Abstract
Mangifera longipes and Quercus gomeziana both is an ethnomedicinally important Asian herb that has been known for numerous healing activity of tribal people. The present research aims to investigate the phytochemical analysis with in vitro, in vivo possibilities of the soluble ethanol extract of M. longipes root (EEMLR) and Q. gomeziana leaves (EEQGL) by an experimental approach. The plant extract of EEMLR and EEQGL was found secondary metabolites, notably steroids, glycosides, tannins, flavonoids, saponins, gums, and alkaloids. Additionally, the extract showed significant activity in antioxidant, antipyretic, anti-inflammatory, membrane stabilization, cytotoxic, thrombolytic, and analgesic activities while no response in antibacterial activity. Our findings reveal that soluble ethanol extract of EEMLR and EEQGL is safe, which can be an effective source for exploring new medicinal products. This research's outcomes may provide potentials for mitigating pyrexia, inflammation, pain, cellular toxicity, and coagulation.
Keywords: Mangifera longipes, Quercus gomeziana, Antipyretic, Anti-inflammatory, Analgesic, Antioxidant
Abbreviations: EEMLR, Ethanol extract of Mangifera longipe leaves root; EEQGL, Ethanol extract of Quercus gomeziana leaves; ASA, Acetyl salicylic acid; IC50, Half maximal inhibitory concentration; LC50, Lethal Concentration 50; DPPH, 2, 2-diphenyl-1-picrylhydrazyl; TLC, Thin-layer chromatography
1. Introduction
Medicinal plants play a significant role in medical science in this artificial age by modulating complex human dysfunctions. It is the respiratory destination of medicinal plants throughout ancient times (Tareq et al., 2020). Notable number of lead compound as a drug synthesized from the natural sources as well as plant origin (J. Rahman et al., 2020). Bioactive ligands having some flavonoids, glycosides, alkaloids, steroids, polyphenol, vitamin C, cholesterol, terpenoids and saponins have been isolated and characterized from the plants and scientists proved that these substances have a different pharmacological and biological activity such as antidepressant, anxiolytic, antimicrobial, antioxidant, thrombolytic, anti-inflammatory, neuroprotective, hepatoprotective, cytotoxic etc. (Islam et al., 2020, Okwu and Uchenna, 2009). These phytochemicals are obtained from various parts of plants that including root, stem, bark, leaves, fruits, and seeds (Sani et al., 2014, Yesmin et al., 2020).
Phytochemicals have been recognized also as origin of traditional medicinal used in the history and in hot demand in parts of the world today (Bussmann and Sharon, 2006). Mangifera has been recorded as belonging to 69 species, classified with three subgenus widely distributed in Asia, namely Bangladesh, Malaysia, Philippines, Thailand, India (Hayati et al., 2018). Traditionally Mangifera longipes Griff. (Family: Anacardiaceae) bark reported for the cure of diarrhea, iron deficiency, syphilis, ringworm, dysentery, leprosy, skin abscesses and infections (Prashanth et al., 2001); leaves for excessive GI (gastrointestinal) motility and wounds (Makare et al., 2001); fruit for the use of xerophthalmia, vitamin A deficiency, skin infection (Khan et al., 2015); roots also used for the sores, leucorrhea, syphilis (Ediriweera et al., 2017) and seeds for the medication of indigestion, cholera, astringency, anthelmintics (Kawamura et al., 2011). However, Quercus had been identified 91 species (Deng et al., 2014), whereas Quercus gomeziana (Family: Fagaceae) and its species were historically used for the treatment of styptic, bacterial infection (Berahou et al., 2007), anthelmintics, cytotoxic, viral infection (Paaver et al., 2010), astringent, larvicidal (Redwane et al., 2002), gastrointestinal (GI) disorder, ulcers (Joshi and Juyal, 2017), wound healing, chronic toxicity (Khattak, 2018), diarrhea, asthma, inflammation (Khennouf et al., 2010), hemostatic, gastropathies, hemorrhoid (Taib et al., 2020). There are currently available drugs that are desensitizing to receptors and immune to microorganisms such as bacteria, which is why new lead compounds are required that will be more sensitive with a definite receptor to give batter action and to overcome resistance (Bristy et al., 2020).
Oxidation, a chemical reaction that has affinity to initiate chain reaction by producing free radicles and deteriorate the food quality as well as color and flavor (Jahan et al., 2020). Antioxidants are any agents that might inhibit the response to oxidation (Matthäus, 2002). There were some phenolic compounds discovered to inhibit oxidation on late 1940 has applied in food industry as synthetic antioxidants mentioned as BHA, BHT, gallates have several severe disadvantages due to highly volatile, highly sensitive to elevated temperatures, and they are all suspicious to have various toxic features (Barua et al., 2020). Natural preservatives are usually used on the food market to avoid pathogenic and spoilage microorganisms that are also responsible for some carcinogenicity, teratogenicity and residual toxicity properties (Chattopadhyay and Bhattacharyya, 2007). Besides, antioxidant assessment, whereas conducted DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging assay for removing oxidation of cell and also reported to cease of un-regularized proliferation (Hridy et al., 2020). Cytotoxicity is the properties of the cells harmful to toxic agents. In this research, brine shrimp lethality bioassay is applied in designing cytotoxic activity determination. The flavonoid unit was exposed to cytotoxicity reductions (Hasanat et al., 2019, Romero-Benavides et al., 2018).
Pain, inflammation including fever are indeed the primary symptoms both of major human and animal disorders (Shanmugam et al., 2019). Inflammation is complex biological system to the vascular tissues responsible for harming stimuli through pathogens, damaged cells or irritants which is individualized by redness, swollen of joints, joint pain and decrement of joint function (Kumar et al., 2013, Rakib et al., 2020a). Membrane stabilizing activity (MSA) experiment is designed to assess the local anesthetic action. Thrombus, a blood clot manifested to the circulatory system of the blood as many available thrombolytic medication regiments officially have some vital flaws together with minimal fibronectin accuracy, substantial bleeding trend and mandate for higher doses (Islam et al., 2013). Furthermore, the Eddy’s hot plate, Brewer’s yeast induced hyperpyrexia, thrombolytic approach was used to investigate the analgesic, antipyretic and thrombotic activity of ethanol extract. The goal of this study was to examine the development of new bioactive lead compounds that will provide batter effects to reduce toxicity and recover diseases.
2. Materials and methods
2.1. Chemicals
The chemicals used for the present study were ethanol, ascorbic acid, acetyl salicylic acid, DPPH purchased from Merck KGaA, (Darmstadt, Germany), streptokinase (Incepta Pharmaceutics Ltd., Dhaka, Bangladesh), diclofenac sodium (ACME Laboratories Ltd., Dhaka, Bangladesh), paracetamol (Square Pharmaceuticals Ltd., Dhaka, Bangladesh), vincristine sulphate (Beacon Pharmaceuticals Ltd., Dhaka, Bangladesh) and others chemicals were organized from local trader through Taj Scientific Ltd, Chittagong, Bangladesh.
2.2. Experimental animals
The study was conducted on Swiss Albino mice purchased from Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong, Bangladesh and Animal Resources Branch (ARB), International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR, B). They were five week of age, weighing about 25–30 gm.
2.3. Plant collection and extract preparation
Fresh root of M. longipes and leaves of Q. gomeziana were selected based on its medicinal uses and collected from the Hill of Khamarpara, Rangamati, Chittagong, Bangladesh. These two plants were identified by Mr. Md. Saidul Alam, Senior Research Officer, Forest Research Institute, Chittagong, Bangladesh. The collected root was sun dried for 17 days and leaves at home dried for 14 days and ground into fine powder and 140 g of each root and leave powder were soaked in 1000 ml ethanol for 10 days at room temperature. The obtained extract was collected, filtered by filter paper and Soxhlet extractor made to evaporate the solvent at 60 °C temperature in the water bath.
2.4. Qualitative phytochemical analysis
Phytochemical measurements have been carried out using standard protocols (Kabir et al., 2016) to evaluate the alkaloids, glycosides, steroids, tannins, flavonoids, saponins, flavanols, carbohydrates, gums and mucilage’s.
2.5. In vitro antioxidant assay
2.5.1. Qualitative test
The crude extract’s free radical scavenging capability was ascertained utilizing DPPH and the solution (0.004 95% w/v) was formulated in 95% ethanol. M. longipes root and Q. gomeziana leaves ethanol extracts was merged in 95% methanol providing stock solution formulation (5 mg/ml). Pharmaceutically accessible thin layer chromatography (TLC) plate was used for DPPH scavenging assessment (Biswas et al., 2014). A sufficiently diluted stock solution was noticed on pre-coated TLC silica gel trays and the discs were formulated in solvent system in various polarities to alleviate the extract’s polar and non-polar parts.
2.5.2. Quantitative test
Serial dilutions have been executed to get 31.25, 62.50, 125, 500 μg/ml from stock solution of the ethanol extract of M. longipes root and Q. gomeziana leaves. Compared to positive control ascorbic acid and negative control 95% ethanol, the half maximum inhibitory concentration (IC50) and half maximum effective concentration (EC50) were calculated graphically (Tadić et al., 2008). Percentage scavenging of the DPPH free radicals was calculated by using the given formula:
where A0 = absorbance of control and Ae = absorbance of sample.
2.6. In vitro cytotoxic assay by brine shrimp lethality bioassay
The crude extracts of M. longipes root and Q. gomeziana leaves were investigated using bioassay of brine shrimp mortality to evaluate the cytotoxic activity. Artemia salina (brine shrimp eggs) was incubated in external water reservoir dissolved in sea salt (Rahman et al., 2020). Inside a warm room (22–29 °C) nauplii were accumulated through the organisms by light to one side of the vessel source and the nauplii from the eggs by pipetting them 2–3 times in small buckets filled with seawater (Pisutthanan et al., 2013). The test dose was animated by serial dilation to 800, 400, 200, 100, 50, 25, 12.5 and 6.25 μg/ml. Positive and negative control labeled vincristine and DMSO were compared with test group. The percentage of lethality of the brine shrimp nauplii for every saturation was estimated from this result (M Atiar Rahman et al., 2013). The mortality was calculated using the following formula:
where Po = observed mortality and Pc = control mortality.
2.7. In vitro antimicrobial activity by disc diffusion assay
Five sets of media were formulated in experiments conducted to analyze the influence of solubilizing agents on the diffusion of elements via the agar (Khanam et al., 2015). Several bacterial strains such as Lactobacillus casei, Lactobacillus coryniformis, Bacillus cereus, Staphylococcus aureus, Bacillus azotoformans inserted into gram positive species and Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Klebsiella pneumoniae, Vibrio cholera implemented into gram negative species were screened. The reported findings were compared with standard azithromycin (Rahman et al., 2013).
2.8. In vitro anti-inflammatory assay
2.8.1. Inhibition of protein denaturation
Anti-inflammatory opportunities of ethanol extract of both the M. longipes root and Q. gomeziana leaves has indeed been studied through minor modifications Sakat et al., (Al Mahmud et al., 2016). The reaction mixture was formulated by 3 ml of 5% egg albumin solution and 3 ml of varying test extract concentrations consisting of pH (5.6 ± 0.2) adjusted by 0.1 N HCl, so that final concentration level would adjust as 125, 250, and 500 μg/ml. Comparable methanol volume served as control. The mixtures were often incubated for 15 min at 37 ± 2 °C in a BOD (biochemical oxygen demand) incubator, then heated for 20 min at 57 °C and absorbance was measured by using blank vehicle at 660 nm (Shah et al., 2017, Ullah et al., 2014). The percentage of protein denaturation suppression was determined by the following formula:
where Ac = absorbance of control and As = absorbance of sample.
2.8.2. Hypotonic solution-induced hemolysis
The recorded approaches for the in vitro membrane stabilizing assay were adopted based on the standard methods. In brief, the suspension of 0.5 ml of HRBC was mixed with 1 ml phosphate buffer (pH 7.4), 2 ml hypo-saline containing either 0.5 ml of both plant extracts and standard drug acetyl salicylic acid of various concentrations (500, 250, 125 µg/ml). Then the extract was incubated at 37 °C for 30 min and centrifuged for 10 min and the supernatant absorbance was recorded at 540 nm respectively. The inhibitory effect of hemolysis from absorbance has been determined by following equation (Sabiu and Ashafa, 2016).
where Ac = absorbance of control and As = absorbance of sample.
2.9. In vitro thrombolytic activity
The thrombolytic activity of ethanol extracts of M. longipes root and Q. gomeziana leaves was investigated by deriving venous blood (5 ml) from study participants and dispersed in 5 separate pre-weighted tubes (500 μl/tube) and incubated for 45 min at 37 °C against positive control streptokinase (Emon et al., 2020). Since creation of the cluster, the serum was permanently removed avoiding upsetting the clot and each tube containing clot was measured and again to estimate the clot weight (Prasad et al., 2006, Shifah et al., 2020). The clot lysis was estimated by following equation:
2.10. In vivo analgesic activity by Eddy's hot plate technique
In this experiment, the root and leaf extracts of M. longipes and Q. gomeziana based on Eddy's hot plate technique applied in Swiss Albino mice (Chavan et al., 2010). Mice were classified into four categories of three animals for each group, as well as treatments were conducted according to tail-flick process. While regrouping, animals were treated orally with the extract in groups two and three, respectively at the dose rate of 400 mg/kg at the same time. Control group (group four) received the reference drug diclofenac (9 mg/kg b.w i.p) and the negative control group (group one) received a corresponding vehicle dose (Aziz, 2015, Uddin et al., 2016). The control and treated animal’s response time after the medication was recorded at 0 and 39 min.
2.11. In vivo antipyretic activity by Brewer's yeast induced pyrexia
This analysis was conducted out with a minor alteration, as described in Turner et al. Extract's antipyretic activity was assessed by using Brewer's yeast induced pyrexia model (Hossain et al., 2016). The rectal temperature is calculated for each of the animal in batch, subcutaneous triggered pyrexia through 10% Brewer yeast suspension injection in the scruff of the neck, 10 ml/kg dose of body weight. Root and leaves extract were administered orally in the study group at a body weight dosage of 500 mg/kg after 18 h compared with the reference drug paracetamol (150 mg/kg) and negative control (distilled water 10 ml/kg).
2.12. Statistical analysis
The values were exposed in mean ± standard error mean (SEM). *p < 0.05, **p < 0.01 and ***p < 0.001 indicated statistical significance compared to different control, calculated by one-way analysis of variance (ANOVA) (Dunnett’s test) using GraphPad Prism version 8.4. (San Diego, CA, USA).
3. Results
3.1. Phytochemical screening
The characterization of chemical properties is important during the analysis of target plant materials for pharmacological effects. The findings of the crude EEMLR and EEQGL phyto-screening assay are stated in Table 1. The EEMLR includes glycosides, tannins, flavonoids, saponins, and gums as well as EEQGL contains steroids, alkaloids, flavonoids, saponins, and gums. These phytochemical group exhibited several pharmacological activities like antibacterial, cytotoxicity, antifungal, antiviral, analgesics, antioxidant, inflammation, cardio protective and anticancer effects etc.
Table 1.
Phytochemical screening of ethanol extract of M. longipes root and Q. gomeziana leaves.
| Examination | Name of the test |
Results |
|
|---|---|---|---|
| EEMLR | EEQGL | ||
| Alkaloids | Mayer’s test | + | + |
| Dragendorff’s test | + | + | |
| Glycosides | Salkowski test | + | – |
| Libermann-burchared test | + | – | |
| Steroids | Salkowski test | + | + |
| Libermann-burchared test | + | + | |
| Tannins | Ferric chloride test | + | – |
| Potassium dichromate test | + | – | |
| Flavonoids | Conc. HCl and alcoholic test | + | + |
| Saponins | Shake test (aq. solution) | + | + |
| Gums | Molisch’s test | + | + |
(+) presence; (-) absence; EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
3.2. Antioxidant activity
3.2.1. Qualitative assay
Ultraviolet (UV)-spectrophotometer obtained light yellow band coated by a purple color shadow for both narrow (254 nm) and broad (360 nm) wavelength range in the qualitative test per DPPH induced TLC plate method, result antioxidant of EEMLR and EEQGL.
3.2.2. Quantitative assay
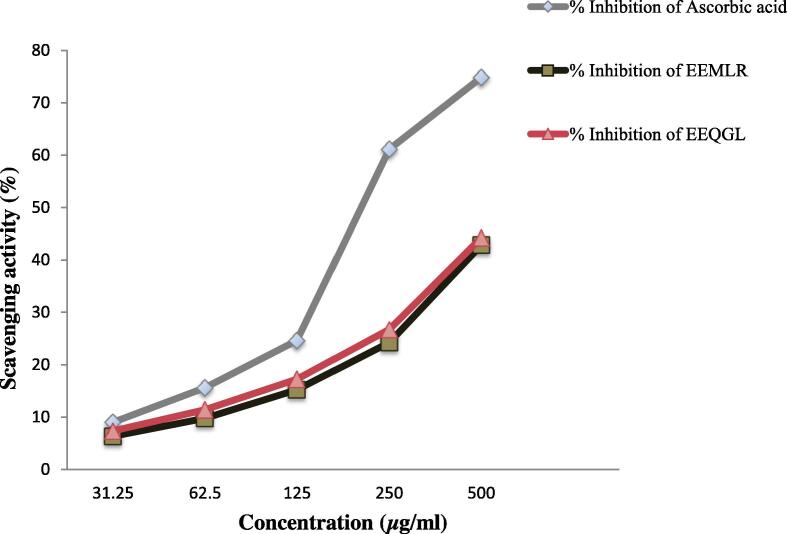
Finding the maximum free radical scavenging activity based on DPPH assay whereas IC50 value of 6.467 and 6.839 μg/ml by an EEMLR and EEQGL compared to the reference ascorbic acid of 3.735 μg/ml as shown in dose-dependent manner as Fig. 1.
Fig. 1.
Antioxidant activity of ethanol extract of M. longipes root and Q. gomeziana leaves. Values are expressed as mean ± SEM (n = 3); EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
3.3. Cytotoxic activity
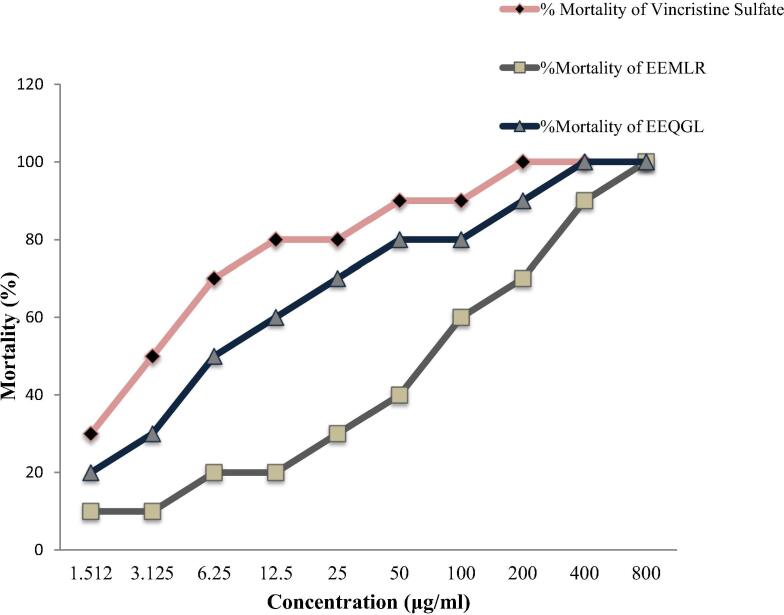
Cytotoxicity activity of EEMLR and EEQGL were screened using hatching of brine shrimp assay where EEMLR exhibited LC50 of 3.49 μg/ml compared with positive control vincristine sulfate. LC50 refers to the concentration of the chemical that represents death in half of the test subjects over a certain period of exposure and calculated by linear regression analysis from plotting percent mortality against the corresponding concentration log expressed in Fig. 2.
Fig. 2.
Cytotoxic activity of ethanol extract of M. longipes root and Q. gomeziana leaves. Values are expressed as mean ± SEM (n = 3); EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
3.4. Antibacterial activity
Disc diffusion method was applied to assess the antibacterial activity of EEMLR and EEQGL against five gram-positive and five gram-negative bacteria using azithromycin as standard. In the screening, both root and leaves extract (500 and 250 μg/disc) displayed negligible antibacterial result compared to azithromycin as standard against all the microorganisms tested.
3.5. Anti-inflammatory activity
3.5.1. Inhibition of protein denaturation assay
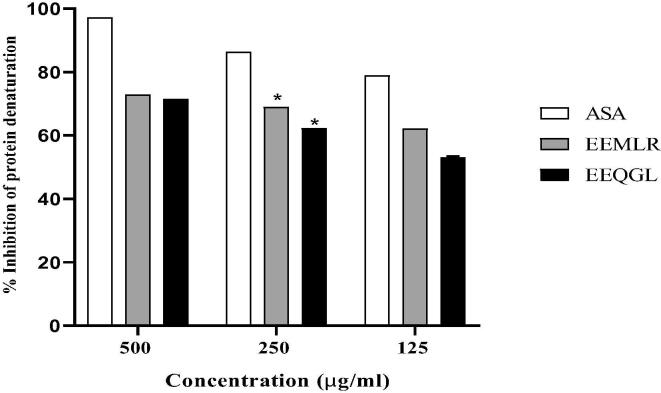
The in vitro anti-inflammatory action of EEMLR and EEQGL was assessed employing concentration-dependent inhibition against denaturation of egg albumin as followed in Fig. 3. Both EEMLR and EEQGL were expressed cabalistic result 72.9657 ± 0.0011 and 71.5686 ± 0.001 whereas, acetyl salicylic acid was found to be 97.2303 ± 0.0008 at dose 500 μg/ml.
Fig. 3.
Anti-inflammatory activity of ethanol extract of M. longipes root and Q. gomeziana leaves. Values are expressed as mean ± SEM (n = 3); *p < 0.05 are statistically significant comparison with acetyl salicylic acid followed by Dunnett’s test. ASA = Acetyl salicylic acid; EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
3.5.2. Hypotonic solution-induced hemolysis assay
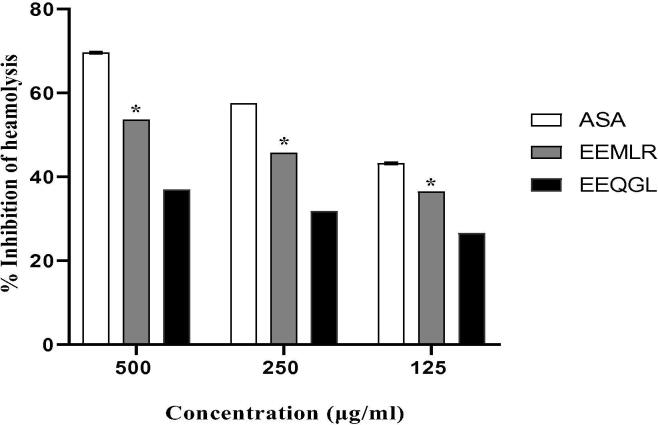
The EEMLR and EEQGL markedly preserved the erythrocyte membrane against denaturation stimulated by the hypotonic solution in the analysis of membrane stabilization function shown in Fig. 4. EEMLR uncovers RBC 's protection against the negative impact of a hypotonic solution at 500, 250 and 125 μg/ml were 53.6567 ± 0.002, 45.6965 ± 0.0029 and 36.492 ± 0.0025 compared with positive control 69.5771 ± 0.0050, 57.8855 ± 0.0219 and 43.2089 ± 0.006 at the same dosages. On the other hand, EEQGL showed 36.990 ± 0.0029, 31.8407 ± 0.0025% and 26.6169 ± 0.0007% inhibition of hypotonic solution-induced hemolysis at the previous corresponding dosages.
Fig. 4.
Membrane stabilizing activity of ethanol extract of M. longipes root and Q. gomeziana leaves. Values are expressed as mean ± SEM (n = 3); *p < 0.05 are statistically significant comparison with acetyl salicylic acid followed by Dunnett’s test. ASA = Acetyl salicylic acid; EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
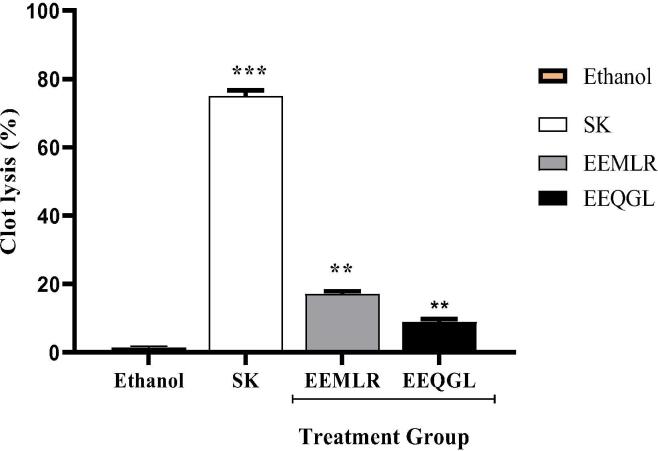
3.6. Thrombolytic activity
The percentage of the EEMLR and EEQGL thrombolysis, indicated by clot lysis, was illustrated in Fig. 5. The figure reported that when compared with negative control, EEMLR displayed a greater inhibition (17.0112 ± 0.8519%) and EEQGL few influence on inhibition (8.8902 ± 0.8519%).
Fig. 5.
Thrombolytic activity of ethanol extract of M. longipes root and Q. gomeziana leaves. Values are expressed as mean ± SEM (n = 3); ***p < 0.001 and **p < 0.01 are statistically significant comparison with control followed by Dunnett’s test. SK = Streptokinase; EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
3.7. Analgesic activity
The EEMLR (500 mg/kg b.w, p.o) and EEQGL (500 mg/kg b.w, p.o) were used to have a pronounced core analgesic effect as reflected by a dramatic improvement comparison with control (1% v/v Tween-80, 1 ml/100 g) in the Eddy's hot plate test represented in Table 3. EEQGL (500 mg/kg b.w, p.o) was reported to be 34.33 ± 0.967 s mean latency, statistically significant (p < 0.01) comparison with control while the mean latency of diclofenac (9 mg/kg b.w, i.p) was 37 ± 0.309 s. The results indicate considerable analgesic response to thermal stimulation.
Table 3.
Analgesic activity of ethanol extract of M. longipes root and Q. gomeziana leaves on Swiss Albino mice.
| Group | Treatment |
Mean Latent (Sec) |
|
|---|---|---|---|
| Initial | After 30 min | ||
| 1 | Control (1% v/v Tween-80, 1 ml/100 g) | 11.66 ± 0.333 | 11.66 ± 0.666 |
| 2 | EEMLR (500 mg/kg, b.w, p.o) | 19 ± 2.516 | 18.66 ± 0.881** |
| 3 | EEQGL (500 mg/kg, b.w, p.o) | 21.33 ± 2.848 | 34.33 ± 0.967*** |
| 4 | Diclofenac (9 mg/kg, i.p) | 16 ± 1 | 37 ± 0.309*** |
Values are expressed as mean ± SEM (n = 3); ***p < 0.001 and **p < 0.01 are statistically significant in comparison with control followed by Dunnett’s test. EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
3.8. Antipyretic activity
EEMLR and EEQGL at the dose of 500 mg/kg resulted in a crucial diminishment in the rectal temperature integrating with hyperthermia up to 3 h. The EEMLR lessened temperature in the 1st, 2nd, and 3rd hours from 102.03 °F to 96.93 °F, 96.96 °F, and 96.23 °F (p < 0.05) independently, and triggered the highest temperature decline in the 3rd-hour corresponding to Brewer’s yeast induced hyperpyrexia method and EEQGL lower temperature from 101.23° F to 97.33 °F, 96.80 °F and 97.13 °F (p < 0.05), respectively, in the 1st, 2nd and 3rd hours, causing higher temperature decline in the 2nd hour. Whereas, paracetamol recorded higher temperature depletion of 97.62 ± 0.34 in the 2nd hour. The results of the antipyretic assay are shown in Table 2.
Table 2.
Antipyretic activity of ethanol extract of M. longipes root and Q. gomeziana leaves on Swiss Albino mice.
| Groups | Oral dose |
Rectal temperature in °F at different hours |
|||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | ||
| Control (DW) | 10 ml/kg | 101.06 ± 0.33 | 100.9 ± 0.20 | 101.26 ± 0.12 | 101.33 ± 0.12 |
| P’tamol | 150 mg/kg | 101.16 ± 0.31 | 97.63 ± 0.29* | 97.62 ± 0.34* | 98.06 ± 0.103* |
| EEMLR | 500 mg/kg | 102.03 ± 0.73 | 96.93 ± 0.40* | 96.96 ± 0.08* | 96.23 ± 0.33* |
| EEQGL | 500 mg/kg | 101.23 ± 0.31 | 97.33 ± 0.31* | 96.80 ± 0.23* | 97.13 ± 0.37* |
Values are expressed as mean ± SEM (n = 3); P’tamol = Paracetamol; *p < 0.05 are significant in comparison with paracetamol followed by Dunnett’s test. P’tamol = Paracetamol; DW = Distilled water; EEMLR = Ethanol extract of M. longipes root; EEQGL = Ethanol extract of Q. gomeziana leaves.
4. Discussion
The phytochemical investigations carried out over the ethanol extract of M. longipes root and Q. gomeziana leaves demonstrated the existence of certain bioactive compounds. In this experiment, seven bioactive compounds were screened with these two extracts, out of which just five in both extracts notably alkaloids, steroids, flavonoids, saponins and gums and two in extract of EEMLR entitled glycosides and tannins were also present (Awoyinka et al., 2007). Alkaloids, tannins, flavonoids were even reported to have vasodilatation, antinociceptive, skin inflammation, antioxidant, antimicrobial, anticancer, antimutagenic activity (Breiterová et al., 2020, Panche et al., 2016, Sieniawska, 2015). The pharmacological experiment based on the phytochemical analysis was planned to determine the biological effect.
Antioxidants appear to mitigate and dissuade damage caused by free radical reactions because of their capacity to contribute radical-neutralizing electrons instead of exchanging radical. Ascorbic acid has a strong antioxidant effect, it is a principle for the ascorbate peroxidase redox enzyme, which is particularly essential for oxidative stress resistance (Rakib et al., 2020b). Not only EEMLR but also EEQGL have had a significant scavenging impact on the DPPH test consisting of IC50 values 6.467 and 6.839 μg/ml, a remarkable association with ascorbic acid due to existence of alkaloids, tannins and gums (Jahan et al., 2020).
The EEMLR and EEQGL showed mortality as in bioassay which implies the biological activity of the chemical substance in the extract. It was found that highly cytotoxic with LC50 values 3.49, 5.97 μg/ml of EEMLR and EEQGL in brine shrimp lethality bioassay suggest prevention of cancer cell proliferation. This result was exposed for the presence of alkaloids, flavonoid group (Howlader et al., 2012).
There are implicit drawback with the use of animals in testing pharmacological research, such as ethical concerns and the lack of justification for their use when other appropriate assay are available or could be reviewed. Hence, the current study is being proposed for anti-inflammatory property through protein denaturation bioassay of EEMLR and EEQGL. Besides, the present result exposed a dose-dependent inhibition of protein (albumin) denaturation by EEMLR and EEQGL, where positive result was noted at high dosages which may due to the presence flavonoids group (Emran et al., 2018, Issa et al., 2006). On the other hand, a numerous studies have shown flavonoids and several plant compounds have analgesic and anti-inflammatory issues as a result of their membrane-stabilizing capacity in various sampling methods (David, 2007, Jorge et al., 2004). EEMLR and EEQGL revealed powerful safeguard resulting from the lysis-induced erythrocyte on dose-dependent and more preventative response close to the standard acetyl salicylic acid due exist of alkaloids (Barbosa-Filho et al., 2006).
There are numerous agents including tissue plasminogen activator (t-PA), streptokinase (SK), urokinase (UK) etc. globally to treat atherothrombotic diseases (Ansari et al., 2012). Analgesics can behave on the central or peripheral nervous system which act by blocking the pathway of impulses at the receiver active site such as COX1 (cyclooxygenase-1) and COX2 (cyclooxygenase-2), while central analgesics not only elevate the threshold for pain but also adjust the physiological response to pain and inhibit depression (Shreedhara et al., 2009). In recent study, mean latency from diclofenac sodium was revealed to be 37 s through Eddy’s hot plate method whereas EEMLR confirmed mean latency of 34.33 s at a concentration of 500 mg/kg, statistically significance (p < 0.05). However, most of the antipyretic drugs act on COX2 impairing the release of PGE2 (prostaglandin E2) from hypothalamus, blocking the neuron-to-neuron inflammation transaction, and boosting anti-inflammatory impulses at the site of action (Dutta et al., 2020, Eldahshan and Abdel-Daim, 2015). The result showed that EEMLR and EEQGL had significant antipyretic impacts in yeast-provoked elevated body temperature following Brewer's yeast-induced pyrexia method and implications for EEQGL in 3 hrs of 97.13 ± 0.37 while EEMLR exercised 96.23 ± 0.33 comparable to paracetamol. The presence of steroids, alkaloids and flavonoids in EEMLR and EEQGL may be a possible reason for this analgesic and antipyretic activity (Hasanat et al., 2019, Rahman et al., 2020).
5. Conclusions
The present study validates that ethanol extracts of M. longipes and Q. gomeziana have great value as a pharmacological source. Potent cytotoxic activity against Brine shrimp was detected, and a maximum percentage of DPPH inhibition was measured. The EEMLR represents the significant antipyretic effect by Brewer's yeast-induced pyrexia method, thrombolytic by clot lysis method, and membrane-stabilizing behaviors against lysis-induced erythrocyte. On the contrary, EEQGL represents the exemplified comparable analgesic responses in Eddy's hot plate test and anti-inflammatory protein denaturation bioassay effect. Bioactive effects may be due to phytochemical groups such as alkaloids, flavonoids, tannins, gums, saponins, steroids, and glycosides. Further evaluations are meant to validate these study hypotheses and to clarify the real purpose of these findings.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to express their gratitude to the Department of Pharmacy, Southern University Bangladesh, 739/A Mehedibag Road, Chittagong-4000, and Department of Pharmacy, International Islamic University Chittagong, Chittagong-4318, Bangladesh for providing their facilities.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical consideration
The study protocol was approved by the Department of Pharmacy, Southern University Bangladesh, Chittagong-4000, Bangladesh (consent approval number: SUB/P&D/0022-2019).
Author’s contributions
BG, MA and MNI employed conceptualization, planning, designed methodology, data analysis, software, manuscript writing, SMT, MMR, SAS, AMT and RM designing, manuscript writing, SMT, MNI, TBE and AMA monitoring, visualization, supervision, MNI, TBE and AMA together with writing—review & editing, correspondence. All authors read, agreed and approved the published version of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammad Nazmul Islam, Email: nazmul@iiuc.ac.bd.
Talha Bin Emran, Email: talhabmb@bgctub.ac.bd.
Ali M. Alqahtani, Email: amsfr@kku.edu.sa.
References
- Al Mahmud Z., Emran T.B., Qais N., Bachar S.C., Sarker M., Uddin M.M.N. Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb) J. Basic Clin. Physiol. Pharmacol. 2016;27(1):63–70. doi: 10.1515/jbcpp-2015-0056. [DOI] [PubMed] [Google Scholar]
- Ansari A.V., Siddiqui H.H., Singh S.P. Antithrombotic and thrombolytic activity of Terminalia belerica fruit extracts. Res. J. Pharm. Biol. Chem. Sci. 2012;3(2):471. [Google Scholar]
- Awoyinka O.A., Balogun I.O., Ogunnowo A.A. Phytochemical screening and in vitro bioactivity of Cnidoscolus aconitifolius (Euphorbiaceae) J. Med. Plant Res. 2007;1(3):63–65. [Google Scholar]
- Aziz M.A. Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J. Integr. Med. 2015;13(3):173–184. doi: 10.1016/S2095-4964(15)60179-0. [DOI] [PubMed] [Google Scholar]
- Barbosa-Filho J.M., Piuvezam M.R., Moura M.D., Silva M.S., Lima K.V.B., da-Cunha E.V.L., Takemura O.S. Anti-inflammatory activity of alkaloids: A twenty-century review. Revista Brasileira de Farmacognosia. 2006;16(1):109–139. [Google Scholar]
- Barua N., Aziz M.A.I., Tareq A.M., Sayeed M.A., Alam N., ul Alam N., Emran T.B. In vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.) Biochem. Biophy. Rep. 2020;23 doi: 10.1016/j.bbrep.2020.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berahou A., Auhmani A., Fdil N., Benharref A., Jana M., Gadhi C.A. Antibacterial activity of Quercus ilex bark's extracts. J. Ethnopharmacol. 2007;112(3):426–429. doi: 10.1016/j.jep.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Biswas N.N., Saha S., Ali M.K. Antioxidant, antimicrobial, cytotoxic and analgesic activities of ethanolic extract of Mentha arvensis L. Asian Pac. J. Trop. Biomed. 2014;4(10):792–797. doi: 10.12980/APJTB.4.2014C1298. [DOI] [Google Scholar]
- Breiterová K., Koutová D., Maříková J., Havelek R., Kuneš J., Majorošová M., Řezáčová M. Amaryllidaceae alkaloids of different structural types from Narcissus L. cv. Professor Einstein and their cytotoxic activity. Plants. 2020;9(2):137. doi: 10.3390/plants9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristy T.A., Barua N., Montakim Tareq A., Sakib S.A., Etu S.T., Chowdhury K.H., Reza A. Deciphering the pharmacological properties of methanol extract of Psychotria calocarpa leaves by in vivo, in vitro and in silico approaches. Pharmaceuticals. 2020;13(8):183. doi: 10.3390/ph13080183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann R.W., Sharon D. Traditional medicinal plant use in Northern Peru: tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2006;2 doi: 10.1186/1746-4269-2-47. 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay R., Bhattacharyya S. Phcog Rev.: Plant review Terminalia chebula: An update. Pharmacog. Rev. 2007;1(1):151–156. [Google Scholar]
- Chavan M., Wakte P., Shinde D. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomed. 2010;17(2):149–151. doi: 10.1016/j.phymed.2009.05.016. [DOI] [PubMed] [Google Scholar]
- David S. Studies force new view on biology of flavonoids. Biol. Med. 2007;541:737–787. [Google Scholar]
- Deng M., Hipp A., Song Y.-G., Li Q.-S., Coombes A., Cotton A. Leaf epidermal features of Quercus subgenus Cyclobalanopsis (Fagaceae) and their systematic significance. Bot. J. Linnean Society. 2014;176(2):224–259. doi: 10.1111/boj.12207. [DOI] [Google Scholar]
- Dutta T., Paul A., Majumder M., Sultan R.A., Emran T.B. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem. Biophy. Rep. 2020;21 doi: 10.1016/j.bbrep.2019.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ediriweera M.K., Tennekoon K.H., Samarakoon S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (mango) Evidence-Based Complement. Altern. Med. 2017:2017. doi: 10.1155/2017/6949835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldahshan O.A., Abdel-Daim M.M. Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnos nux-vomica. Cytotechnol. 2015;67(5):831–844. doi: 10.1007/s10616-014-9723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emon N.U., Kaiser M., Islam M., Kabir M.F.I., Jamir M., Uddin M.A.J., Islam M.N. Anxiolytic and thrombolytic investigation of methanol extract of Piper nigrum L. fruits and Sesamum indicum L. seeds. J. Adv. Biotechnol. Exp. Ther. 2020;3(3):158–164. [Google Scholar]
- Emran T.B., Ahmed S., Zahan S., Rakib A., Hasan M.S., Amin M.N., Uddin M.M.N. Sedative, anxiolytic, antinociceptive, anti-inflammatory and antipyretic effects of a chloroform extract from the leaves of Urena sinuata in rodents. J. Appl. Life Sci. Int. 2018:1–19. [Google Scholar]
- Hasanat A., Kabir M.S.H., Ansari M.A., Chowdhury T.A., Hossain M.M., Islam M.N., Kamal A. Ficus cunia Buch.-Ham. ex Roxb. (leaves): An experimental evaluation of the cytotoxicity, thrombolytic, analgesic and neuropharmacological activities of its methanol extract. J. Basic Clin. Physiol. Pharmacol. 2019;30(4) doi: 10.1515/jbcpp-2016-0140. [DOI] [PubMed] [Google Scholar]
- Hayati I., Mahatma R., Suzanti F. Phylogenetic study of mangifera from sumatra, Indonesia using nuclear and chloroplast DNA sequences. Sabrao J. Breeding Genetics. 2018;50(3) [Google Scholar]
- Hossain M.S., Akter S., Das A., Sarwar M.S. CNS depressant, antidiarrheal and antipyretic activities of ethanolic leaf extract of Phyllanthus acidus L. on Swiss Albino Mice. J. Pharm. Res. Int. 2016;10(5):1–9. [Google Scholar]
- Howlader M.S.I., Sayeed M.S.B., Ahmed M.U., Mohiuddin A.K., Labu Z.K., Bellah S.F., Islam M.S. Characterization of chemical groups and study of antioxidant, antidiarrhoeal, antimicrobial and cytotoxic activities of ethanolic extract of Diospyros blancoi (Family: Ebenaceae) leaves. J. Pharm. Res. 2012;5(6):3050–3052. [Google Scholar]
- Hridy A.U., Shabnaz S., Asaduzzaman M., Shahriar M., Bhuiyan M.A., Islam M.S., Emran T.B. Genetic variations of RAD51 and XRCC2 genes increase the risk of colorectal cancer in Bangladeshi population. Asian Pac. J. Cancer Prev. 2020;21(5):1445–1451. doi: 10.31557/APJCP.2020.21.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.N., Tasnim H., Arshad L., Haque M.A., Tareq S.M., Kamal A.M., Tareq A.M. Stem extract of Albizia richardiana exhibits potent antioxidant, cytotoxic, antimicrobial, anti-inflammatory and thrombolytic effects through in vitro approach. Clin. Phytosci. 2020;6(1):1–9. [Google Scholar]
- Islam S.M.A., Ahmed K.T., Manik M.K., Wahid M.A., Kamal C.S.I. A comparative study of the antioxidant, antimicrobial, cytotoxic and thrombolytic potential of the fruits and leaves of Spondias dulcis. Asian Pac. J. Trop. Biomed. 2013;3(9):682–691. doi: 10.1016/S2221-1691(13)60139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa A.Y., Volate S.R., Wargovich M.J. The role of phytochemicals in inhibition of cancer and inflammation: New directions and perspectives. J. Food Compost. Anal. 2006;19(5):405–419. doi: 10.1016/j.jfca.2006.02.009. [DOI] [Google Scholar]
- Jahan I., Tona M.R., Sharmin S., Sayeed M.A., Tania F.Z., Paul A., Emran T.B. GC-MS phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules. 2020;25(15):3536. doi: 10.3390/molecules25153536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge R.M., Leite J.P.V., Oliveira A.B., Tagliati C.A. Evaluation of antinociceptive, anti-inflammatory and antiulcerogenic activities of Maytenus ilicifolia. J. Ethnopharmacol. 2004;94(1):93–100. doi: 10.1016/j.jep.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Joshi A.K., Juyal D. Traditional and ethnobotanical uses of Quercus leucotrichophora a. Camus (Quercus oblongata D. Don) in Kumaun and Garhwal regions of Uttarakhand, India: a review. Int. J. Herb. Med. 2017;5:06–08. [Google Scholar]
- Kabir M.S.H., Hossain M.M., Kabir M.I., Rahman M.M., Hasanat A., Emran T.B., Rahman M.A. Phytochemical screening, antioxidant, thrombolytic, alpha-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. J. Young Pharm. 2016;8(4):391. [Google Scholar]
- Kawamura F., Ramle S.F.M., Sulaiman O., Hashim R., Ohara S. Antioxidant and antifungal activities of extracts from 15 selected hardwood species of Malaysian timber. Eur. J. Wood Wood Prod. 2011;69(2):207–212. doi: 10.1007/s00107-010-0413-2. [DOI] [Google Scholar]
- Khan A.S., Ali S., Khan I.A. Morphological and molecular characterization and evaluation of mango germplasm: An overview. Scientia Horticulturae. 2015;194:353–366. [Google Scholar]
- Khanam Z., Wen C.S., Bhat I.U.H. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali) J. King Saud Univ. Sci. 2015;27(1):23–30. doi: 10.1016/j.jksus.2014.04.006. [DOI] [Google Scholar]
- Khattak A. University of Peshawar; Peshawar: 2018. Phytochemical Evaluation, Bioassay Screening and Aerial Plant-mediated Silver nanoparticles synthesis using Quercus semecarpifolia Smith. [Google Scholar]
- Khennouf S., Amira S., Arrar L., Baghiani A. Effect of some phenolic compounds and quercus tannins on lipid peroxidation. World Appl. Sci. J. 2010;8(9):1144–1149. [Google Scholar]
- Kumar S., Bajwa B.S., Kuldeep S., Kalia A.N. Anti-inflammatory activity of herbal plants: a review. Int. J. Adv. Pharm. Biol. Chem. 2013;2(2):272–281. [Google Scholar]
- Makare N., Bodhankar S., Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J. Ethnopharmacol. 2001;78(2–3):133–137. doi: 10.1016/s0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Matthäus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002;50(12):3444–3452. doi: 10.1021/jf011440s. [DOI] [PubMed] [Google Scholar]
- Okwu D.E., Uchenna N.F. Exotic multifaceted medicinal plants of drugs and pharmaceutical industries. Afr. J. Biotechnol. 2009;8(25) [Google Scholar]
- Paaver U., Matto V., Raal A. Total tannin content in distinct Quercus robur L. galls. J. Med. Plants Res. 2010;4(8):702–705. [Google Scholar]
- Panche A., Diwan A., Chandra S. Flavonoids: an overview. J. Nnutr. Sci. 2016;5 doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisutthanan S., Plianbangchang P., Pisutthanan N., Ruanruay S., Muanrit O. Brine shrimp lethality activity of Thai medicinal plants in the family Meliaceae. Naresuan Uni. J.: Sci. Technol. (NUJST) 2013;12(2):13–18. [Google Scholar]
- Prasad S., Kashyap R.S., Deopujari J.Y., Purohit H.J., Taori G.M., Daginawala H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006;4:14. doi: 10.1186/1477-9560-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashanth D., Amit A., Samiulla D., Asha M., Padmaja R. α-Glucosidase inhibitory activity of Mangifera indica bark. Fitoterapia. 2001;72(6):686–688. doi: 10.1016/s0367-326x(01)00293-3. [DOI] [PubMed] [Google Scholar]
- Rahman J., Tareq A.M., Hossain M., Sakib S.A., Islam M.N., Ali M., Emran T.B. Biological evaluation, DFT calculations and molecular docking studies on the antidepressant and cytotoxicity activities of Cycas pectinata Buch.-Ham Compounds. Pharmaceuticals. 2020;13(9):232. doi: 10.3390/ph13090232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.A., bin Imran T., Islam S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J. Biol. Sci. 2013;20(3):213–225. doi: 10.1016/j.sjbs.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.A., Sultana R., Emran T.B., Islam M.S., Rahman M.A., Chakma J.S., Hasan C.M.M. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement. Altern. Med. 2013;13(1):25. doi: 10.1186/1472-6882-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakib A., Ahmed S., Islam M.A., Uddin M.M.N., Paul A., Chy M.N.U., Seidel V. Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phytother. Res. 2020 doi: 10.1002/ptr.6725. [DOI] [PubMed] [Google Scholar]
- Rakib A., Sami S.A., Mimi N.J., Chowdhury M.M., Eva T.A., Nainu F., Emon N.U. Immunoinformatics-guided design of an epitope-based vaccine against severe acute respiratory syndrome coronavirus 2 spike glycoprotein. Comput. Biol. Med. 2020:103967. doi: 10.1016/j.compbiomed.2020.103967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwane A., Lazrek H.B., Bouallam S., Markouk M., Amarouch H., Jana M. Larvicidal activity of extracts from Quercus lusitania var. infectoria galls (Oliv.) J. Ethnopharmacol. 2002;79(2):261–263. doi: 10.1016/S0378-8741(01)00390-7. [DOI] [PubMed] [Google Scholar]
- Romero-Benavides J.C., Ortega-Torres G.C., Villacis J., Vivanco-Jaramillo S.L., Galarza-Urgilés K.I., Bailon-Moscoso N. Phytochemical Study and Evaluation of the cytotoxic properties of methanolic extract from Baccharis obtusifolia. Int. J. Med. Chem. 2018;2018 doi: 10.1155/2018/8908435. 8908435–8908435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabiu S., Ashafa A.O.T. Membrane stabilization and kinetics of carbohydrate metabolizing enzymes (α-amylase and α-glucosidase) inhibitory potentials of Eucalyptus obliqua L.Her. (Myrtaceae) Blakely ethanolic leaf extract: An in vitro assessment. S. Afr. J. Bot. 2016;105:264–269. doi: 10.1016/j.sajb.2016.04.007. [DOI] [Google Scholar]
- Sani I., Abdulhamid A., Bello F. Eucalyptus camaldulensis: Phytochemical composition of ethanolic and aqueous extracts of the leaves, stem-bark, root, fruits and seeds. Int. J. Sci. Innov. Res. 2014;3(5):523–526. [Google Scholar]
- Shah M., Parveen Z., Khan M.R. Evaluation of antioxidant, anti-inflammatory, analgesic and antipyretic activities of the stem bark of Sapindus mukorossi. BMC Complement. Altern. Med. 2017;17(1):526. doi: 10.1186/s12906-017-2042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam S., Murugaiyan I., dos Santos Lima B., Serafini M.R., de Souza Araújo A.A., Narain N., Thangaraj P. HPLC–DAD–MS identification of polyphenols from Passiflora leschenaultii and determination of their antioxidant, analgesic, anti-inflammatory and antipyretic properties. Arab. J. Chem. 2019;12(6):760–771. doi: 10.1016/j.arabjc.2016.02.008. [DOI] [Google Scholar]
- Shifah F., Tareq A.M., Sayeed M.A., Islam M.N., Emran T.B., Ullah M.A., Ullah A. Antidiarrheal, cytotoxic and thrombolytic activities of methanolic extract of Hedychium coccineum leaves. J. Adv. Biotechnol. Exp. Ther. 2020;3(1):77–83. [Google Scholar]
- Shreedhara C.S., Vaidya V.P., Vagdevi H.M., Latha K.P., Muralikrishna K.S., Krupanidhi A.M. Screening of Bauhinia purpurea Linn. for analgesic and anti-inflammatory activities. Indian J. Pharmacol. 2009;41(2):75–79. doi: 10.4103/0253-7613.51345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieniawska E. Activities of tannins – from in vitro studies to clinical trials. Nat. Product Commun. 2015;10(11) doi: 10.1177/1934578x1501001118. 1934578X1501001118. [DOI] [PubMed] [Google Scholar]
- Tadić V.M., Dobrić S., Marković G.M., Dordević S.M., Arsić I.A., Menković N.R., Stević T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem. 2008;56(17):7700–7709. doi: 10.1021/jf801668c. [DOI] [PubMed] [Google Scholar]
- Taib M., Rezzak Y., Bouyazza L., Lyoussi B. Medicinal uses, phytochemistry, and pharmacological activities of Quercus Species. Evidence-Based Complement. Altern. Med. 2020;2020:1920683. doi: 10.1155/2020/1920683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareq A.M., Farhad S., Uddin A.N., Hoque M., Nasrin M.S., Uddin M.M.R., Lyzu C. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon. 2020;6(6) doi: 10.1016/j.heliyon.2020.e04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.M.N., Dash R., Kabir M.S.H., Rahman M.M., Islam M.N., Paul A. Cytotoxic, antibacterial and analgesic activities of Rhaphidophora glauca (Wall.) Schott leaves. J. Coast. Life Med. 2016;4(9):750–756. [Google Scholar]
- Ullah H.M.A., Zaman S., Juhara F., Akter L., Tareq S.M., Masum E.H., Bhattacharjee R. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement. Altern. Med. 2014;14(1):346. doi: 10.1186/1472-6882-14-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesmin S., Paul A., Naz T., Rahman A.A., Akhter S.F., Wahed M.I.I., Siddiqui S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba) Clin. Phytosci. 2020;6(1):1–10. [Google Scholar]