Abstract
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a pandemic since WHO made the statement on March 11, 2020. The infection is causing a high mortality in old people, especially those with obesity, type 2 diabetes (T2D) or cardiovascular diseases (CVD). Extra cautions are needed in the treatment of those patients. The CVD drugs ACEIs and ARBs, as well as the T2D drugs GLP-1R agonists, were shown to activate angiotensin-converting enzyme 2 (ACE2) expression in experimental animals. Elevated ACE2 expression may accelerate virus entrance into the host cells during the infection for its replication. However, expression of the soluble ACE2, may neutralize the virus to limit the infection and replication. Given that obese, diabetes and CVD patients often take those medicines in the treatment and prevention of blood pressure and glucose elevation, it remains to be determined whether those medicines represent friend or foe in the treatment of COVID-19. We suggest that retrospective studies should be conducted to determine the exact impact of those medicines in obese, diabetic, or CVD patients who had COVID-19. Results obtained will provide guidance whether those drugs can be utilized in COVID-19 patients with obesity, diabetic, or CVD.
Keywords: COVID-19, ACE2, sACE2, ACEIs, ARBs, GLP-1, GLP-1R, Retrospective studies
1. Introduction
Coronavirus Disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a pandemic since the statement made by WHO on March 11, 2020. By More than 61.5 million people have been infected to date and more than 1.4 million lives have been taken away. Although the pandemic may be under the control with effective vaccines following the global efforts, the high mortality in individuals with obesity, type 2 diabetes (T2D), and cardiovascular diseases (CVD) is currently a challenge in the fight against COVID-19 infection.
The analysis of clinical characteristics of COVID-19 in China and elsewhere have clearly revealed that old people with chronic comorbidities have a higher risk for severe symptoms than younger individuals in the infection (Guan et al., 2020; Mattioli et al., 2020). A recent study indicates that the diabetes drug metformin is able to reduce the mortality rate in the COVID-19 patients (Hariyanto and Kurniawan, 2020). Intensive investigations have been conducted in exploring the relationship between obesity and the severity of COVID-19 (Belancic et al., 2020; Belancic, 2020; AbdelMassih et al., 2020). Here we would like to present a commentary on the use of the two categories of therapeutic agents in COVID-19 patients with metabolic disorders including obesity, T2D and CVD.
2. The hypertension medicines ACEIs and ARBs
Patients with hypertension are commonly treated with the renin angiotensin system (RAS) antagonists, such as angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) in the control of blood pressure. However, these drugs have been reported to increase the expression of angiotensin converting enzyme 2 (ACE2) (Ferrario et al., 2005; Hoffmann et al., 2020), which is recognized as the receptor of SARS-CoV-2 in the cell surface, especially the lung epithelial cells (Hoffmann et al., 2020; Wan et al., 2020). Thus, there has been a speculation that this class of antihypertension drugs might facilitate the infection and replication of the virus (Fang et al., 2020; Diaz, 2020). On the other hand, some researchers believed that the expression may be a beneficial response in terms of limiting the lung injuries by the infection (Cheng et al., 2020; Wu, 2020). Collectively, the exact impact of ACEIs and ARBs in the infection is still under debate. Here we present two lines of justifications supporting the hypothesis that those hypertension medicines may reduce the mortality in COVID-19 patients.
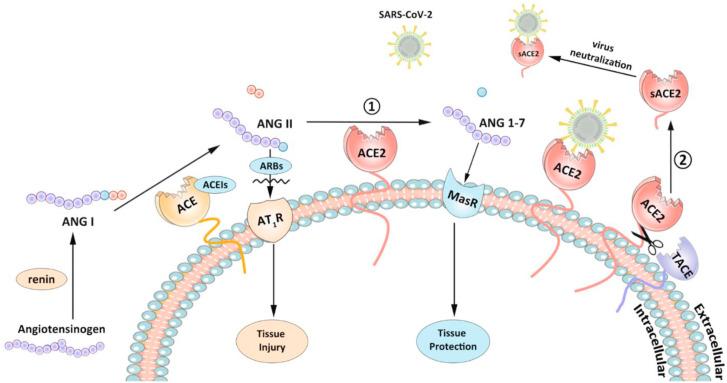
As presented in Fig. 1 , RAS system consists of two reciprocally constrained arms: one is for generation of angiotensin II (Ang II) from angiotensinogen by the enzymes renin and ACE; another is for degradation of Ang II to angiotensin 1-7 by the enzyme ACE2. Therefore, ACE2 plays a counter-regulatory role in the inhibition of RAS system by removing Ang II. It has been reported that ACE2 protected against the severe acute lung failure (Imai et al., 2005). ACE2 was shown to be crucial in the prevention of the development of severe acute respiratory syndrome in the SARS-CoV infection (Kuba et al., 2005). Moreover, ACE2 generates angiotensin 1-7, which has activities including vasodilation, anti-hypertrophy and anti-inflammation (Wosten-van Asperen et al., 2011). Therefore, induction of ACE2 levels by the medicines, ACEIs or ARBs, may generate a protective effect in the COVID-19 patients by reduction of the severe respiratory symptoms risk.
Fig. 1.
Two pathways related to beneficial activity of ACE2 in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Angiotensin I (ANG I) generated from angiotensinogen by renin, can subsequently be catalyzed by angiotensin-converting enzyme (ACE) and produce angiotensin II (ANG II). On the one hand, ANG II binds to angiotensin type 1 receptor (AT1R) to cause tissue injury. On the other hand, ACE2 can convert ANG II to angiotensin 1-7 (ANG 1–7), which can protect against tissue damage through Mas receptor (MasR). Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) can suppress the destructive function of Ang II. ACE2 is also the receptor of SARS-CoV-2. And ACE2 can meanwhile be cleaved by TNF-α converting enzyme (TACE) into soluble forms, namely sACE2, which will be released into extracellular compartments and neutralize SARS-CoV-2.
Conversely, Ang II has a negative feedback in the regulation of ACE2 activity by induction of TNF-α converting enzyme (TACE) leading to ACE2 cleavage (Fig. 1) (Patel et al., 2014). TACE, also known as metalloprotease 17 (ADAM17), is a major protease for ACE2 ectodomain shedding (Lambert et al., 2005). The cleavage happens between amino acids 716 and 741 near the predicted transmembrane domain and residue Lys548 in the ectodomain (Towler et al., 2004), which produces two types of soluble ACE2 (sACE2) with molecular mass of 105 kDa and 95 kDa. It is possible that the shedding ectodomain still possesses the capacity to bind coronavirus but does not mediate the virus entry into the host cell. It has been demonstrated that sACE2 efficiently blocked the association of SARS virus with its cellular receptor (Li et al., 2003), and averted the lung injuries (Kuba et al., 2005). COVID-19 virus replication can be abolished by a recombinant sACE2 protein fused to the Fc portion of immunoglobulin in vitro (Lei et al., 2020). Consistently, the recombinant sACE2 protein has been employed as a potential therapeutic agent for COVID-19 (Zhang et al., 2020; Batlle et al., 2020).
In healthy human subjects, plasma sACE2 level is very low (Lew et al., 2008). However, the level can be significantly induced in patients who are taking ACEIs or ARBs (Epelman et al., 2008, 2009). When ACE2 was cardiac-specifically overexpressed in mice, serum sACE2 level can be increased approximately 20-fold (Donoghue et al., 2003). These observations suggest that the level of extracellular sACE2 can be raised by ACEIs or ARBs, serving as potent decoy receptor for virus neutralization. ACEIs and ARBs may exhibit different activities in the induction of sACE2. In contrast to ACEIs which reduce the level of Ang II and hence deplete the substrate of ACE2, ARBs can promote Ang II accumulation in extracellular compartments towards ACE2, thus inducing the cleavage of ACE2 with increased production of sACE2.
Overall, we agree with others that ACE2 based drugs represent a double-edged sword in coronavirus infection (Onweni et al., 2020; Wang et al., 2020). Although it is an entry for coronavirus invasion, it plays a vital role in protection of the patients against severe tissue damage, especially in the lung. It is unclear whether these RAS antagonists are beneficial or detrimental in COVID-19. There is an urgent demand for clinical studies to resolve this issue.
In addition to ACE2, COVID-19 infection is also dependent on other cellular proteins, such as Furin. The spike protein of SARS-CoV-2 contains a furin cleavage site, the unique RRAR motif; which was not found in any other subtype B betacoronavirus (Wu et al., 2020). Male and elder people, as well as obese and diabetic patients, have increased levels of furin in their blood (AbdelMassih et al., 2020), making this enzyme a potential therapeutic target of COVID-19 in those subjects (AbdelMassih et al., 2020; Wu et al., 2020). It is worth to test whether furin inhibitors, including the flavone Luteolin, isolated from Chinese herbal medicine (Peng et al., 2017), can prevent the COVID-19 infection and virus replication.
3. Diabetic medicine: glucagon-like peptide-1 (GLP-1)
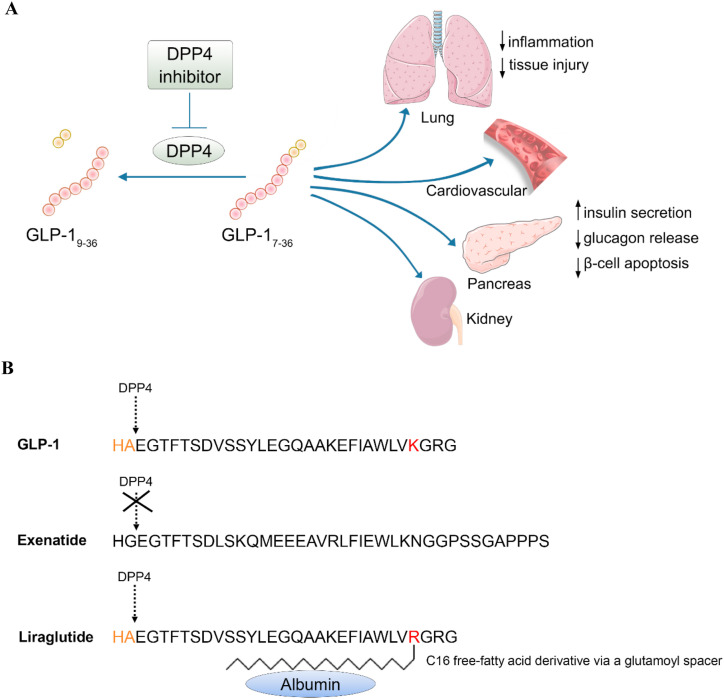
GLP-1 is an incretin (Tian and Jin, 2016; Kieffer and Habener, 1999), a hormone produced in the gut with the capability in the stimulation of insulin secretion postprandially (Kieffer and Habener, 1999; Drucker, 2018). Studies on GLP-1 and another incretin glucose‐dependent insulinotropic polypeptide (GIP) have led to the development of novel therapeutic agents for T2D, known as GLP-1R agonists including Liraglutide; and DPP-4 inhibitors including Sitagliptin (Jin and Weng, 2016). Fig. 2 illustrates main functions of GLP-1 as well as the structure of GLP-1 based diabetes drugs Exenatide (Byetta) and Liraglutide (Victoza).
Fig. 2.
A) Illustration of the process of GLP-1 and target organs of this incretin hormone. B) Structure of Exenatide and Liraglutide, the two GLP-1 based diabetes drugs.
More and more T2D patients are now taking GLP-1R agonists including Exenatide and Liraglutide for both glycemic control and body weight management. Liraglutide, commercially known as Victoza, was developed by Novo Nordisk, approved by the European Medicines Agency (EMA) in 2009, and then by the Food and Drug Administration (FDA) of US in 2010 (Jin and Weng, 2016). In addition to facilitating postprandial insulin secretion, GLP-1 and Liraglutide exert beneficial effects in the treatment of obesity and chronic inflammation (Zhou et al., 2020; M Khroud et al., 2019; Jin and Liu, 2020). Unexpectedly, a previous investigation showed that in type 1 diabetes rats, GLP-1 receptor (GLP-1R) activation by Liraglutide increased ACE2 expression, which reversed the right ventricle hypertrophy in the heart, and improved the production of surfactant proteins (SP-A and SP-B) in the lungs (Romani-Perez et al., 2015). A follow-up study showed that Liraglutide prevented the alteration in lung function induced by in utero growth retardation (IUGR) and promoted the positive effects of ACE2-Ang (Guan et al., 2020; Mattioli et al., 2020; Hariyanto and Kurniawan, 2020; Belancic et al., 2020; Belancic, 2020; AbdelMassih et al., 2020; Ferrario et al., 2005) system in restoring lung function (Fandino et al., 2018).
Those observations turn the GLP-1 based medicines into a double-edged sword as well in the treatment of obese or diabetic patients with COVID-19 infection. Liraglutide treatment may facilitate the infection and replication of SARS-CoV-2 via stimulating ACE2 expression. Alternatively, the treatment may lead to the generation of sACE2, serving as “decoy receptor” in preventing or attenuating virus infection. It is worth to mention that lung epithelia express a high level of GLP-1R (Bullock et al., 1996). Given the metabolic beneficial effect of GLP-1 based drugs and their anti-inflammatory feature demonstrated in animal model (Zhou et al., 2020), the medicine may protect the lung from inflammatory damage through its high level of GLP-1R. These possibilities remain to be assessed in the clinical studies in COVID-19 patients.
4. Summary
We present here that both the hypertension drugs (ACEIs and ARBs) and GLP-1 based T2D drugs may stimulate ACE2 expression, making them double edged sword in the treatment of COVID-19 patients in the population with obesity, diabetes, aging and other metabolic disorders (Jin and Liu, 2020; Monda et al., 2020; Morin, 2020). It remains to be determined whether their treatment also increases the expression of sACE2 in human subjects. We highly suggest that studies should be conducted in advanced animal models generated recently during the combat with COVID-19 (Munoz-Fontela et al., 2020; Dinnon et al., 2020), whether those therapeutic agents are friend or foe. Furthermore, retrospective studies to compare the clinical process and outcomes between COVID-19 patients who have taken and have not taken GLP-1 based drugs, as well as ACEIs and ARBs, should be conducted. Information gained will serve as asset for the improvement of the guideline in the treatment of COVID-19 patients with and without T2D, CVD and other chronic disorders.
Acknowledgements
The authors claim no conflict interest on this short communication. Juan Pang is a recipient of China Scholarship Council (CSC). We thank Dr. Jianping Ye for his valuable comments and suggestions.
References
- AbdelMassih A.F., Ye J., Kamel A., Mishriky F., Ismail H.A., Ragab H.A., El Qadi L., Malak L., Abdu M., El-Husseiny M., Ashraf M., Hafez N., AlShehry N., El-Husseiny N., AbdelRaouf N., Shebl N., Hafez N., Youssef N., Afdal P., Hozaien R., Menshawey R., Saeed R., Fouda R. A multicenter consensus: a role of furin in the endothelial tropism in obese patients with COVID-19 infection. Obes. Med. 2020;19:100281. doi: 10.1016/j.obmed.2020.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. (Lond.) 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- Belancic A. Gut microbiome dysbiosis and endotoxemia - additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes. Med. 2020;20:100302. doi: 10.1016/j.obmed.2020.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belancic A., Kresovic A., Racki V. Potential pathophysiological mechanisms leading to increased COVID-19 susceptibility and severity in obesity. Obes. Med. 2020;19:100259. doi: 10.1016/j.obmed.2020.100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock B.P., Heller R.S., Habener J.F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J. Trav. Med. 2020;27 doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon K.H., 3rd, Leist S.R., Schafer A., Edwards C.E., Martinez D.R., Montgomery S.A., West A., Yount B.L., Jr., Hou Y.J., Adams L.E., Gully K.L., Brown A.J., Huang E., Bryant M.D., Choong I.C., Glenn J.S., Gralinski L.E., Sheahan T.P., Baric R.S. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Wakimoto H., Maguire C.T., Acton S., Hales P., Stagliano N., Fairchild-Huntress V., Xu J., Lorenz J.N., Kadambi V., Berul C.I., Breitbart R.E. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J. Mol. Cell. Cardiol. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabol. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Epelman S., Tang W.H., Chen S.Y., Van Lente F., Francis G.S., Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J. Am. Coll. Cardiol. 2008;52:750–754. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S., Shrestha K., Troughton R.W., Francis G.S., Sen S., Klein A.L., Tang W.H. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J. Card. Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandino J., Vaz A.A., Toba L., Romani-Perez M., Gonzalez-Matias L., Mallo F., Diz-Chaves Y. Liraglutide enhances the activity of the ACE-2/ang(1-7)/mas receptor pathway in lungs of male pups from food-restricted mothers and prevents the reduction of SP-A. Int. J. Endocrinol. 2018;2018:6920620. doi: 10.1155/2018/6920620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China medical treatment expert group for C: clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes. Med. 2020;19:100290. doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T., Liu M. Letter to the editor: comment on GLP-1-based drugs and COVID-19 treatment. Acta Pharm. Sin. B. 2020;10:1249–1250. doi: 10.1016/j.apsb.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T., Weng J. Hepatic functions of GLP-1 and its based drugs: current disputes and perspectives. Am. J. Physiol. Endocrinol. Metab. 2016;311:E620–E627. doi: 10.1152/ajpendo.00069.2016. [DOI] [PubMed] [Google Scholar]
- Kieffer T.J., Habener J.F. The glucagon-like peptides. Endocr. Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C., Qian K., Li T., Zhang S., Fu W., Ding M., Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R.A., Warner F.J., Hanchapola I., Yarski M.A., Ramchand J., Burrell L.M., Smith A.I. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp. Physiol. 2008;93:685–693. doi: 10.1113/expphysiol.2007.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Khroud Z.S., Shao W., Jin T. Moderate preventative effect with intraperitoneal liraglutide injection in high-fat diet induced C57BL/6J obese mouse model. Obes. Med. 2019;16:100153. [Google Scholar]
- Mattioli A.V., Pinti M., Farinetti A., Nasi M. Obesity risk during collective quarantine for the COVID-19 epidemic. Obes. Med. 2020;20:100263. doi: 10.1016/j.obmed.2020.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda V.M., Porcellati F., Strollo F., Gentile S. ACE2 and SARS-CoV-2 infection: might GLP-1 receptor agonists play a role? Diabetes Ther. 2020;11:1909–1914. doi: 10.1007/s13300-020-00898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin N. Response to COVID-19 and diabetes: can DPP4 inhibition play a role? - GLP-1 might play one too. Diabetes Res. Clin. Pract. 2020;164:108160. doi: 10.1016/j.diabres.2020.108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Fontela C., Dowling W.E., Funnell S.G.P., Gsell P.S., Riveros-Balta A.X., Albrecht R.A., Andersen H., Baric R.S., Carroll M.W., Cavaleri M., Qin C., Crozier I., Dallmeier K., de Waal L., de Wit E., Delang L., Dohm E., Duprex W.P., Falzarano D., Finch C.L., Frieman M.B., Graham B.S., Gralinski L.E., Guilfoyle K., Haagmans B.L., Hamilton G.A., Hartman A.L., Herfst S., Kaptein S.J.F., Klimstra W.B., Knezevic I., Krause P.R., Kuhn J.H., Le Grand R., Lewis M.G., Liu W.C., Maisonnasse P., McElroy A.K., Munster V., Oreshkova N., Rasmussen A.L., Rocha-Pereira J., Rockx B., Rodriguez E., Rogers T.F., Salguero F.J., Schotsaert M., Stittelaar K.J., Thibaut H.J., Tseng C.T., Vergara-Alert J., Beer M., Brasel T., Chan J.F.W., Garcia-Sastre A., Neyts J., Perlman S., Reed D.S., Richt J.A., Roy C.J., Segales J., Vasan S.S., Henao-Restrepo A.M., Barouch D.H. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onweni C.L., Zhang Y.S., Caulfield T., Hopkins C.E., Fairweather L., Freeman W.D. ACEI/ARB therapy in COVID-19: the double-edged sword of ACE2 and SARS-CoV-2 viral docking. Crit. Care. 2020;24:475. doi: 10.1186/s13054-020-03195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Peng M., Watanabe S., Chan K.W.K., He Q., Zhao Y., Zhang Z., Lai X., Luo D., Vasudevan S.G., Li G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antivir. Res. 2017;143:176–185. doi: 10.1016/j.antiviral.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Romani-Perez M., Outeirino-Iglesias V., Moya C.M., Santisteban P., Gonzalez-Matias L.C., Vigo E., Mallo F. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156:3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- Tian L., Jin T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J. Diabetes. 2016;8:753–765. doi: 10.1111/1753-0407.12439. [DOI] [PubMed] [Google Scholar]
- Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., Patane M.A., Pantoliano M.W. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142:426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- Wosten-van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel J.B., van der Loos C.M., van Goor H., Kamilic J., Florquin S., Bos A.P. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- Wu Y. Compensation of ACE2 function for possible clinical management of 2019-nCoV-Induced acute lung injury. Virol. Sin. 2020;35:256–258. doi: 10.1007/s12250-020-00205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Zheng M., Yang Y., Gu X., Yang K., Li M., Liu Y., Zhang Q., Zhang P., Wang Y., Wang Q., Xu Y., Zhou Y., Zhang Y., Chen L., Li H. Furin: a potential therapeutic target for COVID-19. iScience. 2020;23:101642. doi: 10.1016/j.isci.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Shao W., Zhang Y., Liu D., Liu M., Jin T. Glucagon-like peptide-1 receptor mediates the beneficial effect of liraglutide in an acute lung injury mouse model involving the thioredoxin-interacting protein. Am. J. Physiol. Endocrinol. Metab. 2020;319:E568–E578. doi: 10.1152/ajpendo.00292.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]