Abstract
Background
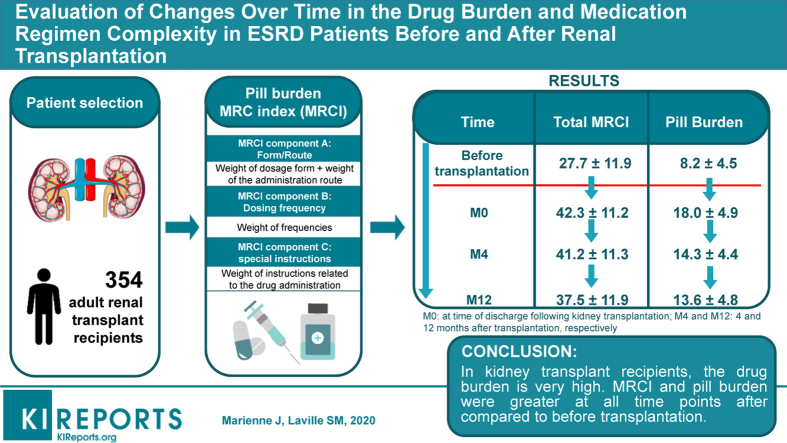
Medication regimen complexity (MRC) has not been characterized in detail in patients with end-stage renal disease (ESRD). The objective of the present study was to quantify changes over time in the prescription drug burden and MRC in patients with ESRD (before transplantation, on discharge after kidney transplantation [M0], and 4 months [M4] and 12 months [M12] afterward).
Methods
We retrospectively studied adult patients having undergone kidney transplantation. The number and types of drug prescribed, the pill burden, and the MRC index (MRCI) at 4 different time points (before transplantation, M0, M4, and M12) were extracted from the patients’ medical records. MRCI was calculated by adding each drug score (calculated according to its formulation, dosing frequency, and additional instructions concerning administration). Hence, the MRCI took account of all prescription drugs. A logistic regression model was used to identify factors associated with an elevated MRCI at M12.
Results
The median (interquartile range) age of the 354 study participants was 52 years (42–62). Respectively 21%, 42%, 53%, and 38% of the patients were taking 10 or more drugs before transplantation and at M0, M4, and M12. At M12, the 3 most frequently prescribed drug classes were immunosuppressants, cardiovascular system drugs, and drugs acting on the alimentary tract and metabolism. The pill burden and MRCI before transplantation were significantly lower (P < 0.001) than at each time point after transplantation. Diabetes and dyslipidemia were independently associated with an elevated MRCI at M12.
Conclusion
In kidney transplant recipients, the drug burden and MRCI were greater at all time points after transplantation than before transplantation. The impact of the drug burden and MRC on medication adherence and clinical outcomes in these patients requires further evaluation.
Keywords: complexity, drugs, end-stage renal disease, kidney transplantation, polypharmacy
Graphical abstract
See Commentary on Page 5
Chronic kidney disease (CKD) is a global health burden.1 A recent study ranked the patients seen by nephrologists as the most complex to manage, in view of the prevalence of polypharmacy and the large number of comorbidities.2 Indeed, recent studies of cohorts of non–end-stage patients with CKD reported a high prevalence of polypharmacy.3, 4, 5 Along with comorbidities and renal complications, the drug burden increases sharply as CKD progresses.4 Indeed, patients on dialysis not only have a large number of comorbidities but also experience specific complications (such as anemia, hyperkaliemia, and bone mineral disorders) requiring specific medications (such as antianemia agents, potassium binders, phosphate binders, and cinacalcet). Various studies have found high levels of polypharmacy (from 10 to 12 drugs per day per individual, on average) in dialysis patients.6, 7, 8 In kidney transplant recipients, immunosuppressive agents are combined with other medications required to manage comorbid conditions. Polypharmacy has been linked to poor quality of life in patients having undergone successful kidney transplantation.9 However, detailed data on changes over time in the drug burden and the types of drugs used by patients with end-stage renal disease (ESRD) are lacking.
The medication burden can be assessed in different ways; these include the number of medications administered and the complexity of the medication regimens. Several validated, non–disease-specific tools for calculating medication regimen complexity (MRC) have been described in the literature.10,11 Some of these tools measure MRC in paper-based, coded medication lists, and have already been applied to various populations, including patients with chronic disease12 and older adults.13 However, a specific tool for patients with ESRD is not available. Regimen complexity is one of the major determinants of medication nonadherence in patients with chronic disease, and nonadherence to immunosuppressants in kidney transplant recipients is a major negative factor for graft survival.14 To the best of our knowledge, MRC in kidney transplant recipients has not previously been assessed with a validated tool. Hence, the objective of the present study was to quantify changes over time in the prescription medication burden and MRC in patients with ESRD before kidney transplantation (during dialysis), on discharge after kidney transplantation (M0), and 4 months (M4) and 12 months (M12) afterward.
Methods
Study Design and Participants
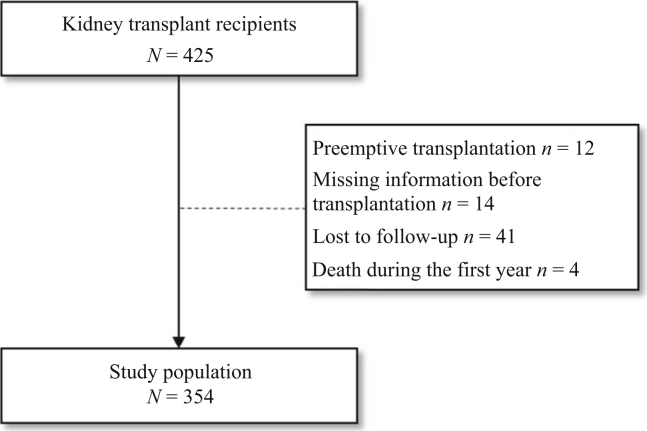
We performed a retrospective study of adult patients (aged 18 and older) having undergone kidney transplantation at Amiens University Medical Center (Amiens, France) between January 1, 2012, and June 15, 2018. We excluded patients (i) with missing data for their clinical status and/or treatments before transplantation, (ii) with missing data for more than 2 of the 4 study time points, (iii) who were lost to follow-up, (iv) who died within a year of transplantation, and (v) who underwent preemptive kidney transplantation (Figure 1).
Figure 1.
Study flowchart.
In line with the French legislation on noninterventional studies, approval by an investigational review board was neither required nor sought. However, the study was registered with the French National Data Protection Commission (Commission Nationale de l'Informatique et des Libertés [Paris, France]; registration number: PI2019_843_0055). Patients were provided with information about the study, and were free to refuse to participate.
Data Collection
The patients’ data were extracted from hospitalization reports at each time point (i.e., just before transplantation, and at M0, M4, and M12 posttransplantation). Before transplantation, all patients were treated by hemodialysis. We recorded the sociodemographic characteristics, the etiology of CKD, smoking status, alcohol consumption, and comorbidities. Patients were classified as having hypertension if this condition was recorded in their medical records or if they were taking antihypertensive medications. Similarly, diabetes was defined as a report in the medical records or the use of antidiabetic drugs, and dyslipidemia was defined as a report in the medical records or the use of lipid-lowering agents. Patients were classified as having cardiovascular disease if they were receiving antiplatelet agents, beta-blockers, and agents acting on the renin-angiotensin system at the same time, or were receiving cardiac therapy drugs (Anatomical Therapeutic and Chemical [ATC] class C01), antithrombotics, or calcium channel blockers. All comorbidities were assessed with reference to prescriptions before kidney transplantation.
As with the patients’ other characteristics, drug prescriptions were recorded at 4 time points: before transplantation, and at M0, M4, and M12. The hemodialysis medication report was used to record drug prescriptions before transplantation, and the hospital’s medical records were used to record drug prescriptions at M0, M4, and M12. The “number of medications” at each time point was defined as the number of distinct drug preparations. Drugs were coded according to the international ATC thesaurus.15 Medication categories were created by reference to the 2 first levels of the ATC codes (e.g., the top-level class C “cardiovascular system” drugs and its subclasses “cardiac therapy” [C01] and “antihypertensives” [C02]). Only prescription medications were recorded. In our descriptive analysis of the number of medications, we defined polypharmacy as 5 or more medications per day per individual and hyperpolypharmacy as 10 or more medications per day per individual; these are the numerical classes most commonly used in the literature.16
The Pill Burden
At each time point, the total pill burden was defined as the total number of pills the subject took daily. The number of pills per day was determined by counting the number of orally administered pills, tablets, or capsules taken per day for each patient. Hence, non–orally administered drugs (powders or granules in food, liquid formulations taken per os, eye drops, and inhaled formulations) were excluded. For tablets taken once or several times a week (but not daily), we divided the pill burden by 7. Hence, a tablet taken once a week was equivalent to taking 0.14 tablets once a day.
Calculation of the MRCI
The MRCI for each patient was calculated at each assessment time point.11 Each drug was weighted according to its formulation, dosing frequency, and additional instructions concerning administration. Hence, the MRCI took account of all prescription drugs (regardless of whether or not they were orally administered) and increased with the number of drugs and the difficulty of administration. The MRCI had 3 different components: (i) the dosage form and administration route, (ii) the dosing frequency, and (iii) additional instructions concerning administration (Supplementary Table S1). For example, an oral tablet medication was given a weight of 1, whereas an eye drop formulation was given a weight of 3. An injectable liquid medication had a weight of 3 if the syringe was prefilled, or 4 if it came in a vial or an ampoule. The weights for Component B ranged from 0.5 for a single daily dose to 12.5 for a dose every 2 hours. Component C quantified the additional instructions for treatment given in the drug’s summary of product characteristics or written on the prescription. Hence, the lowest possible value of the MRCI was 1.5: a capsule or tablet taken by mouth once a day. There was no maximum value because the MRCI increased with the number of drugs (Supplementary Table S2).
Statistical Analyses
Baseline characteristics were described for all participants. The results were expressed as the mean ± SD, the median (interquartile range), or the number (percentages). A 1-way repeated-measures analysis of variance was used to determine whether the MRCI scores, pill burden, and number of daily medications changed over time. Paired Student’s t tests (after Bonferroni’s correction for multiple testing) were also used to compare the mean values of the MRCI score, pill burden, and number of medications per patient at different time points.
Univariate and multivariate logistic regressions were performed to identify factors associated with an MRCI >37.5 12 months after transplantation (37.5 was mean value of MRCI at M12). The multivariable logistic regression analyses were adjusted for possible confounders identified in a review of the literature. Variables with P > 0.10 in the crude model were excluded from the multivariable analysis. Sex, hypertension, and cancer history were not tested because their P value was greater than 0.10. Age, body mass index, diabetes, dyslipidemia, and cardiovascular disease were included in the multivariable analysis.
Statistical analyses were performed with R software (version 3.5.0, Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the Study Population
Data were collected from 354 patients (120 women, 34%) after the exclusion of 71 individuals (including 12 having undergone preemptive transplantation) (Figure 1). The median (interquartile range) age was 52 years (42–62). With regard to comorbidities, 18% of the participants had diabetes, 55% had hypertension, 48% had dyslipidemia, and 52% had a history of cardiovascular disease. The mean ± SD time on dialysis before transplantation was 3.4 years ± 3.4 (Table 1).
Table 1.
Characteristics of the study population
| Total |
|
|---|---|
| (N = 354) | |
| Sex | |
| Men | 233 (65.8) |
| Women | 121 (34.2) |
| Recipient age, yr | 52 (42–62) |
| Body mass index, kg/m2 | 25 (23–29) |
| Cardiovascular disease | 185 (52.3) |
| Hypertension | 194 (54.8) |
| Dyslipidemia | 171 (48.3) |
| Diabetes | 63 (17.8) |
| History of cancer | 25 (7.1) |
| Smoking status | |
| Nonsmoker | 217 (61.3) |
| Smoker | 54 (15.3) |
| Ex-smoker | 83 (23.4) |
| Alcohol consumption | 10 (2.8) |
| Etiology of chronic kidney disease | |
| Diabetic nephropathy | 26 (7.3) |
| Glomerulonephritis | 112 (31.6) |
| Hereditary nephropathy | 4 (1.1) |
| Hypertensive nephropathy | 27 (7.6) |
| Interstitial nephritis | 14 (4.0) |
| Renal and urinary tract malformations | 29 (8.2) |
| Polycystic kidney disease | 59 (16.7) |
| Vascular nephropathy | 17 (4.8) |
| Other nephropathy | 18 (5.1) |
| Indeterminate | 48 (13.6) |
| Previous kidney transplantations | |
| None | 306 (86.4) |
| 1 | 43 (12.1) |
| 2 | 5 (1.4) |
| Time on dialysis, yr | 3.36 (3.35) |
Results are expressed as n (%), median (interquartile range), or mean ± SD.
Patterns of Medication Use and Frequency
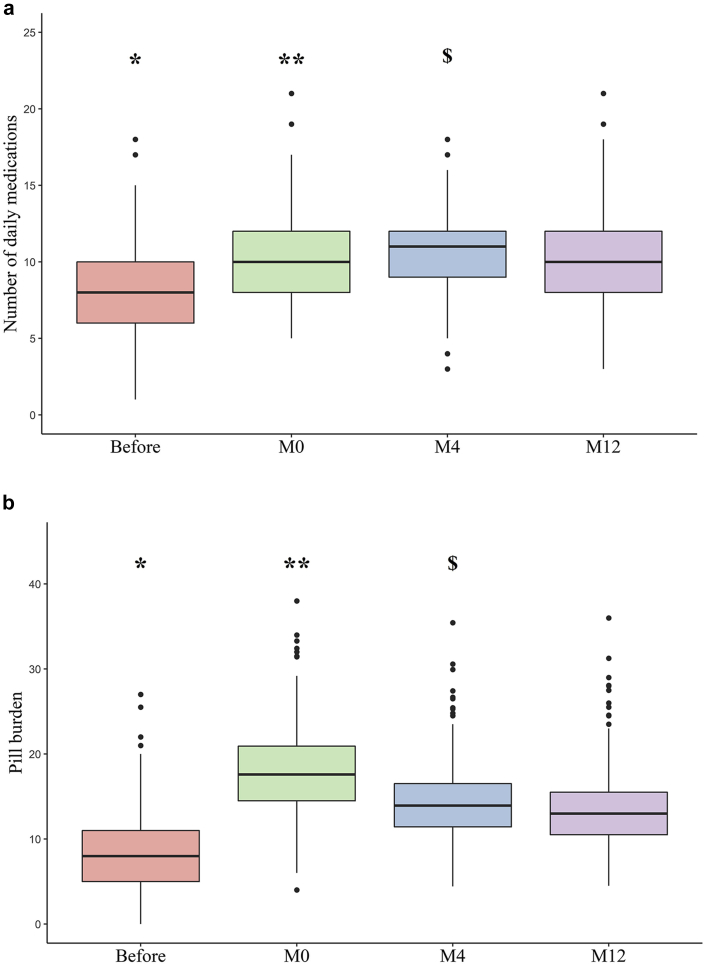
The 354 study participants took a total of 2707 medications before transplantation and 3444 12 months after transplantation. The mean ± SD number of daily prescription medications per individual was respectively 8 ± 3, 10 ± 3, 11 ± 3, and 10 ± 3 before transplantation and at M0, M4, and M12 (Figure 2a). The proportions of patients with polypharmacy and hyperpolypharmacy at M12 were respectively 94% and 38%. Respectively, 21%, 42%, 53%, and 38% of the patients were taking at least 10 drugs before transplantation and at M0, M4, and M12. Before transplantation, the 3 most common ATC medication classes were drugs for the cardiovascular system, drugs acting on the alimentary tract and metabolism, and drugs acting on the blood and blood-forming organs. The 3 most common ATC classes at M12 were immunosuppressants, cardiovascular system drugs, and drugs acting on the alimentary tract and metabolism (Table 2). The use of antihypertensive agents notably varied over the course of ESRD; after transplantation, we observed a significant increase in the use of calcium channel blockers and central antihypertensives, and a decrease in the use of diuretics and in agents acting on the renin-angiotensin system. Unsurprisingly, the use of specific drugs for dialysis care (such as those for the treatment of hyperphosphatemia) decreased after transplantation. Antidiabetic agents were more frequently prescribed 12 months after transplantation than before transplantation. We noted the frequent use of drugs for acid-related disorders.
Figure 2.
Number of daily medications (a) and pill burden (b) during end-stage renal disease (before kidney transplantation, on discharge after kidney transplantation [M0], and 4 and 12 months after kidney transplantation [M4 and M12]). (a) Differences over time were statistically significant (analysis of variance, P < 0.001). ∗P < 0.001 for comparisons between before transplantation and M0, M4, and M12 (after Bonferroni correction). ∗∗Differences between M0 and M4, and M0 and M12 not significant (after Bonferroni correction). $P < 0.001 between M4 and M12 (after Bonferroni correction). (b) Differences over time were statistically significant (analysis of variance, P < 0.001). ∗P < 0.001 for comparisons between before transplantation and M0, M4, and M12 (after Bonferroni correction). ∗∗P < 0.001 between M0 and M4, and between M0 and M12 (after Bonferroni correction). $P = 0.003 between M4 and M12 (after Bonferroni correction).
Table 2.
The most common ATC classes over the course of ESRD: before kidney transplantation, on discharge after kidney transplantation (M0), and 4 and 12 months after kidney transplantation (M4 and M12)
| ATC classes prescribed to patients | ||||
|---|---|---|---|---|
| ATC classes | Before |
M0 |
M4 |
M12 |
| n (%) | n (%) | n (%) | n (%) | |
| Cardiovascular system | 901 (33) | 767 (21) | 896 (24) | 1000 (29) |
| Beta blocking agents | 185 | 195 | 221 | 231 |
| Calcium channel blockers | 113 | 222 | 207 | 199 |
| Central antihypertensives | 78 | 180 | 161 | 172 |
| Lipid-modifying agents | 173 | 48 | 130 | 176 |
| Agents acting on the renin-angiotensin system | 171 | 33 | 85 | 128 |
| Diuretics | 163 | 77 | 78 | 79 |
| Cardiac therapy | 17 | 9 | 12 | 13 |
| Vasoprotectives | 0 | 3 | 2 | 2 |
| Peripheral vasodilators | 1 | 0 | 0 | 0 |
| Alimentary tract and metabolism | 536 (20) | 615 (17) | 751 (20) | 792 (23) |
| Drugs for acid-related disorders | 142 | 323 | 277 | 242 |
| Vitamins | 201 | 37 | 192 | 267 |
| Drugs used in diabetes | 66 | 109 | 152 | 159 |
| Mineral supplements | 98 | 95 | 102 | 94 |
| Antidiarrheals, intestinal anti-inflammatory/anti-infective agents | 9 | 19 | 12 | 13 |
| Drugs for constipation | 7 | 19 | 6 | 9 |
| Drugs for functional gastrointestinal disorders | 6 | 10 | 3 | 3 |
| Digestives, including enzymes | 3 | 2 | 3 | 3 |
| Bile and liver therapy | 2 | 0 | 1 | 2 |
| Stomatological preparations | 0 | 1 | 3 | 0 |
| Other alimentary tract and metabolism products | 2 | 0 | 0 | 0 |
| Antineoplastic and immunomodulating agents | 13 (0.5) | 703 (19) | 678 (18) | 651 (19) |
| Immunosuppressants | 13 | 703 | 678 | 651 |
| Blood and blood-forming organs | 366 (14) | 407 (11) | 386 (10) | 332 (10) |
| Antianemic preparations | 255 | 255 | 220 | 160 |
| Antithrombotic agents | 110 | 149 | 165 | 172 |
| Blood substitutes and perfusion solutions | 0 | 3 | 1 | 0 |
| Antihemorrhagics | 1 | 0 | 0 | 0 |
| Systemic hormonal preparations, excluding sex hormones and insulins | 155 (6) | 314 (9) | 338 (9) | 353 (10) |
| Corticosteroids for systemic use | 42 | 288 | 289 | 300 |
| Calcium homeostasis | 93 | 6 | 32 | 35 |
| Thyroid therapy | 20 | 19 | 17 | 18 |
| Pancreatic hormones | 0 | 1 | 0 | 0 |
| Anti-infectives for systemic use | 7 (0.3) | 469 (13) | 328 (9) | 56 (2) |
| Antibacterials for systemic use | 1 | 319 | 214 | 23 |
| Antivirals for systemic use | 4 | 136 | 103 | 30 |
| Antimycotics for systemic use | 0 | 13 | 8 | 2 |
| Immune sera and immunoglobulins | 1 | 0 | 3 | 1 |
| Antimycobacterials | 1 | 1 | 0 | 0 |
| Nervous system | 157 (6) | 160 (4) | 113 (3) | 122 (4) |
| Psycholeptics | 86 | 92 | 60 | 56 |
| Analgesics | 23 | 35 | 20 | 25 |
| Antiepileptics | 24 | 15 | 15 | 25 |
| Psychoanaleptics | 21 | 13 | 11 | 14 |
| Other nervous system drugs | 2 | 5 | 3 | 2 |
| Anti-Parkinson drugs | 1 | 0 | 0 | 0 |
| Various | 427 (16) | 22 (1) | 32 (1) | 17 (0.5) |
| Drugs for treatment of hyperkalemia and hyperphosphatemia | 420 | 19 | 32 | 16 |
| Detoxifying agents for antineoplastic treatment | 7 | 3 | 0 | 1 |
| Other pharmacological classes | 145 (5) | 165 (5) | 179 (5) | 121 (4) |
| Antiparasitic products, insecticides and repellents | 0 | 101 | 85 | 8 |
| Musculoskeletal system | 48 | 2 | 20 | 42 |
| Sodium bicarbonate | 19 | 23 | 37 | 30 |
| Genito urinary system and sex hormones | 21 | 11 | 23 | 22 |
| Respiratory system | 37 | 12 | 12 | 10 |
| Sensory organs | 4 | 14 | 1 | 5 |
| Dermatologicals | 2 | 2 | 1 | 4 |
| No ATC code | 12 | 0 | 0 | 0 |
| Not marketed in France | 2 | 0 | 0 | 0 |
| Total | 2707 | 3622 | 3701 | 3444 |
ATC, anatomical, therapeutic, and chemical; ESRD, end-stage renal disease.
The main drug classes appear in bold. The denominator is the total number of drug prescriptions.
Immediately after transplantation (M0), the most commonly prescribed combination of immunosuppressive agents was mycophenolate mofetil and tacrolimus (in 240 patients [68%]) (Table 3). A total of 287 patients (81%) were taking corticosteroids (data not shown).
Table 3.
Characteristics of immunosuppressive treatments upon discharge after kidney transplantation (M0)
| Characteristics | Total |
|---|---|
| (n = 354) | |
| Induction therapy, n (%) | |
| Basiliximab | 193 (54.5) |
| Antithymocyte globulin | 160 (45.2) |
| with i.v. Ig | 6 (1.7) |
| Basiliximab + antithymocyte globulin | 1 (0.3) |
| Maintenance therapy, n (%) | |
| MMF + tacrolimus | 240 (67.8) |
| MMF + cyclosporine | 97 (27.4) |
| MMF + everolimus | 1 (0.3) |
| Tacrolimus + everolimus | 14 (4.0) |
| Tacrolimus + azathioprine | 2 (0.6) |
MMF, mycophenolate mofetil.
Pill Burden
The pill burden was significantly different over time (analysis of variance P < 0.001). The mean ± SD pill burden (oral drugs only) before transplantation (8.2 ± 4.5) was significantly lower than at each time point after transplantation (pairwise t test after Bonferroni correction, P < 0.001). The highest value pill burden was recorded 1 month after transplantation (18.0 ± 4.9). At M12, the mean pill burden was 13.6 ± 4.8 (Figure 2b).
Medication Regimen Complexity
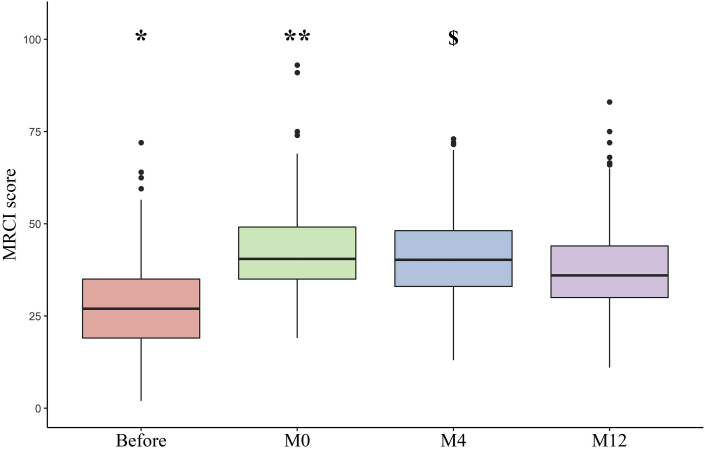
The MRCI score was significantly different over time (analysis of variance P < 0.001). The mean ± SD MRCI before transplantation was 27.7 ± 11.9 (range: 2.0–72.0). The MRCI was significantly higher at M0, M4, and M12 than before transplantation (pairwise t test after Bonferroni correction, P < 0.001). At M12, the mean ± SD MRCI was 37.5 ± 11.9 (range: 11.0–83.0) (Figure 3). The component that contributed the most to the MRCI at each time point was component B (i.e., the dosing frequency) (Table 4).
Figure 3.
The medication regimen complexity index (MRCI) during end-stage renal disease (before transplantation, on discharge after kidney transplantation [M0], and 4 and 12 months after transplantation [M4 and M12]). Differences over time were statistically significant (analysis of variance, P < 0.001). ∗P < 0.001 for comparisons between before transplantation and M0, M4, and M12 (after Bonferroni correction). ∗∗Difference between M0 and M4 not significant, and P < 0.001 between M0 and M12 (after Bonferroni correction). $P < 0.001 between M4 and M12 (after Bonferroni correction).
Table 4.
The change over time in the MRCI components during ESRD (before kidney transplantation, on discharge after kidney transplantation [M0], and 4 and 12 months after kidney transplantation [M4 and M12])
| Before |
M0 |
M4 |
M12 |
|
|---|---|---|---|---|
| (n = 354) | (n = 352) | (n = 348) | (n = 344) | |
| Component A | 10.4 ± 4.76a | 12.9 ± 4.55b | 13.1 ± 4.47e | 12.2 ± 4.52 |
| Component B | 11.6 ± 5.06a | 16.9 ± 4.87c | 16.3 ± 4.84e | 15.0 ± 5.24 |
| Component C | 5.64 ± 3.05a | 12.5 ± 3.67d | 11.7 ± 3.43e | 10.3 ± 3.10 |
| Total MRCI | 27.7 ± 11.9 | 42.3 ± 11.2 | 41.2 ± 11.3 | 37.5 ± 11.9 |
ESDR, end-stage renal disease; MRCI, medication regimen complexity index.
Results are expressed as the mean ± SD.
Differences over time were statistically significant for components A, B, and C (analysis of variance P < 0.001 for each component).
P < 0.001 for comparisons between before transplantation and M0, M4, and M12 (after Bonferroni correction).
Difference between M0 and M4 not significant, P = 0.046 between M0 and M12 (after Bonferroni correction).
Difference between M0 and M4 not significant, P < 0.001 between M0 and M12 (after Bonferroni correction).
P = 0.001 between M0 and M4, P < 0.001 between M0 and M12 (after Bonferroni correction).
P < 0.001 for the comparisons between M4 and M12 (after Bonferroni correction).
Factors Associated With MRC 12 Months After Transplantation
The risk of having an MRCI of more than 37.5 increased significantly when patients had diabetes (odds ratio [95% confidence interval] = 4.97 [2.51–10.45]) or dyslipidemia (2.01 [1.23–3.27]). In the multivariate analysis, we found that age, sex, body mass index, hypertension, and a cardiovascular disease were not significantly associated with the risk of having an MRCI of more than 37.5 (Table 5).
Table 5.
Factors associated with an MRCI >37.5 12 months after kidney transplantation
| Characteristics | Crude model |
Adjusted model |
||||
|---|---|---|---|---|---|---|
| OR | [95% CI] | P value | OR | [95% CI] | P value | |
| Age at the time of kidney transplantation, yr | 1.03 | [1.01–1.05] | 0.001 | 1.01 | [0.99–1.03] | 0.47 |
| Body mass index, kg/m2 | 1.07 | [1.02–1.13] | 0.005 | 1.04 | [0.99–1.10] | 0.15 |
| Cardiovascular disease | ||||||
| No | 1 | |||||
| Yes | 1.90 | [1.23–2.94] | 0.004 | 1.46 | [0.91–2.34] | 0.12 |
| Dyslipidemia | ||||||
| No | 1 | |||||
| Yes | 2.90 | [1.87–4.52] | <0.001 | 2.01 | [1.23–3.27] | 0.005 |
| Diabetes | ||||||
| No | 1 | |||||
| Yes | 7.03 | [3.68–14.42] | <0.001 | 4.97 | [2.51–10.45] | <0.001 |
CI, confidence interval; MRCI, medication regimen complexity index; OR, odds ratio.
Sex, hypertension, and a history of cancer were tested in a univariate analysis but did not meet the criteria for inclusion in the multivariable analysis (P = 0.78, 0.83 and 0.14, respectively).
Discussion
Our present results provide an overview of the patient-level medication burden and the type of medications prescribed in individuals having undergone kidney transplantation in an indication of ESRD. The 3 study endpoints (the number of daily medications, the pill burden, and the MRCI) all changed in the same direction, with a 35% increase of MRCI when comparing between the period before kidney transplantation with the time point 12 months after transplantation. In a multivariate analysis, diabetes and dyslipidemia were associated with MRC.
We have previously evidenced MRC in patients with CKD before kidney transplantation setting.17 The prevalence of polypharmacy in various non-ESRD CKD cohorts varies from 72% to 80%3,4; these values are explained by the patients’ advanced age and multiple comorbidities. In the presented cohort, 53% of the patients (at M4) and 38% (at M12) were classified in the “hyperpolypharmacy” group.
Our findings offer insights into the long-term medications (other than immunosuppressants) commonly prescribed to kidney transplant recipients. On discharge after kidney transplantation (M0), the most common ATC classes (other than immunosuppressants and corticosteroids) were drugs for acid-related disorders, antibacterials for systemic use, and antianemic preparations. Twelve months after transplantation, the most common ATC classes (again other than immunosuppressants and corticosteroids) were beta blocking agents, drugs for acid-related disorders, and vitamins. Indeed, most patients will inevitably receive lifelong immunosuppressive therapy and other medications needed to manage their comorbid conditions (especially cardiovascular disease), prevent viral and bacterial infections, and relieve the gastrointestinal adverse events associated with immunosuppressants.
In parallel with the increase in polypharmacy, we found that daily pill burden increased during the post-transplantation period. The mean daily pill burden ranged from 8.2 (before transplantation) to 18.0 (M0). Furthermore, oral pill burden in the period soon after kidney transplantation exceeded the pill burden before transplantation (on dialysis); this was mainly due to intensive immunosuppressive therapy and prophylaxis for graft complications. Similarly, a 12-month cross-sectional study in India found that the daily pill burden ranged from 10 to 32 immediately after kidney transplantation and from 7 to 28, 12 months later.18 Another retrospective study (of 68 kidney transplant recipients in the United States) found that the daily pill burden was 17, 2 years after transplantation.19
In the literature, polypharmacy has been linked to poor medication adherence.14 The consequences of nonadherence to medication in transplant recipients can be severe: an increased risk of acute or chronic graft rejection, repeat transplantation, and death. Furthermore, a recent analysis of kidney transplant recipients showed that the number of medications and the total weekly pill burden were associated with poor quality of life in general and poor scores for some Kidney Disease Quality of Life-36 subscales (such as the physical functioning and pain severity) in particular.9
The previously validated MRCI may provide more information than the number of medications and the pill burden do, because it accounts for factors such as the administration frequency and the administration route. The present study is the first to have systematically evaluated MRC at multiple time points before and after kidney transplantation. A better understanding of MRC would help to identify the most complicated periods for drug treatment after transplantation.
When we compare our findings with those in the literature on nontransplant cohorts, it is clear that kidney transplant recipients have high medication burdens.20 When studying the MRCI across populations with chronic disease, Libby et al.12 stated that the mean total patient-level MRCI (25.44; range: 6–64) was highest in a cohort of geriatric patients with depression. A cohort of patients with diabetes had the next highest mean patient-level MRCI (22.98; range: 4.0–65.5), followed by patients with HIV (21.76; range: 2.0–67.5) and hypertension (17.8; range: 3–46) cohorts.12 In 157 elderly patients with stage 5 CKD (estimated glomerular filtration rate <15 ml/min per 1.73 m2), the estimated mean ± SD MRCI was 22.8 ± 7.7.21
In comparison, the mean ± SD MRCI in our kidney transplant cohort was highest at M0 (42.3 ± 11.2) and was still high 12 months after transplantation (37.5 ± 11.9). This elevated value is in line with a previous report on a group of heart transplant recipients, in which the MRCI 12 months after transplantation was 30.4.22 We found that the MRCI in our cohort was mainly driven by the dosing frequency.
Dyslipidemia and diabetes are 2 well-known posttransplantation complications related (at least in part) to immunosuppressive agents. Indeed, steroids and calcineurin inhibitors usually lead to quantitative and qualitative abnormalities in levels of very-low-density, low-density, and high-density lipoproteins, and posttransplant diabetes mellitus has emerged as a major adverse effect of immunosuppressive drugs.23, 24, 25 These 2 comorbidities were identified as independent factors associated with an elevated MRCI 12 months after kidney transplantation. Whereas dyslipidemia might reflect sicker patients with a higher number of drugs, one can also reasonably assume that antidiabetic therapy (including multiple injections of insulin and poly-antidiabetic agents) leads to an elevated MRCI, especially because the latter is driven by the administration route and the dosing frequency. As mentioned previously, Libby et al.12 found that diabetes was one of the chronic diseases with an elevated MRCI. In line with a previous report,26 age and sex were not associated with an elevated MRCI in the present study.
The MRCI could be used to simplify medication regimens. Another possible means of simplifying the medication regimen would be to introduce polypills, although this type of formulation is not widely available for medications other than fixed-dose combinations of antihypertensive drugs. The systematic promotion of a “deprescribing” approach (defined as discontinuing medications with the lowest benefit-harm ratio) might also usefully reduce the treatment burden.27,28 However, it is not known whether reducing the MRC has positive effects on clinically relevant health care outcomes such as adherence, overall health, and hospitalization.
The main strength of the present study was its triple assessment of the drug burden (the number of drugs, the oral pill burden, and the MRCI) at different time points in the course of ESRD. Our study had several limitations. First, we retrospectively evaluated prescription medications recorded in the patients’ electronic medical records; hence, we could not determine whether the medications had actually been taken by the patient. This feature also meant that we did not record the use of over-the-counter drugs. Second, our study was performed in a single kidney transplant clinic and thus did not address potential differences in prescribing patterns from one center to another. Last, we did not assess the association between MRC and clinical outcomes. Additional studies are needed to evaluate the relationship among MRC, medication adherence, and clinical outcomes in kidney transplant recipients.
Conclusion
Our present results highlighted a high drug burden and high MRC in transplanted patients. The burden was higher at all time points after the transplantation period than before transplantation. Further evaluations need to assess the impact of the drug burden and MRC on hard outcomes such as acute transplant rejection, medication adherence, quality of life, allograph survival, and hospitalization.
Disclosure
The authors declared no competing interests.
Author Contributions
JM, SML, GC, and SL designed the present project. JM and BB collected data. SML and JM analyzed the data. JM, SML, and SL helped to interpret the results JM, SML, and SL wrote the first draft of the article; and PC, BB, VG-C, KM, GC, and SL provided critical feedback, helped shape the research, the analysis, and the final draft of the manuscript, and approved the version to be published.
Footnotes
Table S1. Medication regimen complexity index calculation
Table S2. Medication regimen complexity index examples. (A) Patient with the highest MRCI at M12. (B) Patient with the lowest MRCI at M12.
Supplementary Material
Table S1. Medication regimen complexity index calculation
Table S2. Medication regimen complexity index examples. (A) Patient with the highest MRCI at M12. (B) Patient with the lowest MRCI at M12.
References
- 1.Bikbov B., Purcell C.A., Levey A.S. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonelli M., Wiebe N., Manns B.J. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt I.M., Hübner S., Nadal J. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clin Kidney J. 2019;12:663–672. doi: 10.1093/ckj/sfz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laville S.M., Metzger M., Stengel B. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol. 2018;84:2811–2823. doi: 10.1111/bcp.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayward S, Hole B, Denholm R, et al. International prescribing patterns and polypharmacy in older people with advanced chronic kidney disease: results from the European Quality study [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfaa064. Accessed June 16, 2020. [DOI] [PubMed]
- 6.St Peter W.L. Management of polypharmacy in dialysis patients. Semin Dial. 2015;28:427–432. doi: 10.1111/sdi.12377. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan B., Mason N.A., Shimp L.A., Ascione F.J. Chronic hemodialysis patients. Part I: Characterization and drug-related problems. Ann Pharmacother. 1994;28:316–319. doi: 10.1177/106002809402800303. [DOI] [PubMed] [Google Scholar]
- 8.Manley H.J., Cannella C.A., Bailie G.R., St. Peter W.L. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney Dis. 2005;46:669–680. doi: 10.1053/j.ajkd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Woźniak I., Kolonko A., Chudek J. Influence of polypharmacy on the quality of life in stable kidney transplant recipients. Transplant Proc. 2018;50:1896–1899. doi: 10.1016/j.transproceed.2018.02.128. [DOI] [PubMed] [Google Scholar]
- 10.McDonald M.V., Peng T.R., Sridharan S. Automating the medication regimen complexity index. J Am Med Inform Assoc. 2013;20:499–505. doi: 10.1136/amiajnl-2012-001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George J., Phun Y.-T., Bailey M.J. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38:1369–1376. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 12.Libby A.M., Fish D.N., Hosokawa P.W. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35:385–398.e1. doi: 10.1016/j.clinthera.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Advinha A., Oliveira-Martins S., Mateus V. Medication regimen complexity in institutionalized elderly people in an aging society. Int J Clin Pharm. 2014;36:750–756. doi: 10.1007/s11096-014-9963-4. [DOI] [PubMed] [Google Scholar]
- 14.Murray M., Kroenke K. Polypharmacy and medication adherence. J Gen Intern Med. 2001;16:137–139. doi: 10.1111/j.1525-1497.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHOCC Guidelines. https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/ Available at:
- 16.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liabeuf S, Laville M. Drug prescription in patients with chronic kidney disease: a true challenge [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfaa164. [DOI] [PubMed]
- 18.Adhikari U.R., Taraphder A., Hazra A., Das T. Pill burden does not influence compliance with oral medication in recipients of renal transplant. Indian J Pharmacol. 2016;48:21–25. doi: 10.4103/0253-7613.174425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardinger K.L., Hutcherson T., Preston D., Murillo D. Influence of pill burden and drug cost on renal function after transplantation. Pharmacotherapy. 2012;32:427–432. doi: 10.1002/j.1875-9114.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- 20.Wimmer B.C., Cross A.J., Jokanovic N. Clinical outcomes associated with medication regimen complexity in older people: a systematic review. J Am Geriatr Soc. 2017;65:747–753. doi: 10.1111/jgs.14682. [DOI] [PubMed] [Google Scholar]
- 21.Parker K., Bull-Engelstad I., Aasebø W. Medication regimen complexity and medication adherence in elderly patients with chronic kidney disease. Hemodial Int. 2019;23:333–342. doi: 10.1111/hdi.12739. [DOI] [PubMed] [Google Scholar]
- 22.Bryant B.M., Libby A.M., Metz K.R. Evaluating patient-level medication regimen complexity over time in heart transplant recipients. Ann Pharmacother. 2016;50:926–934. doi: 10.1177/1060028016657552. [DOI] [PubMed] [Google Scholar]
- 23.Badiou S., Cristol J.-P., Mourad G. Dyslipidemia following kidney transplantation: diagnosis and treatment. Curr Diab Rep. 2009;9:305–311. doi: 10.1007/s11892-009-0047-0. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal A., Prasad G.V.R. Post-transplant dyslipidemia: mechanisms, diagnosis and management. World J Transplant. 2016;6:125–134. doi: 10.5500/wjt.v6.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penfornis A., Kury-Paulin S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006;32:539–546. doi: 10.1016/s1262-3636(06)72809-9. [DOI] [PubMed] [Google Scholar]
- 26.Mansur N., Weiss A., Beloosesky Y. Looking beyond polypharmacy: quantification of medication regimen complexity in the elderly. Am J Geriatr Pharmacother. 2012;10:223–229. doi: 10.1016/j.amjopharm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Hatah E., Braund R., Tordoff J., Duffull S.B. A systematic review and meta-analysis of pharmacist-led fee-for-services medication review. Br J Clin Pharmacol. 2014;77:102–115. doi: 10.1111/bcp.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott I.A., Hilmer S.N., Reeve E. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827–834. doi: 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.