Abstract
Background
The use of microbes that improve plant phosphorus (P) use efficiency is an avenue to boost crop yields while alleviating environmental impacts. We tested three microbial inoculants (Rhizoglomus irregulare alone – designated AMF; Pseudomonas putida alone – designated PSB; and R. irregulare and P. putida in consortium – designated AMF+PSB), combined with chemical fertilizers, in an intensive maize agricultural system.
Results
As hypothesized: (i) despite the native soil microbial community and the application of P fertilizer, the microbial inoculants enhanced plant P uptake from the soil by 14–60%, and consequently improved P acquisition efficiency; (ii) PSB and AMF+PSB plants produced ±50% more biomass per unit of P taken up, and consequently enhanced plant internal P use efficiency (i.e. the biomass produced per unit of P); and (iii) the combined inoculation of AMF and PSB provided the best results in terms of productivity and P use efficiency. Further, the microbial inoculants altered P allocation within the plant, reducing grain P concentration.
Conclusion
By testing the microbial inoculants under field conditions, our study clearly shows that the microbial consortium (AMF+PSB) increased maize productivity, and at the same time improved P use efficiency. Further, the use of these microbial inoculants was shown to be compatible with conventional agricultural management practices.
Keywords: Arbuscular mycorrhizal fungi, Grain phytate, Microbial consortium, Phosphate solubilizing bacteria, Phosphorus acquisition efficiency, Plant internal phosphorus use efficiency
1. Introduction
Phosphorus (P) is an essential macronutrient for all life forms, which greatly limits eco- and agrosystems’ productivity. To meet the needs of a growing human population and their changing consumption patterns, agricultural production was drastically intensified and so was the use of P fertilizers (Childers et al., 2011). Rock phosphate (a high-quality P source used in conventional agriculture) is a non-renewable finite resource whose reserves are quickly being overexploited, and therefore prices are expected to increase dramatically in the near future (Cordell and White, 2011, Reijnders, 2014, Dias et al., 2015). Despite this foreseen limitation to the use of P fertilizers, their application keeps rising (Shepherd et al., 2016) as food demand is forecasted to double by 2050. Further, 60–90% of the P applied to soils as fertilizer is rapidly immobilized, making it unavailable to plants (Richardson and Simpson, 2011, Estrada et al., 2013, Melo et al., 2016). Consequently, P accumulates in agricultural soils and some contain more P than recommended (e.g. European soils), but to ensure high productivity P fertilizers continue to be applied. This excess use of P fertilizers causes severe negative environmental impacts (Reijnders, 2014, Childers et al., 2011), namely eutrophication of water bodies (Hua et al., 2016). This global P paradox creates an urgent need for cleaner agronomic practices capable of boosting crop yields while improving P use efficiency.
Improving P use efficiency in agriculture can be achieved by increasing plant production maintaining a given rate of P fertilizer or by producing the same with lower P-inputs (Rose et al., 2013, Richardson et al., 2009). The improvement of P use efficiency could be accomplished by improving plant internal P use efficiency (IPUE) and/or increasing P uptake from the soil (P-acquisition efficiency; PAE) (Rose and Wissuwa, 2012, Veneklaas et al., 2012, Wang et al., 2010). A combination between these two strategies is desirable in both high- and low-input agricultural systems (Rose and Wissuwa, 2012, Heuer et al., 2017). Since plant IPUE has several definitions (e.g., grain yield per unit of P fertilizer applied; plant biomass per P present in specific tissues), it is not clear how to improve it (Rose and Wissuwa, 2012). By contrast, strategies to improve plant PAE include molecular plant breeding, engineering transgenic plants and inoculating beneficial microbes such as plant growth-promoting rhizobacteria (PGPR) and mycorrhizae (Ramaekers et al., 2010). So far, plant breeding and genetic engineering have not guaranteed higher PAE, and their impact on soil conservation, quality and biodiversity have not been thoroughly explored (Rose et al., 2013). By contrast, inoculating beneficial microbes, combined with technologically advanced agricultural practices, is becoming an important avenue to improve PAE, and consequently P use efficiency.
Beneficial microbes (e.g. in the form of biofertilizers) can control nutrient bioavailability in the soil by establishing more complex interactions with the soil structure, and are more efficient at lower nutrient levels than at higher (Warton et al., 2015, Weltin et al., 2018, Dias et al., 2015). Indeed, microbes play a key role in the P cycle and among the wide diversity of soil microbes, arbuscular mycorrhizal fungi (AMF) and phosphate solubilizing bacteria (PSB) are directly involved in P turnover and plant P acquisition (Zhang et al., 2014). Further, plants naturally interact with both AMF and PSB (Owen et al., 2015). Many studies point out the beneficial effects of AMF (Smith and Read, 2008) and/or PSB inoculation (Zhang et al., 2014, Zhang et al., 2016, Melo et al., 2018, Ordoñez et al., 2016) for plant growth, P uptake and as a biological tool for eco-restoration (Wahid et al., 2016). However, most of these beneficial effects were observed in microcosm experiments or in sterile soil (Rodriguez and Sanders, 2015). These controlled experiments did not take into account how the native soil microbial community shapes AMF’s and PSB’s impact on host plant performance. Therefore, AMF and/or PSB inoculation has to be tested under field conditions and ‘normal’ management practices for a given crop.
Our objective was to test, under field conditions, the effect of three microbial inoculants (with potential to become biofertilizers: AMF alone, PSB alone and AMF+PSB) on improving P use efficiency in an intensive agricultural system. We focused on a cereal crop, maize (Zea mays L.) which provides 15% of the world’s protein and 20% of the world’s calories (Nuss and Tanumihardjo, 2010), and is very P demanding (e.g. fertilizer doses commonly range from 10 to 250 kg ha−1 in Europe) (de Varennes, 2003). Further, as in other cereals, 60–85% of the P acquired by maize plants is allocated to seeds (i.e., grains) in the form of phytate, which is essential for seed germination and seedling vigour (Yamaji et al., 2017, Rose et al., 2013). However, phytate cannot be digested by humans or other monogastric animals, so that all P in the form of phytate results in large quantities of P in animal excrements, greatly contributing to eutrophication (Neset and Cordell, 2012, Cordell and White, 2011). It also decreases other nutrients’ absorption because phytic acid binds with ions, such as zinc, calcium, iron and magnesium (Hurrell et al., 2003) resulting in very insoluble salts with poor bioavailability. Reducing P grain concentration can improve grain quality by increasing its nutritional value and digestibility, and reduce the environmental impacts associated with P losses through excreta (Veneklaas et al., 2012). Therefore, in solving the P paradox, seeds P concentration should be reduced without compromising plant growth or vigour (Rose et al., 2013, Yamaji et al., 2017).
As both AMF (Smith and Read, 2008) and PSB (Melo et al., 2018, Ordoñez et al., 2016, Zhang et al., 2014, Zhang et al., 2016) enhance P bioavailability to plants, we hypothesized that despite the native soil microbial community and the application of P fertilizer, the microbial inoculants would enhance plant P uptake from the soil and, consequently improve PAE. Besides the well-known improvement in P bioavailability, AMF and PSB can alter plant growth and nutrition (Melo et al., 2018) so that we hypothesized that inoculated plants would produce more biomass per unit of P taken up, and consequently enhance plant IPUE, considered as the ratio between biomass and its P content (Veneklaas et al., 2012, Rose et al., 2011, Rose and Wissuwa, 2012, van de Wiel et al., 2016). As a result of enhanced plant IPUE, biomass P concentration tends to decrease, including that of the grain (e.g. reduced phytate concentration). Finally, as AMF produce extensive extraradical hyphae in the soil, which are a habitat for other microbes, and cooperation between AMF and PSB has been shown in vitro (Zhang et al., 2016, Zhang et al., 2018) we hypothesized that the combined inoculation of AMF and PSB would provide the best results in terms of productivity and P use efficiency.
2. Material and methods
2.1. Experimental design
Our experiment consisted of 1 factor: microbial inoculation. The design was a complete randomized block design of 3 blocks, each containing 4 parcels, one for each treatment: control, AMF inoculation (designated AMF), PSB inoculation (designated PSB) and combined inoculation of AMF and PSB (designated AMF+PSB). Each parcel had an area of 2.25 m2 and each block had a total area of 27 m2 (3 × 9 m). To remove boundary effects, plants within in an area of 5.4 m2 in both the east and west side of the experimental area were considered plant guard rows and were not sampled.
The AMF inoculum consisted of propagules and spores of Rhizoglomus irregulare, which was purchased from Symbiom (https://www.symbiom.cz/en). AMF propagules and spores were isolated and counted to correspond to 2.5x106 AMF spores ha−1, in agreement with the recommendations for commercial products trials (Cozzolino et al., 2013). At the time of sowing (T0), AMF inoculum was spread manually on the soil along the planting furrows corresponding to AMF and AMF+PSB treatments.
The PSB inoculum consisted of Pseudomonas putida (and the respective culture medium) which had been isolated from a Portuguese agricultural soil and belongs to the Soilvitae (https://soilvitae.com) PGPR collection. These bacteria were characterized as PSB due to its capacity to solubilize tri-calcium phosphate and phytate in vitro. PSB was inoculated over the area corresponding to PSB and AMF+PSB treatments within the ideal dose range reported by Bashan (1986): 1.5 × 107 CFUs plant−1, which corresponds to 108 CFUs parcel−1 and 1012 CFUs ha−1. The culture broth containing the bacteria was diluted to achieve the desired concentration (1012 CFUs ha−1) and was applied manually together with the seeds along the planting furrows. A second inoculation of PSB (1012 CFUs ha−1) was performed 15 days after sowing (Rakiami et al., 2019, Cipriano et al., 2016).
AMF+PSB was a combination of the two microbial inoculants using the same doses. Control parcels were not inoculated.
2.2. Field site and farming practices
This experiment was conducted in a farm, located in Lourinhã, Lisboa, Portugal (39° 16′ 32.3′’ N 9° 17′ 27.4′’ W), from early June to late September 2016 (110 days). Daily mean air temperature was 24 °C ranging between 16 and 32 °C, relative humidity ranged between 53 and 68%, according to Instituto Português do Mar e da Atmosfera (IPMA). These values represent the average obtained from site equidistant meteorological stations.
The soil had a coarse sand texture, 0.8% of organic matter, pH (H2O) 6.3 and extractable P (Egnér-Riehm method) of 442 ppm (analysis performed by Laboratório de Solos e Plantas, UTAD, Portugal, 2016).
The experimental field is characterized by a regime of intensive agriculture, with rotation: cabbages, potatoes and occasionally maize. Field is fertilized with background fertilizer and top-dressing fertilizer. All cultures are irrigated through a dispersion system.
Zea mays L., cultivar Sincere (Syngenta) can be used for forage and grain production. Seeds were hand sowed on the 4th of June 2016. Plants were grown 0.75 m apart between rows and with 0.20 m spacing along the row, the equivalent of ~67 000 plants ha−1 (da Silva, 2013). Before sowing, a basal fertilization of 16 kg NO3−, 48 kg NHO4+, 96 kg P2O5 and 96 kg K2O ha−1 was applied. This was followed by a top-dressing fertilization, 6 weeks after sowing, of 60 kg NHO4+, 180 kg NH2CONH2, 96 kg P2O5 and 120 kg K2O ha−1 (according to the recommendations of ADP Fertilizantes).
2.3. Sampling and analysis
The experiment was completed when plants reached physiological maturity and grains were on the milk to dough phenological phase. At harvest (22th September), plants were manually cut with loppers. Harvest was limited to a central sampling area of 1.35 m2 per parcel of 16.2 m2, with all border plants being excluded as border plants tend to be more vigorous and more productive than those that grow inside the experimental units, due to the smaller effect of competition between plants and different light exposure.
Maize aboveground tissues were classified as plant shoot. The number of tillers per plant was recorded and shoots were separated in culm (leaves included) and ear maize (cobs and grain) and then weighted. Fresh weight (Fw) of plants from each parcel was measure in the field with a field weighing scale (Kern CXB). A sub-sample of 3 plants per parcel (randomly chosen) were dried to constant mass at 65 °C and the dry weight (Dw) of shoots (shoot biomass), culm and cobs (stover biomass), and grain (grain biomass) were recorded (precision ±0.01 g, model PGW 3502e digital balance). Dried grain and three leaves from each plant were ground using a mill of spheres (Retsch MM 2000). Ground samples were used to determine grain and leaf P concentration, using an Optical Emission Spectroscopy after acid digestion (Huang and Schulte 1985) (analysis performed by Centro de Edafología y Biología Aplicada del Segura – CEBAS-CSIC – Murcia, Spain 2016).
To characterize the soil, samples were collected from bare soil (composite sample, n = 5) until a maximum depth of 15 cm. Samples were air-dried and then analysed for chemical and physical properties: extracted P (Egnér-Riehm method), organic matter quantification and pH (H2O) (analysis performed by Laboratório de Solos e Plantas, UTAD, Portugal, 2016).
2.4. Calculations and statistics
The average Fw of culm and ear maize of each parcel and the average shoot Dw of the 3 sub-samples were represented in tonne per hectare (t ha−1) by multiplying the average parcel value of Fw or Dw by the number of plant density used per hectare (~67 000 plants). Shoot Fw per hectare was defined as green forage and shoot Dw as the biomass.
The dry matter content of the green forage (%) was obtained by multiplying plant biomass (kg) per 100 and dividing by the green forage (kg).
Phosphorus acquisition efficiency (PAE) which represents the amount of P taken up per plant (Wang et al., 2010, Vandamme et al., 2016) was evaluated through shoot P extraction and P fertilizer recovery efficiency, as follows:
| (2.1) |
| (2.2) |
P extraction (kg ha−1) reflects the total P content in plant tissues: shoot, stover and grain. This was calculated by combining grain (g) and stover biomass (g) with respective P concentration (g P/100 g plant) for each treatment and was estimated for a hectare by multiplying the result with plant density, as depicted in formula (2.1). We used leaf concentration to estimate stover concentration (Cavaco and Calouro, 2006). Shoot P extraction was obtained through the sum of grain and stover P extraction, while shoot concentration was obtained by dividing shoot P extraction by shoot biomass. P fertilizer recovery efficiency (kg ha−1) reflects plant’s ability to acquire nutrients applied to the soil (Baligar et al., 2001) and was calculated as shoot P extraction (kg) divided by the amount of P fertilizer applied (kg ha−1), as depicted in formula (2.2).
Plant Internal Phosphorus Utilization Efficiency (IPUE) was evaluated through the amount of biomass (Shoot, Stover or Grain Dw) produced per unit of P present in the shoot (shoot P extraction) (Rose and Wissuwa, 2012), as follows:
| (2.3) |
IPUE (kg Dw kg P extracted ha−1) was expressed in kg of tissue biomass produced per kg of P present in the shoot in a hectare, formula (2.3).
The effect of the inoculants on maize performance was tested using one-way analysis of variance (ANOVA). Differences among treatment means were determinate by Tukey test (p ≤ 0.05). In all cases, preliminary analyses were performed to ensure that there was no violation of statistical assumptions (including the Levene’s test to check for homogeneity of variances). SPSS (version 25·0, IBM, Inc., Chicago, IL, USA) was used for all these analyses. Graphs were developed with GraphPad Prism version 6 (GraphPad Software, San Diego, CA).
3. Results
3.1. Effect of the microbial inoculants on maize productivity
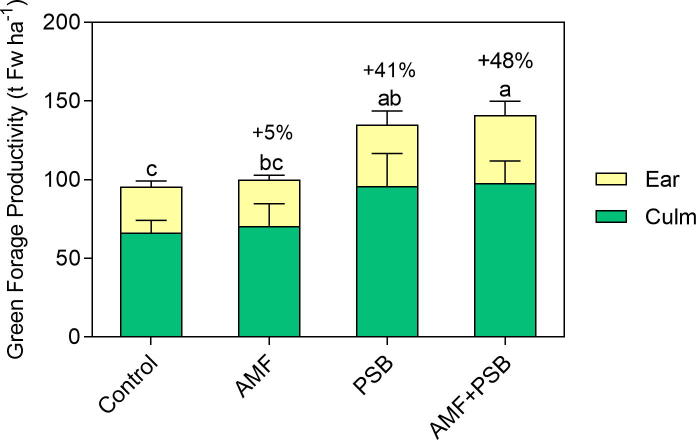
Plant inoculation with PSB alone (PSB) and in combination with AMF (AMF+PSB) promoted forage productivity (i.e. green forage Fw) compared to the control plants (increments of 41% and 48%, respectively). Inoculation with AMF alone (AMF) failed to increase forage productivity (ANOVA green forage productivity F3,6 = 9.90, P ≤ 0.01) (Fig. 1). The microbial inoculants did not affect Fw partitioning between culm and ears (ANOVA ratio Ear/Culm F3,6 = 0.82, P > 0.05).
Fig. 1.
Effect of the microbial inoculants on maize green forage productivity. Stacked bars (green and yellow) show the fresh weight of vegetative (culm) and reproductive (ear) structures. Percentages on top of the bars show the effect in maize green forage promoted by the respective inoculant when compared to control plants. Different letters show significance at 5% level (for total green forage productivity), according to Tuckey’s HSD test. Bars are the mean ± SD (n = 3 plots).
Although not significant, inoculation with PSB alone (PSB) and in combination with AMF (AMF+PSB) tended to produce bigger plants relatively to the control (increment of 53% and 65%, respectively) (ANOVA biomass F3,6 = 3.08, P > 0.05) (Table 1). Microbial inoculants did not change the dry weight partitioning between stover and grain (ANOVA ratio Grain/Stover Dw F3,6 = 0.25, P > 0.05), not even the Dw of each component, when considered separately (ANOVA Stover Dw F3,6 = 2.77, P > 0.05; Grain Dw F3,6 = 3.54, P > 0.05).
Table 1.
Effect of the microbial inoculants on biomass, P extraction at shoot, stover (culm and cobs), and grain level and P fertilizer recovery efficiency. For each line, different letters (mean ± SD of 3 sampling plots, n = 3) show significance at 5% level (no letters mean not significant), according to Tuckey’s HSD test.
| Treatment | Control | AMF | PSB | AMF+PSB |
|---|---|---|---|---|
| Biomass (t Dw ha−1) | 28.8 ± 5.3 | 35.1 ± 9.0 | 44.1 ± 7.7 | 47.6 ± 9.0 |
| Shoot P extraction (kg ha−1) | 85.5 ± 8.8 b | 115.4 ± 11.3 ab | 136.8 ± 5.6 a | 98.4 ± 7.8 ab |
| Stover P extraction (kg ha−1) | 77.8 ± 10.9b | 84.2 ± 10.6 ab | 102.2 ± 6.4 a | 68.4 ± 7.4b |
| Grain P extraction (kg ha−1) | 25.6 ± 5.7 | 18.3 ± 0.8 | 22.0 ± 2.9 | 21.4 ± 0.9 |
| P fertilizer recovery efficiency (kg P content shoot kg−1 P2O5 supplied) | 0.44 ± 0.07b | 0.60 ± 0.11 ab | 0.71 ± 0.11 a | 0.51 ± 0.04 ab |
3.2. Effect of the microbial inoculants on P use
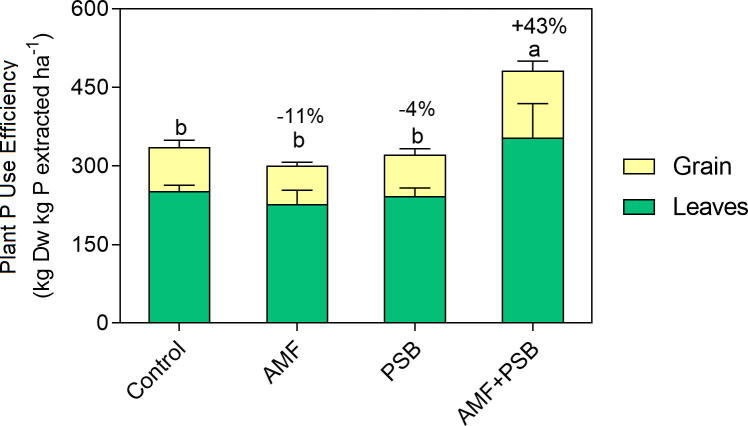
PSB increased P acquisition efficiency (PAE) by increasing P content (Table 1), while AMF+PSB increased plant internal P use efficiency (IPUE – Fig. 2). Only inoculation with PSB alone (PSB) increased shoot P extraction: PSB plants had 60% more P in their shoots (stover and grain) when compared to control plants (ANOVA shoot P extraction F3,6 = 4.88, P ≤ 0.05 – Table 1). Part of this extra P was allocated into the stover, as stover from PSB plants had approximately 31% more P in their shoots than control plants (ANOVA stover P extraction F3,6 = 8.56, P ≤ 0.05). By contrast, grain P extraction showed no differences between treatments (ANOVA grain P extraction F3,6 = 0.36, P > 0.05). Thus, the differences in shoot P extraction reflected the differences in stover P content. Only inoculation with PSB alone (PSB) improved P fertilizer recovery efficiency compared to control (ANOVA P fertilizer recovery efficiency F3,6 = 4.88, P ≤ 0.05).
Fig. 2.
Effect of the microbial inoculants on plant internal P use efficiency (IPUE). Stacked bars (green and yellow) represent the partition of the average IPUE at stover (culm and cobs) and grain level. Each bar represents the mean of 3 sampling plots ± SD (n = 3). Different letters show significance at 5% level (for total IPUE), according to Tuckey’s HSD test.
Only plants inoculated with AMF+PSB produced more shoot biomass (including grain) per unit of P present in the shoot, when compared to the plants treated with the other inoculants (ANOVA IPUEShoot F3,6 = 12.82, P ≤ 0.01; IPUEGrain F3,6 = 13.91, P ≤ 0.01) (Fig. 2). Therefore, AMF+PSB enhanced plants IPUE.
3.3. P concentration in plant tissues
Only AMF+PSB plants had significantly lower shoot P concentration than rest of the plants (ANOVA P concentration shoot F3,6 = 17.13, P ≤ 0.01). Further, no difference in shoot P concentration was detected between control, AMF and PSB plants (Table 2). The microbial inoculants had no effect on stover P concentration (ANOVA P concentration stover F3,6 = 9.60, P ≤ 0.01) but AMF+PSB plants had lower stover P concentration than AMF and PSB plants. Lastly, control plants had the highest P concentration in the grain (ANOVA P Concentration Grain F3,6 = 64.58, P ≤ 0.001). Among inoculated plants, AMF+PSB had lower grain P concentration than AMF inoculated plants. PSB effect did not differ from AMF and AMF+PSB.
Table 2.
Effect of the microbial inoculants on P concentration of shoot, stover (culm and cobs) and grain. For each column, different letters (mean ± SD of 3 sampling plots, n = 3) show significance at 5% level, according to Tuckey’s HSD test.
| Treatment | Shoot [P] (%) | Stover [P] (%) | Grain [P] (%) |
|---|---|---|---|
| Control | 0.30 ± 0.01 a | 0.29 ± 0.02 ab | 0.32 ± 0.02 a |
| AMF | 0.33 ± 0.03 a | 0.37 ± 0.04 a | 0.23 ± 0.01 b |
| PSB | 0.31 ± 0.02 a | 0.34 ± 0.03 a | 0.21 ± 0.03 bc |
| AMF+PSB | 0.21 ± 0.03 b | 0.22 ± 0.04 b | 0.19 ± 0.01 c |
The microbial inoculants were able to reduce grain P concentration (Table 2) and consequently grain phytate. Although, this difference was not detected in the stover, AMF+PSB treated plants had lower stover P concentration than plants treated with the other inoculants. Overall, AMF+PSB plants were the most efficient in reducing shoot P concentration, a decrease of about 30% compared to control plants.
4. Discussion
4.1. Improving plant productivity
The values of productivity we obtained (Fig. 1) are within the range reported for this variety (Cavaco and Calouro, 2006), thus validating the data obtained in this field trial applying ‘normal’ management practices for maize. As hypothesized, and in agreement with other studies (Owen et al., 2015, Ordoñez et al., 2016), inoculation with PSB alone (PSB) or in combination with AMF (AMF+PSB), enhanced plant productivity under field conditions. The microbial effect was significant for green forage (crop Fw – Fig. 1) but not for biomass (Table 1), suggesting that the microbes alone or in combination promoted different effects on plant water uptake and water saving strategies (Richardson et al., 2011). Non-exclusively, the increased productivity we observed may be related with changes in plant hormonal balance induced by plant-microbial interaction (Nadeem et al., 2014). It may also reflect the influence of the inoculants in delaying plant development as in the case of cereals water deficit is a main factor triggering and accelerating grain production (de Varennes, 2003).
4.2. Improving P use efficiency while reducing grain P concentration
Producing plants with lower P concentrations is a way to achieve higher plant IPUE (Veneklaas et al., 2012, Rose et al., 2011). However, if P concentration falls below that recommended for regular plant growth and development (i.e. 0.2–0.5%) (de Varennes, 2003), P deficiency occurs. In our experiment, all inoculated plants showed shoot P concentrations (Table 2) similar to the recommended ones showing that the microbial inoculants were able to reduce P concentration without triggering P limitation.
Another target is reducing grain P concentration and consequently phytate (Yamaji et al., 2017, Rose et al., 2013). Indeed, the microbial inoculants changed P allocation within the plant, resulting in lower grain P concentration (Table 2). The amount of phytate present in plant seeds and grains ranges from 0.5 to 5% of Dw and ideally it should be reduced to 0.025% of Dw or less to minimize P losses (Hurrell et al., 2003). Although PSB inoculation was not the most efficient treatment at improving productivity, PSB inoculated plants were the most efficient at exporting P to the shoot as shown by stover P (Table 1) content. Most P in the stover is in the phosphate form, which is essential for livestock nutrition (Richardson et al., 2011). Since P content is usually low in forages and/or livestock have low P assimilation efficiencies (due to surplus of phytate), dietary P supplements are often required (Sharpley et al., 2000). Therefore, by producing feed of higher P nutritional value and grain with lower phytate (Table 2), inoculation with PSB could contribute to reduce P feed supplements and, consequently, reduce P losses in livestock excreta. This win-win scenario meets farmers’ objectives and is an excellent argument for further studies with this PSB isolate (Pseudomonas putida), and possibly the development of a biofertilizer.
The microbial consortium AMF+PSB reduced shoot P concentration by 30% (Table 2) and produced more green forage (Fig. 1) showing that these plants used less P to produce more green forage (and tended to produce more biomass – Table 1) than the other treatments. Since AMF+PSB also showed the highest plant IPUE (Fig. 2), it could help strengthen food production per unit of area, and sustainably manage the amount of P fertilizer applied in farming systems. AMF+PSB is the most promising microbial inoculant to be used in sustainable agriculture, and should be further tested combined with the 30% reduction in P inputs recommended by the European Union. This microbial consortium (AMF+PSB) could be a key contributor in producing high value food resources with zero increase in land degradation while reducing negative environmental impacts. According to the European agronomic rules, these results are a promising asset towards a bio-economic management agriculture with significant environmental repercussions.
5. Conclusion
By testing the microbial inoculants under field conditions, our study clearly showed that the microbial consortium (AMF+PSB) increased maize productivity, and at the same time improved P use efficiency and reduced grain P concentration. Further, the use of these microbial inoculants was shown to be compatible with conventional agricultural management practices.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We are grateful to João Ferreira for making the experimental site available and to the Reviewers for their comments and suggestions.
Funding
This study was supported by Portuguese funds through Fundação para a Ciência e a Tecnologia (projects PTDC/AGR-PRO/1852/2014 and UIDB/00329/2020, and a postdoc grant SFRH/BPD/85419/2012 to Teresa Dias) and through the project PDR2020-20.2.4-FEADER-055319 (a master grant to Inês Pacheco).
Footnotes
Peer review under responsibility of King Saud University.
References
- Baligar V.C., Fageria N.K., He Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant. 2001;32(7–8):921–950. doi: 10.1081/css-100104098. [DOI] [Google Scholar]
- Bashan Y. Significance of timing and level of inoculation with rhizosphere bacteria on wheat plants. Soil Biol. Biochem. 1986;18(3):297–301. doi: 10.1016/0038-0717(86)90064-7. [DOI] [Google Scholar]
- Cavaco, M., Calouro, F., 2006. Produção integrada das culturas – pastagens e forragens. Direção Geral de Proteção das Culturas, Oeiras.
- Childers D.L., Corman J., Edwards M., Elser J.J. Sustainability challenges of phosphorus and food: solutions from closing the human phosphorus cycle. Bioscience. 2011;61(2):117–124. doi: 10.1525/bio.2011.61.2.6. [DOI] [Google Scholar]
- Cipriano M.A.P., Lupatini M., Lopes-Santos L., da Silva M.J., Roesch L.F.W., Destéfano S.A.L., Freitas S.S., Kuramae E.E., Sessitsch A. Lettuce and rhizosphere microbiome responses to growth promoting Pseudomonas species under field conditions. FEMS Microbiol. Ecol. 2016;92(12) doi: 10.1093/femsec/fiw197. [DOI] [PubMed] [Google Scholar]
- Cordell D., White S. Peak Phosphorus: clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability. 2011;3(10):2027–2049. doi: 10.3390/su3102027. [DOI] [Google Scholar]
- Cozzolino V., Di Meo V., Piccolo A. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. J. Geochem. Explor. 2013;129:40–44. doi: 10.1016/j.gexplo.2013.02.006. [DOI] [Google Scholar]
- da Silva I.O. Universidade dos Açores; 2013. Avaliação de produção de milho doce para consumo em maçaroca em três datas de sementeira diferentes. [Google Scholar]
- de Varennes A. Escolar Editora; Lisboa: 2003. Produtividade dos solos e ambiente. [Google Scholar]
- Dias T., Dukes A., Antunes P.M. Accounting for soil biotic effects on soil health and crop productivity in the design of crop rotations. J. Sci. Food Agric. 2015;95(3):447–454. doi: 10.1002/jsfa.6565. [DOI] [PubMed] [Google Scholar]
- Estrada G.A., Baldani V.L.D., de Oliveira D.M., Urquiaga S., Baldani J.I. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil. 2013;369(1–2):115–129. doi: 10.1007/s11104-012-1550-7. [DOI] [Google Scholar]
- Heuer S., Gaxiola R., Schilling R., Herrera-Estrella L., López-Arredondo D., Wissuwa M., Delhaize E., Rouached H. Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J. 2017;90(5):868–885. doi: 10.1111/tpj.13423. [DOI] [PubMed] [Google Scholar]
- Hua K.K., Zhang W.J., Guo Z.B., Wang D.Z., Oenema O. Evaluating crop response and environmental impact of the accumulation of phosphorus due to long-term manuring of vertisol soil in northern China. Agr. Ecosyst. Environ. 2016;219:101–110. doi: 10.1016/j.agee.2015.12.008. [DOI] [Google Scholar]
- Hurrell R.F., Reddy M.B., Juillerat M.A., Cook J.D. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 2003;77(5):1213–1219. doi: 10.1093/ajcn/77.5.1213. [DOI] [PubMed] [Google Scholar]
- Melo J., Carolino M., Carvalho L., Correia P., Tenreiro R., Chaves S., Meleiro A.I., de Souza S.B., Dias T., Cruz C., Ramos A.C. Crop management as a driving force of plant growth promoting rhizobacteria physiology. SpringerPlus. 2016;5 doi: 10.1186/s40064-016-3232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo J., Carvalho L., Correia P., de Souza S.B., Dias T., Santana M., Carolino M., Aguiar N.O., Canellas L.P., Cruz C., Ramos A.C. Conventional farming disrupts cooperation among phosphate solubilising bacteria isolated from Carica papaya's rhizosphere. Appl. Soil Ecol. 2018;124:284–288. doi: 10.1016/j.apsoil.2017.11.015. [DOI] [Google Scholar]
- Nadeem S.M., Ahmad M., Zahir Z.A., Javaid A., Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014;32(2):429–448. doi: 10.1016/j.biotechadv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Neset T.-S., Cordell D. Global phosphorus scarcity: identifying synergies for a sustainable future. J. Sci. Food Agric. 2012;92(1):2–6. doi: 10.1002/jsfa.4650. [DOI] [PubMed] [Google Scholar]
- Nuss E.T., Tanumihardjo S.A. Maize: a paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. F. 2010;9(4):417–436. doi: 10.1111/j.1541-4337.2010.00117.x. [DOI] [PubMed] [Google Scholar]
- Ordoñez Y.M., Fernandez B.R., Lara L.S., Rodriguez A., Uribe-Velez D., Sanders I.R. Bacteria with phosphate solubilizing capacity alter mycorrhizal fungal growth both inside and outside the root and in the presence of native microbial communities. Plos One. 2016;11(6) doi: 10.1371/journal.pone.0154438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D., Williams A.P., Griffith G.W., Withers P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphorus acquisition. Appl. Soil Ecol. 2015;86:41–54. doi: 10.1016/j.apsoil.2014.09.012. [DOI] [Google Scholar]
- Rakiami A., Bechtaoui N., Tahiri A.I., Anli M., Meddich A., Oufdou K. Use of Rhizobacteria and Mycorrhizae Consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers L., Remans R., Rao I.M., Blair M.W., Vanderleyden J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crop. Res. 2010;117(2–3):169–176. doi: 10.1016/j.fcr.2010.03.001. [DOI] [Google Scholar]
- Reijnders L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014;93:32–49. doi: 10.1016/j.resconrec.2014.09.006. [DOI] [Google Scholar]
- Richardson A.E., Hocking P.J., Simpson R.J., George T.S. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 2009;60(2):124–143. doi: 10.1071/cp07125. [DOI] [Google Scholar]
- Richardson A.E., Lynch J.P., Ryan P.R., Delhaize E., Smith F.A., Smith S.E., Harvey P.R., Ryan M.H., Veneklaas E.J., Lambers H., Oberson A., Culvenor R.A., Simpson R.J. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil. 2011;349(1–2):121–156. doi: 10.1007/s11104-011-0950-4. [DOI] [Google Scholar]
- Richardson A.E., Simpson R.J. Soil microorganisms mediating phosphorus availability. Plant Physiol. 2011;156(3):989–996. doi: 10.1104/pp.111.175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Sanders I.R. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015;9(5):1053–1061. doi: 10.1038/ismej.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T.J., Liu L., Wissuwa M. Improving phosphorus efficiency in cereal crops: is breeding for reduced grain phosphorus concentration part of the solution? Front. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T.J., Rose M.T., Pariasca-Tanaka J., Heuer S., Wissuwa M. The frustration with utilization: why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front. Plant Sci. 2011;2 doi: 10.3389/fpls.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T.J., Wissuwa M. Rethinking internal phosphorus utilization efficiency: a new approach is needed to improve PUE in grain crops. Adv. Agron. 2012;116:185–217. doi: 10.1016/b978-0-12-394277-7.00005-1. [DOI] [Google Scholar]
- Sharpley A., Foy B., Withers P. Practical and innovative measures for the control of agricultural phosphorus losses to water: an overview. J. Environ. Qual. 2000;29(1):1–9. doi: 10.2134/jeq2000.00472425002900010001x. [DOI] [Google Scholar]
- Shepherd J.G., Kleemann R., Bahri-Esfahani J., Hudek L., Suriyagoda L., Vandamme E., van Dijk K.C. The future of phosphorus in our hands. Nutr. Cycl. Agroecosyst. 2016;104(3):281–287. doi: 10.1007/s10705-015-9742-1. [DOI] [Google Scholar]
- Smith S.E., Read D. Third ed. Academic Press, Elsevier Ltd; USA: 2008. Mycorrhizal Symbiosis. [Google Scholar]
- van de Wiel C.C.M., van der Linden C.G., Scholten O.E. Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica. 2016;207(1):1–22. doi: 10.1007/s10681-015-1572-3. [DOI] [Google Scholar]
- Vandamme E., Rose T., Saito K., Jeong K., Wissuwa M. Integration of P acquisition efficiency, P utilization efficiency and low grain P concentrations into P-efficient rice genotypes for specific target environments. Nutr. Cycl. Agroecosyst. 2016;104(3):413–427. doi: 10.1007/s10705-015-9716-3. [DOI] [Google Scholar]
- Veneklaas E.J., Lambers H., Bragg J., Finnegan P.M., Lovelock C.E., Plaxton W.C., Price C.A., Scheible W.-R., Shane M.W., White P.J., Raven J.A. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012;195(2):306–320. doi: 10.1111/j.1469-8137.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- Wahid F., Sharif M., Steinkellner S., Khan M.A., Marwat K.B., Khan S.A. Inoculation of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria in the presence of rock phosphate improves phosphorus uptake and growth of maize. Pak. J. Bot. 2016;48(2):739–747. [Google Scholar]
- Wang X.R., Shen J.B., Liao H. Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci. 2010;179(4):302–306. doi: 10.1016/j.plantsci.2010.06.007. [DOI] [Google Scholar]
- Warton D.I., Blanchet F.G., O'Hara R.B., Ovaskainen O., Taskinen S., Walker S.C., Hui F.K.C. So many variables: joint modeling in community ecology. Trends Ecol. Evol. 2015;30(12):766–779. doi: 10.1016/j.tree.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Weltin M., Zasada I., Piorr A., Debolini M., Geniaux G., Perez O.M., Scherer L., Marco L.T., Schulp C.J.E. Conceptualising fields of action for sustainable intensification – a systematic literature review and application to regional case studies. Agr. Ecosyst. Environ. 2018;257:68–80. doi: 10.1016/j.agee.2018.01.023. [DOI] [Google Scholar]
- Yamaji N., Takemoto Y., Miyaji T., Mitani-Ueno N., Yoshida K.T., Ma J.F. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature. 2017;541(7635):92. doi: 10.1038/nature20610. [DOI] [PubMed] [Google Scholar]
- Zhang L., Fan J.Q., Ding X.D., He X.H., Zhang F.S., Feng G. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol. Biochem. 2014;74:177–183. doi: 10.1016/j.soilbio.2014.03.004. [DOI] [Google Scholar]
- Zhang L., Feng G., Declerck S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018;12(10):2339–2351. doi: 10.1038/s41396-018-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xu M.G., Liu Y., Zhang F.S., Hodge A., Feng G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016;210(3):1022–1032. doi: 10.1111/nph.13838. [DOI] [PubMed] [Google Scholar]