Abstract
Objectives
The efficacy and safety of certolizumab pegol (CZP), an Fc-free, PEGylated anti-TNF, in axial spondyloarthritis (axSpA) has been established in clinical trial settings. We report CZP effectiveness and safety in European clinical practice in patients with axSpA, including radiographic (r-) and non-radiographic (nr-) axSpA.
Methods
CIMAX (NCT02354105), a European non-interventional multicentre prospective study, observed CZP treatment response and safety over 12 months in a real-world axSpA cohort. The primary outcome was change from baseline in BASDAI to week 52, with additional outcomes pertaining to effectiveness and safety. Patients who received ≥1 dose CZP were followed up for adverse events, and those with baseline and ≥1 post-baseline BASDAI assessment were included in effectiveness analyses.
Results
A total of 672 patients (r-axSpA: 469; nr-axSpA: 201; unconfirmed diagnosis: 2) from 101 sites received ≥1 dose of CZP, of whom 564 (r-axSpA: 384; nr-axSpA: 179; unconfirmed: 1) were included in the effectiveness analyses. The mean baseline BASDAI was 6.1 in the overall axSpA population and r-axSpA and nr-axSpA subpopulations. At week 52, the mean (s.d.) change in BASDAI was −2.9 (2.3; n = 439); for r-axSpA and nr-axSpA, it was −2.9 (2.2; n = 301) and −2.8 (2.4; n = 137), respectively (P <0.0001 for all). Similar improvements were seen across other axSpA disease measures. In total, 37.9% (255/672) patients experienced adverse events, and 1.8% (12/672) experienced ≥1 serious adverse events.
Conclusion
Improvements observed in signs and symptoms of axSpA following one year of CZP treatment in real-world clinical practice were similar to those from previous randomized clinical trials, with no new safety concerns.
Keywords: axial spondyloarthritis, certolizumab pegol, non-interventional
Rheumatology key messages
This was a prospective, non-interventional European study of certolizumab pegol for treatment of axial spondyloarthritis.
Over one year, patients newly prescribed certolizumab pegol experienced substantial improvements in symptoms of disease.
Effectiveness and safety outcomes from real-world clinical practice were comparable to those from clinical trials.
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease, predominantly affecting the axial skeleton and entheses. It includes patients with radiographic disease (r-axSpA, also known as ankylosing spondylitis) who have definitive structural damage to the sacroiliac joints on X-ray, normally fulfilling modified New York (mNY) classification criteria [1]; and those with non-radiographic disease (nr-axSpA) in which there are no definitive signs of sacroiliac joint damage on conventional radiographs, but inflammation is often present [1–3].
The main signs and symptoms of axSpA include chronic back pain, which is often inflammatory in nature, morning stiffness, and fatigue, with many patients also showing signs of spinal damage [4]. These symptoms can have a substantial impact on patients’ mobility and function, resulting in reduced quality of life and productivity [5, 6]. The condition is also associated with a number of extra-articular (acute anterior uveitis, psoriasis and inflammatory bowel disease) and peripheral manifestations (arthritis, enthesitis and dactylitis), which further increase the burden of disease [7].
Initial recommendations for management of axSpA include regular exercise and smoking cessation, followed by first-line treatment with NSAIDs and physical therapy [8]. While NSAIDs are effective at treating the major symptoms of disease (pain, stiffness), many patients experience intolerance or inadequate response to these medications. For patients with active disease despite first-line treatment, tumour necrosis factor inhibitors (anti-TNFs) can be effective treatment options [9]. The anti-TNF certolizumab pegol (CZP) has demonstrated long-term efficacy and safety in patients with axSpA in clinical trial settings [10–13]. However, there is a paucity of real-world data on anti-TNF efficacy in patients across the axSpA spectrum, including both r-axSpA and nr-axSpA.
Here, we report results from CIMAX, the first large, non-interventional European study designed to assess the effectiveness and safety of CZP treatment in patients with axSpA in real-world clinical practice.
Methods
Study design
CIMAX/AS0002 (NCT02354105) was a multicentre, prospective, non-interventional European cohort study in patients with axSpA who were newly prescribed CZP as part of routine clinical care. The study was conducted in six countries (Belgium, Germany, Greece, Italy, Spain and the UK), across 101 sites between 12 January 2015 and 9 March 2018. The decision to treat with CZP was made by the treating physician, independent of study participation, during the regular course of practice according to local regulations or guidelines, and was based on the patient’s disease status and clinical diagnosis. CZP dose and administration schedule were made according to the Summary of Product Characteristics (SmPC) [14].
All examinations and investigations such as X-ray or MRI were performed by the treating physician as part of routine care. There were four data collection points (visits), occurring at week 0 (comprising days 1–7, where day 1 was the date of first study medication administration), and approximately week 12 (week 6 through 16), week 24 (17 through 40), and week 52 (41 through 64). No study visits were scheduled according to the study’s observational plan; all data were collected during routine clinical visits scheduled by the treating physician and patient.
In Belgium, Greece and the UK, the study was reviewed by an Independent Ethics Committee; in Spain, Italy and Germany, it was reviewed by the national/regional Regulatory Authority and an Independent Ethics Committee.
Patients
Eligible patients had a clinical diagnosis of active axSpA (r-axSpA or nr-axSpA) according to the decision of their treating physician and had to be newly prescribed CZP according to local regulations or guidelines. All patients had to sign a Patient Data Consent Form and could withdraw from the study at any time. Where possible, the primary reason for withdrawal was recorded by the treating physician.
Study assessments
The primary outcome was change from baseline in BASDAI at week 52 for the overall axSpA population and for the r-axSpA and nr-axSpA subpopulations.
The following secondary variables were assessed in the overall population and both subpopulations: change from baseline in BASDAI at weeks 12 and 24; Assessment of Spondyloarthritis International Society (ASAS) 20% and 40% (ASAS20/40) response at weeks 12, 24 and 52; change from baseline in Bath Ankylosing Spondylitis Functional Index (BASFI) at weeks 12, 24 and 52; change from baseline in Patient’s Global Assessment of Disease Activity (PtGADA) at weeks 12, 24 and 52.
Additional effectiveness variables reported for week 52 include: Ankylosing Spondylitis Disease Activity Score (ASDAS, calculated with CRP or, if unavailable, ESR), total back pain, Physician’s Global Assessment of Disease Activity (PhGADA), concomitant intake of conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) and NSAIDs, and presence of peripheral manifestations (arthritis and enthesitis). In addition, according to the ASAS/EULAR recommendations [8], the following post-hoc analyses were conducted: the proportions of patients achieving BASDAI <4, BASDAI reduction of ≥2.0 from baseline, ASDAS clinically important improvement (ASDAS-CII; reduction of ≥1.1 from baseline), ASDAS-inactive disease (ASDAS-ID; ASDAS <1.3) and ASDAS <2.1 (includes ASDAS-low disease and ASDAS-ID).
Post-hoc analyses of primary and secondary efficacy variables were conducted for patients stratified by prior anti-TNF exposure (naïve vs pretreated) and country.
Adverse events (AEs) were recorded at each study visit by the treating physician and reported using an AE report form for non-interventional studies. All AEs occurring during the study were summarized according to the Medical Dictionary for Regulatory Activities (MedDRA®).
Statistical analysis
For sample size calculation, a mean (s.d.) change from baseline in BASDAI of −3.0 (2.25) was assumed, based on previously reported results from the RAPID-axSpA study [11]. To obtain a maximum extension of the two-sided 95% CI for change from baseline in BASDAI, a total of 152 nr-axSpA patients and 390 r-axSpA patients was required. Assuming an approximate attrition rate of 20%, a total sample size of 678 patients was planned.
Patients who received ≥1 dose CZP were followed up for AEs and included in the Safety Set; those with baseline and ≥1 post-baseline BASDAI assessment formed the Full Analysis Set (FAS) and were included in the effectiveness analyses. For inclusion in the FAS, patients had to have a baseline BASDAI assessment within a predefined window (up to 30 days before or 10 days after the first dose of CZP).
The primary variable, change from baseline in BASDAI at week 52, was analysed using descriptive statistics for the overall population and both r-axSpA and nr-axSpA subpopulations. Categorical variables are reported as the percentage of responders (with 95% CIs for secondary effectiveness variables), and continuous variables are reported as mean (s.d.). Hypothesis testing for the primary variable was performed using the Student’s t test.
All id="344" outcomes are reported using either observed case analysis (with no imputation for missing data) or multiple imputation (MI), in which categorical age (≤/>45 years), subgroup (r-axSpA or nr-axSpA) and prior anti-TNF exposure (naïve or pretreated) were specified as covariates. For the MI analysis, missing data were assumed to be missing at random. Imputed data are reported for week 52 only. When calculating ASDAS (including ASDAS improvement and disease states) using MI, if the CRP value was missing, the observed ESR value was used; if neither were available, imputed CRP values were used.
Statistical analyses were performed using SAS® (SAS-Institute, Cary, NC, USA) Version 9.2.
Results
Patient disposition and baseline characteristics
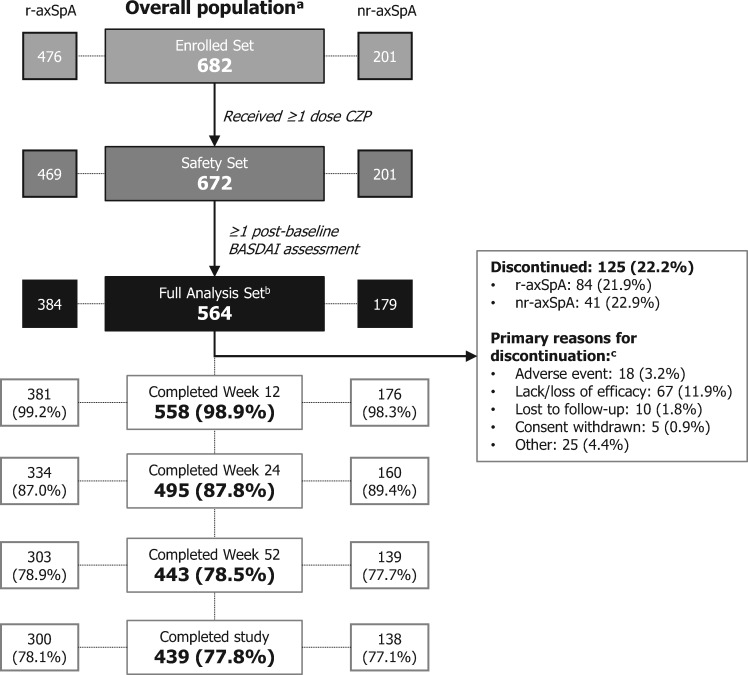
Of 682 enrolled patients, 672 received ≥1 dose of CZP and were included in the safety analyses (Safety Set); this included 469 patients with r-axSpA and 201 with nr-axSpA (two patients did not have a confirmed diagnosis of r- or nr-axSpA). Of these patients, 564 (r-axSpA: 384; nr-axSpA: 179; one unconfirmed diagnosis) had a baseline and ≥1 post-baseline BASDAI assessment and were included in the effectiveness analyses (FAS) (Fig. 1 and Fig. S1, available at Rheumatology online). The mean (s.d.) study duration in the Safety Set was 311.9 (112.3) days [r-axSpA: 312.9 (114.3); nr-axSpA: 310.8 (106.1)].
Fig. 1.
Patient disposition
aThe overall population included patients with an unconfirmed diagnosis of r-axSpA or nr-axSpA. bFor inclusion in the Full Analysis Set, patients had to have a baseline BASDAI assessment within a predefined window (up to 30 days before or 10 days after the first dose of CZP). cPatients may have had multiple reasons for discontinuation. axSpA: axial spondyloarthritis; CZP: certolizumab pegol; nr-axSpA: non-radiographic axSpA; r-axSpA: radiographic axSpA
Baseline characteristics, including disease activity, were comparable between r-axSpA and nr-axSpA patients, although, as expected, in the r-axSpA population there was a higher proportion of males (59.9% vs 47.5%) and longer mean time since diagnosis (5.1 vs 1.9 years) (Table 1). In total, 82.4% (554/672) of patients were also classified as having axSpA using the ASAS classification criteria. Two patients with an unconfirmed diagnosis of r-axSpA or nr-axSpA were included in the overall population (one of whom was included in the FAS). Baseline characteristics were also similar between study completers and non-completers, although a larger proportion of females discontinued the study (Supplementary Table S1, available at Rheumatology online).
Table 1.
Baseline characteristics (Safety Set)
| Overall axSpA (n = 672) | r-axSpA (n = 469) | nr-axSpA (n = 201) | |
|---|---|---|---|
| Age (years) | |||
| Mean (s.d.) | 43.9 (12.7) | 45.1 (12.8) | 41.2 (11.8) |
| Median (range) | 44.0 (18–79) | 45.0 (18–79) | 40.0 (19–71) |
| Female, n (%) | 287 (42.7) | 177 (37.7) | 108 (53.7) |
| Time since diagnosis (years) | |||
| Mean (s.d.) | 4.1 (6.7) | 5.1 (7.6) | 1.9 (3.2) |
| Median (range) | 1.3 (0–56) | 2.2 (0–56) | 0.8 (0–25) |
| Prior anti-TNF use, n (%) | 180 (26.8) | 143 (30.5) | 35 (17.4) |
| Number of prior anti-TNFs, n (%) | |||
| Naïve | 492 (73.2) | 326 (69.5) | 166 (82.6) |
| 1 | 114 (17.0) | 90 (19.2) | 23 (11.4) |
| 2 | 45 (6.7) | 36 (7.7) | 8 (4.0) |
| >2 | 21 (3.1) | 17 (3.6) | 4 (2.0) |
| BASDAI, mean (s.d.) | 6.1 (1.8) | 6.1 (1.8) | 6.2 (1.7) |
| BASFI, mean (s.d.) | 5.3 (2.4) | 5.4 (2.4) | 5.1 (2.3) |
| ASDAS, mean (s.d.) | 3.6 (0.9) | 3.6 (1.0) | 3.4 (0.9) |
| Total back pain, mean (s.d.) | 6.7 (2.2) | 6.8 (2.2) | 6.6 (2.4) |
| CRP (mg/l), geometric mean | 6.97 | 7.70 | 5.55 |
| CRP level, n (%): | |||
| ≤15 mg/l | 385 (57.3) | 251 (53.5) | 134 (66.7) |
| >15 mg/ l | 158 (23.5) | 120 (25.6) | 37 (18.4) |
| Missing | 129 (19.2) | 98 (20.9) | 30 (14.9) |
| ESR (mm/h), geometric mean | 15.92 | 16.75 | 14.34 |
| History of EAMs, n (%) | |||
| Uveitis | 92 (13.7) | 68 (14.5) | 23 (11.4) |
| Inflammatory bowel disease | 41 (6.1) | 34 (7.2) | 7 (3.5) |
| Psoriasis | 64 (9.5) | 46 (9.8) | 18 (9.0) |
| History of peripheral manifestations, n (%) | |||
| Peripheral arthritis | 210 (31.3) | 140 (29.9) | 69 (34.3) |
| Enthesitis | 149 (22.2) | 92 (19.6) | 56 (27.9) |
| Dactylitis | 26 (3.9) | 11 (2.3) | 15 (7.5) |
Safety Set. axSpA: axial spondyloarthritis; ASDAS: Ankylosing Spondylitis Disease Activity Score; axSpA: axial spondyloarthritis; EAM: extra-articular manifestation; nr-axSpA: non-radiographic axSpA; r-axSpA: radiographic axSpA.
Primary variable
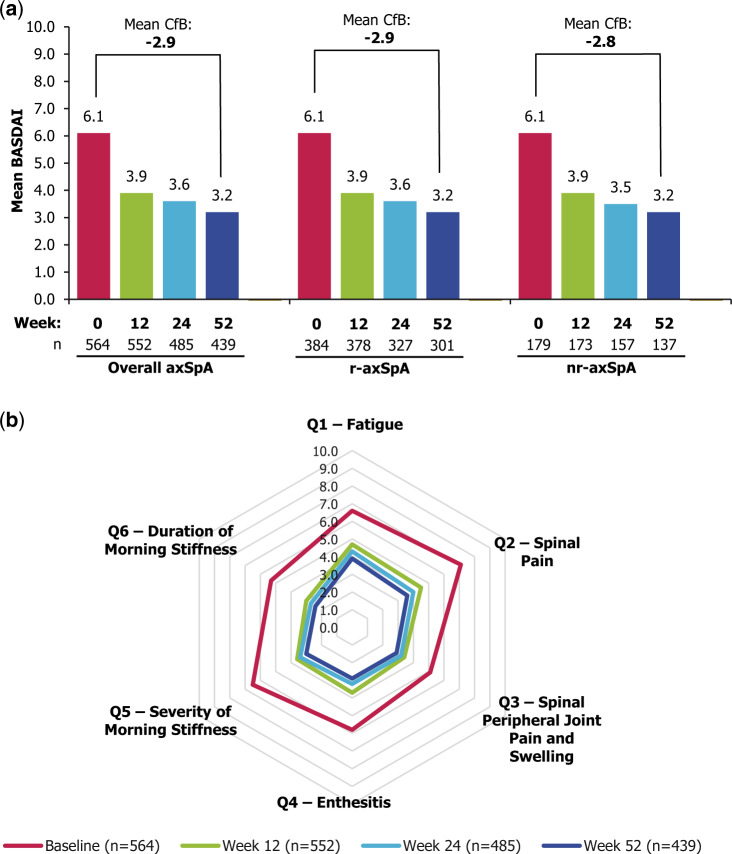
At week 52, for the 439/564 patients who completed the week 52 visit, there was a mean (s.d.) change from baseline in BASDAI of –2.9 (2.3) in the overall axSpA population. Similar improvements were observed in r-axSpA and nr-axSpA patients, who demonstrated a mean (s.d.) change of –2.9 (2.2; n = 301) and –2.8 (2.4; n = 137), respectively (Fig. 2a). Similar changes were observed when missing values were imputed (MI), with a change from baseline of –2.6 (2.4) in the overall population, and –2.7 (2.3) and –2.6 (2.5) in the r-axSpA and nr-axSpA subpopulations.
Fig. 2.
Improvements in BASDAI over 52 weeks’ CZP treatment
(a) BASDAI for overall axSpA and r-axSpA and nr-axSpA subpopulations; (b) breakdown of BASDAI components for overall axSpA population. Full Analysis Set (n = 564). Observed case data. Mean CfB value across all patients with a week 52 BASDAI assessment is reported. *P <0.0001 (Student’s t test). axSpA: axial spondyloarthritis; CfB: change from baseline; CZP: certolizumab pegol; nr-axSpA: non-radiographic axSpA; r-axSpA: radiographic axSpA.
Reductions in BASDAI scores were seen as early as the first visit (week 12), with further improvements at weeks 24 and 52 in the overall population and both subpopulations (Fig. 2a). The mean (s.d.) changes from baseline for weeks 12 and 24 in the overall axSpA population were –2.2 (2.1) and –2.5 (2.2), respectively; similar changes were observed in the r-axSpA [–2.2 (2.0) and –2.5 (2.2)] and nr-axSpA subpopulations [–2.2 (2.2) and –2.5 (2.3)].
In the overall axSpA population, all six items of the BASDAI improved to a similar degree at weeks 12, 24 and 52 (Fig. 2b), including components assessing pain, morning stiffness and fatigue.
Secondary and additional variables
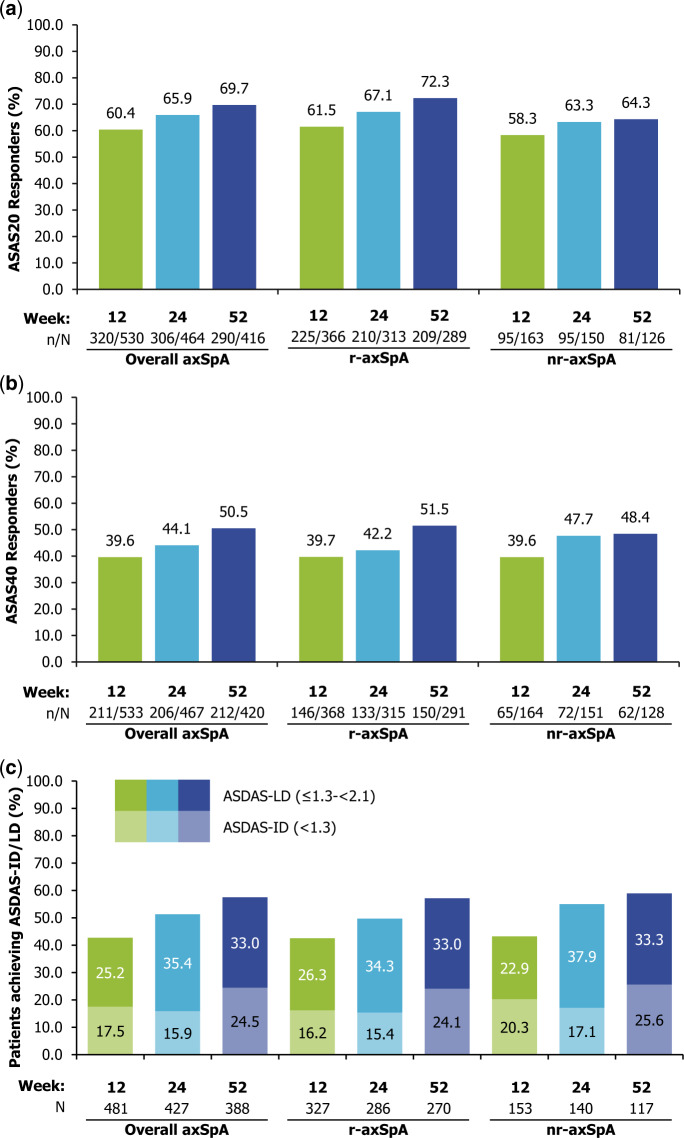
There were overall improvements in secondary variables, including ASAS20 and ASAS40 responses (Fig. 3), BASFI and PtGADA (Table 2) at week 52. ASAS20 was achieved by 69.7% of patients (r-axSpA: 72.3%; nr-axSpA: 64.3%) and ASAS40 by 50.5% (r-axSpA: 51.5%; nr-axSpA: 48.4%). At week 52, the mean (s.d.) change from baseline in BASFI was –2.2 (2.5) in the overall population, and –2.2 (2.4) and –2.1 (2.6) in the r-axSpA and nr-axSpA subpopulations, respectively. For PhGADA, the mean (s.d.) change from baseline at week 52 was –4.1 (2.4) in the overall population, with the same response observed in the r-axSpA and nr-axSpA subpopulations.
Fig. 3.
Responses for key secondary outcomes
Proportion of patients achieving (a) ASAS20, (b) ASAS40 or (c) ASDAS-ID/LD. Full Analysis Set. Observed case data. ASAS20/40: Assessment of SpondyloArthritis international Society 20%/40% response. ASDAS: Ankylosing Spondylitis Disease Activity Score; axSpA: axial spondyloarthritis; ID: inactive disease; LD: low disease; nr-axSpA: non-radiographic axSpA; r-axSpA: radiographic axSpA.
Table 2.
Effectiveness outcomes (Full Analysis Set)
| Overall axSpAa (n = 564) |
r-axSpA (n = 384) |
nr-axSpA (n = 179) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 52(observed) | Week 52(imputed) | Baseline | Week 52(observed) | Week 52(imputed) | Baseline | Week 52(observed) | Week 52(imputed) | |
| BASDAI Mean (s.d.) | 6.1 (1.8) n=564 | 3.2 (2.2) n=439 | 3.5 (2.3) | 6.1 (1.8) n=384 | 3.2 (2.1) n=301 | 3.4 (2.3) | 6.1 (1.7) n=179 | 3.2 (2.2) n=137 | 3.5 (2.4) |
| BASDAI <4 % (n/N) [95% CI] | 10.8 (61/564) [8.3, 13.4] | 68.3 (300/439) [64.0, 72.7] | 62.7 [58.5, 67.0] | 11.2 (43/384) [8.0, 14.4] | 69.1 (208/301) [63.9, 74.3] | 63.4 [58.3, 68.5] | 10.1 (18/79) [5.7, 14.5] | 67.2 (92/137) [59.3, 75.0] | 61.7 [54.0, 69.3] |
| BASDAI CFB ≥2.0 % (n/N) [95% CI] | — | 62.2 (273/439) [57.7, 66.7] | 58.4 [54.0, 62.8] | — | 63.1 (190/301) [57.7, 68.6] | 59.2 [53.8, 64.6] | — | 60.6 (83/137) [52.4, 68.8] | 57.0 [48.9, 65.0] |
| ASDAS (CRP) b Mean (s.d.) | 3.6 (0.9) n=504 | 2.1 (1.0) n=383 | 2.2 (1.0) | 3.7 (0.9) n=345 | 2.1 (1.0) n=267 | 2.2 (1.0) | 3.5 (0.8) n=158 | 2.0 (1.0) n=115 | 2.2 (1.0) |
| ASDAS-CII % (n/N) [95% CI] | — | 63.9 (225/352) [58.9, 68.9] | 57.8 [53.3, 62.3] | — | 63.6 (157/247) [57.6, 69.6] | 58.7 [53.3, 64.2] | — | 65.4 (68/104) [56.2, 74.5] | 56.2 [48.3, 64.1] |
| ASDAS-ID b % (n/N) | 0.8 (4/512) | 24.5 (95/388) | 19.4 [15.9, 22.9] | 0.9 ( 3/351) | 24.1 (65/270) | 19.1 [15.0, 23.2] | 0.6 (1/160) | 25.6 (30/117) | 20.1 [13.9, 26.2] |
| ASDAS-LD % (n/N) | 3.5 (18/512) | 33.0 (128/388) | 30.9 [26.5, 35.4] | 3.7 (13/351) | 33.0 (89/270) | 30.7 [25.4, 36.0] | 3.1 (5/160) | 33.3 (39/117) | 31.6 [23.6, 39.6] |
| ASDAS <2.1 % (n/N) [95% CI] | 4.3 (22/512) [2.5, 6.1] | 57.5 (223/388) [52.6, 62.4] | 50.3 [45.8, 54.9] | 4.6 (16/351) [2.4, 6.7] | 57.0 (154/270) [51.1, 62.9] | 49.9 [44.3, 55.4] | 3.8 (6/160) [0.8, 6.7] | 59.0 (69/117) [50.1, 67.9] | 51.7 [43.6, 59.7] |
| ASAS20 % (n/N) [95% CI] | — | 69.7 (290/416) [65.3, 74.1] | 61.3 [44.3, 78.2] | — | 72.3 (209/289) [67.2, 77.5] | 64.1 [47.0, 81.1] | — | 64.3 (81/126) [55.9, 72.7] | 51.0 [10.1, 91.9] |
| ASAS40 % (n/N) [95% CI] | — | 50.5 (212/420) [45.7, 55.3] | 40.1 [24.1, 56.0] | — | 51.5 (150/291) [45.8, 57.3] | 42.3 [25.6, 58.9] | — | 48.4 (62/128) [39.8, 57.1] | 32.6 [2.3, 62.8] |
| BASFI Mean (s.d.) | 5.3 (2.4) n=563 | 3.1 (2.4) n=439 | 3.3 (2.5) | 5.4 (2.4) n=383 | 3.1 (2.4) n=301 | 3.4 (2.5) | 5.1 (2.3) n=179 | 2.9 (2.5) n=137 | 3.1 (2.6) |
| PtGADA Mean (s.d.) | 6.9 (2.1) n=562 | 3.2 (2.5) n=431 | 3.5 (2.5) | 6.9 (2.2) n=384 | 3.2 (2.4) n=295 | 3.5 (2.4) | 6.9 (1.9) n=177 | 3.3 (2.6) n=135 | 3.5 (2.6) |
| PhGADA Mean (s.d.) | 6.4 (1.7) n=556 | 2.3 (1.9) n=433 | 2.6 (2.0) | 6.5 (1.7) n=378 | 2.4 (1.9) n=295 | 2.6 (2.0) | 6.2 (1.7) n=177 | 2.1 (1.9) n=137 | 2.3 (2.0) |
| Total back pain c Mean (s.d.) | 6.7 (2.3) n=560 | 3.3 (2.4) n=435 | 3.5 (2.4) | 6.8 (2.2) n=382 | 3.3 (2.3) n=297 | 3.6 (2.4) | 6.6 (2.4) n=177 | 3.3 (2.6) n=137 | 3.5 (2.6) |
Full Analysis Set. Week 52 outcomes are reported using observed case analysis or multiple imputation.
Includes 1 patient with unconfirmed AS/nr-axSpA classification;
CRP values <2 were replaced with 2 to compute ASDAS;
Measured using a numeric rating scale where 0 is ‘no pain’ and 10 is ‘worst possible pain’.
ASAS20/40: Assessment of SpondyloArthritis international Society 20%/40% response; ASDAS: Ankylosing Spondylitis Disease Activity Score; ASDAS-CII: ASDAS clinically important improvement (change from baseline of ≥1.1); ASDAS-ID: ASDAS Inactive Disease (<1.3); ASDAS-LD: ASDAS low disease; axSpA: axial spondyloarthritis; BL: baseline; CFB: change from BL; FAS: Full Analysis Set; MI: multiple imputation; nr-axSpA: non-radiographic axSpA; Ph/PtGADA: Physician’s/Patient’s Global Assessment of Disease Activity; r-axSpA: radiographic axSpA.
In addition, patient-reported outcomes, such as PtGADA and total back pain, and post-hoc analyses of clinically important outcomes related to disease activity states, such as achievement of BASDAI < 4, ASDAS-ID or ASDAS < 2.1 (Table 2) showed improvements at week 52 (Table 2). During the 1-year observational period, the proportion of patients with ASDAS-ID increased from 0.8% at baseline to 24.5% at week 52, while the proportion with ASDAS < 2.1 increased from 4.3% to 57.5%; responses were similar between r-axSpA and nr-axSpA (Fig. 3c).
At baseline, peripheral arthritis was recorded in 22.5% (127/564) of the overall population, and in 22.1% (85/384) and 23.5% (42/179) of the r-axSpA and nr-axSpA subpopulations, respectively. By week 52, peripheral arthritis was present in 6.0% (34/564), 7.0% (27/384) and 3.9% (7/179) of patients, respectively. There were similar trends in the improvement of enthesitis: at baseline, enthesitis was present in 17.0% (96/564), 15.1% (58/384) and 20.7% (37/179) patients in the overall, r-axSpA and nr-axSpA populations, respectively; by week 52, enthesitis was recorded in 4.4% (25/564), 2.9% (11/384) and 7.3% (13/179) of patients, respectively.
The mean (s.d.) BASDAI at baseline for those with and without prior anti-TNF exposure was 6.1 (1.8) in both subgroups (n = 165 and n = 399, respectively); at week 52, this had reduced to 3.5 (2.1) in pre-treated patients (n = 127) and 3.1 (2.2) in anti-TNF naïve patients (n = 312), representing a mean change of −2.6 and −3.0, respectively. At week 52, 68.0% (68/100) and 74.6% (141/189) of pre-treated vs anti-TNF naïve patients achieved an ASAS20 response; the proportions achieving an ASAS40 response were 45.0% (45/100) and 55.0% (105/191), respectively.
Improvements in disease activity outcomes stratified by country were similar to that in the overall population, with slightly better improvements observed amongst patients in Greece for ASDAS-CII, ASDAS-MI and ASAS20/40 responses (Supplementary Table S2, available at Rheumatology online).
Safety
Overall, 37.9% (255/672) patients experienced ≥1 AE, and 6.3% (42/672) experienced a serious AE (Table 3). Drug withdrawal due to AEs occurred in 20.5% (138/672) patients.
Table 3.
Safety outcomes (Safety Set)
| MedDRA® v20.1 System Organ Class Preferred Term, n (%) [#] | Overall axSpA (n = 672) | r-axSpA (n = 469) | nr-axSpA (n = 201) |
|---|---|---|---|
| Patient exposure years | 663.9 | 462.5 | 200.1 |
| Any AE | 255 (37.9) [475] | 173 (36.9) [317] | 80 (39.8) [155] |
| EAIR, per 100 PY (95% CI) | 50.7 (44.7, 57.4) | 48.6 (41.7, 56.5) | 54.8 (43.4, 68.2) |
| Drug-related AEs | 139 (20.7) [192] | 87 (18.6) [123] | 50 (24.9) [66] |
| Drug withdrawal due to AE | 138 (20.5) [245] | 96 (20.5) [173] | 41 (20.4) [70] |
| Serious AEs | 42 (6.3) [53] | 29 (6.2) [35] | 13 (6.5) [18] |
| EAIR, per 100 PY (95% CI) | 6.6 (4.7, 8.9) | 6.5 (4.4, 9.3) | 6.8 (3.6, 11.6) |
| Serious infections and infestations | 12 (1.8) [15] | 7 (1.5) [8] | 5 (2.5) [7] |
| Opportunistic infections | 3 (0.4) [3] | 2 (0.4) [2] | 1 (0.5) [1] |
| EAIR, per 100 PY (95% CI) | 0.5 (0.1, 1.3) | 0.4 (0.1, 1.6) | 0.5 (0.0, 2.8) |
| Oral herpes | 2 (0.3) [2] | 2 (0.4) [2] | 0 |
| Ophthalmic herpes zoster | 1 (0.1) [1] | 0 | 1 (0.5) [1] |
| Serious cardiovascular events | 3 (0.4) [3] | 2 (0.4) [2] | 1 (0.5) [1] |
| Cardiac disorders | 2 (0.3) [2] | 1 (0.2) [1] | 1 (0.5) [1] |
| Angina pectoris | 1 (0.1) [1] | 0 | 1 (0.5) [1] |
| Myocardial infarction | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Nervous system disorders | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Paraplegia | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Malignant or unspecified tumours | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Neoplasms benign, malignant and unspecified | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Basal cell carcinoma | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Haematopoietic cytopenias | 1 (0.1) [1] | 0 | 1 (0.5) [1] |
| Blood and lymphatic system disorders | 1 (0.1) [1] | 0 | 1 (0.5) [1] |
| Leukopenia | 1 (0.1) [1] | 0 | 1 (0.5) [1] |
| Serious bleeding events | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Gastrointestinal disorders | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Gastrointestinal haemorrhage | 1 (0.1) [1] | 1 (0.2) [1] | 0 |
| Deaths | 0 | 0 | 0 |
Safety Set. AEs were recorded according to the MedDRA® version 20.1. The overall axSpA population includes two patients with an unconfirmed diagnosis of either r-axSpA or nr-axSpA. #: number of events; AE: adverse event; axSpA: axial spondyloarthritis; EAIR: exposure-adjusted incidence rate; MedDRA®: Medical Dictionary for Regulatory Activities; nr-axSpA: non-radiographic axSpA; PY: patient-years; r-axSpA: radiographic axSpA.
Serious AEs included one malignancy, three serious cardiovascular events and 15 serious infections (eight in r-axSpA and seven in nr-axSpA patients). No specific patterns of infection were observed, and there were no reported cases of tuberculosis.
Two of the 15 cases of serious infection were assessed as related to CZP by the respective treating physicians. One patient experienced sepsis following knee replacement surgery 298 days after the first dose of CZP. The patient had a history of local infection in the left knee, which was assessed as CZP-related, and which worsened leading to sepsis. CZP was withdrawn and the event resolved 84 days after onset. In the second case, a patient experienced liver abscess and sepsis 103 days after the first dose of CZP. The liver abscess, though not the sepsis, was assessed as related to CZP by the treating physician. CZP treatment was consequently interrupted in this patient.
In addition, there were three opportunistic infections, assessed as related to CZP by the treating physician, which included two cases of oral herpes and one case of ophthalmic herpes zoster, none of which were serious. Drug interruption was undertaken in one oral herpes case and in the ophthalmic herpes zoster case; CZP treatment was continued in the other oral herpes case.
Five other serious AEs were assessed as related to CZP by the treating physicians. This included one case of basal cell carcinoma, one of anaphylactic shock and three of pustular psoriasis, all of which occurred in r-axSpA patients. Except in the patient with basal cell carcinoma, CZP was permanently discontinued in all cases.
No deaths were reported during the study.
Discussion
CIMAX is the first observational study to examine the effectiveness and safety of CZP treatment in patients with axSpA in routine clinical practice across multiple European sites. Patients treated with CZP according to the SmPC showed improvements in the primary variable, change from baseline in BASDAI, and in secondary and additional effectiveness variables.
Over one year, improvements were observed in both r- and nr-axSpA patients across multiple measures assessing various aspects of axSpA, including disease activity, pain, morning stiffness, fatigue, inflammation and physical function. In addition, there were reductions in the numbers of patients with peripheral manifestations, including arthritis and enthesitis. The improvements in disease activity and signs and symptoms of disease observed in this study, including BASDAI, ASDAS and patient-reported outcome measures, were comparable to previous clinical trials of CZP in axSpA patients [11, 12]. In line with the results from these trials, patients in CIMAX demonstrated improvements in both primary and secondary measures after ∼12 weeks of CZP treatment, with increasing levels of effectiveness observed at weeks 24 and 52. Improvements were also similar to a previous, smaller non-interventional study examining anti-TNF treatment in 363 r-axSpA and 102 nr-axSpA patients [15].
The BASDAI was used as the primary efficacy variable [16]. This is a widely validated tool for assessment of disease activity in axSpA, comprising questions that evaluate different aspects of disease from the perspective of the patient. Reductions in all components of the BASDAI were seen in this study, indicating that patients experienced improvements in key axSpA symptoms, including pain, fatigue, morning stiffness and inflammation.
Current treat-to-target strategies for axSpA recommend remission or low disease activity as the main goals of treatment [17]. In this study, more than half (57.5%) of patients achieved an ASDAS <2.1, indicating low or inactive disease, and 24.5% achieved ASDAS-ID after one year of treatment. These outcomes were comparable to those in RAPID-axSpA clinical trial, in which ∼30% of patients achieved ASDAS-ID after 6 months of CZP treatment, with responses maintained up to 4 years of treatment [11].
Patients’ baseline characteristics were largely comparable to European cohorts such as the German Spondyloarthritis Inception Cohort (GESPIC) and the Swiss Clinical Quality Management (SCQM) observational studies, both of which included axSpA patients who were prospectively followed up over time [15, 18]. However, baseline disease activity according to BASDAI was higher in the current study (6.1 in both r-axSpA and nr-axSpA, compared with 4.0 and 3.9 in the GESPIC study, and 4.8 and 5.1 in the Swiss study), indicating that patients prescribed CZP in CIMAX had higher disease activity compared with previous cohorts [15, 18]. However, it is also important to note that ∼11% of patients had a BASDAI <4 at baseline. BASDAI >4 is widely used as a cut-off to define active disease and is often specified as an inclusion criterion in randomized clinical trials; as this was an observational study of real-world clinical practice, patients were only required to have a diagnosis of axSpA according to local guidelines used by their treating physician.
Baseline disease activity was comparable between r-axSpA and nr-axSpA subpopulations, as in previous studies [11, 19]. As expected, there was a lower proportion of females in the r-axSpA subpopulation (37.7%) compared with nr-axSpa (53.7%), although this proportion was higher than in RAPID-axSpA13 (27.5%) and the SCQM study (25.9%) [15]. While it has been established that there is a higher proportion of males than females with r-axSpA [20], the lower male-to-female ratio observed in the present study may reflect improvements in diagnosis of r-axSpA in females.
In this study, 26.8% of patients in the overall axSpA population, including 30.5% of r-axSpA and 17.4% of nr-axSpA patients, had previously been exposed to at least one anti-TNF. This was higher than in the RAPID-axSpA study, in which 16% of all axSpA patients had received prior anti-TNF treatment [13]. Interestingly, in these patients we also observed substantial reductions in disease activity, according to the BASDAI and ASAS response criteria. These outcomes suggest that switching to another anti-TNF treatment may be a suitable treatment option for patients who have had previous inadequate response to anti-TNF therapy.
Some differences in baseline characteristics and responses were noted for patients stratified by country; however, the study was not powered to recruit balanced numbers of patients from all six countries. These differences should therefore be interpreted with caution.
The main strength of the study is the inclusion and follow-up of patients in a real-world setting; randomized controlled trials are often conducted in selective patient populations, which may limit their applicability to real-world clinical practice. A further strength is inclusion of both r-axSpA and nr-axSpA subpopulations, although because radiograph and MRIs were evaluated by local readers, there is the possibility that some patients were misclassified. Historically, r-axSpA has received more focus than nr-axSpA despite a similar burden of disease in the two patient populations [21]. Both subpopulations demonstrated similar levels of improvement at week 52, confirming previous findings from RAPID-axSpA indicating that CZP is effective regardless of the presence or absence of radiographic damage [10]. The findings also support the concept of r-axSpA and nr-axSpA as part of the axSpA spectrum without large clinical differences between the two [22].
Limitations of this study include the lack of a comparator arm and missing data due to loss to follow-up, although the latter is a feature inherent to observational studies. A total of ∼22% of patients in the FAS discontinued the study over the 52 weeks: the majority were due to lack or loss of efficacy (n = 67/125), followed by AEs (n = 18/125) and loss to follow-up (n = 10/125). Baseline characteristics between completers and non-completers were comparable; thus, these were unlikely to impact final outcomes. High rates of patient discontinuation are common in real-world studies, in which study visits are not mandated by the protocol. Overall, a large majority of patients completed the study (>75%; 439/564), with similar proportions in the r-axSpA and nr-axSpA subpopulations; the rate of discontinuation was also lower than in previous observational studies (34.0% in a Belgian study of CZP in rheumatoid arthritis [23] and 54.5% in the non-interventional GO-NICE study [24]). Finally, the use of observed data may introduce bias, particularly in long-term studies such as this one, as patients remaining on treatment are more likely to be those for whom the medication is effective and well-tolerated. However, outcomes reported using imputed data to account for patients lost to follow-up supported the results from observed data.
No new safety concerns were identified over the course of treatment. The prevalence of AEs was comparable to that previously reported in a non-interventional study of anti-TNF therapy in patients with axSpA [24]. Drug-related AEs occurred in 21% of the population, lower than in the RAPID-axSpA and C-axSpAnd clinical trials of CZP in axSpA (54% and 30%, respectively) [11, 12]. This may be attributable to the higher rate of discontinuation in CIMAX (22% of the FAS) compared with the RAPID-axSpA (11% at week 48) and C-axSpAnd (9% at week 52; nr-axSpA only) trials [11, 12].
In summary, CZP treatment over one year resulted in demonstrable benefits for patients with axSpA in a European real-world setting, encompassing reductions in disease activity, including key symptoms such as pain, fatigue and morning stiffness, and improvements in function and peripheral manifestations. Effectiveness and safety outcomes were comparable to previous studies of anti-TNFs in axSpA, including both clinical trials and non-interventional studies [11, 12, 15, 18, 19]. Similar reductions in disease activity were observed in both r-axSpA and nr-axSpA subpopulations, demonstrating the benefits of CZP treatment across the full spectrum of axSpA.
Supplementary Material
Acknowledgements
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Tommi Nurminen, UCB Pharma, Monheim am Rhein, Germany, for his contributions to statistical analysis; Susanne Wiegratz, UCB Pharma GmbH, Monheim am Rhein, Germany, for publication coordination; and Jessica Patel, Costello Medical, Cambridge, UK, for medical writing and editorial assistance based on the authors’ input and direction. This study was funded by UCB Pharma. Substantial contributions to study conception and design: X.B., T.W., L.DC., E.C.-E., E.K., B.H., L.B., N.G.; substantial contributions to acquisition, analysis and interpretation of the data: X.B., T.W., L.DC., B.F., E.C.-E., G.K., B.VL., E.K., B.H., L.B., N.G.; drafting the article or revising it critically for important intellectual content: X.B., T.W., L.DC., B.F., E.C.-E., G.K., B.VL., E.K., B.H., L.B., N.G.; final approval of the version of the article to be published: X.B., T.W., L.DC., B.F., E.C.-E., G.K., B.VL., E.K., B.H., L.B., N.G. Data from non-interventional studies is outside of UCB’s data sharing policy. Trial registration: https://clinicaltrials.gov/, NCT02354105.
Funding: This study was sponsored by UCB Pharma. This article was based on the original study AS0002/CIMAX (NCT02354105) sponsored by UCB Pharma. Support for third-party writing assistance for this article, provided by Jessica Patel, PhD, Costello Medical, UK was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosures statement: X.B.: consultancy/speaker fees/research grants from AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, MSD, Novartis, Pfizer and UCB Pharma and grant/research support from AbbVie, Bristol-Myers Squibb, Celgene; T.W.: consultancy/speaker fees/research grants from AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, MSD, Novartis, Pfizer and UCB Pharma; L.DC.: research grants and advisory board fees from AbbVie, MSD, Roche and Pfizer; B.F.: none; E.C.-E.: consultancy fees from UCB Pharma, MSD, AbbVie, Novartis, Janssen; G.K.: honoraria for educational activities and consultancy payments from UCB Pharma, Janssen, AbbVie, Novartis, MSD, Aenorasis, Genesis Pharma, Pfizer, Roche; B.VL., E.K., B.H., L.B.: employees of UCB Pharma; N.G.: honoraria/speaker fees/expenses for attendance at conferences from AbbVie, Janssen and UCB Pharma; and grant/research support from Novartis.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. van der Linden S, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, van der Heijde D, Landewe R. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, Landewe R, van der Heijde D. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 4. Landewe R, Dougados M, Mielants H. et al Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 2009;68:863–7. [DOI] [PubMed] [Google Scholar]
- 5. Kobelt G, Andlin-Sobocki P, Maksymowych WP.. Costs and quality of life of patients with ankylosing spondylitis in Canada. J Rheumatol 2006;33:289–95. [PubMed] [Google Scholar]
- 6. Boonen A, van der Heijde D, Landewe R et al Work status and productivity costs due to ankylosing spondylitis: comparison of three European countries. Ann Rheum Dis 2002;61:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Winter JJ, van Mens LJ, van der Heijde D. et al Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 2016;18:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Heijde D, Ramiro S, Landewé R. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 9. Poddubnyy D. Axial spondyloarthritis: is there a treatment of choice? Ther Adv Musculoskelet Dis 2013;5:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Heijde D, Baraliakos X, Hermann K-GA. et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018;77:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Heijde D, Dougados M, Landewé R. et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology 2017;56:1498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deodhar A, Gensler LS, Kay J. et al. A 52-week randomized placebo-controlled trial of certolizumab pegol in non-radiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landewé R, Braun J, Deodhar A. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Medicines Agency. Summary of Product Characteristics (CIMZIA). https://www.ema.europa.eu/en/documents/product-information/cimzia-epar-product-information_en.pdf (15 April 2020, date last accessed).
- 15. Ciurea A, Scherer A, Exer P. et al. Tumor necrosis factor alpha inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096–106. [DOI] [PubMed] [Google Scholar]
- 16. Garrett S, Jenkinson T, Kennedy LG. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 17. Smolen JS, Schöls M, Braun J. et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rudwaleit M, Haibel H, Baraliakos X. et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 19. Sieper J, Landewé R, Rudwaleit M. et al. Effect of certolizumab pegol over ninety-six weeks in patients with axial spondyloarthritis: results from a phase III randomized trial. Arthritis Rheumatol 2015;67:668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy LG, Will R, Calin A.. Sex ratio in the spondyloarthropathies and its relationship to phenotypic expression, mode of inheritance and age at onset. J Rheumatol 1993;20:1900–4. [PubMed] [Google Scholar]
- 21. Baraliakos X, Braun J.. Non-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences? RMD Open 2015;1:e000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deodhar A, Strand V, Kay J, et al The term ‘non-radiographic axial spondyloarthritis’ is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann Rheum Dis 2016;75:791–4. [DOI] [PubMed] [Google Scholar]
- 23. Westhovens R, Ravelingien I, Vandevyvere K et al. Improvements in productivity and increased participation in daily activities over 52 weeks of certolizumab pegol treatment of rheumatoid arthritis: results of a Belgian observational study. Acta Clin Belg 2019;74:342–50. [DOI] [PubMed] [Google Scholar]
- 24. Kruger K, Burmester GR, Wassenberg S. et al Effectiveness and safety of golimumab in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis under real-life clinical conditions: non-interventional GO-NICE study in Germany. BMJ Open 2018;8:e021082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.