Abstract
The isolation and identification of beneficial bacteria from the active phase of composting is considered to be a key bio-quality parameter for the assessment of the process. The aim of this work was the selection and identification of beneficial bacteria from a co-composting experiment of vegetable waste (VW), olive oil mill waste (O2MW), and phosphate sludge (PS). Phosphate-solubilizing strains were isolated from the thermophilic phase using Pikovskaya (PVK) solid medium supplemented with tricalcium phosphate Ca3(PO4) (TCP) as the sole source of phosphorus (P). Therefore, the selected isolate Alcaligenes aquatilis GTE53 was tested to tolerate abiotic stresses (different levels of temperature, variable pH, high salinity and water stress). The isolate was also assessed for indole acetic acid (IAA) and siderophores synthesis, nitrogen fixation, phenol degradation and pathogens inactivation. The quality of the co-composting process was also investigated by monitoring the physico-chemical parameters. The obtained results showed that A. aquatilis GTE53 displayed a higher solubilization index of 2.4 and was efficiently dissolved, up to 162.8 and 247.4 mg·mL−1 of inorganic phosphate from PS and phosphate rock (PR), respectively. A. aquatilis GTE53 exhibited siderophores and IAA release, along with atmospheric nitrogen fixation. In addition to that, A. aquatilis GTE53 showed a high resistance to heat and tolerance to acidic and alkaline pH, high salinity and water stress. Moreover, A. aquatilis GTE53 could degrade 99.2% of phenol from a high-concentrated medium (1100 mg·L−1 of phenol) and can inactivate the most abundant pathogens in industrial wastes: Escherichia coli, Streptococcus sp., Salmonella sp., and Fusarium oxysporum albedinis. Analysis of temperature, pH, electrical conductivity, carbon/nitrogen (C/N) ratio, indicated successful co-composting. An efficient transformation of P to the available form and a great abatement of polyphenols, were also recorded during the process. The findings of this study will help to advance the understanding of A. aquatilis GTE53 functions and will facilitate its application to promote beneficial microbial organisms during composting, thus obtaining a high-quality product.
Keywords: Alcaligenes aquatilis, Phosphate solubilisation, Phenol degradation, Bioremediation, Pathogens
1. Introduction
Recently, the high demand for food from the growing population has implied that agro-food industries have multiplied their production capacity, resulting in an increase in the consumption of synthetic fertilisers. In Morocco, the phosphate operator Office Chérifien des Phosphates (OCP) developed its industry by building a slurry pipeline, which revolutionised the transport of phosphate ore from extraction and enrichment sites to the factories (Geissler et al., 2018). This strategy tripled the production of phosphate ore and its derivatives (i.e chemical fertilisers) in 2018, according to estimates by Pufahl Peir and Lee (2017), and has led to increased amounts of by-products such as PS. In fact, PS production is estimated to be 28.1 million tons per year (Loutou et al., 2013). The elimination of PS by storage around phosphate laundries represents a major economic and environmental issue, disfiguring landscapes and reducing arable land. However, PS contains a significant amount of phosphate in the form of carbonate fluorapatite Ca5(PO4)3F, which is a hard and insoluble crystalline solid (Takahashi and Anwar, 2007, Chang and Yang, 2009, Haouas et al., 2020). This low-grade material is difficult to use as fertiliser, and its chemical processing is not considered economically viable (Haouas et al., 2020).
Up to now, rare studies have been performed on the co-composting of PS with other organic resources, although this would be a suitable process to transform these materials into a valuable product with stable organic matter (OM) and no harmful effects (Cerda et al., 2018). During the composting process, numerous microbiological processes take place, leading to OM degradation and causing quantitative and qualitative changes (Ayilara et al., 2020). Assessing microbial species is among the key tasks for understanding the composting mechanism. Nowadays, not the number, but the efficiency of the bacterial strains is considered important; suitable strains can perform multiple functions and can tolerate processing stressors during the composting of industrial wastes including adverse temperature, texture, humidity, pH levels, and organic pollutants (Geng et al., 2006, Vargas-García et al., 2010, Stella and Sashikala, 2016). Only a few studies have concentrated on the identification of bacterial strains that are multifunctional, capable to survive under harsh environments, and involved in producing high quality compost (Egelkamp et al., 2017, Saffari et al., 2017, Kutu et al., 2019, Lutz et al., 2020).
In the present work, a thermophilic bacterium A. aquatilis GTE53 with a broad range of beneficial effects was isolated from a successful co-composting of PS and organic residues (O2MW and VW). Alcaligenes aquatilis is a rare bacterium, which was found for the first time in an aquatic environment (Van Trappen et al., 2005), has been able to live and grow in overly stressful environments such as marine ecosystems. Furthermore, owing to its ability to use aromatic hydrocarbons as a source of carbon and nitrogen (Durán et al., 2019a), this bacterium was able to live in urban areas contaminated by petrol and its derivatives.
To the best of our knowledge, one report evaluated the biotechnological traits of Alcaligenes aquatilis, which could dissolve inorganic phosphate contributing to the enhancement of maize plant growth (Pande et al., 2017). Thus, no previous study of Alcaligenes aquatilis isolated from composting plant. The objective of this study was to remove the knowledge gap about the beneficial functions of Alcaligenes aquatilis for probable use in biotechnological purposes. For this reason, A. aquatilis GTE53 was assayed for the production of substances that promote plant growth, tolerance to environmental stressors, phenol degradation, and antagonism potential toward pathogenic microorganisms.
2. Materials and methods
2.1. Feeding materials
PS was sampled from phosphate washing plants operated by OCP, Khouribga City (Morocco). O2MW is divided into two fractions, which are the olive oil mill pomace (O2MP) and olive oil mill wastewater (O2MW2) and were provided by olive oil mills located in Beni Mellal’s region (Morocco). VW was collected from the university campus of Beni Mellal and the wheat straw was collected from a local farm. Table 1 represents the physico-chemical characteristics and the elemental composition of main composted wastes (O2MW, VW, and PS).
Table 1.
Physico-chemical characteristics and elemental composition of composted materials.
| Characteristics | PS | VW | O2MP | O2MW2 |
|---|---|---|---|---|
| Humidity% | 2.1 ± 0.1 | 87.1 ± 3 | 7.4 ± 0.12 | 94.3 ± 0.1 |
| pH | 8.2 ± 0.3 | 4.41 ± 0.09 | 5.75 ± 0.1 | 4.85 ± 0.02 |
| EC (mS·cm−1) | 0.8 ± 0.03 | 8.72 ± 0.07 | 12.9 ± 0.2 | 12 ± 0.1 |
| TOC% | 0.38 ± 0.05 | 60.67 ± 2.3 | 25.1 ± 0.1 | 68.3 ± 1.2 |
| TNK% | 0.07 ± 0.01 | 1.176 ± 0.1 | 1.02 ± 0.03 | 2.16 ± 0.01 |
| Al-P (mg·g−1) | 0.75 ± 0.03 | 0.64 ± 0.04 | 0.036 ± 0.003 | 0.022 ± 0.001 |
| Ca-P (mg·g−1) | 82.60 ± 2.1 | 1.86 ± 0.21 | 0.170 ± 0.01 | 0.134 ± 0.024 |
| Fe-P (mg·g−1) | 0.07 ± 0.001 | 0.47 ± 0.05 | 0.076 ± 0.007 | 0.187 ± 0.0028 |
| Organic P (mg·g−1) | 0.98 ± 0.21 | 2.45 ± 0.7 | 2.2 ± 0.61 | 3.11 ± 0.44 |
| P Olsen (mg·g−1) | 0.07 ± 0.001 | 0.66 ± 0.01 | 0.204 ± 0.004 | 0.577 ± 0.002 |
| SiO2% | 12.21 | 0.14 | 0.33 | 0.42 |
| TiO2% | 0.23 | 0.04 | 0.05 | 0.03 |
| Al2O3% | 2.98 | 0.29 | 0.13 | 0.22 |
| Fe2O3% | 1.18 | 0.314 | 0.18 | 0.07 |
| MgO% | 2.33 | 0.987 | 0.56 | 0.1 |
| CaO% | 39.72 | 10.55 | 3.25 | 1.4 |
| Na2O% | 0.66 | 1.23 | 2.41 | 3.03 |
| K2O% | 0.41 | 8.45 | 6.87 | 10.52 |
| P2O5% | 20.01 | 0.55 | 0.73 | 0.69 |
2.2. Composting
The dimensions of the compost pile were 1.5 × 1.2 × 6 m (width × height × length). Compost was prepared by using 200 kg of O2MP, 70 L of O2MW2, 500 kg of VW and 180 kg of PS pulverised over the pile. In addition, 30 kg of wheat straw was supplemented to the composting pile to enhance the aeration. The optimization of the doses and volumes of waste in this experiment is related to the level of humidity and the OM content of each waste in order to ensure optimum conditions for the aerobic decomposition. During 150 days of the process, a manual turning every 7 days was made for aeration and homogenisation of the composting mixture. The temperature was measured daily and the humidity was maintained by addition of O2MW2 in the first week and then by water for the remaining processing time.
2.3. Physico-chemical and elementary analysis
To measure the humidity content, the sample was oven-dried at 105 °C for 24 h. Levels of pH and EC were determined in a mixture of the dried sample and the distilled water with a ratio of 1:10, according to the French standard (AFNOR, 1975). In addition, total organic carbon (TOC) and total nitrogen kjeldahl (TNK) were measured following the method reported by Tallou et al., 2020a; the ash content was determined after calcining the sample at 550 °C for 6 h, and the decomposition rate was calculated following Eq. (1) (Atif et al., 2020).
| (1) |
Ai: ash content in the sample at the initial phase of composting; Af: ash content in the sample at the corresponding phase of composting.
Polyphenol was measured in the compost sample using Folin-Ciocalteu reagent as described by Vazquez-Roncero et al. (1974). The speciation of the major forms of inorganic phosphate: calcium phosphate (Ca-P), aluminum phosphate (Al-P), and iron phosphate (Fe-P), was conducted according to the Chang and Jackson process (1957). Bray and Kurtz (1945) method was used to measure organic phosphate. While, the available phosphate was determined following the Olsen procedure (Du et al., 2013).
For detection of major elements, the Energy dispersive X-ray fluorescence (EDXRF) Epsilon 4 (Malvern Panalytical, Almelo, The Netherlands) was used.
2.4. Screening, isolation, and characterisation of the bacterial strain
The screening has been conducted using the following procedure: 1 g of the compost sample taken at the thermophilic phase was dispersed in 900 μl of sterilised distilled water, from which several dilutions was carried out. Subsequently, a 100 μl solution aliquot of 10−5, 10−6, and 10−7 was plated on PVK agar medium (pH 7) containing TCP as the only source of P (Yu et al., 2019). After 7 days of incubation at 28 ± 2 °C, colonies with a clear halo were marked positive for phosphate solubilisation (Wei et al., 2018, Yu et al., 2019). Therefore, the colonies of the isolated strains were purified 2–3 times on the PVK agar solid medium until pure colonies were obtained. The selected isolate GTE53 showed a large halo zone than the other isolates in the PVK agar medium and its solubilization index (SI) was determined via Eq. (2) (Premono et al., 1996):
| (2) |
Dc is the colony diameter; Dh is the halo zone diameter.
Phenotypic characterisation of the isolate was performed using the method reported by Benjelloun et al. (2019). Except for water stress tolerance, all tests were realised on agar medium of Yeast Extract Mannitol (YEM) inoculated with bacterial preculture and then incubated at 28 °C for 7 days. For the tolerance to temperature variations, the inoculated strain was incubated at 28, 45, 50, 55, and 60 °C. For pH tolerance, the agar plates were adjusted at the different values of 3, 5, 6, 7, 8, 9 and 10 using NaOH and HCl. While, for NaCl tolerance, the media were prepared using increasing concentrations of NaCl of 1, 2, 3, 4, 5, 6, 7, and 8%. Water stress was performed at concentrations of polyethylene glycol 6000 (PEG 6000) of 5, 10, 15, 20, and 25% in YEM broth. In addition, the Gram staining method and the observation of cell morphology were carried out as described in the Bergey Manual on determining bacteriology (1923).
2.5. Phenol biodegradation
A 5 mL aliquot of freshly grown inoculum of the strain (DO600 nm = 0,67) was transferred into a flask containing 100 mL of the minimal salt medium (MSM) (Yang et al., 2020). Phenol as the sole carbon source was added to the medium for the preparation of 200; 400; 800; 1100; 1400; 1700; 2000 and 2500 mg·L−1. After 48 h of incubation at 28 °C, cell growth and phenol removal percentage (Eq. (3)) were measured (Li et al., 2019).
| (3) |
Ci: initial concentration of phenol; Cr: remaining concentration of phenol after incubation period.
2.6. Inhibitory activity against pathogens
Anti-pathogenic effect of the strain GTE53 was investigated using the method of agar diffusion, where the isolate was spotted on the surface of Luria-Bertani agar (LB) plates previously sprayed with a broth culture of one of the pathogenic standard test organisms: Escherichia coli CCMM B4, Streptococcus sp. CCMM B24, and Salmonella sp. CCMM B7. Indeed, the effect was determined by measuring the diameter of the halo zone of inhibition surrounding the test strain (GTE53) (Zahir et al., 2013). As well as, the inhibitory effect of the isolate GTE53 on the mycelial growth of Fusarium oxysporum albedinis (Foa A27) was evaluated after co-culturing using the Potato Dextrose Agar plate (PDA) method. The percentage of inhibition was calculated over control by using the Eq. (4) (El Hassni et al., 2007).
| (4) |
C0 is the colony diameter in the control; Ct is the colony diameter in the treatment.
All pathogen bacteria and the fungi have been provided by the Moroccan Coordinated Collections of Microorganisms (CCMM).
2.7. Plant growth promotion factors
Siderophores release was tested on an agar plate, prepared using Chrome Azurol S (CAS). The isolate was inoculated by spot method on the CAS plate and incubated at 28 °C for 48 h. The apparition of a yellow halo around the colony marked as positive for siderophores production (Louden et al., 2011). Therefore, the production of IAA was determined according to Bric et al. (1991) using LB medium containing 0.5 g·L−1 of L-tryptophan and inoculated at 28 °C until bacterial colony reached a diameter of 1–2 mm. The colony was incubated after being covered with filter paper saturated with Salkowski reagent; the formation of red halos indicates IAA production. Nitrogen fixation was determined by the observation of bacterial strain growth in Burk’s solid N-free medium (Castellano Hinojosa and Bedmar, 2017).
The GTE53′s efficacy in phosphate solubilization has been measured in liquid culture supplemented with PS and Moroccan PR. Firstly, a 5% bacterial size inoculum with an OD600 nm of 0.5 (v:v) was inoculated into 100 mL of PVK medium containing 0.5 g of PS and another containing PR. For 6 days, the isolate was incubated on a shaking incubator of 150 rpm at 28 °C and 1 mL of the supernatant was sampled on days 2, 4, and 6. In the supernatant of liquid PVK, soluble phosphate was determined using the blue molybdenum method and pH values were also registered at the same time intervals (Paul and Sinha, 2017, Yu et al., 2019).
2.8. 16S rRNA gene sequencing and analysis
2.8.1. Extraction of total DNA
Total DNA of the bacterial isolate was obtained by phenol-chloroform extraction from exponentially growing bacterial culture (Chen and Kuo, 1993). The cells were centrifuged at 13,000 rpm for 5 min, and then suspended in the lysis buffer. Then, 100 µl of 5 M NaCl was added to the solution and the tubes were centrifuged at 13,000 rpm for 10 min at 4 °C. After that, equal volume of phenol-chloroform: isoamyl alcohol (24: 1, v/v) was added to the solution supernatant. The DNA was precipitated in absolute ethanol and washed twice with 70% ethanol, then dried and redissolved in pure sterile water. The concentration and purity of the DNA were evaluated using a NanoDropTM spectrophotometer. The DNA was diluted to a standard concentration of 100 ng·µl−1, and was stored at −20 °C for later use, such as for amplification of the PCR template.
2.8.2. Amplification of 16S rRNA
Almost 1500 bp of the 16S rRNA gene were amplified using the two universal primers rD1/fD1, the sequences of which are (5′AAGGAGGTGATCCAGCCGCA3 ')/(5′GGAGAGTTAGATCTTGGCTC3′) (Weisburg et al., 1991). In a reaction volume of 25 μl, the mixture containing: 1 μl of DNA with a concentration of 100 ng·μl−1, 1 μl of each primer at 10 μM, 9.5 μl of pure sterile water and 12.5 μl of MytTaqTM Mix, 2x (BIOLINE) ready to use, containing Taq polymerase enzyme, reaction buffer, MgCl2 and dNTPs. The thermal cycles were used under the optimal conditions supplied with the MytTaqTM Mix.
The amplification of the DNA fragments was verified by horizontal electrophoresis on 1% agarose gel containing 0.5 μg·mL−1 of ethidium bromide (BET) in 1X TAE buffer. The 1 Kb (HyperLadder, BIOLINE ™) was used as a molecular weight marker during each migration performed. The visualization of the migration of the bands was made by Ultra-violet using a UV transilluminator (Enduro GDS).
2.8.3. Sequencing and analysis of the 16S rRNA gene
The 16S rRNA amplification product was purified using the Quiagen PCR product purification system. The sequencing was carried out on a BIOSYSTEME 3130 automatic sequencer (Cité de l'Innovation de Fès, Morocco). The sequence obtained was compared with those from the GenBank database using the program available at NCBI (www.ncbi.nlm.nih.gov).
The nucleotide sequence generated is compared with the reference strains provided by GENBANK database in National Center for Biotechnology Information (NCBI) using the BLASTN software (Altschul et al., 1990). Nucleotide sequence was aligned, verified, and corrected manually using Chromas 2 (Version 2.6.5) and MEGA 7 (Version 7.0.26). The phylogenetic analysis were established by MEGA 7 software. The distances were quantified according to the two-parameter model of Kimura (1980). The phylogenetic tree was established using the Neighbor Joining method (Saitou and Nei, 1987). Node support has been estimated with 1000 bootstrap replicas. The obtained sequence was deposited and identified in the GENBANK database via an accession number.
2.9. Statistical analysis
Physico-chemical and elemental analysis carried out in triplicate and reported as mean ± SD (standard deviation). Means were analysed by Tukey test HSD using the SPSS 25 software with a significance level of 0.05.
3. Results
3.1. Composting monitoring
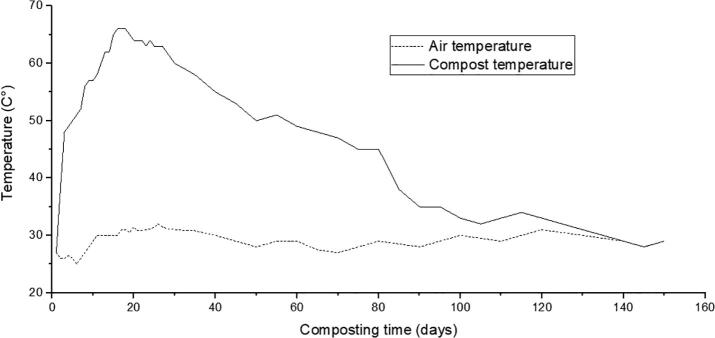
After 5 months of co-composting of PS, VW, and O2MW, the various analyses carried out showed significant changes in the composting parameters (p < 0.05) (Table 2). The temperature increased vigorously in the first 20 days of the process to reach its maximum of around 66 °C (Fig. 1). At this period, the pH became alkaline and electrical conductivity dropped significantly to 2.58 mS·cm−1 after 30 days. After that, the temperature began to decrease during the maturation phase, marking the mesophilic conditions for the last 30 days and then turned to the ambient temperature at the final stage of the process. The pH demonstrated a significant improvement to 8.6, while the electrical conductivity continued to decrease to a value of 1.61 mS·cm−1.
Table 2.
Physico-chemical parameters of the composting trial at different stages (0: the initial stage, 30: after 30 days, 90: after 90 days, and 150: after 150 days).
| Parameters | Composting stages (days) |
F | p value | |||
|---|---|---|---|---|---|---|
| 0 | 30 | 90 | 150 | |||
| Humidity% | 60 ± 1.5 | 57.6 ± 3.8 | 50.9 ± 5.4 | 27.9 ± 1.1 | 54.717 | <0.05 |
| pH | 5.91 ± 0.08 | 8.02 ± 0.07 | 8.6 ± 0.01 | 8.56 ± 0.02 | 1639.008 | <0.05 |
| EC (mS·cm−1) | 5.53 ± 0.18 | 2.58 ± 0.19 | 2.07 ± 0.1 | 1.61 ± 0.11 | 628.552 | <0.05 |
| TOC% | 42.5 ± 0.2 | 40.5 ± 3 | 35.5 ± 1.2 | 28.4 ± 0.8 | 42.593 | <0.05 |
| TNK% | 0.65 ± 0.02 | 1.25 ± 0.01 | 2.6 ± 0.01 | 2.92 ± 0.02 | 14029.2 | <0.05 |
| C/N | 65 ± 2.11 | 34 ± 1.8 | 14 ± 1.2 | 10 ± 1.3 | 786.85 | <0.05 |
| Decomposition% | 0 | 21.4 ± 1.3 | 58.4 ± 0.1 | 77.7 ± 1.1 | 5090.41 | <0.05 |
| Al-P (mg·g−1) | 0.04 ± 0.01 | 0.23 ± 0.02 | 0.37 ± 0.02 | 0.51 ± 0.03 | 268.611 | <0.05 |
| Ca-P (mg·g−1) | 68.7 ± 1.58 | 73.53 ± 2.48 | 71.2 ± 1.87 | 57.4 ± 1.34 | 43.999 | <0.05 |
| Fe-P (mg·g−1) | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.25 ± 0.01 | 0.34 ± 0.02 | 161.571 | <0.05 |
| Organic P (mg·g−1) | 7.30 ± 1.36 | 6.59 ± 0.84 | 6.66 ± 0.74 | 5.15 ± 0.22 | 3.14 | 0.087 ns |
| Olsen P (mg·g−1) | 0.75 ± 0.02 | 0.90 ± 0.01 | 1.14 ± 0.11 | 1.41 ± 0.34 | 7.807 | <0.05 |
| Polyphenols% | 0.59 ± 0.03 | 0.52 ± 0.02 | 0.07 ± 0.00 | 0.02 ± 0.00 | 810.445 | <0.05 |
ns: not significant at p value < 0.05.
Fig. 1.
Temperature of the compost pile during 150 days of composting in comparison with the air ambient temperature. (…) Air temperature and (—) Compost pile temperature.
TNK gradually increased throughout the composting time from 0.65 on the first day to 2.92% on the last day. While a slight decrease in TOC (from 42.5 to 40.5%) was registered in the first month, resulting in a low decomposition rate of 21% at this stage. After that, decomposition was accelerated and reached 77.7% and TOC accounted for 28.4% at the maturation phase. The final C/N ratio of the PS and O2MW2 co-composting has achieved 10. As a direct consequence of the OM decomposition, the concentration of polyphenol decreased significantly from 0.5885 to 0.0157%.
Regarding the dynamics of P inside the composting pile, the P associated with calcium (Ca-P) had the largest percentage share of P fractions, with 82.6 and 68.7 mg·g−1 in the PS and in the starting mixture of compostable materials, respectively. The concentration of Ca-P augmented during the first 30 days and decreased steadily, into 57.4 mg·g−1 after 150 days of composting (Table 2). During the same period of composting, organic P reported a marginal decline from an initial value of 7.30 mg·g−1 to a final value of 5.15 mg·g−1. The Fe-P and Al-P fractions reported a small rise during the composting cycle, achieving 0.34 and 0.51 mg·g−1, respectively, on the 150th day of composting. In fact, bioavailable P was increased to a substantial volume of up to 1.41 mg·g−1.
3.2. Different functions of the isolated bacterium
3.2.1. Plant growth promotion factors
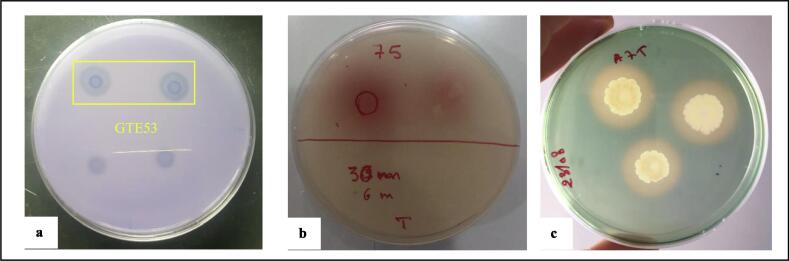
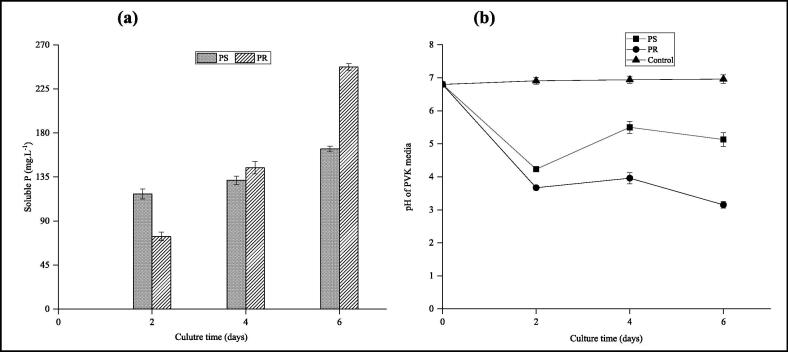
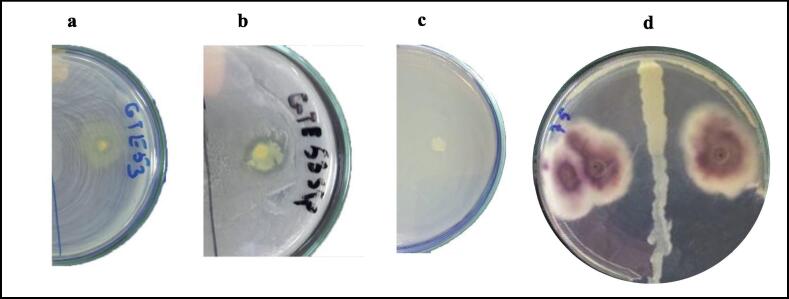
Twelve phosphate-solubilising isolates from the thermophilic phase of the composting trial. The isolated strain GTE53 was selected as a performant phosphate-solubilising bacterium, showing a clear halo zone with a diameter of 17.00 mm around its colony and with an SI of 2.4 (Fig. 2a). As well as, the phosphate solubilisation test of GTE53 in PVK liquid medium indicated that the bacterium effectively solubilised the phosphate complexed in PS and PR (Fig. 3a). Strain GTE53 produced 162.8 and 247.4 mg·L−1 of soluble phosphate, respectively, from PS and PR after 6 days of incubation. In the first 2 days, pH dropped from 6.8 to 3.96 and 3.86 in the PS and PR media, respectively, which led to PS and PR dissolution. In addition, GTE53 was capable of producing IAA (Fig. 2b) and siderophores (Fig. 2c) and was also capable of fixing atmospheric nitrogen.
Fig. 2.
Phosphate solubilisation in TCP solid medium (a); AAI production test (b); and siderphores production test (c) in GTE53.
Fig. 3.
Change in soluble P (a) and pH (b) by isolated GTE53 in liquid broth media supplemented with PS and with PR during 6 days of incubation.
3.2.2. Bioremediation
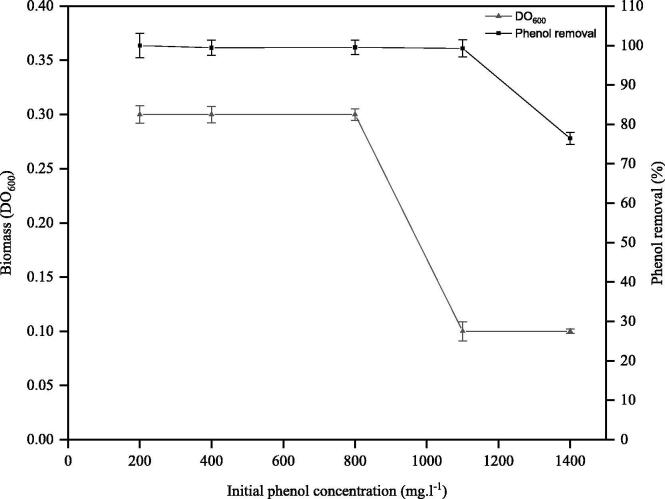
Biodegradation of phenol and microbial biomass of isolate GTE53 at different phenol concentrations was determined after 48 h of culture time (Fig. 4). Isolate GTE53 demonstrated an effective degradation of phenol, resulting in the removal of almost 100% of phenol in a solution of 200 mg·L−1. As well as, 99.4, 99.5 and 99.2% of phenol removal in solutions containing 400, 800 and 1100 mg·L−1 of phenol, respectively. However, the biomass of the isolate began to decrease from 1100 mg·L−1 of phenol. However, the isolate may tolerate a phenol concentration of up to 1400 mg·L−1, and still leading to an elimination rate of 76.4% of this amount of phenol. When the phenol concentration exceeded 1400 mg·L−1, the biomass growth dropped vigorously until no growth was detected at a maximum concentration of 2000 and 2500 mg·L−1.
Fig. 4.
Profile of bacterial cell of strain GTE53 growth with DO = 600 nm and phenol removal at various initial concentrations of phenol: 200, 400, 800, 1100 and 1400 mg·L−1.
3.2.3. Inhibitory activity against pathogens
The isolate GTE53 inhibited the growth of E. coli CCMM B4, Streptococcus sp. CCMM B24, and Salmonella sp. CCMM B7 by showing higher inhibition zones having 20.50 ± 0.21 mm, 18.30 ± 0.23 mm and 15.10 ± 0.31 mm in diameter, respectively (Fig. 5a–c). In addition, the antifungal effect of GTE53 was studied. Thus, the bacterium showed a strong antagonistic potential by inhibiting 43.75% of the mycelial growth of Foa A27 (Fig. 5d).
Fig. 5.
The antimicrobial effect of isolated GTE53 against Escherichia coli B4 (a), Streptococcus sp. B24 (b), Salmonella sp. CCMM B7 (c); and Foa A27 (d).
3.2.4. Phenotypic and molecular characterization of strains using 16S rRNA
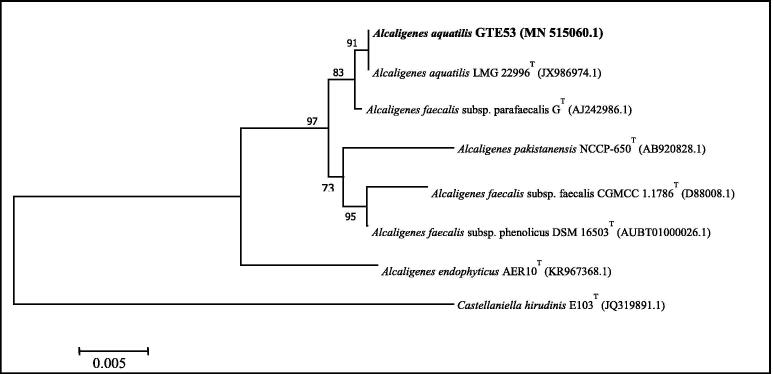
Based on a PCR analysis of the 16 S RNA gene followed by sequencing (99.66% similarity), the isolate GTE53 was identified as Alcalegenes aquatilis. Additional confirmation of the genus Alcaligenes was obtained by submitting the FASTA sequence to GenBank with the access number of MN515060. After that, the BLASTN analysis showed the similarity of the 16S rRNA sequence with various sequences of Alcaligenes species and based on the phylogenetic tree (Fig. 6), it was evident that the isolated organism GTE53 is more closely linked to Alcaligenes aquatilis. The results of the phenotypic analysis indicate that the isolate GTE53 was a gram-negative and short rod. Furthermore, strain GTE53 can tolerate stressful conditions similar to those found in the composting pile; it can grow well in extreme temperatures up to 60 °C, within a pH range of 3–10, at high salinity 8% of NaCl, and water stress (25% PEG 6000).
Fig. 6.
Phylogenetic tree based on 16S rRNA gene sequences by Neighbor Joining method (using MEGA 7.0), showing the relationship between strain GTE53 and other members of the Alcaligenes sp. The Genbank nucleotide accession numbers are listed next to the strain names. The scale bars represent 0.005 substitution/site.
4. Discussion
The raw materials used in the composting mixture have different chemical compositions (Table 1). These materials contain a large amount of OM in O2MP, O2MW2 and VW as well as minerals incorporated in the PS. This has ensured the supply of vital nutrients for microbial development, such as phosphorus, carbon and nitrogen (Vishan et al., 2014). During the composting process, the microbes broke down carbon-rich compounds such as carbohydrates and polyphenols into simpler metabolites used as an energy source (Amir et al., 2010, Cesaro et al., 2019). Due to intensive microbial activity, a high OM decomposition rate was associated with an increase in compost pile temperature (Amir et al., 2005). At the same time, organic carbon was transformed into more complexed compounds (humic substances) and a part was lost as CO2, as was the mineralisation of organic nitrogen into ammonium ions (NH4+) and nitrate (NO3−) (Zhou et al., 2018, Cesaro et al., 2019). Consequently, the values of C/N ratio of about 10, pH of 8.56 and EC of 1.61 mS·cm−1 after 150 days of the process indicated well-composted materials according to many authors (Alburquerque et al., 2009, Gigliotti et al., 2012, Vázquez et al., 2015).
Otherwise, living microorganisms during co-composting of PS, VW, and O2MW meet their P requirements by converting the organic P and Ca-P into a bioavailable form (Olsen P), which doubled between the initial and final stages of composting. Several mechanisms involved in the P biotransformation, including the mechanism of acidification by organic acids, chelation with organic ligands (e.g. siderophores), redox reactions, phosphatase synthesis, and the production of extracellular polymers (Sharma et al., 2013, Bargaz et al., 2018, Yu et al., 2019). The results indicate that the solubilisation of Ca-P and the mineralisation of organic P was accompanied by secondary reactions in which the soluble P was complexed by Fe and Al ions (Jakubus, 2016). In fact, P-solubilizing microorganisms play a crucial role in advancing the solubilisation response throughout the composting process, rendering the final product rich in available P (Adnan et al., 2017).
The strain GTE53 was selected as a specific bacterium that led to the closure of nutrients cycles during composting. Indeed, the phenotypic characteristics of GTE53 were consistent with the phenotypic features of the genus Alcaligenes (Durán et al., 2019b). A. aquatilis GTE53 had the capacity to dissolve the inorganic phosphate contained in PS by reducing the pH of the cell’s environment owing to its acidification mechanisms (Li et al., 2019). Organic acids released by phosphate-solubilising bacteria have been reported as the key mechanism used to solubilize Ca-P by replacement between H+ and Ca2+ cations (Alori et al., 2017). After that, the P released during composting can be fixed to free ions Fe2+. In this context, A. aquatilis GTE53 can play a vital role in the P cycle by controlling the binding of P to the surface of iron ions via the production of iron-specific chelators, so-called siderophores (Hamdali et al., 2008, Castellano Hinojosa and Bedmar, 2017). In addition, by relating the increase in N during composting to the contribution of microbes, it was possible to define the role of A. aquatilis GTE53 in this increase by the fixation of atmospheric nitrogen; hence, mitigating nitrogen loss occurred especially in the thermophilic phase. Furthermore, it was observed that the isolated strain could release IAA in the presence of L-tryptophan; this phytohormone was involved in many reactions during the vegetative growth and development (Bargaz et al., 2018).
In addition, GTE53 showed potent inhibitory effect towards three Gram-positive and Gram-negative bacteria, which are the most abundant pathogens in organic wastes compost according to several authors (Krzyściak et al., 2013, Jones et al., 2014, Atif et al., 2020, Ramos et al., 2019). This effect could be associated with the production of bioactive substances by the genus Alcaligenes that have antimicrobial activity against antibiotic-resistant bacteria (Zahir et al., 2013, Zahir et al., 2014, Paul and Sinha, 2017). In addition to their role in fostering plant growth, siderophores produced by the bacterial isolate GTE53 may be involved in the antagonistic activity by depriving the iron of other bacterial competitors. Indeed, Iron is crucial for all living organisms and as observed in this study its concentration during the co-composting of PS, VW, and O2MW was reduced due to the formation of Fe-P complexes (Alori et al., 2017). This insufficiency of iron in the compost medium provokes the bacterium to confiscate iron from pathogens, thereby limiting their development (Syed-Ab-Rahman et al., 2018). For antifungal activity, this beneficial strain has been shown to be effective in limiting the development of Foa A27, which is reported as the most harmful fungal disease (Bayoud) of date palm in Morocco and Algeria (Dihazi et al., 2012, Alwahshi et al., 2019).
The tolerance of A. aquatilis GTE53 to high temperatures, high salt, pH, and water deficit, indicates its ability to create rapid physiological responses to changes in environmental stresses. In fact, thermophilic bacteria, owing to specific genes can stabilize the enzyme activities or protein structures in their native configurations against high temperatures (Koga, 2012, Wang et al., 2015). Moreover, the bacterium A. aquatilis GTE53 is capable of acidifying or alkalizing the pH of the cytoplasm in order to make the cell function and the structure compatible with the pH of the environment and therefore promote bacterial growth (Liu et al., 2015). Generally, homeostasis in bacteria occurs through two main mechanisms. In a very acidic environment the bacteria tend to maintain a low intracellular proton concentration, while in a strongly alkaline environment, alkaline compounds are produced to neutralize the acid generated during extracellular metabolism (Padan et al., 2005, Xu et al., 2020). This is consistent with the pH results recorded during phosphate solubilization by A. aquatilis GTE53 (Fig. 3b)
The tolerance to acidic pH and also to high salinity, allowing for A. aquatilis GTE53 to be a valuable bioremediation agent for the treatment of acidic and/or saline effluents from agro-food industries such those from olive oil mills (Tallou et al., 2020b). Therefore, A. aquatilis GTE53 has shown a great potential to degrade phenol efficiently, which is highly toxic chemical widely found in industrial wastewaters (Li et al., 2019). During the experiment, A. aquatilis GTE53 decomposed phenol into simpler compounds H2O and CO2 by cleavage of the aromatic ring of phenol to use it as a source of carbon and energy. Furthermore, compared to the results found by Liu et al. (2016), isolate GTE53 appeared to be more effective than Acinetobacter calcoaceticus PA, which removed 99.5% of the phenol in a solution of 800 mg·L−1 within 48 h instead of 91.6% by Acinetobacter calcoaceticus PA. As well as, Acinetobacter calcoaceticus PA was less tolerant than A. aquatilis GTE53 by phenol concentrations greater than 1100 mg·L−1. This reveals that A. aquatilis GTE53 is capable of functioning in severe environments and highly concentrated of organic impurities such as phenol.
5. Conclusion
To gain a better and deeper understanding of the compost system, it is advantageous to target beneficial microbes and identify their specific functions. Accordingly, the isolated strain A. aquatilis GTE53 has been shown to play a pivotal role in bioremediation by degrading phenol and by tolerating abiotic stressors also can exhibits plant growth-promoting attributes. In addition, its inhibitory action against many pathogenic bacteria and fungi leads us to think about performing more investigations to find out the bioactive substances involved. The findings of this study will be of substantial value for further use of A. aquatilis GTE53 to activate the co-composting of phosphate source materials with organic residues come from industrial plants.
CRediT authorship contribution statement
Ayoub Haouas: Writing - original draft, Formal analysis. Cherkaoui El Modafar: Funding acquisition, Project administration. Allal Douira: Validation. Saâd Ibnsouda-Koraichi: Validation. Abdelkarim Filali-Maltouf: Data curation. Abdelmajid Moukhli: Validation. Soumia Amir: Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Authors would like to acknowledge the support through the R&D Initiative – Appel à projets autour des phosphates APPHOS – sponsored by OCP (OCP Foundation, R&D OCP, Mohammed VI Polytechnic University, National Center of Scientific and technical Research CNRST, Ministry of Higher Education, Scientific Research and Professional Training of Morocco MESRSFC) under the project entitled ’Procédés biotechnologiques pour la valorisation des boues et des déchets miniers de phosphate : Formulation d’un Phospho-compost bio-fertilisant pour application directe en agriculture productive et respectueuse de l’environnement » (Réf. BIO-MOD-01/2017).
Footnotes
Peer review under responsibility of King Saud University.
References
- Adnan M., Shah Z., Fahad S., Arif M., Alam M., Khan I.A., Mian I.A., Basir A., Ullah H., Arshad M. Phosphate-solubilizing bacteria nullify the antagonistic effect of soil calcification on bioavailability of phosphorus in alkaline soils. Sci, Rep. 2017;7:1–13. doi: 10.1038/s41598-017-16537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association Française de normalisation AFNOR, 1975. Dosage de l’azote Ammoniacal. NF-T 90-015.
- Alburquerque J.A., Gonzálvez J., Tortosa G., Baddi G.A., Cegarra J. Evaluation of “alperujo” composting based on organic matter degradation, humification and compost quality. Biodeg. 2009;20:257–270. doi: 10.1007/s10532-008-9218-y. [DOI] [PubMed] [Google Scholar]
- Alori E.T., Glick B.R., Babalola O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Alwahshi K.J., Saeed E.E., Sham A., Alblooshi A.A., Alblooshi M.M., El Tarabily K.A., Abu Qamar S.F. Molecular identification and disease management of date palm sudden decline syndrome in the united arab emirates. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S., Hafidi M., Merlina G., Revel J.C. Sequential extraction of heavy metals during composting of sewage sludge. Chemosphere. 2005;59:801–810. doi: 10.1016/j.chemosphere.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Amir S., Jouraiphy A., Meddich A., El Gharous M., Winterton P., Hafidi M. Structural study of humic acids during composting of activated sludge-green waste: elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010;177:524–529. doi: 10.1016/j.jhazmat.2009.12.064. [DOI] [PubMed] [Google Scholar]
- Atif K., Haouas A., Aziz F., Yasser Jamali M., Tallou A., Amir S. Pathogens evolution during the composting of the household waste mixture enriched with phosphate residues and olive oil mill wastewater. Waste Biomass Valorizat. 2020;11:1789–1797. doi: 10.1007/s12649-018-0495-3. [DOI] [Google Scholar]
- Ayilara M.S., Olanrewaju O.S., Babalola O.O., Odeyemi O. Waste management through composting: challenges and potentials. Sustainability. 2020;12:1–23. [Google Scholar]
- Bargaz A., Lyamlouli K., Chtouki M., Zeroual Y., Dhiba D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front Microbiol. 2018;9:1606. doi: 10.3389/fmicb.2018.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjelloun I., Thami Alami I., Douira A., Udupa S.M. Phenotypic and genotypic diversity among symbiotic and non-symbiotic bacteria present in chickpea nodules in morocco. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey's manual of determinative bacteriology Am J Public Health. 1923;13(12):1042. [Google Scholar]
- Bray R.H., Kurtz L.T. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1945;59:39–45. doi: 10.1097/00010694-194501000-00006. [DOI] [Google Scholar]
- Bric J.M., Bostock R.M., Silverstone S.E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991;57(2):535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano Hinojosa A., Bedmar E.J. Methods for evaluating plant growth-promoting rhizobacteria traits. In: Singh H.B., Sharma B.K., Keswani C., editors. Advances in PGPR Research. CABI Internation; Oxford: 2017. pp. 255–274. [Google Scholar]
- Cerda A., Artola A., Font X., Barrena R., Gea T., Sánchez A. Composting of food wastes: status and challenges. Bioresour. Technol. 2018;248:57–67. doi: 10.1016/j.biortech.2017.06.133. [DOI] [PubMed] [Google Scholar]
- Cesaro A., Conte A., Belgiorno V., Siciliano A., Guida M. The evolution of compost stability and maturity during the full-scale treatment of the organic fraction of municipal solid waste. J. Environ. Manage. 2019;232:264–270. doi: 10.1016/j.jenvman.2018.10.121. [DOI] [PubMed] [Google Scholar]
- Chang C.H., Yang S.S. Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour. Technol. 2009;100:1648–1658. doi: 10.1016/j.biortech.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Chang S.C., Jackson M.L. Fractionation of soil phosphorus. Soil Sci. 1957;84:133. [Google Scholar]
- Chen W.P., Kuo T.T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993;21(9):2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihazi A., Jaiti F., Wafa T., Kilani-Feki O., Jaoua S., Driouich A., Baaziz M., Daayf F., Serghini M.A. Use of two bacteria for biological control of bayoud disease caused by Fusarium oxysporum in date palm (Phoenix dactylifera L) seedlings. Plant Physiol. Biochem. 2012;55:7–15. doi: 10.1016/j.plaphy.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Du Z.Y., Wang Q.H., Liu F.C., Ma H.L., Ma B.Y., Malhi S.S. Movement of phosphorus in a calcareous soil as affected by humic acid. Pedosphere. 2013;23(2):229–235. doi: 10.1016/S1002-0160(13)60011-9. [DOI] [Google Scholar]
- Durán R.E., Barra-Sanhueza B., Salvà-Serra F., Méndez V., Jaén-Luchoro D., Moore E.R.B., Seeger M. Complete genome sequence of the marine hydrocarbon degrader Alcaligenes aquatilis QD168, isolated from crude oil-polluted sediment of Quintero Bay, central Chile. Microbiol Resour Announc. 2019;8 doi: 10.1128/MRA.01664-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán R.E., Méndez V., Rodríguez-Castro L., Barra-Sanhueza B., Salvà-Serra F., Moore E.R.B. Genomic and physiological traits of the marine bacterium Alcaligenes aquatilis QD168 isolated from quintero bay, central Chile, reveal a robust adaptive response to environmental stressors. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelkamp R., Schneider D., Hertel R., Daniel R. Nitrile-degrading bacteria isolated from compost. Front. Environ. Sci. Eng. 2017;5 doi: 10.3389/fenvs.2017.00056. [DOI] [Google Scholar]
- El Hassni M., El Hadrami A., Daayf F., Chérif M., Barka E.A., El Hadrami I. Biological control of bayoud disease in date palm: selection of microorganisms inhibiting the causal agent and inducing defense reactions. Environ. Exp. Bot. 2007;59:224–234. doi: 10.1016/j.envexpbot.2005.12.008. [DOI] [Google Scholar]
- Geissler B., Hermann L., Mew M.C., Steiner G. Striving toward a circular economy for phosphorus: the role of phosphate rock mining. Minerals. 2018;8:395. doi: 10.3390/min8090395. [DOI] [Google Scholar]
- Geng A., Soh A.E.W., Lim C.J., Loke L.C.T. Isolation and characterization of a phenol-degrading bacterium from an industrial activated sludge. Appl. Microbiol. Biotechnol. 2006;71(5):728–735. doi: 10.1007/s00253-005-0199-z. [DOI] [PubMed] [Google Scholar]
- Gigliotti G., Proietti P., Said-Pullicino D., Nasini L., Pezzolla D., Rosati L., Porceddu P.R. Co-composting of olive husks with high moisture contents: organic matter dynamics and compost quality. Int. Biodeterior. Biodegradat. 2012;67:8–14. doi: 10.1016/j.ibiod.2011.11.009. [DOI] [Google Scholar]
- Hamdali H., Hafidi M., Virolle M.J., Ouhdouch Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl. Soil Ecol. 2008;40(3):510–517. doi: 10.1016/j.apsoil.2008.08.001. [DOI] [Google Scholar]
- Haouas A., El Modafar C., Douira A., Ibnsouda-koraichi S., Filali-maltouf A., Moukhli A., Amir S. The effect of phosphate and organic additives on the stability of food waste in the full-scale composting. PLANT CELL BIOTECHNOLOGY AND MOLECULAR BIOLOGY. 2020;21(39–40):17–28. https://ikprress.org/index.php/PCBMB/article/view/5428 [Google Scholar]
- Jakubus M. Estimation of phosphorus bioavailability from composted organic wastes. Chem. Spec. Bioavailab. 2016;28:189–198. doi: 10.1080/09542299.2016.1227687. [DOI] [Google Scholar]
- Jones L.A., Worobo R.W., Smart C.D. Plant-pathogenic oomycetes, Escherichia coli strains, and salmonella spp. frequently found in surface water used for irrigation of fruit and vegetable crops in New York State. Appl. Environ. Microbiol. 2014;80:4814–4820. doi: 10.1128/AEM.01012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Koga Y. Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea. 2012;2012:789652. doi: 10.1155/2012/789652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyściak W., Pluskwa K.K., Jurczak A., Kościelniak D. The pathogenicity of the Streptococcus genus. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:1361–1376. doi: 10.1007/s10096-013-1914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutu F.R., Mokase T.J., Dada O.A., Rhode O.H.J. Assessing microbial population dynamics, enzyme activities and phosphorus availability indices during phospho-compost production. Int. J. Recycl. Org. Waste Agricult. 2019;8:87–97. doi: 10.1007/s40093-018-0231-9. [DOI] [Google Scholar]
- Li H., Meng F., Duan W., Lin Y., Zheng Y. Biodegradation of phenol in saline or hypersaline environments by bacteria: a review. Ecotoxicol. Environ. Saf. 2019;184:109658. doi: 10.1016/j.ecoenv.2019.109658. [DOI] [PubMed] [Google Scholar]
- Liu Y., Tang H., Lin Z., Xu P. Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Biotechnol. Adv. 2015;33:1484–1492. doi: 10.1016/j.biotechadv.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Liu Z., Xie W., Li D., Peng Y., Li Z., Liu S. Biodegradation of phenol by bacteria strain Acinetobacter Calcoaceticus PA isolated from phenolic wastewater. Int. J. Environ. Res. Publ. Health. 2016;13 doi: 10.3390/ijerph13030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louden B.C., Haarmann D., Lynne A.M. Use of blue agar CAS assay for siderophore detection. JMBE. 2011;12(1):51–53. doi: 10.1128/jmbe.v12i1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutou M., Hajjaji M., Mansori M., Favotto C., Hakkou R. Phosphate sludge: thermal transformation and use as lightweight aggregate material. J. Environ. Manage. 2013;130:354–360. doi: 10.1016/j.jenvman.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Lutz S., Thuerig B., Oberhaensli T., Mayerhofer J., Fuchs J.G., Widmer F. Harnessing the microbiomes of suppressive composts for plant protection: from metagenomes to beneficial microorganisms and reliable diagnostics. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Bibi E., Ito M., Krulwich T.A. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta. 2005;1717:67–88. doi: 10.1016/j.bbamem.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A., Pandey P., Kaushik S. Co-inoculation of Burkholderia cepacia and Alcaligenes aquatilis enhances plant growth of maize (Zea mays) under green house and field condition. Korean J. Environ. Agric. 2017;44(2):196–210. doi: 10.7744/kjoas.20170019. [DOI] [Google Scholar]
- Paul D., Sinha S.N. Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann. Agrar. Sci. 2017;15:130–136. doi: 10.1016/j.aasci.2016.10.001. [DOI] [Google Scholar]
- Premono M.E., Moawad A.M., Vleck L.G. Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop. Sci. 1996:13–23. [Google Scholar]
- Pufahl Peir K., Lee A.G. Sedimentary and igneous phosphate deposits: formation and exploration: an invited paper. Econ Geol. 2017;112(3):483–516. doi: 10.2113/econgeo.112.3.483. [DOI] [Google Scholar]
- Ramos C.P., Santana J.A., Morcatti Coura F., Xavier R.G.C., Leal C.A.G., Oliveira Junior C.A., Heinemann M.B., Lage A.P., Lobato F.C.F., Silva R.O.S. Identification and characterization of Escherichia coli, Salmonella Spp., Clostridium perfringens, and C. difficile isolates from Reptiles in Brazil. Biomed. Res. Int. 2019 doi: 10.1155/2019/9530732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffari H., Pourbabaee A.A., Asgharzadeh A., Besharati H. Isolation and identification of effective cellulolytic bacteria in composting process from different sources. Arch. Agron. Soil Sci. 2017;63(3):297–307. doi: 10.1080/03650340.2016.1198006. [DOI] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sharma S.B., Sayyed R.Z., Trivedi M.H., Gobi T.A. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus. 2013;2:587. doi: 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella M., Sashikala M. Beneficial microorganisms isolated from vegetable compost. J. Trop. Agric. Food Sci. 2016;44(2):277–293. [Google Scholar]
- Syed-Ab-Rahman S.F., Carvalhais L.C., Chua E., Xiao Y., Wass T.J., Schenk P.M. Identification of soil bacterial isolates suppressing different Phytophthora spp. and promoting plant growth. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Anwar M.R. Wheat grain yield, phosphorus uptake and soil phosphorus fraction after 23 years of annual fertilizer application to an Andosol. Field Crops Res. 2007;101:160–171. doi: 10.1016/j.fcr.2006.11.003. [DOI] [Google Scholar]
- Tallou A., Haouas A., Jamali M.Y., Atif K., Amir S., Aziz F. Review on cow manure as renewable energy. In: Patnaik S., Sen S., Mahmoud M.S., editors. Smart Village Technology: Concepts and Developments, Modeling and Optimization in Science and Technologies. Springer International Publishing; Cham: 2020. pp. 341–352. [DOI] [Google Scholar]
- Tallou A., Salcedo F.P., Haouas A., Jamali M.Y., Atif K., Aziz F., Amir S. Assessment of biogas and biofertilizer produced from anaerobic co-digestion of olive mill wastewater with municipal wastewater and cow dung. Environ. Technol. Innov. 2020;20:101152. doi: 10.1016/j.eti.2020.101152. [DOI] [Google Scholar]
- Van Trappen S., Tan T.L., Samyn E., Vandamme P. Alcaligenes aquatilis sp. nov., a novel bacterium from sediments of the Weser Estuary, Germany, and a salt marsh on Shem Creek in Charleston Harbor, USA. Int. J. Syst. Evol. Microbiol. 2005;55(Pt 6):2571–2575. doi: 10.1099/ijs.0.63849-0. [DOI] [PubMed] [Google Scholar]
- Vargas-García M.C., Suárez-Estrella F., López M.J., Moreno J. Microbial population dynamics and enzyme activities in composting processes with different starting materials. Waste Manag. 2010;30:771–778. doi: 10.1016/j.wasman.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Vázquez M.A., Sen R., Soto M. Physico-chemical and biological characteristics of compost from decentralised composting programmes. Bioresour. Technol. 2015;198:520–532. doi: 10.1016/j.biortech.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Vazquez-Roncero A., Graciani-Constante E., Maestro-Duran R. Componentes fenolicos de la aceituna. I. Polifenoles de la pulpa. Grasas y Aceites. 1974;25:269–279. [Google Scholar]
- Vishan I., Kanekar H., Kalamdhad A. Microbial population, stability and maturity analysis of rotary drum composting of water hyacinth. Biologia. 2014;69:1303–1313. doi: 10.2478/s11756-014-0450-0. [DOI] [Google Scholar]
- Wang Q., Cen Z., Zhao J. The survival mechanisms of thermophiles at high temperatures: an angle of Omics. Physiology. 2015;30:97–106. doi: 10.1152/physiol.00066.2013. [DOI] [PubMed] [Google Scholar]
- Wei Y., Zhao Y., Shi M., Cao Z., Lu Q., Yang T., Fan Y., Wei Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018;247:190–199. doi: 10.1016/j.biortech.2017.09.092. [DOI] [PubMed] [Google Scholar]
- Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhao Z., Tong W., Ding Y., Liu B., Shi Y., Wang J., Sun S., Liu M., Wang Y., Qi Q., Xian M., Zhao G. An acid-tolerance response system protecting exponentially growing Escherichia coli. Nat. Commun. 2020;11:1496. doi: 10.1038/s41467-020-15350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Pratap Singh R., Song D., Chen Q., Zheng X., Zhang C., Zhang M., Li Y. Synergistic effect of Pseudomonas putida II-2 and Achromobacter sp. QC36 for the effective biodegradation of the herbicide quinclorac. Ecotoxicol. Environ. Saf. 2020;188:109826. doi: 10.1016/j.ecoenv.2019.109826. [DOI] [PubMed] [Google Scholar]
- Yu L.Y., Huang H.B., Wang X.H., Li S., Feng N.X., Zhao H.M., Huang X.P., Li Y.W., Li H., Cai Q.Y., Mo C.H. Novel phosphate-solubilising bacteria isolated from sewage sludge and the mechanism of phosphate solubilisation. Sci. Total Environ. 2019;658:474–484. doi: 10.1016/j.scitotenv.2018.12.166. [DOI] [PubMed] [Google Scholar]
- Zahir I., Abdellah H., Bahafid W., Iraqui M.H., Ibnsouda S. A novel Alcaligenes faecalis antibacterial-producing strain isolated from a Moroccan tannery waste. Afr. J. Microbiol. Res. 2013 doi: 10.5897/ajmr2013.6029. [DOI] [Google Scholar]
- Zahir I., Houari A., Iraqui M.H., Ibnsouda S. Partial purification and antimycobacterial screening of the ethyl acetate extract of Alcaligenes faecalis BW1. Br. Microbiol. Res. J. 2014 doi: 10.9734/bmrj/2014/10595. [DOI] [Google Scholar]
- Zhou H., Zhao Y., Yang H., Zhu L., Cai B., Luo S., Cao J., Wei Z. Transformation of organic nitrogen fractions with different molecular weights during different organic wastes composting. Bioresour. Technol. 2018;262:221–228. doi: 10.1016/j.biortech.2018.04.088. [DOI] [PubMed] [Google Scholar]