Abstract
Liver disorders may occur as a result of exposure to chemical compounds capable of inducing the oxidative stress and hepatic injuries. The aim of present study was to investigate the effects of flower extracts of B. Variegata for the treatment of liver injury induced by the CCl4. About 1 ml/kg body weight (b.w) of CCl4 was induced to experimental mice by intraperitoneal way for 14 days. The methanol and chloroform extracts (100, 200 and 300 mg/kg b.w) were administered to experimental animals for 14 days along with standard drug Silymarine (100 mg/kg b.w). The extracts alone showed no evidence of hepatic toxicity but animals exposed to CCl4 without the treatment with B. Variegata presented variations in levels of liver enzymes, antioxidant enzymes, proteins and blood cells as well as injuries in liver cells were also observed during histopathological study. However, after the treatments especially with 300 mg/kg b.w of methanol flower extracts levels of liver markers (ALT, AST and ALP), antioxidant enzymes and blood cells decreases and turned towards normal levels. Whereas level of total proteins and bilirubin was improved and damaged liver cells were repaired. The curative activity of flower extracts can be correlated to the higher potential of antioxidants and occurrence of Quercetin and some other organic compounds those were investigated from flower extracts of B. Variegata during HPLC and GC-MS analysis. The finding of this study supports the use of B. Variegata flower formulation in folk medicines.
Keywords: B. Variegata, Phytochemicals, HPLC, Antioxidants, Hepatoprotective effect
1. Introduction
Medicinal plants play significant role as raw material for important drugs; those are providing remedies against many human disorders. Report of world health organization (WHO) interpret that almost 80% of the world’s population rely on traditional system of folk remedies for primary health care and that is providing considerable economic benefit for the treatment of various diseases (Igoli et al., 2005, Munazir et al., 2012). Medicinal plants produce diversity of secondary metabolites like polyphenols (flavonoids, tannins), saponins, alkaloids and carotenoids which are acts as potent antioxidant compounds (Kaur et al., 2006). Even though flavonoids possess different biochemical activities however the preeminent defined property of flavonoids is the capability to act as antioxidant compounds (Cody et al., 1986).
Oxidative stress is triggered by free radicals that deplete the sources of antioxidant enzymes and decline the functioning of the liver cells. Liver is prone to oxidative stress induced damage caused by free radicals. To scavenge free radicals, antioxidant defense system can reduce their harmful effects (Choudhary and Tandon, 2009). Natural antioxidants either in the form of raw extracts or their chemical constituents are very effective to prevent the destructive processes caused by oxidative stress which is accountable for a lot of disorders in humans such as infections, cancer, diabetes, Alzheimer’s diseases, arthritis, cardiovascular diseases and AIDS (Oluyemi et al., 2007, Zengin et al., 2011). Drugs derived from natural sources play a considerable role in the prevention and treatment of human diseases (Farnsworth, 1993).
About 6,000 diverse varieties of plants are found in Pakistan’s flora and assert enormous exotic and intuitive plant species as the possible resource for improving health standards of local occupants in long term future across numerous cultures. Rural communities rely on medicinal plants for curing their ailments due to their quick accessibility (Mustafa et al., 2016, Irum et al., 2019).
Bauhinia Variegata Linn.is an edible plant belongs to family (Fabaceae/Leguminosae) consists of approximately 300 species. The plant is broadly dispersed in most tropical countries, including Africa, Asia and South America. The leaves, flowers and stem-bark of Bauhinia have been used over and over again in folk medicine as a remedy for different kinds of pathologies (da Silva and Filho, 2002, Cavalcanti and Favoretto, 2005, Filho, 2009). Young flowers have been used by inhabitants of hilly areas from northern east of Punjab as vegetable to get relief from different diseases including liver disorders. It is a common practice to isolate compounds from plant extracts, a number of different separation techniques including HPLC and GC–MS are widely to get possible information about pure compounds. In present work flower extracts of B. Variegata were assessed for their possible phytochemicals contents as well as for in vivo and in vitro bioactivities.
2. Material and methods
2.1. Plant samples collection
The fresh young flowers of Bauhinia Variegata Linn were collected from Tehsil Kotli Sattian (District Rawalpindi) in the flowering period of March - April 2016. The flower samples were identified by experienced taxonomist Dr. Mushtaq Ahmad, Associate Professor in the Department of Plant sciences, Quaid-i- Azam University Islamabad Pakistan. The voucher specimen was maintained in the Herbarium of Pakistan (ISL) Department of Plant sciences, Quaid-i-Azam University for future reference (BV115).
2.2. Plant extraction
The fresh flowers of Bauhinia Varigata Linn (BV) were washed with double distilled water and dried in the shade for 4–5 days. The dried flowers were crushed into fine powder by using Willy machine. There has been no mesh sizing done and the coarse powder was used for extract preparation using organic solvents. A 10% solution of flower powder in methanol (BVM) and chloroform (BVC) was prepared followed by solvent extraction. The obtained extract was dried and evaporated in the rotary evaporator. Then the dried extracts were stored at 4 °C for more chemical analysis.
2.3. Phytochemicals estimation
The dried flower extracts were used for the quantitative assessment of phytochemicals to quantify the total phenols (Harborne, 1998), total flavonoids (AOAC 2003), saponins (Marinova et al., 2005), alkaloids and tannins (Abbasi et al., 2015).
2.4. Antioxidants assays
2.4.1. DPPH assay
The free radical scavenging ability of the plant extracts were assessed by using 1,1 diphenyl 1–2 -picryl-hydrazyl (DPPH) assay. Plant concentrations (20 to 100 µg/ml) with DPPH solution (2 ml) were incubated in darkness (30 min). Measurement of test sample was recorded at 517 nm against blank mixture (DPPH). Standard or positive control gallic and ascorbic acid were used for comparison. Inhibition concentration (IC50) was obtained from linear regression equation. Antioxidant potential is achieved by following formula:
x = Absorbance without plant extract
y = Absorbance with plant extract
2.4.2. ABTS scavenging activity
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) method is defined by (Ashafa et al., 2010). Oxidation of ABTS solution (3 mM) was done by potassium persulfate (2.5 mM) and the above mixture solution was placed in dark (12 h). After incubation, mixture was diluted with the help of distilled water to get an absorbance of 2.5. Concentrations of plant extract (20–100 µg/ml) and positive controls (standard) were analyzed at 734 nm. The Scavenging activity was determined by using formula:
x = Absorbance without plant extract
y = Absorbance with plant extract
2.4.3. H2O2 assay
The oxidant scavenging of hydrogen peroxide (H2O2) was used according to the method of (Ruch et al., 1989). H2O2 solution (4 mM) was formulated in phosphate buffer (50 mM) with 7.4 pH. Put the mixture in plant concentrations to get their volume up to 4 ml followed by shaking and incubation (10 min). Absorbance of blank (phosphate buffer), test samples and standards were taken at 230 nm. Scavenging capacity was assessed by the formula:
2.4.4. Estimation of flavonoids with high performance liquid Chromatography (HPLC)
HPLC analysis was performed via Shimadzu HPLC system (Tokyo, Japan) specified with C18 column (250 × 4.5 mm, 5 m), gradient pump with detector (UV/Visible). Biocompounds were extracted by different mobile phases (Acetonitrile & 0.1% phosphoric acid with the ratio of 36:64), and the taken volume of injection was 20 µl. Quercetin from the samples was quantified at 285 nm per flow rate (1 ml/min). The standard used in above analysis was Quercetin and all the measurements were taken in triplicates.
2.4.5. Gas chromatography-Mass Spectrometry analysis (GC–MS)
GC–MS model QP2010 (Shimadzu®) equipped with following specifications; RTx- 5MS capillary column (cross bond 5% diphenyl – 95% dimethylpolysiloxane), film thickness (30 m × 0.25 mm × 0.25 μm) and carrier gas (helium) in split less inject mode @ 250 °C. Provisions of column include 1.2 ml/min flow rate @ linear velocity mode, oven temperature programmed@150 °C for 1 min and then at 4 °C /min to 150 °C for10 min. Volume of injection (0.2 µl), 50:1 split ratio and injector temperature (275 °C) with N2 carrier gas (1.0 ml/min).A mass spectrum was operated at 70 eV electron ionization in SIM (Selected Ion Monitoring) manner and ions were taken (m/z). Peaks obtained were tinted and compared with NIST library’s spectral data base for compound identification.
2.4.6. Identification of compounds
GC–MS mass spectrum interpretation was performed by using NIST (National Institute of Standard and Technology). Compound name, structure, molecular weight and percentage were estimated by the comparison of an average area with total area. Spectra of unidentified components were matched with Software Turbo mass 5.2 (2005 version).
2.4.7. Hepatoprotective effect
Total 50 albino male mice weighing (50 ± 2.0 g) were obtained from NIH (National institute for health) Islamabad. Experimental work followed all precautions and guidelines proposed by ethical committee of an Institute (National Veterinary Lab, Islamabad). Mice were kept in the animal house with following conditions: Standard animal house conditions were observed with special monitoring and maintenance. commercial chow (Feed Mills, Islamabad), water ad libitum and proper.
2.4.8. Acute oral toxic study
The study was performed to standardize the dose of botanicals for animals as previously manifested by (Gulfraz et al., 2008).
2.4.9. In-vivo study
Animals were categorized into ten groups and each group consists of five mice. GroupI and II were named “Normal group” and “Olive oil group”. They were provided normal feed till 14 days with 1 ml olive oil to olive group. Group III were given CCl4 (1 ml/kg b.w) by intraperitoneal way for 14 days to induce toxicity. Group IV, V and VI were given methanol extracted plant drug @ 100 mg/kg b.w, @ 200 mg/kg b.w and @ 300 mg/kg b.w when toxicity is created by CCl4.Group VII, VIII and IX were delivered 100 mg/kg b.w, 200 mg/kg b.w and 300 mg/kg b.w chloroform extracted plant drug after CCl4 induction. Group X was provided standard control drug “Silymarine”100 mg/kg b.w to cure toxicity of CCl4. All the plant and standard drug doses given by gavage. On fifteenth day animals were initially weighed and then sacrifice for the removal of blood and liver for different examinations. Blood was centrifuged @3000 rpm (10 min) for serum separation. Liver was removed, washed and weighed for the comparison. Subsequently, blood, serum and liver were stored at −20°C prior to analysis.
2.4.10. Biochemical analysis
The biochemical markers (AST, ALP, ALT and Bilirubin) were verified with kits (AMS diagnostic, Italy). Red Blood Cells (RBCs), White Blood Cells (WBCs) and Platelets were calculated with the procedures by (Dacie, 2006). Liver derived antioxidant enzymes i.e. SOD, GPx, CAT & total protein were assessed by the procedures of (Winterbourn and Metodiewa, 1999, Misra and Fridovich, 1972, Flohe and Günzler, 1984, Lowry et al., 1951).
2.4.11. Histopathology assessment
Mice liver was wisely handled after slaughtering and saved in formalin (10%) to protect it from oxidation or damage. Tested specimens were dehydrated in alcohol then passed into different xylene changes and fix into paraffin section. For histopathological investigations, specimen was cut by microtome with 5 µm thickness, mounted at glass slides and after that stained through Ehrlich’s hematoxylin as well as eosin counter stain.
2.4.12. Statistical analysis
Statistical software Graph pad prism 7 was used to calculate mean (±), SE (standard error), probability and Pearson coefficient correlation.
3. Results and discussion
3.1. Quantitative phytochemical screening
The beneficial properties of medicinal plants are probably due to occurrence of various secondary metabolites, those serves as defense mechanism against many microorganisms (Britto and Sebastian, 2012).Therefore due to strong evidence obtained during screening of phytochemicals various secondary metabolites like flavonoids, phenols, tannins, saponins, alkaloids and carotenoids were quantified from various flower extracts of B. Variegata (Table 1). B. Variegata possesses significantly higher quantity of total phenols 21.34 ± 2.63 and 17.66 ± 1.3 mg/g in both DVM and DVC. Subsequently 18.16 ± 3.1 and 12.18 ± 0.21 mg/g flavonoid contents are present in DVM and DVC. Methanol extracted more quantity of phytochemicals as compare to chloroform. Methanol has a potential for the diverse richness of phytochemical extraction and found to be more effective for isolating numerous phyto-constituents (Maqsood et al., 2017). The initial approximation may be useful in uncovering the bioactive compounds and subsequently may lead to the drug discovery and development (Bhuiyan et al., 2009).
Table 1.
Quantification of various phyto-constituents from flower extracts of Bauhinia Variegata.
| Sample extracts | Total Flavonoids (mg QE/g) |
Total phenols (mg GAE/g |
Alkaloids (mg/g) |
Tannins (mg/g) |
Saponins (mg/g) |
|---|---|---|---|---|---|
| Methanol extracts | 18.16 ± 3.1Þ | 21.34 ± 2.63° | 11.89 ± 0.25° | 9.63 ± 0.35Þ | 5.29 ± 0.85Þ |
| Chloroform extracts | 12.18 ± 0.21Þ | 17.66 ± 1.3Þ | 8.18 ± 0.24° | 7.38 ± 0.56° | 3.38 ± 0.24° |
All the values were taken in triplicates, Mean ± standard deviation (SD) while = p < 0.01,Þ= p < 0.05°, QE = Quercetin equivalent and GAE = Gallic acid equivalent.
3.2. Antioxidant assays
In the present study, three different antioxidant methods have been used for evaluation of free radical scavenging activity of methanol and chloroform extracts of B. Variegata (Table 2). It was observed that flower extracts of B. Variegata has produced strong antioxidant activities (BVM: IC50 = 21.93 ± 0.43, 47.25 ± 0.39 and 47.88 ± 0.16 µg/ml) (DVC: IC50 = 34.85 ± 0.33, 48.2 ± 0.61 and 59.02 ± 0.80 µg/ml) against DPPH, ABTS and hydrogen peroxide antioxidant assay. BV extracts were more active against DPPH scavenging assay. Antioxidant potential of medicinal plant is thought to be accredited by the presence of their active constituents like flavonoids and phenols. Excessive production of reactive oxygen species or insufficient antioxidant resistance induces oxidative stress in human body and cause degenerative diseases. Antioxidant activity control series of mechanisms e.g. scavenges free radicals, decomposes peroxides, reducing capacities, prevent binding of transition metal ions and inhibit chain initiation. The achieved results in the present study concord the previous findings described for the root and stem analysis of B. variegata (Rajani and Ashok, 2009) (see Table 3).
Table 2.
IC50 values of radical scavenging potential of various extracts of B. Variegata.
| Extracts | DPPH Assay | H2O2 Assay | ABTS Assay | Ascorbic acid | Gallic acid |
|---|---|---|---|---|---|
| Methanol | 21.93 ± 0.43Þ | 47.88 ± 0.16° | 47.25 ± 0.39° | 7.22 ± 0.38° | 2.434 ± 0.74 Þ |
| Chloroform | 34.85 ± 0.33 Þ | 59.02 ± 0.80° | 48.2 ± 0.61° | 8.69 ± 0.39 Þ | 5.87 ± 0.22 Þ |
Results are Means ± SD, (n = 3), expressed in µg/ml whereas ° = p < 0.01,Þ= p < 0.05
Table 3.
GC–MS analysis of Organic compounds identified in Bauhinia Variegata.
| Peak | R.T | Area (%) | Molecular Formula | Molecular weight | Name of compound |
|---|---|---|---|---|---|
| 1 | 1.136 | 4.213 | C4NiO4 | 170 | Nickel tetracarbonyl |
| 2 | 1.178 | 10.321 | C3H6O | 58 | Propanone |
| 3 | 15.776 | 35.092 | C38H68O8 | 652 | 1-(+)-Ascorbic acid 2, 6-dihexadecanoate |
| 4 | 19.215 | 35.740 | C21H40O4 | 356 | 2,3-Dihydroxypropyl elaidate SS 2,3-Dihydroxy |
| 5 | 19.626 | 10.314 | C18H36O2 | 284 | Stearic acid |
| 6 | 21.907 | 1.580 | C44H86O3 | 662 | Docosanoic anhydride |
| 7 | 27.710 | 0.616 | C23H40O4 | 380 | Sebacic acid |
| 8 | 27.940 | 0.710 | C60H122 | 842 | Hexacontane |
| 9 | 29.510 | 0.271 | C36H70O2 | 534 | Hexadecenoic acid |
| 10 | 30.750 | 1.140 | C54H108Br2 | 914 | Tetrapentacontane |
Organic compounds along with retention time (R.T) and Peak area (%) obtained by GC–MS analysis.
3.3. Quercetin analysis by HPLC
Analysis of methanol flower extracts of B. Variegata with HPLC revealed that significant quantity of quercetin (3.5 mg/g) at peak area of 248,178 and 12.5 retention time (Fig. 1, Fig. 2).Flavonoids being an important group of secondary metabolites play a dynamic role for the protection against various diseases. Plant phytochemicals such as flavonoids control the activity of enzymes and human cells, and found to have antitumor, anti-hepatotoxic, anti-inflammatory and anti-allergic activities (Simpson et al., 2011). Moreover flavonoids provide health benefits and have oxidant quenching activities in both in vivo and in vitro systems by their role as dietary component (Cook and Samman, 1996). Quercetin is a known bioflavonoid has an antioxidant & anti-inflammatory activity and exhibited unique anticancer properties.
Fig. 1.
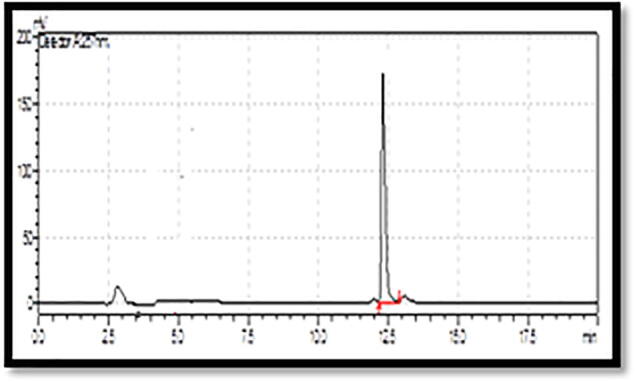
Chromatogram of Quercetin standard.
Fig. 2.
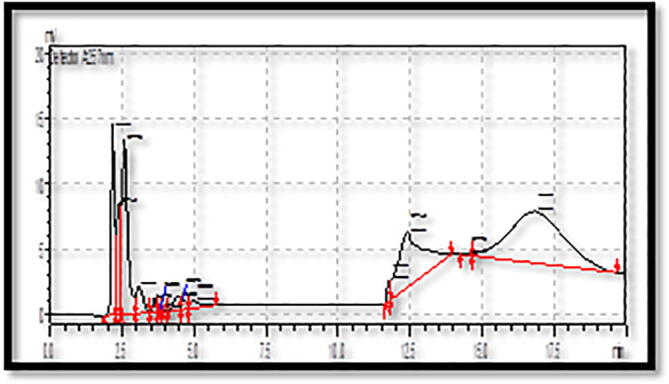
HPLC analysis of Quercetin from B. variegata.
Quercetin (C15 H10 O7) is a poly-phenolic heterocyclic flavonoid compound contains 302.235 g/mol molecular weight and commonly found in fruits and vegetables as most abundant dietary flavonols with average intake of 25–50 mg (Formica and Regelson, 1995) (see Table 4).
Table 4.
Molecular structure of the compounds identified by GC–MS.
| Compounds | Molecular formula | Molecular Structures |
|---|---|---|
| 1-(+)-Ascorbic acid 2, 6-dihexadecanoate | C38H68O8 | 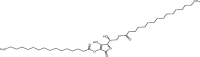 |
| 2,3-Dihydroxypropyl elaidate SS 2,3-Dihydroxy | C21H40O4 | |
| Stearic acid | C18H36O2 | |
| Docosanoic anhydride | C44H86O3 |  |
3.4. Identification of compounds by GC–MS
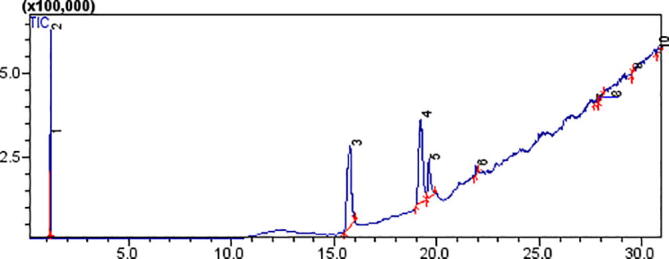
Methanol flower extracts of B. Variegata was subjected to Gas Chromatography Mass Spectrometry analysis and results are demonstrated in Fig. 3 and Table 2. Comparison of chromatogram and compounds gave peak area (%) and further information was attained from the data base of NIST library. GC–MS spectrum showed 10 compounds and out of which 4 compounds illustrated higher substantial peaks. Results showed that 1-(+)-Ascorbic acid 2, 6-dihexadecanoate (35.092%), Dihydroxypropylelaidate (35.740%), Stearic acid (10.314%) and Docosanoic anhydride (1.580%) was observed on Peak 3, 4, 5 and 6 in BV flower extract.
Fig. 3.
Chromatogram showing analysis of methanol flower extracts of B. Variegata by using GC–MS.
After comprehensive survey of literature it was known that these organic compounds were not earlier reported from leaves, flower and root extracts of B. Variegata, therefore our claim for first time investigation of these compounds from flower of extracts of B. Variegata is strongly justified. Hence according to our results presence of biologically important organic compounds in B. Variegata is a good proof for the use of this extract in folk medicines against human disorders (Pinheiro et al., 2017). Furthermore 1-(+)-Ascorbic acid 2, 6-dihexadecanoate (C38H68O8) is an essential fatty acid and ascorbic acid is a strong antioxidant compound and being use as food additive, whereas 2,3-Dihydroxypropylelaidate SS 2,3-Dihydroxy (C21H40O4) is a very important organic compound that is not reported largely from plant extracts but scientists believed this compound have very important role to prevent many human disease. Fatty acids reduce oxidative stress by playing an important role in the functionality of liver cells during injury (Beeharry et al., 2003, Hernandez et al., 2016).
3.5. Hepatoprotective effect
3.5.1. Acute toxicity
Study showed no mortality or noticeable behavioral changes in the animals (mice) of all groups. Methanol flower extracts of B. Variegata were observed innocuous up to 500 mg/kg body weight as our earlier study reported same dose for other plant (Gul et al., 2017).
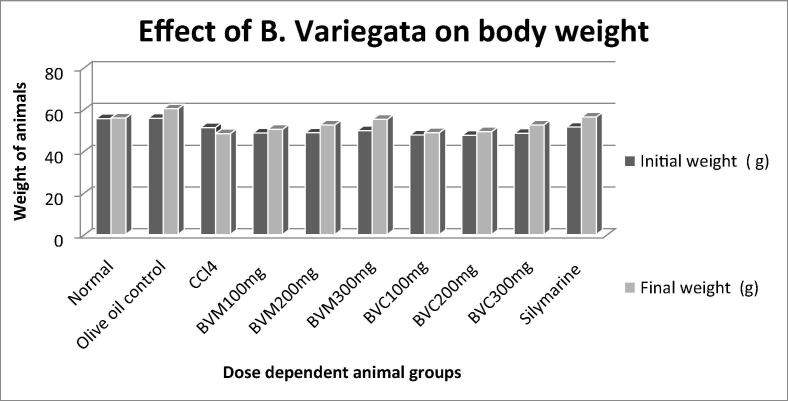
3.5.2. Response of plant extracts on body weight
The weight of mice was suddenly reduced (50 ± 2.0 to 48.3 ± 0.5) when CCL4 was induced in mice from all groups. However with the treatment of plant extracts particularly 300 mg/kg body weight (b.w) methanol flower extract has shown increased in the body weight which was comparable with animals from normal control group (Fig. 4). Carbon tetrachloride is readily absorbed from the gastrointestinal tract causes vomiting gastrointestinal pain, cell injury and liver necrosis followed by cell degeneration and decreased in body weight (Carlberg and Mannervik, 1975). The recovery of body weight of animals proved the protective effects of B. Variegata extracts and due to its treatment weight of animals became normal after passage of time.
Fig. 4.
Comparison of initial and final body weight of mice.
3.5.3. Liver and antioxidant enzymes
The levels of different biochemical markers i.e. (ALT, AST and ALP) and total direct Bilirubin in the blood serum of trial animals were assessed. It was observed that after administration of CCl4, intensities of various parameters were increased (Table 5). While by the consumption of plant drug, ranges of enzymes and Bilirubin became normal and significant results were noted for 300 mg/kg b.w of methanol flower extracts. The effects of flower extracts on hepatic enzymes such as CAT, SOD, GPx and total proteins were also evaluated (Table 6).
Table 5.
Role of flower extracts on Hepatic enzymes.
| S # | Trial Animals | ALT U/L |
AST U/L |
ALP U/L |
Direct bilirubin g/dl |
|---|---|---|---|---|---|
| 1 | Normal control | 38.5 ± 0.5 |
80.6 ± 0.5 | 110.6 ± 1.2 | 0.2 ± 0.01 |
| 2 | Olive oil | 40.6 ± 1.2 |
63.5 ± 3.6 | 175.2 ± 1.5 | 0.3 ± 0.01 |
| 3 | CCl4 | 120.4 ± 0.7 |
127.6 ± 2.5 | 294.5 ± 2.6 | 1.9 ± 0.2 |
| 4 | Methanol flower extract(100 mg/kg b.w) + CCl4 | 70.6 ± 3.5 | 84.5 ± 4.7 | 319.7 ± 4.5 | 0.9 ± 0.1 |
| 5 | Methanol flower extract(200 mg/kg b.w) + CCl4 | 57.4 ± 2.6 | 79.5 ± 3.5 | 301.5 ± 2.5 | 0.7 ± 0.1 |
| 6 | Methanol flower extract(300 mg/kg b.w) + CCl4 | 56.5 ± 3.1 | 63.5 ± 2.8 | 238.3 ± 1.5 | 0.7 ± 0.02 |
| 7 | Chloroform flower extract (100 mg/kg b.w) + CCl4 |
74.1 ± 3.7 | 110.5 ± 2.4 | 337.7 ± 4.6 | 1.1 ± 0.3 |
| 8 | Chloroform flower extract (200 mg/kg b.w) + CCl4 |
72.2 ± 3.2 | 89.4 ± 4.2 | 295.6 ± 5.3 | 1.2 ± 0.4 |
| 9 | Chloroform flower extract (300 mg/kg b.w) + CCl4 |
64.5 ± 1.9 | 81.3 ± 3.5 | 275.6 ± 2.8 | 0.8 ± 0.4 |
| 10 | Silymarine (100 mg/kg b.w) + CCl4 | 66.4 ± 2.1 | 76.1 ± 2.5 | 185.6 ± 2.6 | 0.5 ± 0.01 |
Effect of dosage of flower extract on liver markers were recorded (n = 5).
Table 6.
Assessment of hepatic anti-oxidant enzymes and total proteins.
| Sr.No. | Test animals | CAT (m mol/min mg protein) |
SOD (USOD/mg Protein) |
GPx (µmol/min/mg Protein) |
Total protein g/dl |
|---|---|---|---|---|---|
| 1 | Normal group | 8.2 ± 0.3 | 10.15 ± 0.6 | 32.10 ± 3.3 | 3.15 ± 0.4 |
| 2 | Olive oil | 7.9 ± 0.4 | 10.22 ± 0.7 | 34.5 ± 3.2 | 3.1 ± 0.5 |
| 3 | CCl4 | 4.75 ± 0.6 | 5.75 ± 0.4 | 17.83 ± 1.5 | 0.75 ± 0.1 |
| 4 | Methanolic flower extract(100 mg/kg b.w) + CCl4 | 7.42 ± 0.3 | 8.29 ± 0.5 | 24.1 ± 2.6 | 1.84 ± 0.5 |
| 5 | Methanolic flower extract(200 mg/kg b.w) + CCl4 | 7.91 ± 0.7 | 9.95 ± 0.6 | 28.72 ± 2.8 | 2.29 ± 0.7 |
| 6 | Methanolic flower extract(300 mg/kg b.w) + CCl4 | 8.15 ± 0.8 | 11.03 ± 0.8 | 32.51 ± 3.6 | 3.10 ± 0.8 |
| 7 | Chloroform flower extract (100 mg/kg b.w) + CCl4 |
7.05 ± 0.6 | 8.04 ± 0.7 | 22.4 ± 2.1 | 1.76 ± 0.6 |
| 8 | Chloroform flower extract (200 mg/kg b.w) + CCl4 |
7.61 ± 0.4 | 9.52 ± 0.8 | 26.25 ± 2.5 | 2.35 ± 0.3 |
| 9 | Chloroform flower extract (300 mg/kg b.w) + CCl4 |
7.99 ± 0.6 | 10.78 ± 1.5 | 30.63 ± 3.1 | 2.92 ± 0.2 |
| 10 | Silymarin (100 mg/kg b.w) + CCl4 |
8.11 ± 1.2 | 11.62 ± 0.6 | 33.7 ± 1.5 | 3.22 ± 0.8 |
Values were taken (n = 5)
When CCl4 was incorporated in the body of mice it decreased the level of all enzymes when compared to normal level (normal group). However, after treatment of 300 mg/kg b. w methanol flower extracts, levels of these parameters remained increased on dose depended way. It was reported earlier that flavonoids improved viability of cells and repressCCl4causedhepatocyte’s cellular leakage (Wu et al., 2006). Similarly, several doses of flower extracts have significantly moderated the ranges of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) together with direct Bilirubin.
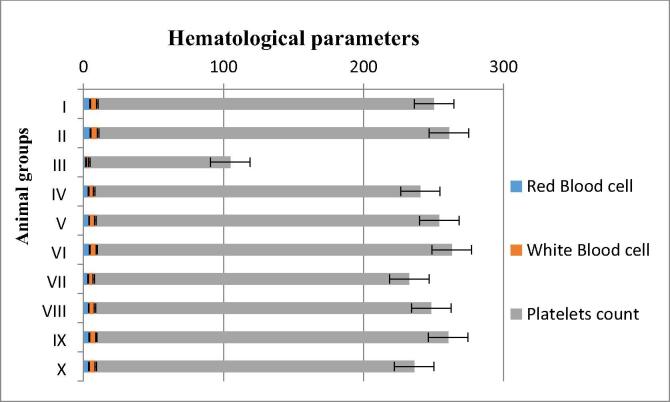
3.5.4. Hematological study
Effects of B.V extracts on RBC, WBC and Platelets levels were assessed. After administration of CCl4 to experimental animals, levels of RBC, WBC and Platelets were decreased as relate to normal (Fig. 5). Current findings specify that the flower extracts and silymarine standard drug increased above parameters to normal ranges (normal group) and further improvement can depends on amount of dose given. Rapid rise or decline in hematological parameters is the source to weaken animal immune system and these persistent conditions end in severe consequences (Carvalho et al., 2011).
Fig. 5.
Response of the animal group against different doses were recorded n = 5.Whereas RBC, WBC and Platelets count is measured in 106 /µl, 103 /µl and 103 /µl.
3.5.5. Histopathology
Alteration observed in the symmetry of liver cells was created by CCL4 shown in (Fig. 6a) and healing property of methanol flower extract of B. variegate is presented in (Fig. 6b). In CCL4 group impaired liver tissues with altered cellular organization, inflammation and hepatocyte pycnosis were noticed. Animals treated with 100and 200 mg/kg b.w recovered the cellular organization to some extent, while the flower extract 300 mg/kg b.w has reinstated the structural arrangement of tissues which verified its curative potential and was analogous to silymarine effect. Histological studies discovered the dynamic role of flavonoids for the treatment of damaged cells of liver (Hollman et al., 1997, Wu et al., 2006).
Fig. 6.
a: CCl4 induced toxicity in the liver by changing its symmetry. b:Damaged liver treated with B.V flowers tried to cure the injury.
Based on the existing results, it is concluded that B. Variegata young flower can be used as a natural antioxidants source and its consistent ingestion could provide health benefits to human population by protecting against oxidative stress, hepatic injury and similar other liver disorders. Further detailed in vitro and in vivo correlation based studies along with separation of active constituents are required to unravel the novel approaches for curing free radical induced diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Grant provided by department of Plant sciences Quaid-I-Azam University for conducting this experiment is highly appreciated. We are also very thankful to the administration of NVL Islamabad for providing their facilities for animal model. Moreover, The Higher Education Commission Pakistan's grant number 55830-2BM2-153 was utilized for funding this research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Eilaf Ahmed, Email: sahmed64@uclan.ac.uk.
Muhammad Asghar Khan, Email: asghar.khan@numspak.edu.pk.
Muhammad Gulfraz, Email: gulfrazsatti@uaar.edu.pk.
References
- Abbasi, A.M., M.H. Shah, T. Li, X. Fu, X. Guo and R.H. Liu. 2015. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J. ethnopharmacol.,162(l):333-345. [DOI] [PubMed]
- AOAC. 2003. Official methods of analysis of the association of official’s analytical chemists, 17th edn. Association of official analytical chemists, Arlington, Virginia.
- Ashafa A.O.T., Grierson D.S., Afolayan A.K. In vitro antioxidant activity of extracts from the leaves of Felicia muricata Thunb an underutilized medicinal plant in the Eastern Cape Province, South Africa. Afr. J. Complement&Altern.Med. 2010;7(4) doi: 10.4314/ajtcam.v7i4.56695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeharry, N., J. E. Lowe, A. R. Hernandez, J. A. Chambers, F. Fucassi, P.J. Cragg, M. H. GreenandI.C. Green. 2003. Linoleic acid and antioxidants protect against DNA damage and apoptosis induced by palmitic acid. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis., 530(1-2): 27-33. [DOI] [PubMed]
- Bhuiyan M.N.I., Begum J., Bhuiyan M.N.H. Analysis of essential oil of eaglewood tree (Aquilaria agallocha Roxb.) by gas chromatography mass spectrometry. Bang. J. Pharmacol. 2009;4(1):24–28. [Google Scholar]
- Carlberg I.N.C.E.R., Mannervik B.E.N.G.T. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. chem. 1975;250(14):5475–5480. [PubMed] [Google Scholar]
- Carvalho A.L.N., Annoni R., Silva P.R.P., Borelli P., Fock R.A., Trevisan M.T.S., Mauad T. Acute, subacute toxicity and mutagenic effects of anacardic acids from cashew (Anacardium occidentale Linn.) in mice. J. Ethnopharmacol. 2011;135(3):730–736. doi: 10.1016/j.jep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Cavalcanti, K.M.P.H., and R.F. Favoretto.2005. Bauhinia forficate Link. Coletânea Científica de Plantas de Uso Medicinal, Amaral ACF, Simões EV, Ferreira KaLP (eds). Fiocruz: Rio de Janeiro, 1-17.
- Choudhary R., Tandon R.V. Consumption of functional food and our health concerns. Pak J. Physiol. 2009;5(1) [Google Scholar]
- Cody, V., E. Middleton and J. B. Harborne. 1986. Plant flavonoids in biology and medicine: biochemical, pharmacological, and structure-activity relationships: proceedings of a symposium held in Buffalo, New York, July 22-26, 1985. Progress in clinical and biological research (USA). [PubMed]
- Cook, N. C. andS.Samman.1996. Flavonoids—chemistry, metabolism, cardio protective effects, and dietary sources. J. Nutr. Biochem., 7(2), 66-76.
- da Silva, K. L., and V. C. Filho.2002. Plantas do gênero Bauhinia: composição química e potencial farmacológico. Quim. Nova., 25(3): 449-454.
- Dacie, J. V. 2006. Dacie and Lewis practical haematology. Elsevier Health Sciences.
- Farnsworth Norman R. Ethnopharmacology and future drug development: the North American experience. J. Ethnopharmacol. 1993;38(2-3):137–143. doi: 10.1016/0378-8741(93)90009-t. [DOI] [PubMed] [Google Scholar]
- Filho, V. C. 2009. Chemical composition and biological potential of plants from the genus Bauhinia. Phytother res., An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives., 23(10):1347-1354. [DOI] [PubMed]
- Flohe L., Günzler W.A. Vol. 105. Academic Press; 1984. [12] Assays of glutathione peroxidase; pp. 114–120. (Metho. Enzymol.). [Google Scholar]
- Formica J.V., Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995;33(12):1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gul H., Ahmad M., Zafar M., Ahmad M.S., Abid A., Hira S., Shah I., Gulfraz M. The In Vitro and In Vivo Biological Activities of the Leaf of Cape Myrtle Myrsine africana L. Phytother Res. 2017;31(9):1305–1309. doi: 10.1002/ptr.5853. [DOI] [PubMed] [Google Scholar]
- Gulfraz M., Mehmood S., Ahmad A., Fatima N., Praveen Z., Williamson E.M. Comparison of the antidiabetic activity of Berberis Lyceum root extract and berberine in alloxan-induced diabetic rats. Phytother Res. 2008;22(9):1208–1212. doi: 10.1002/ptr.2438. [DOI] [PubMed] [Google Scholar]
- Harborne A.J. Springer science & business media; 1998. Phytochemical methods a guide to modern techniques of plant analysis. [Google Scholar]
- Hernandez Teri L., Van Pelt Rachael E., Anderson Molly A., Reece Melanie S., Reynolds Regina M., de la Houssaye Becky A., Heerwagen Margaret, Donahoo William T., Daniels Linda J., Chartier-Logan Catherine, Janssen Rachel C., Friedman Jacob E., Barbour Linda A. Women With Gestational Diabetes Mellitus Randomized to a Higher–Complex Carbohydrate/Low-Fat Diet Manifest Lower Adipose Tissue Insulin Resistance, Inflammation, Glucose, and Free Fatty Acids: A Pilot Study. Dia Care. 2016;39(1):39–42. doi: 10.2337/dc15-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman P.C., van Trijp J.M., Buysman M.N., Mengelers M.J., de Vries J.H., Katan M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418(1–2):152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- Igoli J.O., Ogaji O.G., Tor-Ayiin T.A., Igoli N.P. Traditional medicine practice amongst the Igede people of Nigeria. Part II. Afr. J. Complement Altern Med. 2005;2(2):134–152. [Google Scholar]
- Irum S., Tabassum S., Qureshi R., Gulfraz M., Anwar P. Phytochemical analysis of medicinally important constituents of Teucrium stocksianum boiss. Pak. J. Bot. 2019;51(3):1067–1074. [Google Scholar]
- Kaur G., Alam M.S., Jabbar Z., Javed K., Athar M. Evaluation of antioxidant activity of Cassia Siamea flowers. J. Ethnopharmacol. 2006;108(3):340–348. doi: 10.1016/j.jep.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maqsood M., Qureshi R., Arshad M., Ahmed M.S., Ikram M. Preliminary phytochemical screening, antifungal and cytotoxic activities of leaves extract of Moringa oleifera Lam. from Salt range, Pakistan. Pak. J. Bot. 2017;49(1):353–359. [Google Scholar]
- Marinova D., Ribarova F., Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metallurgy. 2005;40(3):255–260. [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- Munazir M., Qureshi R., Arshad M., Gulfraz M. Antibacterial activity of root and fruit extracts of Leptadenia pyrotechnica (Asclepiadaceae) from Pakistan. Pak. J. Bot. 2012;44(4):1209–1213. [Google Scholar]
- Mustafa G., Ahmed S., Ahmed N., Jamil A. Phytochemical and antibacterial activity of some unexplored medicinal plants of Cholistan desert. Pak. J. Bot. 2016;48(5):2057–2062. [Google Scholar]
- Oluyemi, K. A., U. C.Okwuonu,D. G. Baxter andT. Oyesola. 2007. Toxic effects of methanolic extract of Aspilia africana leaf on the estrous cycle and uterine tissues of Wistar rats. Int. J. Morphol., 25(3): 609-614..
- Pinheiro, M. S., L., S. Rodrigues, L. E. I. L. A. S NETO,R. Q. Moraes-Souza, T. S. Soares, M. F. Américo, K.E. Campos, D. C. DamascenoandG. T. Volpato.2017. Effect of Bauhinia holophylla treatment in Streptozotocin-induced diabetic rats. Anais da Academia Brasileira de Ciências., 89(1): 263-272. [DOI] [PubMed]
- Rajani G.P., Ashok P. In vitro antioxidant and antihyperlipidemic activities of Bauhinia variegata Linn. Ind. J. pharmacol. 2009;41(5):227. doi: 10.4103/0253-7613.58513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Simpson Bradley S., Claudie David J., Smith Nick M., Gerber Jacobus P., McKinnon Ross A., Semple Susan J. Flavonoids from the leaves and stems of Dodonaea polyandra: A Northern Kaanju medicinal plant. Phytochemistry. 2011;72(14-15):1883–1888. doi: 10.1016/j.phytochem.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Winterbourn Christine C., Metodiewa Diana. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radical Biol. Med. 1999;27(3-4):322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Wu Yihang, Wang Fang, Zheng Qunxiong, Lu Longxi, Yao Hongtian, Zhou Changxin, Wu Xiumei, Zhao Yu. Hepatoprotective effect of total flavonoids from Laggera alata against carbon tetrachloride-induced injury in primary cultured neonatal rat hepatocytes and in rats with hepatic damage. J. Biomed. Sci. 2006;13(4):569–578. doi: 10.1007/s11373-006-9081-y. [DOI] [PubMed] [Google Scholar]
- Zengin G., Aktumsek A., Guler G.O., Cakmak Y.S., Yildiztugay E. Antioxidant Properties of Methanolic Extract and Fatty Acid Composition of Centaurea urvillei DC. subsp. Hayekiana Wagenitz. Rec. Nat Prod. 2011;5(2) [Google Scholar]