Abstract
Research background
Red beet (Beta vulgaris L.) is commercially grown in Punjab and Khyber Pakhtunkhwa (KPK) regions while cultivated as vegetable in Baluchistan and Sindh regions of Pakistan. It is well known for its health-promoting role in several clinical and pathologic outcome due to abundance of betalains and other bioactive compounds. The purpose of study was to interpret bioactivity of of B. vulgaris leaves (BvLM) and roots (BvRM) extracts for finding natural cure of neurodegenerative diseases (NDs).
Experimental approach
BvLM and BvRM extracts were evaluated for phytochemical composition, antioxidant, anti-inflammatory and anticholinesterase potential using standard protocols with modifications.
Results
Phytochemicals analysis of BvLM and BvRM extracts depicted the presence of flavonoids, phenols, alkaloids, saponins and glycosides. The IC50 values for free radical scavenging activity for BvRM and BvLM showed that BvLM (DPPH: 2.20 ± 0.72 μg/mL, : 0.0519 ± 1.02 μg/mL) is more significant as compared to BvRM (DPPH: 2.312 ± 0.72 μg/mL, : 2.668 ± 0.49 μg/mL). BvLM showed significant protection against heat induced hemolysis of HRBCs and protein denaturation (2.322 ± 0.1 > 2.324 ± 0.06 μg/mL) as compared to BvRM (8.572 ± 0.2 > 50.18 ± 1.0 μg/mL). Both extracts found to exhibit strong inhibitory potential against acetylcholinesterase enzyme.
Discussion/Conclusion
Our study highlighted B. vulagris extracts as rich and nutritious source of antioxidants, anti-inflammatory and cholinesterase inhibitors that could be helpful in preventing and treating neurodegenerative disorders (NDs). In consideration of multifactorial and complex etiology of NDs, BvLM and BvRM extracts would be apt candidates for development of therapeutic strategy for management of multiple cognitive disorders.
Keywords: Red beets (Beetroots, Beta vulgaris); Phytochemical analysis; Acetylcholinesterase (AChE) inhibitory activity; Anti-inflammatory; Antioxidant; Neurodegenerative disorders
1. Introduction
Exploring enhanced disease ameliorating therapeutic strategies against cognitive disorders like Alzheimer’s disease (AD) is a leading challenge of recent era. Cognitive impairment and neuropathological etiology is in consistent with cholinergic deficiency due to presence of neurofibrillary tangles and senile plaques besides the degeneration of cholinergic and cortical neurons in basal forebrain (Davies and Maloney, 1976, Varadarajan et al., 2000). World Health Organization (WHO) estimated approximately 71% of the dementia cases occur in developing countries (Ferri et al., 2005, Goverdhan et al., 2012, Mathew and Subramanian, 2014). According to an estimate, by 2050, prevalence of dementia is expected to rise around 46.8 million people (Association, 2015). Therefore, cognitive disorders have become primary research areas due to their huge economic, social and healthcare impacts upon society. Financial burden along with social stigma linked with the loss of cognitive capabilities and lead to psychological sufferings in patients (Borsje et al., 2016). Loss of acetylcholine (ACh) neurotransmitter is critically important in causing dysfunction of cholinergic neurotransmission in aging or age-related disorders, responsible for cognitive deficits (Vinutha et al., 2007). Acetylcholinesterase (AChE) is primarily responsible for ACh breakdown within cerebral cortex synapses (Bierer et al., 1995). Complex and multifaceted pathophysiology in neurodegeneration includes inflammation (Heneka et al., 2015), accretion of amyloid peptide (Aβ), highly phosphorylated tau protein, oxidative stress, mitochondrial dysfunction (Federico et al., 2012), neuronal apoptosis and cholinergic signaling deficit (Mufson et al., 2008). Strategies to improve cholinergic function include the cholinergic receptors activation or providing long term ACh availability to neuronal synaptic cleft via suppressing ACh hydrolysis by AChE (Palop and Mucke, 2016). Release of oxidative and inflammatory stress and ACh stimulation via inhibition of AChE seem imperative potential targets for neurodegeneration therapeutics. Commercially available AChE inhibitors for treatment of mild to moderate neurodegenerative disorders (NDs) include donepezil, tacrine, galanthamine, huperizine A, physostigmine (eserine) and rivastigmine. Synthetic drugs exhibit limited efficacy and significant side effects including gastrointestinal disturbances, hepatotoxicity, low bioavailability, short term biological activity (Chopra et al., 2011, Lee et al., 2011). These drugs also reported to temper only cholinergic symptoms, not to design for targeting specific mechanistic pathways involved in NDs pathogenesis (Lovell and Markesbery, 2007, Nunomura et al., 2001). To counteract these side effects, natural therapeutic options are considered for enhancing NDs treatment efficiency (Li et al., 2014). Natural antioxidants and anti-inflammatory bioactive components possessed by medicinal plants extracts are reported to prevent cell damage and exhibit neuroprotective effects for the management of NDs (Aguiar and Borowski, 2013, Chen et al., 2016). Animal studies for improving cognitive functions using curcumin, a natural compound, indicated the recovery in memory, improved cholinergic function and reversal of stress-induced loss in neurogenesis (Poulose et al., 2017). Another study indicated the anti-cholinesterase activity of saffron ultimately modulating the cholinergic functions (Bukhari et al., 2018).
Beta vulgaris commonly known as beetroot is an herbaceous biennial crop belonging to Chenopodiaceae family. B. vulgaris contains saponins, carotenoids, glycine betaine, folates, betanin, betacyanines, polyphenols and flavonoids (Lechner et al., 2010, Socaciu, 2007). B. vulgaris poses a variety of health benefits and aids in combating various disorders and health conditions (Clifford et al., 2015).
Current research study is an attempt to extract mixture of bioactive components from B. vulgaris leaves and roots and to compare their antioxidant, anti-inflammatory and anti-acetylcholinesterase activities in order to persuade their relevance for therapy of cognitive disorders. This study is to find a promising and natural treatment for neurodegenerative disorders and to what extent the plant shows inhibition activity of certain enzymes most dominantly AChE. To detect if the plant poses any anti-inflammatory and antioxidant activities and whether it is acceptable to pursue and conduct the research further in in vivo in order to find a better put treatment of neurodegenerative disorders.
2. Materials and methods
2.1. Ethics statement
A part of current research study involved the utilization of human blood samples. This study was approved by ethical review board of Department of Biosciences, COMSATS University Islamabad (CUI), Islamabad, Pakistan. Moreover, blood samples from healthy volunteers who fulfilled the selection criteria were collected after a signed informed consent.
2.2. Chemicals and reagents
Chemicals such as NaCl, chloroform, concentrated sulfuric acid, ferric chloride, lead acetate etc were collected from the general laboratory of the campus. 25 g of AlbuMAX™| Lipid-Rich BSA Cat#11020021(Gibco™ United States) and 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB; Ellman’s Reagent) Cat# 22582 (Gibco™ United States) were purchased from Thermo Fisher Scientific. Acetylthoicholine chloride (ATCCl) Cat # A5626 (Sigma Aldrich, St. Louis, United States), Gelatin solution Cat # G1393 (Sigma Aldrich, United States), Acetylcholinesterase (AChE) from Electric eel Cat # C3389 (Sigma Aldrich, United States) were purchased from Sigma.
2.3. Study area, plant material collection and identification
Fresh Red beets (B. vulgaris) plant species were collected from a local vendor on Park Road Near cultivation point and transported to laboratory (Fig. 1a). The plant was identified by a taxonomist and a voucher specimen was deposited. The stems joining the roots and leaves were detached and discarded. The leaves and roots were washed with fresh water. The roots were peeled and sliced into fine and thin pieces. Both the leaves and thinly sliced roots were shade dried at room temperature until all the water content had evaporated and the sample had become fit for grinding. Once the sample had completely dried, both the roots and leaves were separately grinded into powder using a mechanical blender and placed in containers for later use.
Fig. 1.
Snapshot of beta vulgaris plant along (1a) with its hydromethanolic leaves (BvLM) (1b) and roots (BvRM) (1c) extracts.
2.4. Preparation of extracts
Soxhlet’s extraction method was used for the preparation of crude leaves and roots extracts. The procedure described by De Castro and Priego-Capote (2010) and RNS Yadav (Yadav and Agarwala, 2011) was followed with slight modifications. The leaf and root powder was collected in the apparatus, separately and was treated with extraction solvents i.e. n-hexane and hydromethanol solution. Both the solvents were heated at 67 ℃, until the siphon tube of the Soxhlet’s apparatus showed a colorless extract. The n-hexane and hydromethanol solution treated leaves and roots extracts were dried using rotary dryer and fan drying method respectively (Fig. 1b & c).
2.5. Percentage yield of the extracts
Percentage yield of extracts was calculated using the following formula:
2.6. Qualitative phytochemical screening
Following biochemical tests were performed for the detection of bioactive compounds.
2.6.1. Tests for flavonoids
B. vulgaris hydromethanolic extracts of roots (BvRM) and leaves (BvLM) were treated with few drops of diluted sodium hydroxide. Indication of intense yellow color after the addition of few drops of dilute acid, lead to the presence of flavonoids (Shah and Hossain, 2014).
2.6.2. Tests for phenols
Ferric Chloride Test. The BvRM and BvLM extracts were dissolved and boiled separately in distilled water and filtered. Neutral ferric chloride (5% FeCl3) solution was added to the mixture and observation of dark green coloration indicated the presence of phenolic compounds (Usman et al., 2009).
2.6.3. Lead acetate test
A bulky white precipitation was observed which ensured the presence of phenols, when BvRM and BvLM extracts were dissolved separately in distilled water to which 10% lead acetate solution (3 mL) was added (Singh and Bag, 2013).
2.6.4. Tests for alkaloids
1% HCl (2 mL) was added to BvRM and BvLM extracts separately. The mixture was heated gently followed by the addition of Mayer's and Wagner's reagents. Precipitate’s turbidity indicated the presence of alkaloids (Shrestha et al., 2015).
2.6.5. Test for saponins
BvRM and BvLM extracts (1 mL) were added to 1 mL of deionized water, separately, the mixture was shaken for 30 s vigorously. The tubes were made to stand for 15 min to indicate the presence and absence of persistent frothing. If the frothing was persistent it indicated the presence of saponins (Shrestha et al., 2015).
2.6.6. Test for glycosides
Salkowski’s Test. Chloroform (2 mL) was added to BvRM and BvLM extracts (1 mL). Concentrated (2 mL) was added carefully and was mixed gently. Indication of reddish brown color in chloroform lower layer lead to the presence of glycosides (Yadav and Agarwala, 2011).
2.7. Quantitative phytochemical analysis
2.7.1. Total phenolic content
Folin-Ciocalteu (FC) Reagent method was used for the determination of polyphenols in the aqueous BvRM and BvLM extracts. In 1 mL of roots and leaves extract separately, FC-reagent (0.5 mL) and 20% (w/v) solution of Na2CO3 was added. Mixture was incubated at room temperature for 15 min and absorbance was recorded at 650 nm along with standard (gallic acid) (Baba and Malik, 2015).
2.8. Determination of antioxidant activities of BvRM and BvLM extracts
2.8.1. DPPH (2, 2-Diphenyl 1–2 picrylhydrazyl) assay
BvRM and BvLM extracts were analyzed for their antioxidant properties. For this purpose, DPPH free radical scavenging assay was performed. Multiple concentrations (20–250 μg/mL) of BvRM and BvLM extracts were used. Briefly, reaction mixture contained 1 mL (0.3 mM) of DPPH (D9132, Sigma Aldrich, United States), 1 mL extract sample and 1 mL methanol. Sample mixture was allowed to react at room temperature and then incubated at 37 °C for 45 min in dark. Absorbance was noted at 517 nm using blank, negative control and gallic acid as positive control (Dhanasekaran, et al., 2015). The percent radical scavenging efficacy was determined using formula:
The experiments were performed in triplicates. The half maximal inhibitory concentration (IC50) values were calculated using Graphpad Prism Version 7.04.
2.8.2. Hydrogen peroxide radical () scavenging assay
BvRM and BvLM extracts were evaluated for their ability to scavenge hydrogen peroxide by a method described by Ruch et al. (1989). Briefly, H2O2 (2 mM) solution was prepared in phosphate buffered saline (PBS, 50 mM; pH 7.4). In 0.3 mL of PBS, 0.1 mL of test sample was added followed by addition of H2O2 (0.6 mL) and vortexed. BvRM and BvLM in multiple concentrations (20–250 µg/mL) were used to analyze IC50 value. The absorbance of each concentration of tested sample along with blank and standard (gallic acid) was measured at 230 nm. Following equation was used to determine and calculate the hydrogen peroxide scavenging ability of the extracts:
2.9. Anti-inflammatory potential of extracts
2.9.1. Inhibition of protein denaturation method
BvRM and BvLM extracts were prepared in multiple seria dilutions (25–200 μg/mL). Aspirin was used as a standard drug and prepared in the concentrations from 1 to 500 μg/mL in methanol. 1 g of Bovine Serum Albumin (BSA) was dissolved in PBS to obtain 1% of solution of BSA. The pH of the solution was adjusted to 6.3 by adding few drops of 1 N HCl. 1 mL of the extracts of different concentrations were taken in the test tubes and 1% BSA (1 mL) was added to each tube. Similarly 1 mL of aspirin of multiple concentrations (1–500 µg/mL) was taken in different tubes and 1% BSA (1 mL) was added to them. The samples were heated in water bath at 37 °C for 20 min and then at 57 °C for 20 min. After heating the samples were allowed to cool at room temperature. The absorbance of the samples was measured at 660 nm using UV-spectrophotometer. The entire experiment was performed in triplicates and the percentage inhibition was calculated:
2.9.2. Human red blood cells stabilization (HRBC) test
HRBC test was performed in two steps. The first step involved preparation of erythrocyte suspension. The suspension was prepared by collecting fresh human blood (10 mL) from a healthy volunteer who had not taken any NSAIDS for 2 weeks prior to the experiment. The blood was collected in heparinized centrifuge tubes and was centrifuged for 10 min at 3000 rpm. After centrifugation the sample was washed with equal volume of normal saline solution 2 times and centrifuged for 3 min at 3000 rpm until a clear supernatant was obtained. The supernatant was discarded and the volume of blood was measured and made to 10% (v/v) with normal saline solution. The second step of this test involved heat induced hemolysis where reaction mixture of 2 mL was prepared comprising of 1 mL of BvRM and BvLM extracts concentration ranging from (20–200 μg/mL) and 1 mL of 10% RBC suspension. Saline solution was used instead of the extracts in the case of control. Aspirin was used as a positive control. The samples were incubated for 30 min at 56 °C then centrifuged for 5 min at 2500 rpm. The absorbance of supernatant was measured at 560 nm. The test was performed in triplicates. Percentage of hemolysis of human red blood cell membrane was calculated:
Percentage of membrane stabilization was also calculated:
2.10. Acetylcholinesterase inhibition assay
The method described by Md. Abul Hasnat et al. (2013) was used to determine the inhibitory activity of BvRM and BvLM extracts of acetyl-cholinesterase. 20 μL of 0.2 units/mL of enzyme solution, 120 μL of 0.1 M sodium phosphate buffer and 20 μL of BvRM and BvLM extracts were mixed and incubated in microplate for 15 min at 37 °C. After 15 min the substrate (ATCCl) and DTNB were added to each well in the volume of 20 μL. Formation of colored product 5-thio-2-nitrobenzoate anion indicated the hydrolysis of ATCCl. The anion was formed because of the reaction of DTNB and thiochloine which is released because of enzyme hydrolysis. After the formation of colored product, the absorbance was measured at 410 nm after 10 min. Donepazil was used as a positive control. The experiment was performed in duplicates. AChE percentage inhibition was calculated using the equation:
Multiple serial dilutions of BvRM, BvLM and donepezil (400 µg/mL to 12.5 µg/mL) were prepared for calculation of 50% inhibitory concentration (IC50).
2.11. Statistical analysis
The statistical analysis of data was expressed in mean standard deviation of triplicate readings. IC50 values were calculated using nonlinear regression in GraphPad Prism (Prism, 1994). P values were calculated by performing Kruskal-Wallis test (P < 0.05) in order to find the significance of difference between means of different extracts. GraphPad Prism 7 was used for performing statistical analysis. Microsoft Excel 2007 was also used for graphical evaluations.
3. Results
3.1. Yield calculation for extracts
The percentage yield of BvRM, BvLM, and BvLH extracts were calculated. Out of three extracts, BvRM showed the highest percentage yield (Table 1).
Table 1.
Table showing percentage yield of various extracts of Beta vulgaris.
| Serial # | Extracts | Abbreviations | Percentage yield (%age) |
|---|---|---|---|
| 01 | Roots extract in hydromethanol solvent | 54.06% | |
| 02 | Leaves extract in hydromethanol solvent | 4.89% | |
| 03 | Leaves extract in n-hexane solvent | 1.46% |
3.2. Estimation of qualitative analysis of phytochemicals present in B .vulgaris extracts
Phytochemical screening tests of BvLM and BvRM extracts depicted the presence of flavonoids, phenols, saponins, alkaloids and glycosides (Table 2).
Table 2.
Table showing various phytochemicals present in the hydromethanolic extracts of Beta vulgaris.
| Serial # | Phytochemical tests | Characteristics found | Findings | Results |
|---|---|---|---|---|
| 01 | Phenolic compounds in BvLM | Green coloration in FeCl3 test | +++ |  |
| 02 | Phenolic compounds in BvRM | Light green coloration in FeCl3 test | ++ |  |
| 03 | Flavonoids in BvLM | Formation of intense yellow color which became colorless after the addition of few drops of dilute acid | + |  |
| 04 | Flavonoids in BvRM | Formation of intense yellow color which became colorless after the addition of few drops of dilute acid | +++ |  |
| 05 | Saponins in BvLM | Mild frothing | + | |
| 06 | Saponins in BvRM | Mild frothing | + | |
| 07 | Alkaloids in BvLM | Precipitate’s turbidity | ++ |  |
| 08 | Alkaloids in BvRM | Precipitate’s turbidity | ++ |  |
| 09 | Glycosides in BvLM | Reddish brown color indicated the presence of glycosides in the lower layer of chloroform | +++ |  |
| 10 | Glycosides in BvRM | Reddish brown color indicated the presence of glycosides in the lower layer of chloroform | ++ |  |
+: present ++: moderately present +++: highly present.
3.3. Quantitative phytochemical analysis of extracts
3.3.1. Total phenolic content determination
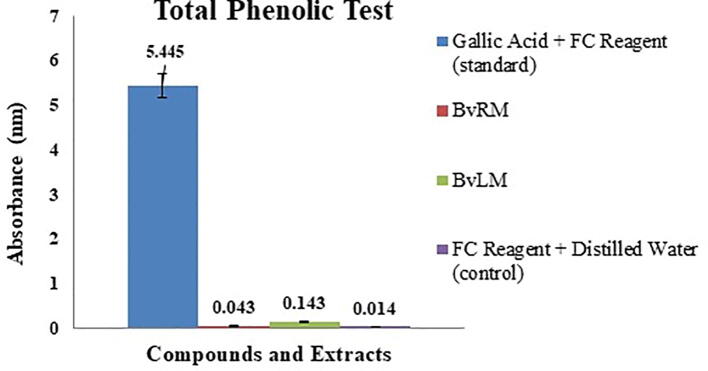
To evaluate the presence of total phenolic content in the BvRM and BvLM extracts, Folin-Ciocalteu Reagent method was performed. Gallic acid being phenolic in nature was used as a standard. BvLM showed a higher value of 0.143 ± 0.00 as compared to BvRM (0.041 ± 0.003), indicating that leaves have a higher phenolic content as compared to the roots of B. vulgaris (Fig. 2).
Fig. 2.
Total phenolic content of BvRM and BvLM extract in terms of absorption measured along with the standard Gallic Acid.
3.4. Antioxidant activities of BvRM and BvLM extracts
3.4.1. DPPH (2, 2-Diphenyl 1-2 picrylhydrazyl) assay
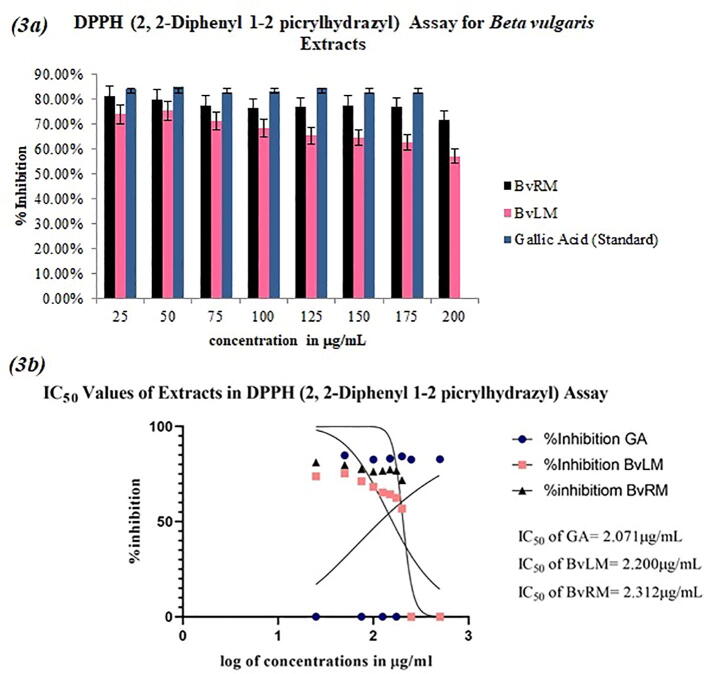
Fig. 3a has shown the radical scavenging potential of different concentrations of B. vulgaris extracts. BvRM extract at 25 μg/mL concentration attained the highest scavenging activity of 81.09% as compared to BvLM (73.81%). These results illustrated the competitive antioxidative potency of BvRM and BvLM extracts with purified standard. For the interpretation of results, 50% inhibitory concentration (IC50) for each extract was calculated. The results showed that BvRM exhibit IC50 value of 2.32 ± 0.72 μg/mL as compared to BvLM (2.20 ± 0.72 μg/mL) (Fig. 3b). The reason why BvLM has lower IC50 value may be that it has more phenolic content as compared to the BvRM extract. Lower IC50 with higher scavenging activity depicted the significant therapeutic potential of bioactive constituents found in extracts. However, we can say that both the extracts can possibly act as primary antioxidants. The scavenging activity of these extract was found to be comparable to gallic acid (IC50: 2.071 ± 0.72 μg/mL) (Fig. 3b).
Fig. 3.
(3a) A bar plot showing DPPH radical scavenging activity of the different extracts of Beta vulgaris in comparison to Gallic acid as standard along with their respective error amount as a percentage (3b) IC50 values of the selected plant extracts for DPPH radical scavenging activity along with the standard Gallic acid.
3.4.2. Hydrogen peroxide radical () scavenging assay
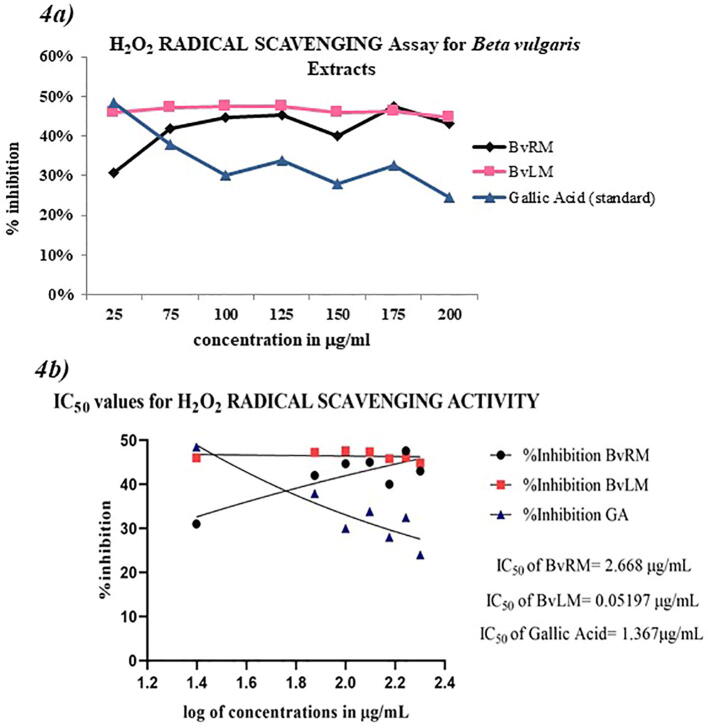
Hydrogen peroxide Radical (H2O2) Scavenging Assay was performed to evaluate BvRM and BvLM extracts for their antioxidant activity. Both extracts showed significant radical scavenging activity when compared to the gallic acid. Comparatively, BvLM showed higher scavenging activity than BvRM. The scavenging activity of BvLM (100 μg/mL) with percentage inhibition being found 47.60% (Fig. 4a) on the other hand BvRM showed 44.69% scavenging activity at 100 μg/mL as compared to standard Gallic acid (100 μg/mL) illustrating 51% scavenging activity.
Fig. 4.
(4a) A linear plot showing H2O2 radical scavenging activity of the different extracts of Beta vulgaris in comparison to Gallic acid as standard (4b) IC50 values for H2O2 radical scavenging assay shown by different extracts of Beta vulgaris along with the standard Gallic acid.
Depicted IC50 values of BvLM (0.0519 ± 1.02 μg/mL) and BvRM (2.668 ± 0.49 μg/mL) illustrated BvLM as stronger radical scavenger than BvRM. BvLM (IC50: 0.0519 ± 1.02 μg/mL) was found to be more significant than the standard Gallic acid (IC50: 1.367 ± 0.1 μg/mL) (Fig. 4b). The scavenging activity for H2O2 of plant extracts is given in the following order BvLM > BvRM (0.0519 ± 1.02 > 2.668 ± 0.49 μg/mL).
3.5. Estimation of Anti-inflammatory activity of BvRM and BvLM extracts
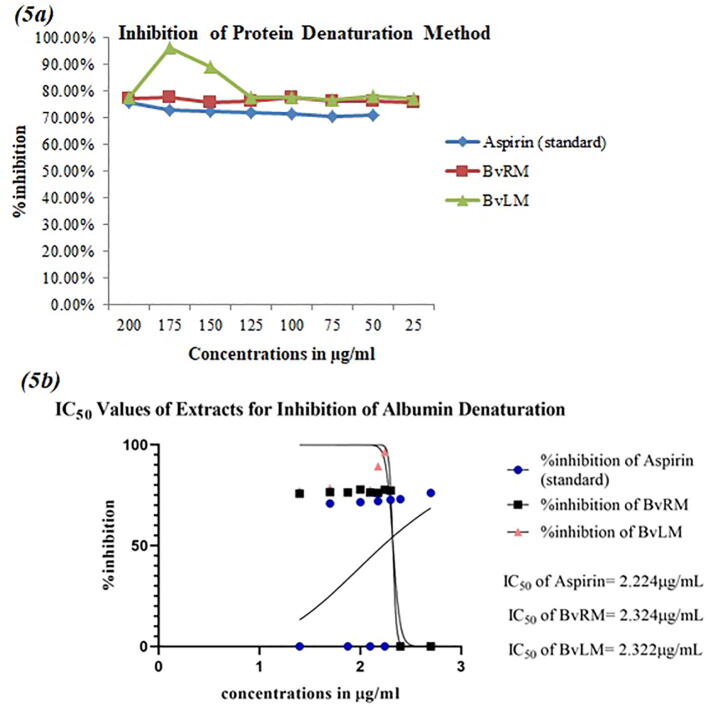
3.5.1. Estimation of inhibition of protein denaturation
The BvRM and BvLM extracts were analyzed for their inhibition of protein denaturation using Bovine Serum Albumin (BSA) as a protein of interest. Both extracts showed significant inhibition of protein denaturation. BvLM showed 96.45% inhibitory activity at concentration of 175 μg/mL. Both extracts displayed anti-inflammatory activity as compared to standard drug, aspirin (Fig. 5a) possibly proving the higher protein denaturation inhibitory activity. BvRM depicted relatively constant inhibition potential (~80%) at multiple dilution concentrations ranging from 25 to 200 µg/mL while BvLM showed the same inhibitory pattern with the deviation of increased protein protective potency at 150 µg/mL and 175 µg/mL concentrations. Both extracts exhibited the same but higher inhibitory potency of protein denaturation as compared to standard drug. The P values of both the standard and extracts were significant (P = 0.0063).
Fig. 5.
(5a) A linear plot showing inhibition of protein denaturation by different extracts of Beta vulgaris in comparison to aspirin as a standard drug (5b) IC50 values for log of concentrations of Beta vulgaris extracts for the inhibition of albumin denaturation along with aspirin as a standard drug.
The IC50 values of BvRM and BvLM were almost similar (2.324 ± 0.06 μg/mL and 2.322 ± 0.1 μg/mL, respectively) with BvLM showing a slightly higher IC50 value than BvLM (Fig. 5b).
3.5.2. Estimation of human red blood cells (HRBCs) membrane stabilization test
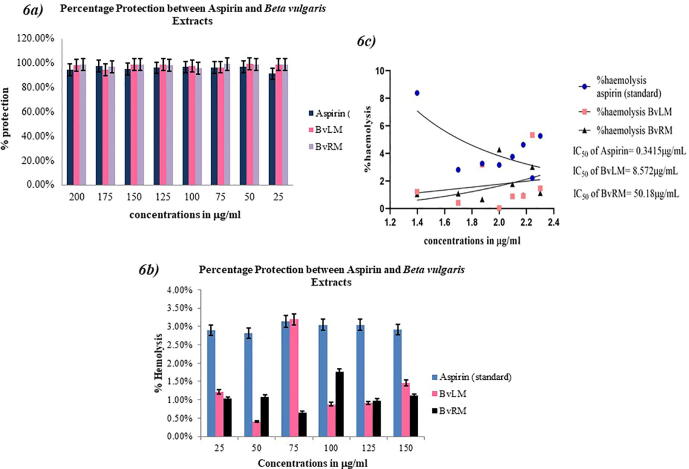
The BvRM and BvLM extracts were investigated for their anti-inflammatory activity using HRBC stabilization test. Aspirin, an anti-inflammatory drug was used as standard. BvLM extract at 50 μg/mL concentration showed the highest percentage of protection (99.58%) from hemolysis (Fig. 6a). In heat induced hemolysis assay, the BvLM extract showed the minimum percentage of hemolysis at 100 μg/mL with percentage being 0.4% (Fig. 6b). Statistical analysis showed that the values were significant (P = 0.0185). BvRM and BvLM showed IC50 values of 50.18 ± 1.0 μg/mL and 8.572 ± 0.2 μg/mL, respectively while aspirin showed IC50 value of 0.341 ± 0.47 μg/mL indicating that percentage protection of hemolysis by selected plant extracts is significantly higher than standard drug (Fig. 6c).
Fig. 6.
(6a) A bar plot showing percentage protection of HRBCs by different extracts of Beta vulgaris in comparison to aspirin as standard drug along with their respective error amount as a percentage (6b) A bar plot showing percentage inhibition of hemolysis of HRBCs by different extracts of Beta vulgaris in comparison to aspirin as standard drug along with their respective error amount as a percentage (6c) IC50 values for log of concentrations of Beta vulgaris extracts for the inhibition of hemolysis of HRBCs along with aspirin as a standard drug.
3.6. Estimation of acetylcholinesterase inhibition activity
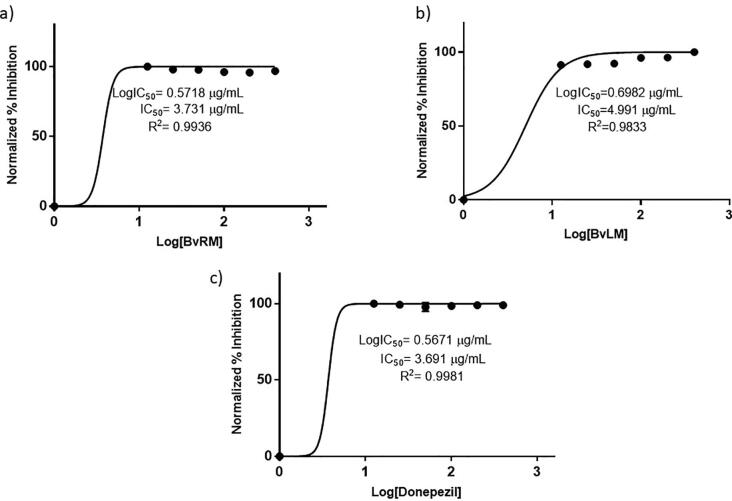
The inhibitors of AChE are being used widely to find targeted therapy for neurodegenerative diseases. The BvRM and BvLM extracts were also investigated for their AChE inhibition activity. Both extracts depicted significant inhibition activity along with the standard drug, donepezil. BvRM and BvLM depicted 89.8% and 93.3% inhibition of AChE in comparison to 94.2% inhibition by donepezil at 100 μg/mL (Fig. 7). Dose response analysis illustrated the low IC50 values for both extracts along with donepezil depicting their high efficacy for AChE inhibition (Fig. 8).
Fig. 7.
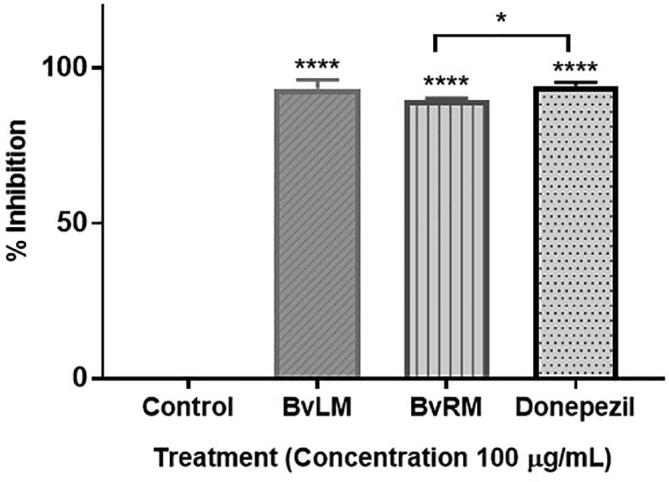
A bar plot is depicting percentage inhibition of acetylcholinesterase enzyme by BvRM and BvLM extracts in comparison to standard drug, donepezil. Extracts with 100 µg/mL and 200 µg/mL concentrations were used in conjugation with standard drug.
Fig. 8.
IC50 values calculation using dose response analysis. (a) Serial dilutions of BvRM extract were used and 3.731 µg/mL dose was found as IC50. (b) Serial dilutions of BvLM extract were used and 4.991 µg/mL dose was counted as IC50. (c) Serial dilutions of standard drug (donepezil) were used and 3.691 µg/mL dose was found as IC50.
4. Discussion
From botanical and health perspective, the importance of B. vulgaris is highly appreciated. However, proving its importance in the treatment of neurodegenerative diseases is under consideration. It is now known that beetroots do have the ability to cure dementia – a symptom shown by cognitive disorders, by providing oxygenation to the somatomotor cortex of the brain. In the current study, elaborative phytochemical investigation and pharmacognostic potential of B. vulgaris extracts is reported. When subjected to in vitro testing, BvRM and BvLM extracts showed a plethora of characteristics. Presence of multiple secondary metabolites like saponins, flavonids, alkaloids, glycosides and phenolic compounds in preliminary phytochemical screening assays depicted extracts as rich nutritious source. Interestingly, quantitative analysis indicated the presence of phenolic compounds specifically in leaves extract (BvLM) which directly correlate with multiple bioactivities of extract. The phenolic compounds of BvLM extracts contain potential bioactive secondary metabolites (Edziri et al., 2019). However if not a lot, in comparison to the leaves, the roots also contain secondary metabolites formed by the phenolic compounds.
Free radicals are produced during various metabolic processes in body and are associated with diverse range of disorders like diabetes, neurodegenerative diseases, cancer, coronary heart disease, immunosuppression and many others (Kumar and Pandey, 2013, Kumar and Pandey, 2015). Singlet oxygen and hydrogen peroxide are considered in the category of non-free radicals whilst hydroxyl, superoxide, lipid peroxyl and nitric oxide are general free radicals (Halliwell and Gutteridge, 2015). Generally, natural body defense mechanisms like chain breaking antioxidants generation and protective antioxidant systems neutralize the destructive effect of free radicals (Jacob, 1995). Moreover devastative tissue injury occurs when rate of free radical generation exceeds the limit of natural antioxidant mechanisms. Therefore, various diseases like neurodegenerative disorders can be treated with drugs/agents (natural or synthetic) having radical scavenging potential. Natural flora is a valuable source of antioxidants which have been reported to provide protection from radicals induced damage (Kumar et al., 2013). The significant anti-radical activities of BvLM and BvRM extracts were observed using DPPH (with IC50 values of 2.20 ± 0.72 and 2.32 ± 0.72 μg/mL respectively) and H2O2 radical scavenging assays (with IC50 values of 0.0519 ± 1.02 and 2.668 ± 0.49 μg/mL respectively). The antioxidant ability of BvLM and BvRM may be correlated with the presence of phenols especially BvLM since it showed a better radical scavenging ability as compared to BvRM due to the presence of phenols in a greater amount. These results are in concordance with the study done by Irshad et al. (2012).
Inflammation is considered as a beneficial process under normal conditions. Inflammation being an immune response can also have deteriorating impact on its host, at times. The traditional treatment for inflammation involves the use of non-steroidal and anti-inflammatory drugs commonly known as NSAIDS (Non-Steroidal Anti-Inflammatory Drugs). These drugs do pose severe side effects in the case of chronic doses (Varga et al., 2017). The BvRM and BvLM extracts were evaluated for their anti-inflammatory action potential by conducting protein denaturation and HRBCs stabilization assays. Both extracts, BvLM and BvRM had the ability to inhibit protein/albumin denaturation when compared with the standard anti-inflammatory drug aspirin. Results of anti-inflammatory assays illustrated that B. vulgaris, commonly used as functional food, can highly be utilized for the cure of inflammation and related symptoms instead of synthetic conventional drugs which poses severe side effects. They also have the ability to boost immunity because of their anti-inflammatory properties and their richness in Vitamin C (Schlueter and Johnston, 2011). BvLM and BvRM extracts inhibited protein denaturation significantly exhibiting IC50 values of 2.322 ± 0.1 and 2.324 ± 0.06 μg/mL respectively. Results from current study presented evidence for membrane stabilization and inhibition of heat induced hemolysis by extracts as additive mechanism of their anti-inflammatory potential.
In neurological disorders treatment, strong inhibitors of acetylcholinesterase enzyme (AChE) are of great importance. This enzyme is involved in catalyzing the acetylcholine (ACh), therefore having potential for treatment of dementia (Nawaz and Choudhary, 2004) in addition to many other neuronal disorders (Kandiah et al., 2017). Decrease in ACh concentration via dysfunction of multiple biochemical pathways eventually leads to NDs (Callahan et al., 2017). AChE causes the termination of signal transmission in synapses via ACh. Therefore inhibition of this metabolizing enzyme may provide effective therapy for many other neurological disorders (Xiao et al., 2017). Unfortunately, currently available drugs are significantly associated with severe side effects including hepatotoxicity and are potent to use only in mild condition of disorder (McEneny-King et al., 2017). Therefore, prime importance of current study is to explore novel remedies for NDs treatment. Various research groups including current study are scrutinizing indigenous flora for finding potential bioactive compounds for treatment of neurological disorders. Present study illustrated the very significant AChE inhibitory activity by B. vulgaris extracts. Results demonstrated that percentage inhibition values of BvLM and BvRM are not significantly different from in comparison to donepezil (standard drug) at the same concentration. BvLM and BvRM extracts exhibited IC50 values of 4.99 μg/mL and 3.731 μg/mL in comparison to donepezil (IC50: 3.691 μg/mL) against AChE. Results have shown the significant therapeutic potency of extracts for cholinesterase. Previously Carpolobia lutea and many other plants were analyzed for their antioxidant activity and found therapeutically effective against AChE (Nwidu et al., 2017). Although beetroots show promising properties and activities, yet further studies regarding isolation will authenticate its therapeutic potential as neuroprotective agent. In future, our research group will continue study for the exploration of pharmacological activities of purified phytochemicals of B. vulgaris using in vitro and in silico techniques.
5. Conclusion
The current research work is an exclusive study for finding phytochemical composition, radical scavenging, anti-inflammatory and anti-acetylcholinesterase activity of methanolic extracts of B. vulgaris roots and leaves part. The results of AChE inhibition and radical scavenging assays were highly significant. Research data indicate that B. vulgaris is greatly enriched with antioxidants which can be implemented as potential therapeutic agents for the treatment of various neurological disorders. In the light of above mentioned pharmacognostic activity of B. vulgaris extracts, further studies on bioassay guided separation of bioactive compounds and their mechanism of action is recommended.
Funding
Current research work has received no specific grant from any funding agency in the public, commercial or not for-profit section.
Availability of data and materials
The data presented in this manuscript belong to research work of Shifa Shah and has not been deposited in any repository yet. However, the materials are available to the researchers upon request.
Authors’ contributions
SS carried out experimental work, data collection and literature search. SR supervised research work, helped in study design, data evaluation and drafted the final version of the manuscript. AMB and SMS helped in plant collection, provision of laboratory reagents and processing data interpretation. ZJ and AN reviewed the manuscript and statistical work. Authors approved the final manuscript for publication.
Declaration of Competing Interest
The authors declare that they have no competing interest.
Acknowledgements
The authors are grateful to the Department of Biosciences, COMSATS University Islamabad (CUI), Islamabad, Pakistan for providing laboratory facilities to conduct research activity.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aguiar S., Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013;16:313–326. doi: 10.1089/rej.2013.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A.s., 2015. 2015 Alzheimer's disease facts and figures. Alzheimers Dement 11, 332–384. [DOI] [PubMed]
- Baba S.A., Malik S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah. Univ. Sci. 2015;9:449–454. [Google Scholar]
- Bierer L.M., Haroutunian V., Gabriel S., Knott P.J., Carlin L.S., Purohit D.P., Perl D.P., Schmeidler J., Kanof P., Davis K.L. Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of the cholinergic deficits. J. Neurochem. 1995;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- Borsje P., Hems M.A., Lucassen P.L., Bor H., Koopmans R.T., Pot A.M. Psychological distress in informal caregivers of patients with dementia in primary care: course and determinants. Fam. Pract. 2016;33:374–381. doi: 10.1093/fampra/cmw009. [DOI] [PubMed] [Google Scholar]
- Bukhari S.I., Manzoor M., Dhar M. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018;98:733–745. doi: 10.1016/j.biopha.2017.12.090. [DOI] [PubMed] [Google Scholar]
- Callahan P.M., Bertrand D., Bertrand S., Plagenhoef M.R., Terry A.V., Jr Tropisetron sensitizes α7 containing nicotinic receptors to low levels of acetylcholine in vitro and improves memory-related task performance in young and aged animals. Neuropharmacology. 2017;117:422–433. doi: 10.1016/j.neuropharm.2017.02.025. [DOI] [PubMed] [Google Scholar]
- Chen, L.-E., Wu, F., Zhao, A., Ge, H., Zhan, H., 2016. Protection efficacy of the extract of Ginkgo biloba against the learning and memory damage of rats under repeated high sustained. Evid. Based Complement. Alternat. Med. 2016. [DOI] [PMC free article] [PubMed]
- Chopra K., Misra S., Kuhad A. Current perspectives on pharmacotherapy of Alzheimer's disease. Expert Opin. Pharmacother. 2011;12:335–350. doi: 10.1517/14656566.2011.520702. [DOI] [PubMed] [Google Scholar]
- Clifford T., Howatson G., West D.J., Stevenson E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7:2801–2822. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Maloney A. Selective loss of central cholinergic neurons in Alzheimer's disease. The Lancet. 1976;308:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- De Castro M.L., Priego-Capote F. Soxhlet extraction: past and present panacea. J. Chromatogr. 2010;1217:2383–2389. doi: 10.1016/j.chroma.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Edziri H., Jaziri R., Haddad O., Anthonissen R., Aouni M., Mastouri M., Verschaeve L. Phytochemical analysis, antioxidant, anticoagulant and in vitro toxicity and genotoxicity testing of methanolic and juice extracts of Beta vulgaris L. S. Afr. J. Bot. 2019;126:170–175. [Google Scholar]
- Federico A., Cardaioli E., Da Pozzo P., Formichi P., Gallus G.N., Radi E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012;322:254–262. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M., Hall K., Hasegawa K., Hendrie H., Huang Y. Global prevalence of dementia: a Delphi consensus study. The lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverdhan, P., Sravanthi, A., Mamatha, T., 2012. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis 2012. [DOI] [PMC free article] [PubMed]
- Halliwell B., Gutteridge J.M. Oxford University Press; USA: 2015. Free radicals in biology and medicine. [Google Scholar]
- Hasnat M., Pervin M., Lim B.O. Acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of Ganoderma lucidum grown on germinated brown rice. Molecules. 2013;18:6663–6678. doi: 10.3390/molecules18066663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad, M., Zafaryab, M., Singh, M., Rizvi, M., 2012. Comparative analysis of the antioxidant activity of Cassia fistula extracts. Int. J. Med. Chem. 2012. [DOI] [PMC free article] [PubMed]
- Jacob R.A. The integrated antioxidant system. Nutr. Res. 1995;15:755–766. [Google Scholar]
- Kandiah N., Pai M.-C., Senanarong V., Looi I., Ampil E., Park K.W., Karanam A.K., Christopher S. Rivastigmine: the advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson’s disease dementia. Clin. Interv. Aging. 2017;12:697. doi: 10.2147/CIA.S129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Pandey A.K. Free radicals: health implications and their mitigation by herbals. J. Adv. Med. Med. Res. 2015:438–457. [Google Scholar]
- Kumar S., Mishra A., Pandey A.K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern. Med. 2013;13:120. doi: 10.1186/1472-6882-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J.F., Wang L.-S., Rocha C.M., Larue B., Henry C., McIntyre C.M., Riedl K.M., Schwartz S.J., Stoner G.D. Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N-nitrosomethylbenzylamine-treated rats. J. Med. Food. 2010;13:733–739. doi: 10.1089/jmf.2008.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Sancheti S.A., Bafna M.R., Sancheti S.S., Seo S.-Y. Acetylcholineterase inhibitory and antioxidant properties of Rhododendron yedoense var. Poukhanense bark. J. Med. Plants Res. 2011;5:248–254. [Google Scholar]
- Li S., Chen G., Zhang C., Wu M., Wu S., Liu Q. Research progress of natural antioxidants in foods for the treatment of diseases. Food Sci. Hum. Well. 2014;3:110–116. [Google Scholar]
- Lovell M.A., Markesbery W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew M., Subramanian S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0086804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEneny-King A., Osman W., Edginton A.N., Rao P.P. Cytochrome P450 binding studies of novel tacrine derivatives: predicting the risk of hepatotoxicity. Bioorg. Med. Chem. Lett. 2017;27:2443–2449. doi: 10.1016/j.bmcl.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Counts S.E., Perez S.E., Ginsberg S.D. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev. Neurother. 2008;8:1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S.A., Choudhary M.I. New cholinesterase inhibiting bisbenzylisoquinoline alkaloids from Cocculus pendulus. Chem. Pharm. Bull. (Tokyo) 2004;52:802–806. doi: 10.1248/cpb.52.802. [DOI] [PubMed] [Google Scholar]
- Nunomura A., Perry G., Aliev G., Hirai K., Takeda A., Balraj E.K., Jones P.K., Ghanbari H., Wataya T., Shimohama S. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nwidu L.L., Elmorsy E., Thornton J., Wijamunige B., Wijesekara A., Tarbox R., Warren A., Carter W.G. Anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea. Pharm. Biol. 2017;55:1875–1883. doi: 10.1080/13880209.2017.1339283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016;17:777. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose S.M., Miller M.G., Scott T., Shukitt-Hale B. Nutritional factors affecting adult neurogenesis and cognitive function. Adv. Nutrit. 2017;8:804–811. doi: 10.3945/an.117.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prism G. San Diego; CA, USA: 1994. Graphpad software. [Google Scholar]
- Ruch R.J., Cheng S.-J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Schlueter A.K., Johnston C.S. Vitamin C: overview and update. J. Evid. Based Complementary Altern. Med. 2011;16:49–57. [Google Scholar]
- Shah M.D., Hossain M.A. Total flavonoids content and biochemical screening of the leaves of tropical endemic medicinal plant Merremia borneensis. Arab. J. Chem. 2014;7:1034–1038. [Google Scholar]
- Shrestha P., Adhikari S., Lamichhane B., Shrestha B.G. Phytochemical screening of the medicinal plants of Nepal. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015;1:11–17. [Google Scholar]
- Singh K.L., Bag G. Phytochemical analysis and determination of total phenolics content in water extracts of three species of Hedychium. Int. J. PharmTech. Res. 2013;5:1516–1521. [Google Scholar]
- Socaciu C. CRC Press; 2007. Food colorants: chemical and functional properties. [Google Scholar]
- Usman H., Abdulrahman F.I., Usman A. Qualitative phytochemical screening and in vitro antimicrobial effects of methanol stem bark extract of Ficus thonningii (Moraceae) Afr. J. Tradit. Complement. Altern. Med. 2009;6 doi: 10.4314/ajtcam.v6i3.57178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan S., Yatin S., Aksenova M., Butterfield D.A. Alzheimer's amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- Varga, Z., rafay ali Sabzwari, S., Vargova, V., 2017. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: an under-recognized public health issue. Cureus 9. [DOI] [PMC free article] [PubMed]
- Vinutha B., Prashanth D., Salma K., Sreeja S., Pratiti D., Padmaja R., Radhika S., Amit A., Venkateshwarlu K., Deepak M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007;109:359–363. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Xiao S., Wang T., Ma X., Qin Y., Li X., Zhao Z., Liu X., Wang X., Xie H., Jiang Q. Efficacy and safety of a novel acetylcholinesterase inhibitor octohydroaminoacridine in mild-to-moderate Alzheimer's disease: a phase II multicenter randomised controlled trial. Age Ageing. 2017;46:767–773. doi: 10.1093/ageing/afx045. [DOI] [PubMed] [Google Scholar]
- Yadav R., Agarwala M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this manuscript belong to research work of Shifa Shah and has not been deposited in any repository yet. However, the materials are available to the researchers upon request.