response [9] and we have found more that patients achieved remission at 6 and 12 months following the interventions, suggesting better outcomes. These data only relate to patients initiating MTX and the lack of a prescribing database at the outset of the project means we do not have similar information for patients established on MTX. Second, this QI programme was carried out in a single centre and the pathways and interventions described may not be directly translatable to other NHS clinics. However, initial non-adherence rates are consistent with previous reports, which estimate that at least one in four people with long-term conditions do not take their medication as prescribed. We have created a resource pack that could be adapted by other centres (https://www.musculoskeletal.manchester.ac.uk/); this is currently being trialled in two other UK NHS Trusts. In particular, it should be noted that the advice given about alcohol and MTX differs from that in some other guidance; we recommend a maximum of 6 units per week, and this consensus was reached to mitigate against the concerns of medical staff about the impact of combined MTX and alcohol on liver function. It is important to recognize that the population screened for adherence was not the same at each time point; hence, we cannot say for certain which of any specific intervention was responsible for the decrease, which would therefore suggest that the package of interventions should be used if implementing at other centres. The process for initiating MTX will differ across settings and so not all the suggestions will be appropriate; for example, few centres have a virtual DMARD clinic and so the approach of highlighting concerns about adherence when initiating MTX via the virtual DMARD form will require a different approach. The Hawthorne effect of behaviour modification as a result of being observed is likely to have played a role in improving adherence rates, and one of the most important culture changes in the department was that clinicians asked about adherence more often; indeed, previous research related to hand washing shows that observation alone can be very effective [14]. We see this as a positive benefit, as the results show that improved adherence correlated with improved remission rates over the same time periods. Finally, this work was restricted to MTX adherence, but the same process could be applied to other drugs where non-adherence is a potential issue; for example, a previous UK study showed 27% non-adherence to biologic therapies [15].
The use of the biochemical test of MTX drug levels confirmed objectively that non-adherence rates fell: from 56% to 17% between the two time points in 2016 and 2019. It is interesting that self-reported non-adherence was lower than that detected biochemically before the introduction of the interventions (56% biochemical non-adherence vs 24% self-report) and even after the QI interventions, 17% who self-reported to be adherent were biochemically non-adherent. The assay has been validated and found to be 95% sensitive, but we cannot exclude measurement error as a cause for the discrepant results; however, the fact that biochemical and self-report adherence has improved shows that adherence behaviour has changed since the introduction of the programme. The testing of blood samples was performed only at the two time points described, which were over 30 months apart, and patients were not told that the blood would be tested for MTX drug levels; it is unlikely therefore that performing the blood test was responsible for the change in adherence.
In hypertension management, biochemical testing of urine for commonly prescribed anti-hypertensives has been reported to improve adherence, improve control of blood pressure and to result in health economic benefits [16]. This QI project shows that adherence can also be improved by comprehensively tackling multiple points in the prescribing pathway to remove and address barriers to adherence; ultimately, this may have health economic implications by delaying progression to biologic or targeted therapies, and improving drug survival and subsequent patient outcomes in those requiring such treatments.
Supplementary Material
Acknowledgements
A.B. and I.B. are National Institute for Health Research (NIHR) Senior Investigators and are supported by the NIHR Manchester Biomedical Research Centre. M.J. was funded by an NIHR academic clinical lectureship and currently a Presidential Fellowship. S.M. is funded by the NIHR Manchester Biomedical Research Centre (BRC).
Funding: Funding for the QI training programme was provided by Manchester Academic Health Sciences Centre (MAHSC). Funding for the biochemical assay was provided by the Manchester Molecular Pathology Node (MMPathic, grant ref. MR/N00583X/1), MAHSC and Versus Arthritis Centre for Genetics and Genomics (grant ref. 21754). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosure statement: C.B. has received honoraria from Abbvie, Amgen, Almirall, Janssen, Novartis and Pfizer. K.H. has received grants/research support from Pfizer, Bristol Myers Squibb (BMS) and UCB, and honoraria from Pfizer. P.H. has received consultancy fees from Abbvie and Novartis. M.J. has received honararia from Grifols and travel support from Pfizer. I.B. has received grant support from Genzyme Sanofi GlaxoSmithKline (GSK) and speaker fees from UCB, Eli Lilly, Astrazaneca, IlTOO, Aurinia and Merck Sorono. A.B. has received grant funding from BMS and speaker/consultancy fees from Abbvie, Eli Lilly and Celgene. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Gwinnutt JM, Symmons DPM, MacGregor AJ. et al. Twenty-year outcome and association between early treatment and mortality and disability in an inception cohort of patients with rheumatoid arthritis: results from the Norfolk Arthritis Register. Arthritis Rheumatol 2017;69:1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ledingham J, Gullick N, Irving K. et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2017;56:865–8. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 4. Breedveld FC, Weisman MH, Kavanaugh AF. et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. [DOI] [PubMed] [Google Scholar]
- 5. Bijlsma JWJ, Welsing PMJ, Woodworth TG. et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388:343–55. [DOI] [PubMed] [Google Scholar]
- 6. Donahue KE, Schulman ER, Gartlehner G. et al. Comparative effectiveness of combining MTX with biologic drug therapy versus either MTX or biologics alone for early rheumatoid arthritis in adults: a systematic review and network meta-analysis. J Gen Intern Med 2019;34:2232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sergeant JC, Hyrich KL, Anderson J. et al. Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res Ther 2018;20:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burkhart PV, Sabate E.. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh 2003;35:207. [PubMed] [Google Scholar]
- 9. Hope HF, Bluett J, Barton A. et al. Psychological factors predict adherence to methotrexate in rheumatoid arthritis; findings from a systematic review of rates, predictors and associations with patient-reported and clinical outcomes. RMD Open 2016;2:e000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jani M, Chinoy H, Warren RB. et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol 2015;67:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller WR, Rollnick S.. The effectiveness and ineffectiveness of complex behavioral interventions: impact of treatment fidelity. Contemp Clin Trials 2014;37:234–41. [DOI] [PubMed] [Google Scholar]
- 12. Bluett J, Riba-Garcia I, Verstappen S. et al. Development and validation of a methotrexate adherence assay. Ann Rheum Dis 2019;78:1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marengo MF, Suarez-Almazor ME.. Improving treatment adherence in patients with rheumatoid arthritis: what are the options? Int J Clin Rheumtol 2015;10:345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eckmanns T, Bessert J, Behnke M, Gastmeier P, Rüden H.. Compliance with antiseptic hand rub use in intensive care units: the Hawthorne effect. Infect Control Hosp Epidemiol 2006;27:931–4. [DOI] [PubMed] [Google Scholar]
- 15. Bluett J, Morgan C, Thurston L. et al. Impact of inadequate adherence on response to subcutaneously administered anti-tumour necrosis factor drugs: results from the biologics in rheumatoid arthritis genetics and genomics study syndicate cohort. Rheumatology (Oxford) 2015;54:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Schoonhoven AV, van Asselt ADI, Tomaszewski M. et al. Cost-utility of an objective biochemical measure to improve adherence to antihypertensive treatment. Hypertension 2018;72:1117–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.