Abstract
Pathogenic and spoilage fungi cause enormous challenges to food related fatal infections. Plant essential oil based classical emulsions can functions as antifungal agents. To investigate the antifungal spectrum, that is the scope of the nanoemulsion composed of Cleome viscosa essential oil and Triton-x-100 fabricated by ultrasonication method. Minimum inhibitory and fungicidal concentration of essential oil nanoemulsion (EONE) was tested against food borne pathogenic C. albicans. The MIC and MFC values ranged from 16.5 to 33 µl/ml with significant reduction on biofilm of C. albicans isolates. The alteration of molecular fingerprints was confirmed by Fourier transformed infrared spectroscopy and subsequent reduction of chitin levels in cell walls was noted by spectroscopic analysis. The EONE and their bioactive compounds cause collateral damage on C. albicans cells.
Keywords: Nanoemulsion, Food borne pathogen, Fungal cell wall inhibitor, Anti-fungal activity Anti biofilm activity
1. Introduction
Essential oils (EOs) derived from plant without any artificial additives, which consists plant derived secondary metabolites of natural aromatic hydrophobic liquids with many single bioactive compounds. EOs have a broad spectrum of biological activities and have been utilized as antimicrobials and antifungals, antioxidants, antiallergy and anticancer agents (Seow et al., 2013). Generally EOs contain 20–60 compounds like alkaloids, terpenoids, flavonoids, isoflavones, polyphenols, biogenic aldehydes and fat soluble pigments (Bakkali et al., 2008, Calo et al., 2015). Currently, the food industry has demonstrated that the growing demand for novel food preservatives developed from natural compounds against food borne pathogenic microorganisms (Donsì and Ferrari, 2016). The use of EOs currently, focused considerable attention as a preservative agent in the modern food industry due to their high reactivity and hydrophobicity. Also, emulsion based EO delivery system can maintain natural quality of food and their biological activity, which is appropriate with many food applications.
Nanoemulsion (NE) refers to a stable colloidal system of nanometric droplets with diameters ranging from 5 to 100 nm. The utilization of NE system attracts modern food, cosmetics and pharmaceutical industries due to functional properties such as high physical stability, bioavailability and low turbidity (Mou et al., 2008). The nanometric size of the droplets increases bioavailability and enhance the diffusion due to the subcellular size. Hence EO-based NE dexterously penetrates into food materials as food grade ingredients, where microorganisms grow and proliferate. Also the hydrophilic and hydrophobic ability of surfactants and emulsifiers along with bioactive compound in EOs collectively contribute antimicrobial and antibiofilm activity (Ferreira et al., 2010). Hence the objective of the present study was to prepare NE from food grade plant essential oil and to analyze its efficacy against food-borne pathogenic Candida albicans.
Cleome viscosa linn, is a subtropical bushy aromatic herb, is commonly called dog mustard or wild mustard. C. viscosa oil is widely used in traditional systems of medicine and possesses various bioactive phytochemicals that exhibit various biological activities (Mali, 2009, Williams et al., 2003). In this study, stable nanoemulsions were prepared by ultrasonic emulsification using C. viscosa essential oil, with droplet diameters below 20 nm. The prepared essential oil nanoemulsion (EONE) was evaluated to characterize its In vitro antifungal activity.
2. Materials and methods
2.1. C. albicans strains
The pathogenic C. albicans and C. albicans ATCC-90028 (Control) strains were used in this study. The pathogenic C. albicans were isolated from spoiled food samples; the isolated strains were identified using a HiCandida Identification Kit (KB006, HiMEDIA, Mumbai, India). Biofilm production phospholipase and proteinase production of each isolate was analyzed by standard method (Shin et al., 2002, Wiebusch et al., 2017, Price et al., 1982). The positive isolates were selected and named individually as CA1 to 7.
2.2. Essential oil extraction and nanoemulsion preparation
The C. viscosa essential oil was extracted as per protocol described in our previous research (Krishnamoorthy et al., 2018). The extracted essential (EO) oil and Triton-x-100 in distilled water was used for nanoemulsion synthesize. Triton-x-100 (polyoxyethylene isooctylphenyl ether) is a nonionic surfactant that has a lipophilic aromatic hydrocarbon and hydrophilic polyethylene oxide chain, and it was utilized as a surface active agent due to its water absorbing and water repellent balance value (HLB = 13.6) (Liu et al., 2019). EO with a concentration of 6% v/v was used for all nanoemulsion (NE) preparations. The EO and surfactant were mixed in diverse proportions [1:1, 1:2 and 1:3 (v/v)] and added to water for preparation of a stubbly emulsion. The mixture was sonicated using a probe sonicator (Ultrasonics, USA) at 20 kHz and a power output of 750 W. The sonicator created the rigorous disrupting forces to reduce the droplet diameter of the stubbly emulsion. The sonication-assisted emulsification process was performed over several intervals (5, 10, 15, 20, 25 and 30 min) and the potential energy was quashed by holding ice reservoir on sample. Then, the droplet size and its durability of the EONE was analyzed at room temperature.
2.3. Antifungal activity
2.3.1. Determination of minimal inhibitory and minimal fungicidal concentrations (MIC/MFC)
The MIC and MFC of the EONE was analyzed using broth microdilution method (CLSI, 2006). Briefly, mid-log-phase of isolated pathogenic C. albicans strains 10 µl (1 × 103 CFU/mL, 600 nm, Abs 0.09–0.1) and EONE concentration ranging from 396–16.5 µl/ml. For positive control the antifungal agent Nystatin (Sigma Aldrich, USA) was used at concentrations ranging from 148.5–33 µg/ml. The microplate was incubated at 37 °C for 24 h. After the incubation, 50 µl aliquots from each well were separately plated on Sabouraud Dextrose Agar (Hi-Media, Mumbai, India) and visible fungal growth on medium were assessed. The MIC value was noted as no visible growth on YPD broth was recorded as MIC and no fungal growth on the SDA agar plates was recorded as MFC. All experiments were performed in triplicate.
2.3.2. Kinetics of killing
The fungicidal dynamics of EONE was performed by the method reported by Ramalingam et al. (2012). Briefly, different dilutions of EONE (1, 5, 30 and 60 dilutions) along with overnight test cultures (1 × 103) were added to a well. Kinetic killing effects were recorded OD at 600 nm at 1, 2, 5, 10 and 15 h interval and each assay was carried out in triplicates. The negative and Positive controls were maintained in YPD broth.
2.4. Biofilm inhibitory assay
The inhibitory effect of EONE against the biofilm formation by pathogenic C. albicans was evauated by semiquantitative plate assays as explained by Stepanović et al. (2007). A mass of OD 600–0.8 overnight cultures were statically incubated in polystyrene 96-well plates along with fresh YPD medium and incubated at 37 °C for 6 h and after incubation unbounded cells were removed by washing with Phosphate-buffered saline (PBS, pH 7.4). The sub lethal concentrations of EONE (70, 60, 50, 40, 30 and 20%), were added to each well along with fresh YPD medium, and plate was incubated for 24 h at 37 °C. Finally, dyed with an aqueous solution of 0.4% crystal violet and microplate reader was used to measure the absorbance at 600 nm. Untreated well was used as negative control and nystatin tread wells was used as positive control.
2.5. Spectroscopic analysis of chitin
Fungal cell wall chitin inhibitory effects of EONE was evaluated using Trypan Blue (TB) quantification method reported by Liesche et al. (2015). The treated and untreated cells were centrifuged at 3000 rpm for 15 min, supernatant was discarded. The unwanted debris from the pellets was removed by washing with phosphate buffer saline (PBS, pH 7.4). The collected pellets were in sterile distilled water, subjected to sonication using ultra sonicator for 1 h, and then centrifuged at 3000 rpm for 15 min. The pellet was subjected to thermal treatment for 1 hr at 80 °C along with 6% NaOH. Then the suspension was washed twice with PBS and the pellet was resuspended along with TB at a final concentration of 10 μgml−1. The unbounded stains were removed by washing three times with PBS, and the fluorescence emission spectrum was determined by a spectrofluorometer (PerkinElmer LS 55, United Kingdom). Excitation spectra were taken with the above instrument over a range 200–450 nm, while fluorescence spectra were recorded over a span of 300–700 nm using excitation at 620. Absorption spectra were recorded using a spectrophotometer (PerkinElmer Lambda 950, United Kingdom) over a range of 200–700 nm and NaOH served as a baseline interference control.
2.6. Fourier-transformed infrared spectroscopy (FT-IR)
The EONE action on treated C. albicans cells were analyzed for molecular modifications using IR spectra (Ghosh et al. 2013). The inoculum size 2 × 103 CFU/mL was treated with EONE concentrations corresponding to pre-determined MIC values and tubes were incubated 8 h at 30 °C. After the incubation, the EONE treated and untreated cells were freeze dried for 8 h under vacuum pressure (0.01 mm). The freeze dried cells (1 mg) were mixed with potassium bromide crystals then pressed under vacuum in a vibratory ball mill. The IR spectra were obtained using FT-IR instrument (Waltham, MA, USA) and 400–4000 cm−1 scanning rang was fixed for both treated and untreated cells with typical resolution of 1.0 cm.
2.7. Scanning electron microscopy analysis
Scanning electron microscope (JSM-6380LA, Tokyo, Japan) was used to visualize the both treated and untreated cells. The cells were collected by centrifugation at 1500 rpm for 15 min. The collected cells were spreaded on a glass cover slip and immediately placed in a freshly prepared 2.5% glutaraldehyde solution for 4 h to fix the cells. The fixed specimen was placed in a 1% Zetterquist’s osmium tetroxide for 30 min, finally dehydrated with series of ethanol and dried using desiccator. Finally, sputter coated (Denton Vacuum, Moorestown, NJ, USA) with gold–palladium (40%/60%) and then samples were examined at 20 kV in a high vacuum mode.
3. Results and discussion
3.1. Pathogenicity of the isolates
All 7 isolated C. albicans strains grew in hypertonic Sabouraud dextrose broth and formed smooth, white, creamy-appearing colonies on tobacco agar, thus providing phenotypic confirmation. Assessment of the pathogenicity of all isolates indicated that they were positive for phospholipase and proteinase production and biofilm production. Biofilm production initiates the adherence and colonization of fungal to host cells and production phospholipase and proteinase enzyme cause host cell damage (Lahkar et al., 2017). These results in accordance with previous study (Mohandas and Ballal, 2011).
3.2. Synthesis of essential oil nanoemulsion
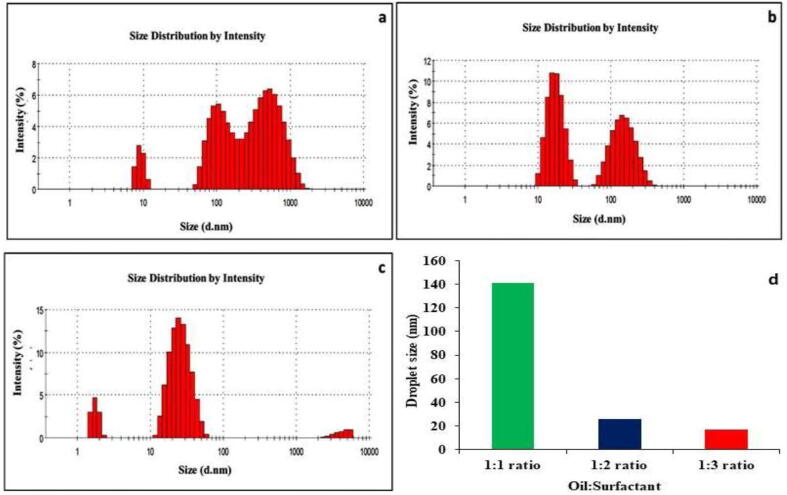
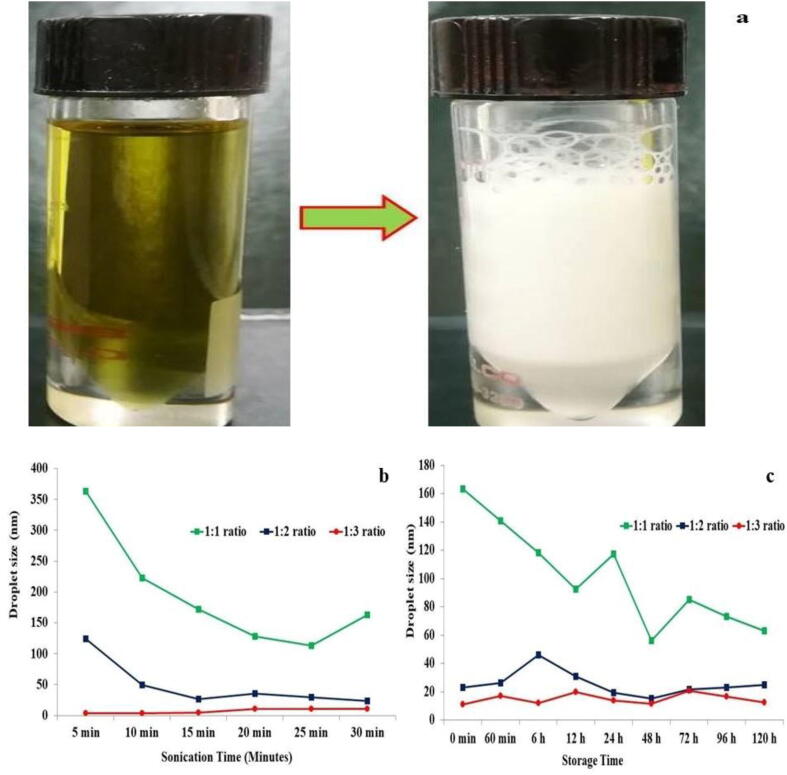
The non-ionic surfactant Triton-x-100 with essential oil and ultrasonication were employed to synthesize the EONE. The high hydrophile-lipophile balance (HLB-13.6) value of the Triton x-100 is highly suitable for preparing nanoemulsions. Different proportions of oil:surfactant (1:1, 1:2, and 1:3 v/v) are utilized to prepare a nanoemulsion. Fig. 1 shows an EONE histogram of the average prepared droplet size 17, 26 and 140 nm from different ratio of oil: surfactant (Fig. 1a–c). The droplet size of EONE decreased tremendously with increases in the concentration of surfactant (Fig. 1d). Liu et al, reported that surfactant concentration plays an important role in droplet size of the basil oil nanoemulsion (Liu et al., 2019). We further analyzed the role of ultrasonic emulsification in nanoemulsion. Fig. 2b depicts the changes in droplet size of prepared nanoemulsions using ultrasonication with different intervals. The nanoemulsion results clearly suggested that the nanosize of the droplet is decreased with increases in the sonication time. However, after 25 min of sonication, the droplet size suddenly increased due to either the Ostwald ripening mechanism or coalescence. Similarly, previous studies reported that emulsification period increase with decrease the droplet size of Nigella sativa L. essential oil and basil oil nanoemulsion (Urbánková et al., 2019). Additionally, an effect of EONE droplet size due to concentration of non-ionic surfactant was evaluated. Low concentrations of Triton x-100 (10%) could result in the average nanoemulsion droplet sizes being very large (164 nm) compared with 20% (24 nm) and 30% (11 nm) Triton x-100, because the excess amount of the surfactants could bind the surface of the nanoemulsion and reduce the interfacial interaction of the colloids. Thus, the nanosize of the EONE was decreased with increases in the surfactant ratio. A high amount of surfactant could stabilize the EONE system. The concentration and nature of the surfactant could alter the stability of the nanoemulsion through coalescence or Ostwald ripening. The prepared EONE stability is an important factor for their widespread application. For this reason, we further analyzed the life span of the droplet size on influence of storage time in NE. As shown in Fig. 2c, we found considerable alterations in the droplets size of NE storage at room temperature, ranging from 10 to 19 nm, 23 to 24 nm, and 163 to 63 nm for 1:3, 1:2, and 1:1 (oil: surfactant (v/v)) EONE, respectively. These results clearly indicate that droplet size gradually increased up to 12 h and subsequently slowly decreased due to the Ostwald ripening. As similar, Periasamy et al. reported that Ostwald ripening process exist during Nigella sativa L. essential oil nanoemulsion formulation using non-ionic surfactant polysorbate 80 (Periasamy et al., 2016). Also, Wooster et al. demonstrated that oil phase and surfactant nature were cause an influence on formation and stabilization of nanoemulsion (Wooster et al., 2008).
Fig. 1.
Droplet size analysis of prepared EONE using different ratios of oil and surfactant. a. 1:1, b. 1:2, and c. 1:3. d. Influence of surfactant and oil ratio on nanoemulsion droplet size.
Fig. 2.
a. C. viscosa essential oil and prepared EONE b. Prepared EONE stability at room temperature during storage, c. Effects of ultrasonication on nanoemulsion droplet size.
3.3. Determination of MIC and MFC values
MIC and MFC values of the EONE in comparison with nystatin (control) antifungal agent against the planktonic pathogenic C. albicans strains are reported in Table 1. The MIC of EONE was 16.5 µl/ml, inhibiting all the isolated C. albicans stains except for CA1 and CA6 (66 µl/ml). This variation may be due to the resistance phenomenon related to exposure via preservatives or antibiotics (Hecker et al., 2003, Tscherner et al., 2011 Jan 11, Zuza et al., 2017). The positive control nystatin antifungal agent showed a MIC of 33 µg/ml against all pathogenic strains. Interestingly, our results showed that EONE was more active than the nystatin. The antifungal activity of EONE enhanced the transport mechanisms through disruption of the cell wall formation during the growth period (Donsì and Ferrari, 2016). The non-ionic surfactant concentration which was used for the synthesis of EONE did not inhibit the growth of C. albicans cells (Hwang et al., 2013). The reference ATCC 90028 strain and the isolated pathogenic strains CA2, CA3, CA4, CA5 and CA7 showed a reduced MIC and MFC (33 µl/ml and 49.5 µl/ml, respectively) values against nystatin and EONE. This was likely due to its nonresistant character; its antifungal activity is well-established and reported by other studies (Espinel-Ingroff et al., 1995, Liao et al., 1999). Interestingly, the isolated strains CA1 and CA6 showed increased MIC and MFC values (82.5 µl/ml and 99 µl/ml, respectively) against both nystatin and EONE, due to drug resistance/mutant characteristics (Luu et al., 2001). The EONE MFC ratio against these pathogenic C. albicans strains entirely coincided with the MIC (33–99 µl/ml). MIC and MFC assessment confirmed that EONE has fungicidal effects against pathogenic planktonic yeast cells. Recently, several reports have indicated that nanoemulsions are active against bacterial pathogens due to their high levels of interaction with bacterial cell membranes, thereby resulting in enhancement of antibacterial activity (Serra et al., 2018). Our investigation suggested the impact of the nanodroplet size and mixture of bioactive components in the EONE influence the antifungal activity. These results consistent with previous reports (Pedro et al., 2009).
Table 1.
Minimal inhibitory and Fungicidal Concentrations (MIC/MFC) of EONE against C. albicans.
| Strains | Nystatin (µg/ml) |
EONE (µl/ml) |
||
|---|---|---|---|---|
| MIC | MFC | MIC | MFC | |
| ATCC 90028 | 33 | 49.5 | 16.5 | 33 |
| CA1 | 82.5 | 99 | 66 | 132 |
| CA2 | 33 | 49.5 | 16.5 | 33 |
| CA3 | 33 | 49.5 | 16.5 | 33 |
| CA4 | 33 | 49.5 | 16.5 | 33 |
| CA5 | 33 | 49.5 | 16.5 | 33 |
| CA6 | 82.5 | 99 | 66 | 132 |
| CA7 | 33 | 49.5 | 16.5 | 33 |
CA1 to CA6 - isolated pathogenic C. albicans and C. albicans ATCC90028 - control stain.
EONE - Essential oil nanoemulsion, Nystatin - Antifungal agent.
3.4. Kinetics of killing
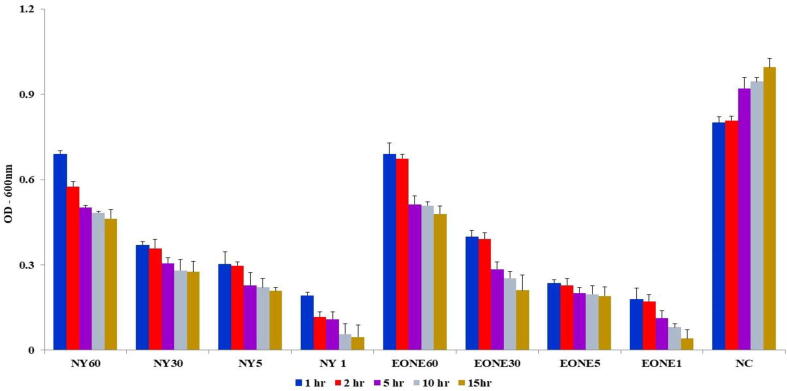
The fungicidal action of EONE against the C. albicans was determined in comparison to untreated (negative control) cells. Fig. 3 shows the time required to reduce the load of surviving fungal pathogens by an order of magnitude. The untreated negative control and cells treated with water and surfactant did not cause any measurable fungal inactivation. Interestingly, there was more rapid inactivation with EONE than the nystatin antifungal agent, and the obtained values were significantly different (P value < 0.001) when compared to untreated negative controls. Nanoemulsions at biocidal concentrations are nontoxic to mucosal membranes and gastrointestinal tract tissues (Hamouda et al., 1999, Sonneville et al., 2004, Baker et al., 2000). The nanoemulsion showed significant activity at dilutions of 1 and 5 (P value < 0.001), whereas nystatin exhibited the highest activity at dilutions of 1. Statistically significant values were calculated in comparison to negative untreated controls. Thus, the killing kinetics of the current study revealed that EONE has resilient fungicidal effects. These results can be explained by the synergism between EO bioactive substances and nanodroplets, resulting in significant antifungal activity against fungal pathogens (Bala et al., 2010, Ajaiyeoba, 2000).
Fig. 3.
Kinetics of fungicidal activity of EONE on C. albicans treated with different concentrations for a variable period of time (1, 2, 5, 10 and 15 h) at 37 °C. NY -Nystatin, EONE – Essential oil nanoemulsion (1, 5, 30 and 60 dilutions), NC – Negative control.
3.5. Effects of an EONE nanoemulsion on biofilm
Biofilms represent syntrophic microbial colonies that are attached to the surface and encapsulated by the extracellular matrix produced by microbial communities (Mah and O'Toole, 2001). The isolated pathogenic C. albicans are able to form biofilms, which are important characteristics of virulence (Lewis, 2001). The present nanoemulsion was evaluated to determine its potential to suppress and diminish the biofilm formation (Fig. 4). EONE act efficiently against the biofilm formation of pathogenic C. albicans. The highest reduction in biofilm formation was observed at 70% MFC. The results showed that C. viscosa EONE has more potential targets against pathogenic C. albicans compared to the commonly used nystatin antifungal agent. This may be due to the presence of high levels of bioactive flavonoids, alkaloids and tannins in C. viscosa EO (Bala et al., 2010).
Fig. 4.
The anti-biofilm activity of EONE against C. albicans strains.
3.6. Spectroscopic analysis of chitin levels
Chitin level reflects the cell wall deformation of pathogenic fungi due to inactive metabolic activity. Levels of both chitin and ergosterol have been found to be similar under identical nutritional growth conditions (Nylund and Wallander, 1992, Kitajima, 2000). To confirm the cell wall disruption or deformation, spectroscopic analysis of chitin was performed. TB has a strong affinity and emits fluorescence intensity for chitin of the fungal cell wall. For TB steady-state excitation and fluorescence spectra, the experimental configuration, such as slit width (10 nm), the voltage of the xenon lamp (750 V) and scan rate (1000 nm/min), were the same for all experiments. Fig. 5a (untreated cells) shows the excitation (green solid line) and emission spectra (red and blue dotted lines) of untreated cells. The excitation spectrum showed two peaks, one at 285 nm (due to transitions) and the other at 225 nm (due to transitions). The fluorescence spectra were obtained by excitation with two peak wavelengths. The fluorescence spectra had a peak approximately 350 nm, and the profile remained the same for both excitation wavelengths with proportional intensities, indicating that there is only one emitting species. The samples were excited at 225 and 285 nm for all experiments. However, for simplicity, we discuss only the 225 nm results because the fluorescence spectra excited with 285 gives similar result but with low intensity. Fig. 5b shows the excitation and emission spectra of nystatin (NS) and NE; when compared to control, both NS and NE intensities were low, indicating both had inhibited chitin levels and thus lower amounts of TB bound to the cell wall. The obtained fluorescence spectra of TB staining in treated cells matched with previous studies of TB staining for pure chitin (Liesche et al., 2015, Merzendorfer, 2012), indicating fluorescent binding of chitin in the treated cells. The inhibitory effect of NE was at least two-fold greater than NS, which is evident in the fluorescence intensities where the intensity of NS was 795 a.u. This indicates greater chitin presence when compared to the intensity of NE (339 a.u.). Therefore we speculate that the EONE inhibit chitin synthesis thereby exhibit antifungal activity.
Fig. 5.
Excitation and emission spectra of cell wall components (Chitin) stained by Trypan Blue with C. albicans cells. a. Fluorescence spectra of untreated cell wall components; b. Fluorescence spectra of EONE- and Nystatin-treated cell wall components.
3.7. Fourier-transformed infrared spectroscopy (FT-IR)
FTIR (Fourier transformed infrared spectroscopy) is a vibrational spectroscopic technique used to confirm molecular fingerprints. FTIR spectroscopic analysis was performed to confirm the changes in EONE-treated cells at a molecular fingerprint level. Fig. 6a shows untreated control cells, b shows EONE spectra, and c shows EONE-treated cells. Their entirely different band confirms that changes occurred at the molecular level. The band between 550 and 1300 cm−1 is characteristic of macromolecules such as polysaccharides (Novák et al., 2012, Galichet et al., 2001, Križková et al., 2001); decreased intensity peaks at 663, 1102, and 1251 cm−1 were observed on NE-treated cells that are reflective of a reduction in the amount of polysaccharides in the cell wall (Rumengan et al., 2014). The band at 2926 cm−1 represents C-H stretching and bands for chitin (Teng et al., 2001). NE-treated cells showed very broad absorption at 3443, 2926, 2863 and 1743 cm−1 due to decreases in chitin (ν ( CH—)) levels in membranes of the treated cells (Kamnev, 2008). Results for this assay showed peak patterns consistent with standard chitin peaks of previous reports (Ospina Álvarez et al., 2014). The bands at 1743 cm−1 correspond to amide stretching of C O, while the bands at 1251 cm−1 correspond to the N—H deformation of amides. The FTIR spectral reports in the present study confirmed the wide range of molecular changes in EONE-treated C. albicans cells when compared to untreated cells.
Fig. 6.
Superimposed FTIR spectra of a. Untreated control C. albicans cells, b. EONE and c. EONE-treated C. albicans cells.
3.8. SEM analysis of morphological characteristics
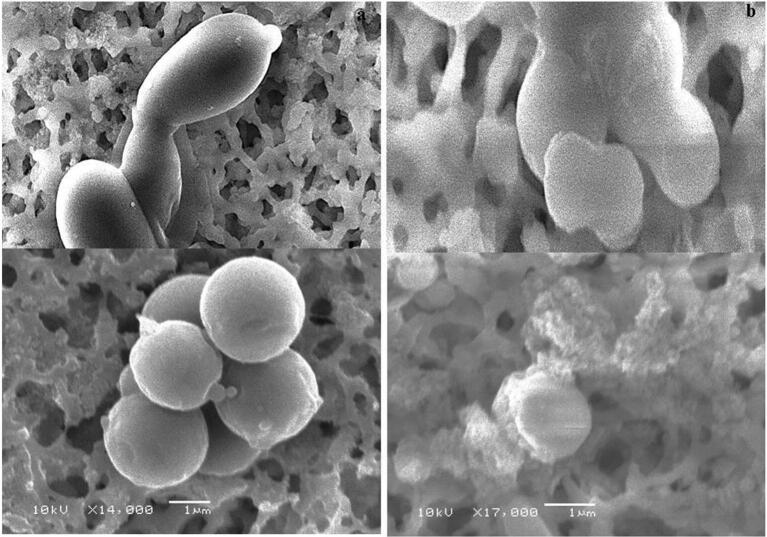
The structural modification effect of EONE on C. albicans cells was examnined by SEM analysis. SEM analysis showed that on the surface of C. albicans treated with the MIC of EONE (Fig. 7b), the cell surfaces were aberrant compared to those of untreated control cells (Fig. 7a). In addition, treated cells showed loss of their linear structure, irregularly located scars on the cell surface and abnormal cells due to disrupted cell walls (Escalante et al., 2008, Freires et al., 2014). The untreated control cell surfaces appeared clear and smooth. These results speculate that EONE act via inhibition of cell wall synthesis and cause collateral damage on C. albicans.
Fig. 7.
SEM imaging of Morphological changes. a. Untreated C. albicans cells; b. EONE-treated C. albicans cells.
4. Conclusion
In conclusion, a stable food-grade nanoemulsion using essential oils of C. viscosa with droplet sizes below 20 nm demonstrated remarkable inhibitory effects against foodborne pathogenic C. albicans. The synthesized nanoemulsion showed significant fungicidal effects on C. albicans and several morphological disruption effects, at least partially by interfering with cell wall biosynthesis. These EONE-based safe, natural products by a bio-based approach hold potential for providing quality and safety benefits for food preservation against microbial spoilage with negligible toxicity, according to the FDA.
Acknowledgements
The authours acknowledge the support from the Researchers supporting project number (RSP-2020/178), King Saud University, Riyadh, Saudi Arabia. Also, we thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ajaiyeoba E.O. Phytochemical and antimicrobial studies of Gynandropsis gynandra and Buchholzia coriaceae. Afric. J. Biomed. Res. 2000;3:161–165. [Google Scholar]
- Baker, J.R., Wright, D.C., Hayes, M.M., Hamouda, T., Brisker, J.M., 2000. Methods of inactivating bacteria including bacterial spores. US patent no. 6,015,832.
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – A review. Food Chem. Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bala A., Kar B., Haldar P.K., Mazumder U.K., Bera S. Evaluation of anticancer activity of Cleome gynandra on Ehrlich's Ascites Carcinoma treated mice. J. Ethnopharmacol. 2010;129(1):131–134. doi: 10.1016/j.jep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Calo J.R., Crandall P.G., O'Bryan C.A., Ricke S.C. Essential oils as antimicrobials in food systems – A review. Food Control. 2015;54:111–119. [Google Scholar]
- CLSI Performance standards for antimicrobial susceptibility; sixteenth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA, USA, 2006:M100–S16. [Google Scholar]
- Donsì F., Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Escalante A., Gattuso M., Pérez P., Zacchino S. Evidence for the Mechanism of Action of the Antifungal Phytolaccoside B Isolated fromPhytolacca tetrameraHauman. J. Nat. Prod. 2008;71(10):1720–1725. doi: 10.1021/np070660i. [DOI] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Rodríguez-Tudela J.L., Martinez-Suárez J.V. Comparison of two alternative microdilution procedures with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro testing of fluconazole-resistant and -susceptible isolates of Candida albicans. J. Clin. Microbiol. 1995;33:3154–3158. doi: 10.1128/jcm.33.12.3154-3158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J., Alves D., Neves O., Silva J., Gibbs P., Teixeira P. Effects of the components of two antimicrobial emulsions on food-borne pathogens. Food Control. 2010;21(3):227–230. [Google Scholar]
- Freires I.D., Murata R.M., Furletti V.F., Sartoratto A., Alencar S.M., Figueira G.M., Rosalen P.L. Coriandrum sativum L. (Coriander) Essential Oil: Antifungal Activity and Mode of Action on Candida spp., and Molecular Targets Affected in Human Whole-Genome Expression. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0099086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galichet A., Sockalingum G., Belarbi A., Manfait M. FTIR spectroscopic analysis ofSaccharomyces cerevisiaecell walls: study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Lett. 2001;197(2):179–186. doi: 10.1111/j.1574-6968.2001.tb10601.x. [DOI] [PubMed] [Google Scholar]
- Ghosh V., Mukherjee A., Chandrasekaran N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013;20(1):338–344. doi: 10.1016/j.ultsonch.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Hamouda T., Hayes M., Cao Z., Tonda R., Johnson K., Wright D., Baker J. A Novel Surfactant Nanoemulsion with Broad-Spectrum Sporicidal Activity againstBacillusSpecies. J. Infect. Dis. 1999;180(6):1939–1949. doi: 10.1086/315124. [DOI] [PubMed] [Google Scholar]
- Hecker M.T., Aron D.C., Patel N.P., Lehmann M.K., Donskey C.J. Unnecessary Use of Antimicrobials in Hospitalized Patients. Arch. Intern. Med. 2003;163(8):972. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- Hwang Y.Y., Ramalingam K., Bienek D.R., Lee V., You T., Alvarez R. Antimicrobial Activity of Nanoemulsion in Combination with Cetylpyridinium Chloride in Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013;57(8):3568–3575. doi: 10.1128/AAC.02109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamnev A.A. FTIR spectroscopic studies of bacterial cellular responses to environmental factors, plant-bacterial interactions and signalling. Spectroscopy. 2008;22(2–3):83–95. [Google Scholar]
- Kitajima Y. Structural and Biochemical Characteristics of Pathogenic Fungus: Cell Walls, Lipids and Dimorphism, and Action Modes of Antifungal Agents. Nippon Ishinkin Gakkai Zasshi. 2000;41(4):211–217. [PubMed] [Google Scholar]
- Križková L., Ďuračková Z., Šandula J., Sasinková V., Krajčovič J. Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat. Res./Gen. Toxicol. Environ. Mutagen. 2001;497(1–2):213–222. doi: 10.1016/s1383-5718(01)00257-1. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy R., Athinarayanan J., Periasamy V.S., et al. Antimicrobial activity of nanoemulsion on drug-resistant bacterial pathogens. Microb. Pathog. 2018;120:85–96. doi: 10.1016/j.micpath.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Lahkar, V., Saikia, L., Patgiri, S.J., Nath, R., Das, P.P., 2017. Estimation of biofilm, proteinase & phospholipase production of the Candida species isolated from the oropharyngeal samples in HIV-infected patients. Indian J. Med. Res. 145(5), 635–640. [DOI] [PMC free article] [PubMed]
- Lewis K. Riddle of Biofilm Resistance. Antimicrob. Agents Chemother. 2001;45(4):999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R.S., Rennie R.P., Talbot J.A. Assessment of the Effect of Amphotericin B on the Vitality of Candida albicans. Antimicrob. Agents Chemother. 1999;43(5):1034–1041. doi: 10.1128/aac.43.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesche, J., Marek, M., Günther-Pomorski, T., 2015. Cell wall staining with Trypan blue enables quantitative analysis of morphological changes in yeast cells. Front. Microbiol. 6. [DOI] [PMC free article] [PubMed]
- Liu, Q., Huang, H., Chen, H., Lin, J., Wang, Q., 2019. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 24(23), 4242. [DOI] [PMC free article] [PubMed]
- Luu L.N., Cowen L.E., Sirjusingh C., Kohn L.M., Anderson J.B. Multilocus Genotyping Indicates that the Ability to Invade the Bloodstream Is Widespread among Candida albicans Isolates. J. Clin. Microbiol. 2001;39(4):1657–1660. doi: 10.1128/JCM.39.4.1657-1660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah T.C., O'Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Mali R.G. Cleome viscosa (wild mustard): A review on ethnobotany, phytochemistry, and pharmacology. Pharm. Biol. 2009;48(1):105–112. doi: 10.3109/13880200903114209. [DOI] [PubMed] [Google Scholar]
- Merzendorfer H. Chitin synthesis inhibitors: old molecules and new developments. Insect Sci. 2012;20(2):121–138. doi: 10.1111/j.1744-7917.2012.01535.x. [DOI] [PubMed] [Google Scholar]
- Mohandas V., Ballal M. Distribution of Candida species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in Southern India. J. Glob. Infect. Dis. 2011;3:4–8. doi: 10.4103/0974-777X.77288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou D., Chen H., Du D., Mao C., Wan J., Xu H., Yang X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int. J. Pharm. 2008;353(1–2):270–276. doi: 10.1016/j.ijpharm.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Novák M., Synytsya A., Gedeon O., Slepička P., Procházka V., Synytsya A., Čopíková J. Yeast β(1–3), (1–6)-d-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012;87(4):2496–2504. [Google Scholar]
- Nylund J., Wallander H. 5 Ergosterol Analysis as a Means of Quantifying Mycorrhizal Biomass. Meth. Microbiol. 1992:77–88. [Google Scholar]
- Ospina Álvarez S.P., Ramírez Cadavid D.A., Escobar Sierra D.M., Ossa Orozco C.P., Rojas Vahos D.F., Zapata Ocampo P., Atehortúa L. Comparison of extraction methods of chitin from Ganoderma lucidum mushroom obtained in submerged culture. Biomed Res Int. 2014;2014:169071. doi: 10.1155/2014/169071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro A.S., Cabral-Albuquerque E., Ferreira D., Sarmento B. Chitosan: An option for development of essential oil delivery systems for oral cavity care? Carbohydr. Polym. 2009;76(4):501–508. [Google Scholar]
- Periasamy V.S., Athinarayanan J., Alshatwi A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem. 2016;31:449–455. doi: 10.1016/j.ultsonch.2016.01.035. [DOI] [PubMed] [Google Scholar]
- Price M.F., Wilkinson I.D., Gentry L.O. Plate method for detection of phospholipase activity inCandida albicans. Med. Mycol. 1982;20(1):7–14. doi: 10.1080/00362178285380031. [DOI] [PubMed] [Google Scholar]
- Ramalingam K., Amaechi B.T., Ralph R.H., Lee V.A. Antimicrobial activity of nanoemulsion on cariogenic planktonic and biofilm organisms. Arch. Oral Biol. 2012;57(1):15–22. doi: 10.1016/j.archoralbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumengan F.M., Suryanto E., Modaso R., Wullur S., Tallei T.E., Limbong D. Structural Characteristics of Chitin and Chitosan Isolated from the Biomass of Cultivated Rotifer, Brachionus rotundiformis. Int. J. Fish. Aquat. Sci. 2014;3(1):12–18. [Google Scholar]
- Seow Y.X., Yeo C.R., Chung H.L., Yuk H. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2013;54(5):625–644. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- Serra E., Hidalgo-Bastida L., Verran J., Williams D., Malic S. Antifungal Activity of Commercial Essential Oils and Biocides against Candida Albicans. Pathogens. 2018;7(1):15. doi: 10.3390/pathogens7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.H., Kee S.J., Shin M.G., Kim S.H., Shin D.H., Lee S.K., Ryang D.W. Biofilm Production by Isolates of Candida Species Recovered from Nonneutropenic Patients: Comparison of Bloodstream Isolates with Isolates from Other Sources. J. Clin. Microbiol. 2002;40(4):1244–1248. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville A.O., Simonnet J., L'Alloret F. Nanoemulsions: a new vehicle for skincare products. Adv. Colloid Interface Sci. 2004;108–109:145–149. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Stepanović, S., Vuković, D., Hola, V., Bonaventura, G.D., Djukić, S., Ćirković, I., Ruzicka, F., 2007. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115(8), 891–899. [DOI] [PubMed]
- Teng W.L., Khor E., Tan T.K., Lim L.Y., Tan S.C. Concurrent production of chitin from shrimp shells and fungi. Carbohydr. Res. 2001;332(3):305–316. doi: 10.1016/s0008-6215(01)00084-2. [DOI] [PubMed] [Google Scholar]
- Tscherner M., Schwarzmüller T., Kuchler K. Pathogenesis and Antifungal Drug Resistance of the Human Fungal Pathogen Candida glabrata. Pharmaceuticals (Basel). 2011;4(1):169–186. [Google Scholar]
- Urbánková L., Kašpárková V., Egner P., Rudolf O., Korábková E. Caseinate-Stabilized Emulsions of Black Cumin and Tamanu Oils: Preparation, Characterization and Antibacterial Activity. Polymers (Basel) 2019;11(12):1951. doi: 10.3390/polym11121951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebusch L., De Almeida-Apolonio A.A., Rodrigues L.M., De Paula Bicudo B., Dos Santos Silva D.B., Lonchiati D.F., De Oliveira K.M. Candida albicans isolated from urine: Phenotypic and molecular identification, virulence factors and antifungal susceptibility. Asian Pacif. J. Trop. Biomed. 2017;7(7):624–628. [Google Scholar]
- Williams L., Vasques E., Reid W., Porter R., Kraus W. Biological activities of an extract from Cleome viscosa L. (Capparaceae) Naturwissenschaften. 2003;90(10):468–472. doi: 10.1007/s00114-003-0460-1. [DOI] [PubMed] [Google Scholar]
- Wooster T.J., Golding M., Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24(22):12758–12765. doi: 10.1021/la801685v. [DOI] [PubMed] [Google Scholar]
- Zuza A.D.L., Silva-Rocha W.P., Chaves G.M. An Update on Candida tropicalis Based on Basic and Clinical Approaches. Front. Microbiol. 2017;13(8):1927. doi: 10.3389/fmicb.2017.01927. [DOI] [PMC free article] [PubMed] [Google Scholar]