Abstract
The Andean tree Schinus areira L. has multiple traditional uses, from the treatment of bronchitis and rheumatic diseases to menstrual cycle regulation and wound healing. With reported hypotensive, analgesic, antitumoral and anti-inflammatory properties, it acts predominantly against diseases related to oxidative stress. This study focuses on the antioxidant activity and phytochemical profile of the extracts of Schinus areira L.
Serial extraction of the fruits was performed both by maceration and by Soxhlet. Total phenols and flavonoids were measured using the Folin-Ciocalteu method and AlCl3, respectively. In vitro antioxidant activity was determined by FRAP and DPPH.
Results were similar for both extraction methods. Primary metabolites detected included carbohydrates, proteins and amino acids; secondary metabolites included tannins, flavonoids, saponins, steroids and triterpenes. Antioxidant activity was confirmed for ethyl acetate, methanolic and aqueous extracts. The methanolic extract had both the highest polyphenol content (>195 mg GAE/ g dry weight) and the highest antioxidant activity [EC50 > 476 μg/mL; >273 mg AA/g dry weight (DPPH); >301 mg AA/ g dry weight (FRAP)]. The extract does not produce macrophage cytotoxicity in RAW 264.7, which is indicated by an average cytotoxicity of 2% over 24 h.
Our study serves as a starting point for future research on the pharmacological properties of Schinus areira L.
Keywords: Antioxidant activity, Flavonoids, Schinus areira L., Polyphenols
1. Introduction
Since their earliest origins, humans have benefited from the properties of dietary plant species in order to preserve and better their health. The perennial Andean tree Schinus molle var areira (L.) DC. is a synonym of Schinus areira L. (Anacardiaceae) belongs to a South American genus comprising 30 species (Dellacassa, 2010, Murray and Murray, 2017). Common names (in English: California peppertreee, Californian pepper tree, Californian peppertree, pepper tree, peppercorn, peppercorn tree, pepperina, Peruvian mastic, Peruvian mastic tree, Peruvuian mastictree, Peruvian pepper tree, Peruvian peppertree; in Spanish: pimiento boliviano (Bolivia), aguaribay (Uruguay), pimentero, falsa pimienta (Chile), anacahuita, mulli and cuyash (Perú), molle (Bolivia and Argentina), aroeira (Brazil and Colombia) (García & Ormazabal, 2008). It grows up to 25 m tall and produces red or pink berries with a spicy taste and a strong smell resembling that of pepper (García and Ormazabal, 2008, Ministerio de Salud de Chile, 2007). Its traditional uses in medicine and diet include applications as a purging agent, mouth wash, diuretic, disinfectant, hypotensor and sedative. The volatile oil obtained by steam distillation of the berries is used as a substitute of black pepper in spice mixes. In Peru, the berries are soaked in water with sugar to produce chicha de molle for consumption at traditional festivities (Dellacasa, 2010). In Chile, infusions of leaves and fruits are used to treat bronchitis and asthma, rheumatic diseases, liver and stomach complaints, sciatica, the swelling of arms and legs, and to promote wound healing. Several studies have demonstrated biological activity for the control of insect and microorganism pests: Sitophilus oryzae (Benzi et al., 2009), Nezara veridula (Werdin et al., 2008, Werdin et al., 2011), Tribolium castaneum Herbst (Descamps et al., 2008), Botrytis cinerea Pers. (Hapon et al., 2017), Gonipterus platensis Marelli (Chiffelle et al., 2017), Blatella germanica L. (Sanchez et al., 2006), Helicoverpa zea (Boddie) (Guevara et al., 2018), Xanthogaleruca luteola Müller (Huerta et al., 2010), Apis mellifera (Guala et al., 2014), Rhipicephalus microplus (Torres et al., 2012), Triatoma infestans Klug (Ferrero et al., 2006) and Staphylococcus aureus ATCC 25923 (Alfaro-Perez & Ruiz-Barrueto, 2018). There are two varieties in Chile, S. areira L. and S. molle var. rusbyi Barkley (Muñoz et al., 2004); here, we focus on S. areira L.
Previous studies have demonstrated various biological effects of the extracts of S. molle L., including as a hypotensor, analgesic, antispasmodic, antifungal, antitumoral, anti-inflammatory and diuretic (Quiroga et al., 2001, Yueqin et al., 2003, Ruffa et al., 2002, Bras et al., 2010, Bello et al., 1996, Barrachina et al., 1997); anti-rheumatic, antiseptic, anti-inflammatory, antifungal, antimicrobial, in the treatment of disorders related to skin and anti-depressive trealtment (Silva-Junior et al., 2015). These effects are in line with the presence of a range of biologically active compounds, such as essential oils (including α and β-felandrene, (±)camphene, myrcene, (-)α and (+)β-pinene, carvacrol, (−)-limonene), flavonoids, phenolic compounds, tannins, triterpenes and steroids, and quinones (Dellacassa, 2010, Muñoz et al., 2004, Fuertes et al., 2014). Many studies have demonstrated the antioxidant effect of S. molle essential oil, and their components. (Guala et al., 2009, Bendaoud et al., 2010, do Rosário Martins et al., 2014).
Likewise, despite a potential role in the demonstrated biological activity of the plant, the antioxidant activity of the fruits from Chilean species has never been investigated. Finally, the traditional use of this species in diseases related to oxidative stress needs to be supported by scientific evidence.
We thus obtained a phytochemical profile of the fruit extracts of S. areira L., including total phenols and flavonoids, as well as testing their antioxidant activity by FRAP and DPPH. A serial extraction was performed both by maceration and by Soxhlet, and the results of these two protocols were compared. Our study is the first to investigate at depth the spectrum of primary and secondary metabolites presents in S. areira L., and to examine the antioxidant activity of this species of wide use in traditional medicine and diet.
2. Materials and methods
2.1. Plant material
Fruits of S. areira L. were collected in the Municipality of La Florida, Santiago, Chile, in the month of September and confirmed in the Department of Pharmacy at Universidad Andres Bello (herbarium specimen SQF-22501). Fruits were dried in a forced air oven at 30 °C over five days, until reaching a constant weight, and shredded with a Braun Multiquick 7 immersion blender prior to extraction.
2.2. Serial extraction
40 g of fruits of S. areira L. were used in a serial extraction with solvents of increasing polarity: hexane, dichloromethane, ethyl acetate, methanol and water. The extraction was performed using two methods, by maceration and by Soxhlet. Extraction by maceration was performed by leaving the plant material in contact with the solvent for 48 h (solvent:plant material = 10:1) at room temperature, leaving the material to dry before adding the next solvent. Soxhlet extraction was done over five hours with each solvent, before air-drying the plant material at room temperature and continuing to the next solvent. All extracts were concentrated using a rotary evaporator at low pressure and stored in amber bottles.
2.3. Phytochemical profile
Standard tests for primary and secondary metabolites were carried out according to the Phytochemical screening standard, which also contains the results of these tests (see Results section, Table 2). Each test for each primary or secondary metabolite included a reference compound.
Table 2.
Quantitative phytochemical analysis of S. areira L. extracts.
| Metabolite | Reference compound | Assay | Reference | RC | Maceration |
Soxhlet |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEX | DCM | EA | MET | AQ | HEX | DCM | EA | MET | AQ | |||||
| Carbohydrates | Glucose | Molisch | Morsy, 2014 | +++ | – | – | – | +++ | +++ | – | – | – | +++ | +++ |
| Benedict | Morsy, 2014 | ++ | – | – | ± | +++ | ++ | – | – | – | +++ | ++ | ||
| Proteins and amino acids | Albumin | Ninhydrin | Morsy, 2014 | ++ | – | – | – | ++ | +++ | – | – | – | ++ | +++ |
| Biuret | Obasi et al. 2010 | +++ | – | – | – | – | – | – | – | – | – | – | ||
| Alkaloids | Atropine | Dragendorff | Obasi et al. 2010 | +++ | – | – | – | – | – | – | – | – | – | – |
| Mayer | Obasi et al. 2010 | +++ | – | – | – | – | – | – | – | – | – | – | ||
| Wagner | Obasi et al. 2010 | +++ | – | – | – | – | – | – | – | – | – | – | ||
| Steroids and terpenoids | Cholesterol | Liebermann-Burchard | Morsy, 2014 | +++ | + | +++ | ++ | – | – | +++ | ++ | + | – | – |
| Salkowski | Morsy, 2014 | +++ | – | – | – | – | – | – | – | – | – | – | ||
| Saponins | Saponin | Foam | Morsy, 2014 | +++ | – | – | – | ++ | + | – | – | – | ++ | + |
| Cardiac glycoside | Digoxin | Keller-Killiani | Morsy, 2014 | +++ | – | – | – | – | – | – | – | – | – | – |
| Coumarin | Warfarin | Fluorescence | Morsy, 2014 | +++ | – | – | – | – | – | – | – | – | – | – |
| Naphtho- and anthraquinones | Bark of Rhamnus frangula, leaves of Cassia angustifolia M. Vahl | Bornträger | Morsy, 2014 | +++ | – | – | – | – | – | – | – | – | – | – |
| Bornträger (modified) | Morsy, 2014 | + | – | – | – | – | – | – | – | – | – | – | ||
| Flavonoids | Quercetin | Aluminium chloride | Morsy, 2014 | +++ | – | – | ++ | ++ | ++ | – | – | + | ++ | + |
| Shinoda | Sarla et al., 2012 | +++ | – | – | – | + | ++ | – | – | – | + | ++ | ||
| Alkaline reagent | Roopalatha and Vijay Mala, 2013 | +++ | – | – | + | ++ | +++ | – | – | + | ++ | ++ | ||
| Lead acetate | Morsy, 2014 | +++ | – | – | – | +++ | + | – | – | – | +++ | +++ | ||
| Tannins and phenolic compounds | Grape seed extract (Vitis vinifera) | Iron(III) chloride | Sarla et al., 2012 | +++ | – | – | ± | +++ | ++ | – | – | ± | +++ | ++ |
| Gelatine | Sarla et al., 2012 | +++ | – | – | ± | ++ | – | – | – | ± | ++ | – | ||
HEX, hexane extract; DCM, dichloromethane extract; EA, ethyl acetate extract; MET, methanolic extract; AQ, aqueous extract.
+++ = High, ++ = Moderate, + = Low ± = inconclusive; RC = result for the reference compound.
2.4. Total polyphenols
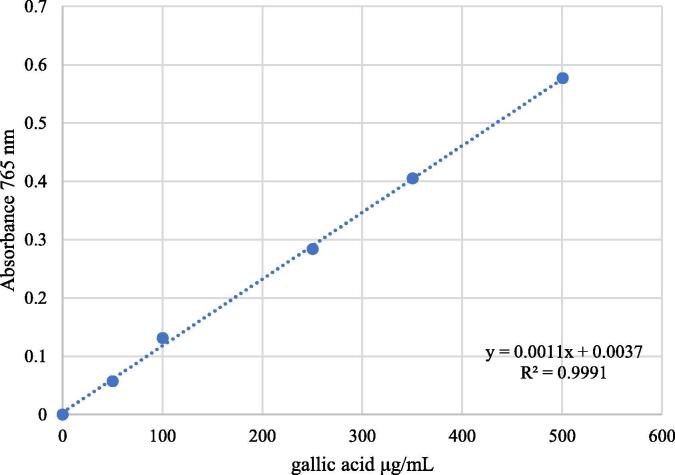
Total polyphenols were measured in triplicate using the Folin-Ciocalteu method, as described in the literature (Singleton and Rossi, 1965, Waterhouse, 2002), based on a calibration curve of gallic acid in distilled water at concentrations of 50, 100, 250, 350 and 500 μg/mL (Fig. 1). Absorbance was measured at 765 nm.
Fig. 1.
Standard calibration curve of gallic acid.
2.5. Total flavonoids
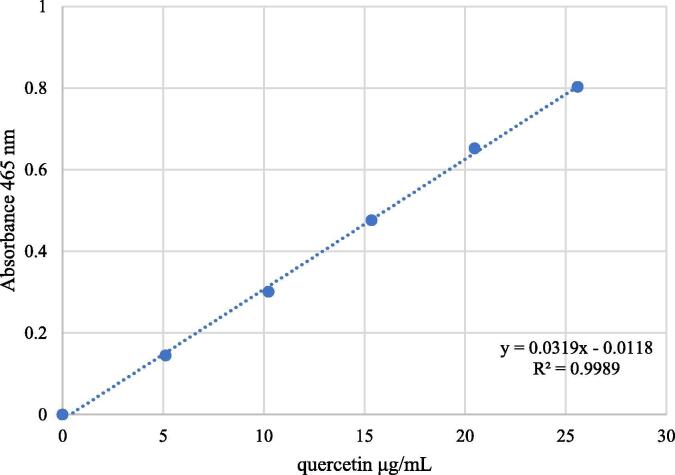
Total flavonoids were measured in triplicate based on flavonoid-AlCl3 complex formation, as described in the literature (Kumazawa et al., 2004), based on a calibration curve of quercetin in ethanol at concentrations of 5, 10, 15, 20 and 25 μg/mL (Fig. 2). Absorbance was measured at 420 nm.
Fig. 2.
Standard calibration curve of quercetin.
2.6. DPPH free radical scavenging
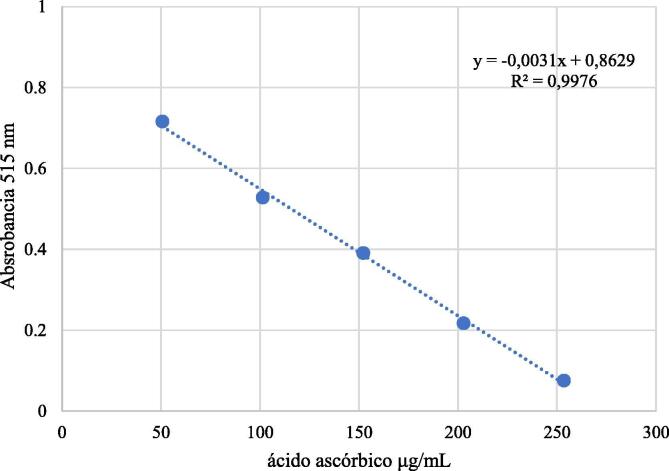
Free radical scavenging was measured in triplicate using DPPH (2,2-diphenyl-1-picrylhydrazyl) as described in the literature (Brand-Williams et al., 1995), based on a calibration curve of ascorbic acid in methanol at concentrations of 50, 100, 150, 200 and 250 μg/mL (Fig. 3). DPPH solutions were prepared daily. As a reference compound, BHA was used in methanol at a concentration of 50 μg/mL. Absorbance was measured at 515 nm.
Fig. 3.
Standard calibration curve of ascorbic acid (•DPPH method).
DPPH scavenging was monitored at 515 nm until the reaction reached a plateau, over a minimum of 30 min and a maximum of 120 min. The remaining percentage of DPPH (% DPPH rem) was calculated once the reaction had reached a plateau, or after 30 min; it served as the basis to determine the EC50. This was again done in triplicate.
2.7. Ferric reducing antioxidant power (FRAP)
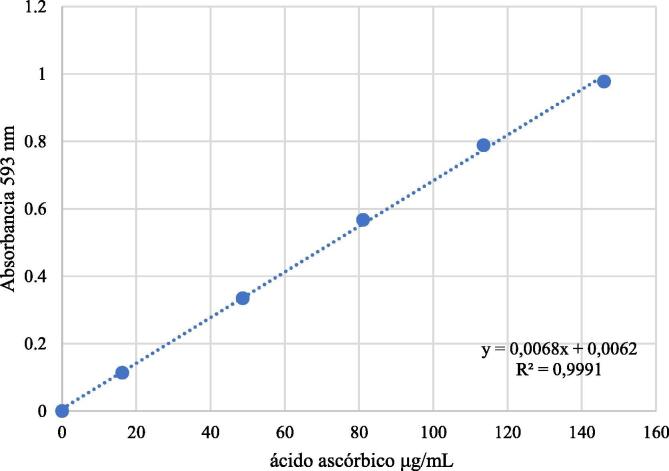
The FRAP was determined in triplicate as described in the literature (Benzi & Strain, 1996), based on a calibration curve of ascorbic acid in methanol at concentrations of 16.22, 48.66, 81.1, 113.54 and 146.0 μg/mL (Fig. 4). FRAP solutions were prepared daily. As a reference compound, BHA was used in methanol at a concentration of 30 μg/mL. Absorbance was measured at 593 nm.
Fig. 4.
Standard calibration curve of ascorbic acid (FRAP method).
2.8. Cell culture
RAW 264.7 cell line was used during the experiments (murine macrophage, ATCC TIB-71 ™). Cells were kept in bottles TR6002 (Trueline) with RPMI-1640 (Gibco, Life Technologies) containing 4 mM L-glutamine, FCS 10% (v/v), 100 U/mL of antibiotic–antimycotic and 3.7 g/L of sodium bicarbonate at pH 7.4 and maintained in incubator at 37 °C and 5% CO2. Cells were counted by trypan blue using an automated cell counter (Countess™, Invitrogen). Viability percentage was always ≥95%.
2.9. Cytotoxicity assay
Cell viability was carried out through Lactate Dehydrogenase (LDH) assay. In brief, cells (5x105 cells/well) were incubated with the following extracts: Dichloromethane, Methanol and Hexane at concentrations of 0.01 to 0.05 and 0.1 μM. Cells were incubated for 24 hrs at 37 °C and 5% CO2. Then, 10X of lysis buffer was added to one well as a positive control. Supernatants were collected and centrifuged at 14,000 rpm for 5 min. In a 96 well plate aliquots of 50 uL of centrifuged supernatants were transferred and mixed with 50 μL of substrate mix for 30 min at room temperature in dark conditions. Then 50 μL of stop buffer were added to stop the enzyme reaction and absorbance at 490 nm was measured on a spectrophotometric reader (Synergy H1 Hybrid Reader, Biotek®).
The absorbance are used to calculate the percent cytotoxicity of each sample in the following equation:
2.10. Enzyme linked immunosorbent assay (ELISA)
In a 96 well plate (Nunc MaxiSorp™ ELISA Plates, Uncoated), 100 μL of capture antibody (1:200) was incubated in loading buffer (0.1 M NaHCO3, Na2CO3 0.03 M). The plate was sealed and left overnight at 4 °C. Then, the plate was washed 3 times with wash buffer (PBS + 0.05% Tween 1x-20) and 100 μL of blocking buffer (1x PBS + 10% FBS) and was incubated for 2 hrs at room temperature. Then, the plate was incubated with 100 μL of supernatants obtained in each treatment (Dichloromethane, Hexane and Methanol extracts (0.05; 0.01 0,1 μg/ml)) previously centrifuged at 14,000 rpm and diluted (1:10) in dilution buffer (10% FBS in 1X PBS). In parallel standard samples for calibration curve (Mouse MAX ™ Deluxe ELISA Set, Biolegend®) was performed and the plate was sealed and left overnight at 4 °C. Then, the plate was washed 3 times and the detection antibody was incubated with biotinylated conjugate (1: 200) in dilution buffer for 1 hr at room temperature. Then, the plate was washed 3 times and incubated with 100 μL of avidin HRP (1: 1000) in dilution buffer for 30 min at room temperature. The plate was washed 5 times and 100 μL of enzyme substrate TMB-HS (Biolegend) was added for 10–15 min (blue color). Then, the reaction was stopped by adding 100 μL of stop buffer (2 N H2SO4) (yellow color) and the colorimetric reaction was measured at 450 nm in a spectrophotometric reader (Synergy H1 Hybrid reader, Biotek®).
2.11. Statistic analysis
All analyzes were performed by GraphPad Prism software version 5.03. Results were analyzed using one-way ANOVA with Bonferroni post-test. n.s. = Not significant, * p < 0.05, ** p < 0.01, *** p < 0.001.
3. Results
The yields of all extracts are listed in Table 1.
Table 1.
Yield of the serial extraction of S. areira L. in solvents of increasing polarity and two extraction methods.
| Extract | Yield (% w/w) |
|
|---|---|---|
| Soxhlet | Maceration | |
| HEX | 6.9 | 5.6 |
| DCM | 3.0 | 2.7 |
| EA | 1.0 | 0.8 |
| MET | 11.8 | 12.1 |
| AQ | 11.9 | 12.4 |
HEX, hexane extract; DCM, dichloromethane extract; EA, ethyl acetate extract; MET, methanolic extract; AQ, aqueous extract.
3.1. Phytochemical profile
The results of the phytochemical screening are shown in Table 2. No differences in the presence or absence of metabolites were observed between the different extraction methods. The spectrum of secondary metabolites was greater in methanolic and aqueous extracts than in the extracts obtained with non-polar solvents. Phenolic compounds, tannins and flavonoids were predominantly found in the methanolic and aqueous extracts, and only to a minor degree in the ethyl acetate extract. Only methanolic, aqueous and ethyl acetate extracts were thus tested for polyphenols and flavonoids Table 2.
3.2. Total polyphenols
Total polyphenols of S. areira L. extracts were expressed as gallic acid equivalents (GAE) following the calibration curve equation y = 0.0011x + 0.0037, R2 = 0.9991 (Fig. 1, showed above), where y is the absorbance at 765 nm, and x is the total polyphenol content of S. areira L. extracts in mg GAE/g.
The results are summarized in Table 3. No notable differences were observed between the two extraction methods. Polyphenol content was highest in the methanolic extracts (Soxhlet: 195.787 ± 3.292 mg GAE/g; maceration: 197.023 ± 3.353 mg GAE/g) and lowest in the aqueous extracts (Soxhlet: 84.703 ± 6.766 mg GAE/g; maceration: 88.535 ± 3.317 mg GAE/g) Table 3.
Table 3.
Total polyphenols, total flavonoids and antioxidant activity of S. areira L. extracts.
| Extract | Total polyphenols (mg GAE/ g d.w. ± SD) |
Total flavonoids (mg QE/ g d.w. ± SD |
DPPH |
FRAP (mg AA/ g d.w. ± SD) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 μg/mL |
mg AA/ g d.w. ± SD |
|||||||||
| Soxhlet | Maceration | Soxhlet | Maceration | Soxhlet | Maceration | Soxhlet | Maceration | Soxhlet | Maceration | |
| EA | 88.747 ± 2.207 | 92.443 ± 6.109 | 8.937 ± 0.034 | 9.281 ± 0.182 | 848.638 | n/d | 189.384 ± 0.400 | 137.002 ± 1.736 | 193.978 ± 2.486 | 135.392 ± 10.444 |
| MET | 195.787 ± 3.292 | 197.023 ± 3.353 | 12.226 ± 0.430 | 12.032 ± 0.390 | 476.560 | 485.636 | 273.597 ± 3.158 | 283.051 ± 5.062 | 301.609 ± 3.024 | 309.048 ± 4.066 |
| AQ | 84.703 ± 6.766 | 88.535 ± 3.317 | 13.631 ± 0.111 | 13.257 ± 0.206 | n/d | n/d | 88.522 ± 1.567 | 93.968 ± 1.378 | 115.730 ± 0.536 | 101.942 ± 3.491 |
| BHA | n/d | n/d | n/d | 1824.640 ± 93.983 | 1953.305 ± 78.850 | |||||
| AA | n/d | n/d | 124.126 | n/d | n/d | |||||
EA, ethyl acetate extract; MET, methanolic extract; AQ, aqueous extract.
d.w. = extract dry weight; SD = standard deviation; n/d = not determined; GAE = gallic acid equivalents; QE = quercetin equivalents; AA = ascorbic acid; BHA = Butylated hydroxyanisole.
3.3. Total flavonoids
Total flavonoids were expressed as quercetin equivalents (QE), following the calibration curve equation y = 0.0319x 0.0118, R2 = 0.9989 (Fig. 2, showed above), where y is the absorbance at 420 nm, and x is the total flavonoid content of the extracts of S. areira L. in mg QE/g.
The results are summarized in Table 3. No notable differences were found between the two extraction methods. The flavonoid content was highest in the methanolic extracts (Soxhlet: 12.226 ± 0.430 mg QE/g; maceration: 12.032 ± 0.390 mg QE/g), and lowest in the ethyl acetate extracts (Soxhlet: 8.937 ± 0.034 mg QE/g; maceration: 9.281 ± 0.182 mg QE/g).
3.4. In vitro antioxidant activity: kinetics of DPPH scavenging
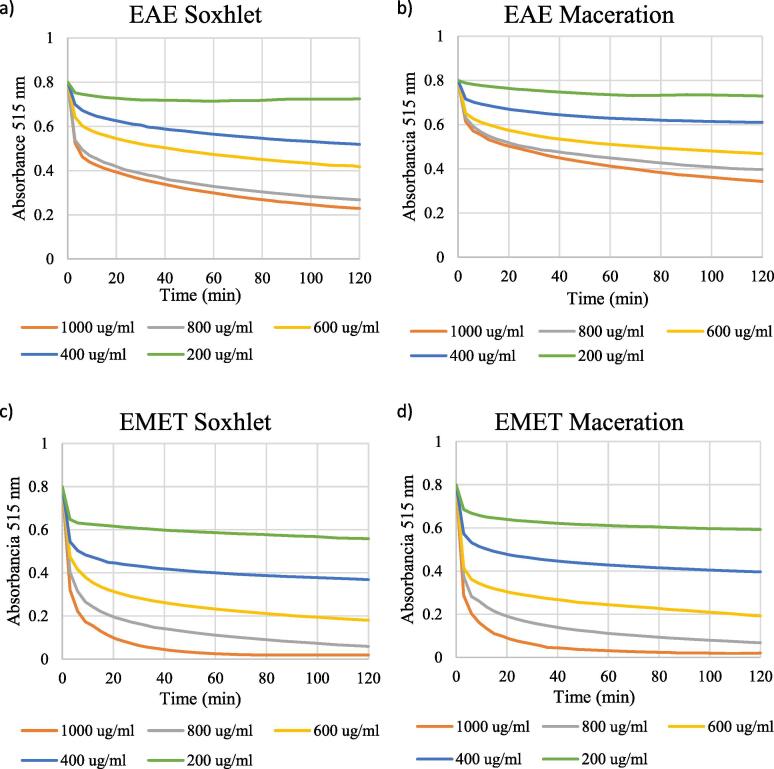
DPPH scavenging capacity was examined for ethyl acetate, methanolic and aqueous extracts obtained by both maceration and Soxhlet. All extracts showed antioxidant activity in a dosis-dependent manner (Fig. 5), where initial DPPH levels decreased faster with increasing extract concentrations. The reaction of ascorbic acid with DPPH was completed within three min, while for S. areira L. extracts, the reaction continued beyond 30 min; it did not reach a plateau within the time of the experiment. %DPPHrem and EC50 were thus calculated after 30 min, as frequently done in the literature (Lim et al., 2006, Iqbal et al., 2015, Benites et al., 2018, Benites et al., 2019a, Benites et al., 2019b, Araya et al., 2020).
Fig. 5.
Kinetics DPPH for extracts obtained with the soxhlet method (panels a, c, e) and with the maceration method (panels b, d, f).
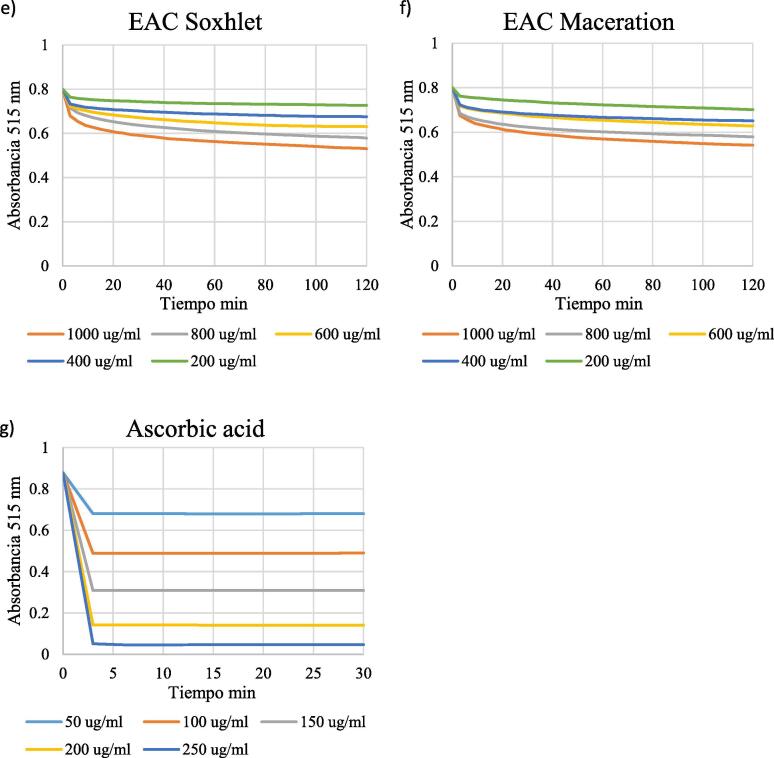
3.5. Determination of the EC50
EC50 values were determined from the plots of extract concentrations vs %DPPHrem (Fig. 6).
Fig. 6.
Percentage of DPPH remaining after 30 min for different concentrations of the extracts obtained by soxhlet (panels a, c, e) and maceration (panels b, d, f). Panel g) shows the percentage of DPPH remaining after 30 min for different concentrations of ascorbic acid, compound with rapid kinetics.
All samples examined showed antioxidant activity (see Table 3). EC50 values could not be determined for the aqueous extracts and for the ethyl acetate extract obtained by maceration, since they did not decrease DPPH levels by 50% within the time of the experiment.
The lowest EC50 values were observed for the methanolic extracts (Soxhlet: 476.560 μg/mL; maceration: 485.636 μg/mL). The EC50 for the standard compound ascorbic acid was 124.126 μg/mL.
3.6. Antioxidant capacity by DPPH scavenging
Antioxidant capacity of S. areira L. extracts was expressed as ascorbic acid equivalents (AAE), following the calibration curve equation y = −0.0031x + 0.8629, R2 = 0.9976 (Fig. 3. Showed above), where y is the absorbance at 515 nm, and x is the antioxidant capacity of S. areira L. extracts in mg AAE/g. The results are shown in Table 3. No notable differences in antioxidant capacity were found between extraction methods. For both methods, antioxidant capacity was highest in the methanolic extracts (Soxhlet: 273.597 ± 3.158 mg AAE/g; maceration: 283.051 ± 5.062 mg AAE/g) and lowest in the aqueous extracts (Soxhlet: 88.522 ± 1.567 mg AAE/g; maceration: 93.968 ± 1.378 mg AAE/g). The antioxidant capacity of the standard compound BHA was 1824.640 ± 93.983 mg AAE/g.
3.7. FRAP xxx
The FRAP of S. areira L. extracts was expressed as AAE, following the calibration curve equation y = 0.0068x + 0.0062, R2 = 0.9991 (Fig. 4, showed above), where y is the absorbance at 593 nm, and x is the FRAP of S. areira L. extracts in mg AAE/g.
The results are shown in Table 3. The FRAP was highest in methanolic extracts (Soxhlet: 301.609 ± 3.024 mg AAE/g; maceration: 309.048 ± 4.066 mg AAE/g) and lowest in ethyl acetate extracts (Soxhlet: 115.730 ± 0.536 mg AAE/g; maceration: 101.942 ± 3.491 mg AAE/g). The FRAP of the standard compound BHA was 1953.305 ± 78.850 mg AAE/g.
3.8. S. areira L. extract do not produce cytotoxicity in RAW 264.7 macrophages
Prior to evaluating anti-inflammatory activity of S. areira L. different extracts we investigated the possible cytotoxic effect that might generate dichloromethane, methanol, hexane extracts at different concentrations. In this assay the integrity of the cell membrane was evaluated by measuring LDH activity enzyme. Results in Fig. 7 indicate average cytotoxicity levels of 2% for 24 h, demonstrating that increasing concentrations of the respective extracts, showed no cytotoxicity levels with respect to its control (p > 0.05).
Fig. 7.
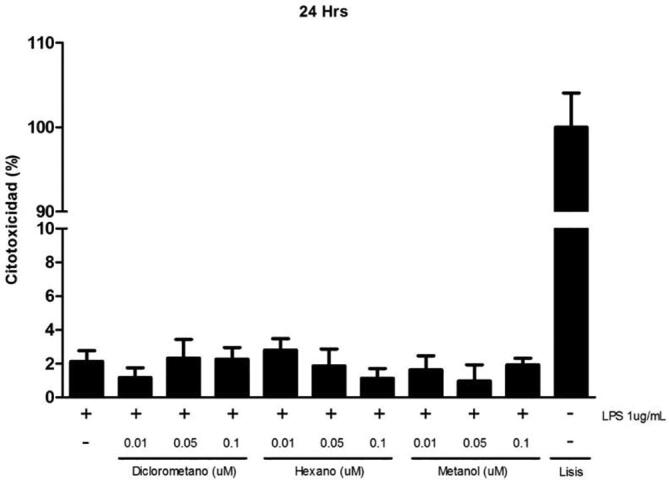
Cytotoxic effect of S. areira L. extracts on RAW 264.7 cells. 5 × 105 cells/well were seeded and treated with different concentrations of dichloromethane, methanol and hexane extracts (0.01–0.05 and 0.1 μM) for 24 hrs. Culture medium was used as negative control and lysis buffer was added to the cells as a positive control. LPS (1 ug/mL) was used as a proinflammatory agent. Cytotoxicity was determined by LDH assay. Results are expressed as the mean ± standard deviation of three independent experiments with duplicate determinations. One-way ANOVA with Bonferroni test as statistical analysis was performed. n.s. = not significant.
3.9. S. areira L. extracts presents anti-inflammatory activity in RAW 264.7 macrophages
Since different concentrations of the obtained extracts do not generate cytotoxic effects on RAW 264.7 cells, we proceeded to determine whether these concentrations could trigger anti-inflammatory response. For this, we quantify the secretion of two proinflammatory cytokines (IL-6 and TNF-α) by ELISA. Results indicated that only the dichloromethane extract in a concentration of 0.1 μM causes a significant decrease in the IL-6 levels (Fig. 8A), whereas TNF-α was decreased with all extracts at concentrations of 0.1 μM with respect to its control (Fig. 8B). LPS was used as an inducer of proinflammatory cytokine secretion in RAW 264.7 cells (Xiang et al., 2018) Fig. 2, Fig. 8.
Fig. 8.
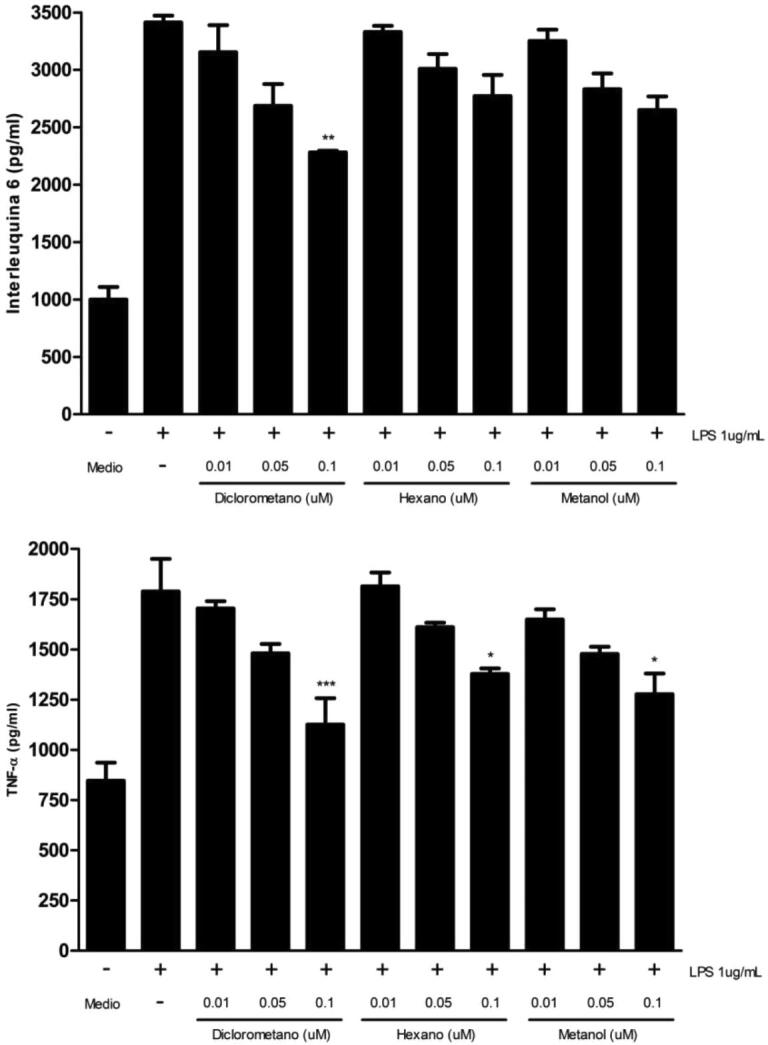
Proinflammatory cytokines quantification in RAW 264.7. 5x105 cells/well were plated and incubated with culture medium (control), LPS 1 ug/ml (positive control), and dichloromethane, hexane and methanol extracts (0.01–0.05 and 0.1 μM) for 24 h at 37 °C and 5% CO2. IL-6 (A) and TNF-α (B) concentrations were analyzed by ELISA. Values are expressed as the mean ± standard deviation of three independent experiments with duplicate determinations. One-way ANOVA with Bonferroni test as statistical analysis was performed. * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
This study is the first to combine a phytochemical screening with an investigation of the antioxidant capacity of S. areira L. extracts.
There were no major differences in the yield of the different extracts when comparing the two extraction methods. Yields were greatest for methanolic and aqueous extracts, suggesting a greater content of polar than non-polar compounds. Phytochemical analyses of the same S. areira L. variety studied here have identified sesquiterpenes, triterpenes, flavonoids, tannins, saponins, gums, resins, and essential oils (Dellacassa, 2010, Yueqin et al., 2003, Ferrero et al., 2006). In line with these previous findings, we found carbohydrates, proteins, flavonoids, tannins, terpenes and saponins in S. areira L. extracts.
Many studies about propierties and the fitocomponents obteained from S. areira L. have been published and it showed the great potential of antioxidants isolated compounds and their activity in Schinus areira leaves and fruits. (Murray and Murray, 2017, Celaya et al., 2016).
The breadth of secondary metabolites can be linked to potential biological effects. Flavonoids are known to have anti-inflammatory, antimicrobial, antithrombotic, antiallergic, antitumoral, anticancerogenic, and antioxidant properties (Venegas, 2012). Likewise, the properties of polyphenols include antioxidant, antibacterial, antiviral, anticancerogenic and anti-inflammatory activities. Tannins inhibit the growth of pathogenic fungi, as well as having antibacterial, antioxidant and diuretic properties, and acting as an insecticide. Saponins have antifungal, anticancer, anti-inflammatory, hemolytic, antiprotozoal and hypocholesterolemic properties, as well as acting as a spermicide. Finally, terpenoids are known to possess analgesic, antioxidant and antidiabetic properties (Pranoothi et al., 2014, Samatha et al., 2012, Poongothai et al., 2011). S. areira L. is widely used in traditional medicine as an anti-inflammatory, antispasmodic and analgesic (Ministerio de Salud de Chile, 2007); these uses might be attributed to the presence of some of the metabolites identified here. The results of our study thus represent a first step towards the isolation and characterization of the diverse active principles present in S. areira L., contribute to a better understanding of this plant and can be extended to pharmacological studies.
There is no classification system allowing establishing whether the polyphenol contents of a plant should be considered high or low. However, in a study of the extracts of various species of the genus Schinus, the polyphenol content of S. areira L. extract did not exceed 15 mg GAE/g dry weight (Rhouma et al., 2009). The variety studied was not specified, complicating a comparison. Nevertheless, we found a much higher polyphenol content than what has previously been reported for S. areira L., in particular in the methanolic extracts (see Table 3).
Antioxidant activity is generally determined over a fixed period of time, which varies considerably among studies. Most studies measure %DPPH rem after 15 min and/or 30 min, and only rarely after 2 h (Lim et al., 2006, Iqbal et al., 2015, González et al., 2007, Tepe et al., 2007, Fadda et al., 2014). Nevertheless, the use of a fixed end point does often not take into account the kinetic behavior of the antioxidants tested. As a consequence, shorter reaction times might lead to lower antioxidant activity values; likewise, very potent antioxidants require a shorter reaction time. Here, we monitored the reaction of the extracts with DPPH over time until they reached a plateau, i.e. until absorbance values remained constant. We found that free radical scavenging continued beyond 30 min, thus showing that the compounds present in the extracts examined here showed a slow kinetics in their reaction with DPPH, and demonstrating that the time interval necessary to complete the reaction depends significantly on the sample examined. Consequently, standard time limits cannot be set to replicate this assay.
We found that the methanolic extracts had the lowest EC50, and hence the greatest antioxidant effect; however, their effect was much lower than that of the standard ascorbic acid.
The highest FRAP and DPPH were found in the methanolic extract obtained by maceration; however, these values were again much lower than those of the synthetic antioxidant BHA. To the best of our knowledge, there are no reports in the literature on the antioxidant activity of S. areira L. extracts (of either variety), making our study a starting point for future investigations.
With the highest flavonoid content of all extracts examined, the aqueous extracts might be expected to also have the highest antioxidant capacity. Nevertheless, their antioxidant capacity is lower than that of the ethyl acetate extracts, whose flavonoid content is the lowest of all extracts. Flavonoid antioxidant activity depends on their chemical structure, and in particular on the position and number of substitutions in the flavonoid nucleus and B-ring (Gonzalez et al., 2007). Antioxidant activity can thus not be related to flavonoid content in a straightforward way. The methanolic extracts, which have the highest level of antioxidant activity, might contain flavonoids with free hydroxyl groups in strategic positions, conferring them a particularly high antioxidant activity.
In contrast, there was a positive correlation between antioxidant activity and total polyphenol content, in line with the literature (Dudonné et al., 2009). This might indicate that the phenolic compounds are the main contributors to antioxidant activity in the extracts examined. It should also be taken into account that the assays used here are only part of the various assays available to test antioxidant activity, and although we have shown that S. areira L. possess electron transfer activity, this does not preclude the presence of other mechanisms of antioxidant action. Furthermore, antioxidant activity was not evaluated in hexane and dichloromethane extracts due to their low solubility in polar solvents, which makes them incompatible with FRAP and DPPH assays. However, these extracts showed the presence of terpenes, which are also known to possess antioxidant effects (Pranoothi et al., 2014, Celaya et al., 2016). Also, Bendaoud et al., in 2010 evaluated in vitro antioxidant and antiradical scavenging properties of essential oils from S. molle by using 1,1-diphenyl-2-picrylhydrazyl (DPPH), they showed hat this antioxidant effect was correlated with anticancer activity also evaluated in the study (Bendaoud, et al., 2010). Many studies have demonstrated the antioxidant effect of S molle essential oil, inferring that the terpenes and components present in these oils may be part of the phytochemicals responsible for this effect (Bendaoud et al., 2010, do Rosário Martins et al., 2014, Guala et al., 2009).
In conclusion, our study is the first to combine an in-depth phytochemical screening with the assessment of the antioxidant capacity of the extracts of the South American tree S. areira L. The spectrum of compounds identified and the antioxidant activity found are compatible with the pharmacological activity of this species, as well as with its traditional use as a remedy and dietary ingredient. Our study represents a starting point for the future biochemical and pharmacological investigation of this species.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Special thanks to staff of Biopub, in particular to Dra. Barbara Kremeyer for assistance in writing and translating this manuscript.
Funding
Funded by the Proyecto Nucleo UNAB DI_622_14N (Universidad Andres Bello) of Maite Rodriguez-Diaz and patially by the Proyecto 001616_Dicyt - Universidad de Santiago de Chile of José L. Martínez.
Author contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used: Conceptualization, M.R.-D.; methodology, V.R. and M.C.O.; validation, V.R.; formal analysis, M.H. and J.M.D.; investigation, M.R.-D. and V.R.; resources, F.T. and M.R.-D.; data curation, M.H. and M.R.-D.; writing-original draft preparation, M.R.-D. and M.H.; writing-review, and editing, M.R.-D. and J:L.M.; visualization, J.M.D. and M.R.; supervision, M.R.-D., and M.C.O.; funding acquisition, M.R.-D. and M.C.O.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
José L. Martinez, Email: joseluis.martinez@usach.cl.
Maité Rodríguez-Díaz, Email: maite.rodriguez@unab.cl.
References
- Alfaro-Perez M.Y., Ruiz-Barrueto M.A. Efecto antibacteriano in vitro del extracto acuoso de Schinus molle (molle) sobre Staphylococcus aureus ATCC25923. REBIOL. 2018;38:4–16. [Google Scholar]
- Araya A., Luna J., Ramírez L., Leiva A., Valdivia F., Vega S., Sánchez A., Flores L., Peracchio L., Mera F., Cosmelli D., Ahumada J., Fajardo M., Otero C., Delgado J.M., Martinez J.L., Rodríguez-Diaz M. Importancia del conocimiento por la población de las propiedades antioxidantes en plantas nativas de Chile. Contrib. Científ. Tecnol. 2020;41:29–33. doi: 10.35588/cdicyt.v44i1.4628. [DOI] [Google Scholar]
- Barrachina M.D., Bello R., Martínez-Cuesta M.A., Primo-Yúfera E., Esplugues J. Analgesic and central depressor effects of the dichloromethanol extract from Schinus molle L. Phyther. Res. 1997;11:317–319. doi: 10.1002/(sici)1099-1573(199706)11:4<317::aid-ptr91>3.0.co;2-m. [DOI] [Google Scholar]
- Bello R., Barrachina M.D., Moreno L., Primo-Yúfera E., Esplugues J. Effects on arterial blood pressure of the methanol and dichloromethanol extracts from Schinus molle L. in rats. Phyther. Res. 1996;10:634–635. doi: 10.1002/(sici)1099-1573(199611)10:7<634::aid-ptr917>3.0.co;2-w. [DOI] [Google Scholar]
- Bendaoud H., Romdhane M., Souchard J.P., Cazaux S., Bouajila J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010;75:C466–C472. doi: 10.1111/j.1750-3841.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- Benites J., Guerrero-Castilla A., Salas F., Martinez J.L., Jara-Aguilar R., Venegas-Casanova E.A., Suarez-Rebaza L., Guerrero-Hurtado J., Buc Calderon P. Chemical composition, in vitro cytotoxic and antioxidant activities of the essential oil of Peruvian Minthostachys mollis Griseb. Bol. Latinoam. Caribe. Plant Med. Aromat. 2018;17:566–574. [Google Scholar]
- Benites J., Ybañez-Julca R.O., Ganoza-Yupanqui M.L., Mantilla-Rodriguez E., Zavala E., Velasquez S., Gajardo S., Morales B., de Albuquerque R.D.D.G., Rocha L., Martinez J.L. Antioxidant effect and chemical composition of Ananas comosus [L.] Merr. peels from Peruvian Northern. Bol. Latinoam. Caribe. Plant Med. Aromat. 2019;18:577–585. [Google Scholar]
- Benites J., Asunción-Alvarez H.D., Ybañez-Julca R.O., Ganoza-Yupanqui M.L., Jacinto-Fernandez J.J., Reyes-De la Vega J.B., Zavaleta-Cruz H.J., Pinedo-Alcántara A.N., Lavado-Fonseca A.M., Medina-Mejia C.A., Catalan M., Morales B., de Albuquerque R.D.D.G., Rocha L., Martinez J.L. Chemical composition by HPLC-ESI-QTOF-MS/MS: Estrogenic and antioxidant effects of Mangifera indica L. cv. “Kent” leave extracts on ovariectomized rats. Bol. Latinoam. Caribe. Plant Med. Aromat. 2019;18:336–346. [Google Scholar]
- Benzi I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Benzi V., Stefanazzi N., Ferrero A.A. Biological activity of essential oils from leaves and fruits of pepper tree (Schinus molle L.) to control rice weevil (Sitophilus oryzae L.) Chil. J. Agric. Res. 2009;69:154–159. doi: 10.4067/s0718-58392009000200004. [DOI] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28:25–30. doi: 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- Bras C., Domínguez S., Codón S., Minetti A., Ferrero A. Consequences of subchronic exposure to ethanolic extract from fruits and leaves of Schinus molle var. areira L. in mice. J. Ethnopharmacol. 2010;132:321–327. doi: 10.1016/j.jep.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Celaya L.S., Viturro C.I., Silva L.R., Moreno S. Natural antioxidants isolated from Schinus areira leaves by ultrasound-assisted extraction. Int. J. Food Stud. 2016;5:167–179. doi: 10.7455/ijfs.v5i2.329. [DOI] [Google Scholar]
- Chiffelle I., Huerta A., Sandoval A.A., Araya J.E. Insecticide effect of leaf extract from Schinus molle on larvae of Gonipterus platensis. Rev. Fac. Agron. Medellin. 2017;70:8263–8270. doi: 10.15446/rfna.v70n3.58272. [DOI] [Google Scholar]
- Dellacassa E. Edipucrs; Porto Alegre, Brasil: 2010. Normalización de productos naturales obtenidos de especies de la Flora Aromática Latinoamericana: proyecto CYTED IV.20. [Google Scholar]
- Descamps L.R., Stefanazzi N., Sanchez C., Ferrero A.A. Actividad biológica de extractos vegetales de Schinus molle var. Areira (Anacardiaceae) en Tribolium castaneum Herbst. (Insecta, Coleoptera, Tenebrionidae), plaga de grano almacenado. Bol. Sanidad Veg. 2008;34:585–606. [Google Scholar]
- do Rosário Martins, M., Arantes, S., Candeias, F., Tinoco, M. T., Cruz-Morais, J. 2014. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 151, 485–492. 10.1016/j.jep.2013.10.063. [DOI] [PubMed]
- Dudonné S., Vitrac X., Coutière P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- Fadda A., Serra M., Molinu M.G., Azara E., Barberis A., Sanna D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. J. Food Compos. Anal. 2014;35:112–119. doi: 10.1016/j.jfca.2014.06.006. [DOI] [Google Scholar]
- Ferrero A.A., Werdin J.O., Sánchez C. Biological activity of Schinus molle on Triatoma infestans. Fitoterapia. 2006;77:381–383. doi: 10.1016/j.fitote.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Fuertes C.M., Jurado B., Gordillo G.C., Negrón L.P., Núñez E., Esteban M., Távara A. Estudio integral de plantas Biocidas del algodonero. Cienc. Invest. 2014;13:34–41. [Google Scholar]
- García N., Ormazabal C. Enersis S.A; Santiago, Chile: 2008. Árboles nativos de Chile; p. 196. [Google Scholar]
- González M.C., Kite G., Martínez M., Soto M. Actividad antioxidante de flavonoides del tallo de orégano mexicano (Lippia graveolens HBK var. berlandieri Schauer) Rev. Fitotec. Mex. 2007;30:43–49. [Google Scholar]
- Guala M., Elder H., Perez G., Chiesa A. Evaluation of the antioxidant power of fractions of Schinus molle L. essential oil obtained by vacuum distillation. Inf. Tecnol. 2009;20:83–88. doi: 10.4067/s0718-07642009000200011. [DOI] [Google Scholar]
- Guala M.S., Lapissonde M.O., Elder H.V., Perez G.A. Efecto acaricida del aceite esencial de aguaribay (Schinus molle L.) y sus fracciones en colmenares de abejas (Apis mellifera) en relación con la composición química. Inf. Tecnol. 2014;25:151–156. doi: 10.4067/s0718-07642014000200017. [DOI] [Google Scholar]
- Guevara D.J., Araya J.E., Huerta A., Chiffelle I. Extract of Schinus molle and Artemisia absinthium against Helicoverpa zea on fresh ear corn in Ecuador. Chil. J. Agric. Anim. Sci. 2018;34:216–225. doi: 10.4067/s0719-38902018005000505. [DOI] [Google Scholar]
- Hapon M.V., Boiteux J.J., Fernández M.A., Lucero G., Silva M.F., Pizzuolo P.H. Effect of phenolic compounds presents in Argentinian plant extracto n mycelial growth of the plant pathogen Botrytis cinérea Pers. Phyton. 2017;86:270–277. [Google Scholar]
- Huerta A., Chiffelle I., Puga K., Azúa F., Araya J.E. Toxicity and repellence of aqueous and ethanolic extract from Schinus molle on elm leaf beetle Xanthogaleruca luteola. Crop Protect. 2010;29:1118–1123. doi: 10.1016/j.cropro.2010.04.010. [DOI] [Google Scholar]
- Iqbal E., Salim K.A., Lim L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. – Sci. 2015;27:224–232. doi: 10.1016/j.jksus.2015.02.003. [DOI] [Google Scholar]
- Kumazawa S., Hamasaka T., Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–339. doi: 10.1016/s0308-8146(03)00216-4. [DOI] [Google Scholar]
- Lim Y.Y., Lim T.T., Tee J.J. Antioxidant propierties of guava fruit: Comparison with some local fruits. Sunway Acad. J. 2006;3:9–20. [Google Scholar]
- Ministerio de Salud de Chile. 2007. Medicamentos herbarios tradicionales. 103 especies vegetales. Santiago, Chile.
- Morsy N. Phytochemical analysis of biologically active constituents of medicinal plants. Main Green Chem. 2014;13:7–21. doi: 10.3233/mgc-130117. [DOI] [Google Scholar]
- Muñoz O., Montes M., Wilkomirsky T. Editorial Universitaria; Santiago, Chile: 2004. Plantas medicinales de uso en Chile: Química y farmacología. [Google Scholar]
- Murray A.P., Murray M.G. Phytochemistry, traditional uses and bioactivity of the medicinal plant Schinus areira L. (Anacardiaceae): a review. Nat. Prod. J. 2017;7:97–103. doi: 10.2174/2210315507666170117145728. [DOI] [Google Scholar]
- Obasi N.L., Egbuonu A.C., Ukoha P.O., Ejikeme P.M. Comparative phytochemical and antimicrobial screening of some solvent extracts of Samanea saman (Fabaceae or Mimosaceae) pods. African J. Pure Appl. Chem. 2010;4:206–212. [Google Scholar]
- Poongothai A., Sreena K.P., Sreejith K., Uthiralingam M., Annapoorani S. Preliminary phytochemicals screening of Ficus racemosa Linn. Bark. Int. J. Pharma Bio. Sci. 2011;2:432–434. [Google Scholar]
- Pranoothi E.K., Narendra K., Joshi D.S., Swathi J., Sowjanya K.M., Rathnakarreddi K.V.N., Emmanuel S., Padmavathi S., Satya A.K. Studies on qualitative, quantitative, phytochemical analysis and screening of in vitro biological activities of Leucas indica (L) var. nagalapuramiana. Int. J. Herb. Med. 2014;2:30–36. [Google Scholar]
- Quiroga E.N., Sampietro A.R., Vattuone M.A. Screening antifungal activities of selected medicinal plants. J. Ethnopharmacol. 2001;74:89–96. doi: 10.1016/s0378-8741(00)00350-0. [DOI] [PubMed] [Google Scholar]
- Rhouma A., Daoud H.B., Ghanmi S., Salah H.B., Romdhane M., Demak M. Antimicrobial activities of leaf extracts of Pistacia and Schinus species against some plant pathogenic fungi and bacteria. J. Plant Pathol. 2009;91:339–345. [Google Scholar]
- Roopalatha U.C., Vijay Mala G.N. Phytochemical analysis of successive reextracts of the leaves of Moringa oleifera Lam. Int. J. Pharm. Pharm. Sci. 2013;5:629–634. [Google Scholar]
- Ruffa M.J., Ferraro G., Wagner M.L., Calcagno M.L., Campos R.H., Cavallaro L. Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J. Ethnopharmacol. 2002;79:335–339. doi: 10.1016/s0378-8741(01)00400-7. [DOI] [PubMed] [Google Scholar]
- Samatha T., Srinivas P., Shyamsundarachary R., Marka R., Nanna R.S. Phytochemical analysis of seeds, stem bark and root of an endangered medicinal forest tree Oroxylum indicum (l) kurz. Int. J. Pharma Bio. Sci. 2012;3:1063–1075. [Google Scholar]
- Sanchez C., Alzogaray R., Ferrero A. Repellency assay with Schinus molle var. areira (L.) (Anacardiaceae) essential oils against Blattella germanica L. (Blattodea: Blattellidae) BioAssay. 2006;6:1. [Google Scholar]
- Sarla S., Abhay P.M., Bhawana S., Hemlata S. Pharmacognostic, phytochemical and antimicrobial screening of Aphanamixis polystachya, an endangered medicinal tree. Int. J. Pharm. Pharm. Sci. 2012;4:235–240. [Google Scholar]
- Silva-Junior E.F., Aquino P.G.V., Santos-Junior P.F.S., Nascimento I.J.S., Gomes E.A., Silva A.L.L., Verissimo R.C.S.S., Aquino T.M., Araújo-Junior J.X. Phytochemical compounds and pharmacological properties from Schinus molle Linnaeus and Schinus terebinthifolius Raddi (Anacardiaceae) J. Chem. Pharmaceut. Res. 2015;7:389–393. doi: 10.1515/znc-1987-1-203. [DOI] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965;16:144–158. [Google Scholar]
- Tepe B., Daferera D., Tepe A.S., Polissiou M., Sokmen A. Antioxidant activity of the essential oil and various extracts of Nepeta flavida Hub.-Mor. from Turkey. Food Chem. 2007;103:1358–1364. doi: 10.1016/j.foodchem.2006.10.049. [DOI] [Google Scholar]
- Torres F.C., Machado Lucas A., Sardá Ribeiro V.L., Martins J.R., von Poser G., Guala M.S., Elder H.V., Cassel E. Influence of essential oil fractionation by vacuum distillation on acaricidal activity against the Cattle Tick. Braz. Arch. Biol. Technol. 2012;55:613–621. doi: 10.1590/s1516-89132012000400018. [DOI] [Google Scholar]
- Venegas E. Cuantificación de flavonoides totales y taninos presentes en el extracto acuoso de hojas de Thea sinensis L. y su capacidad antioxidante. UCV-Scientia. 2012;4:161–174. [Google Scholar]
- Waterhouse A. Current Protocols in Food Analytical Chemistry. John Wiley & Sons Ltd.; New York, USA: 2002. Determination of total phenolics. [Google Scholar]
- Werdin J.O., Murray A.P., Ferrero A.A. Bioactividad de aceites esenciales de Schinus molle var areira (Anacardiaceae) en ninfas II de Nezara viridula (Hemiptera: pentatomidae)- Bol. Sanidad Veg. 2008;34:367–375. [Google Scholar]
- Werdin J., Gutiérrez M.M., Ferrero A.A. Repellency assays with plant extracts and essential oils from Schinus molle var. areira (L) (Anacardiaceae) and DEET against Nezara viridula L. (Hemiptera: Pentatomidae) BioAssay. 2011;6:8. [Google Scholar]
- Xiang L., Hu Y.F., Wu J.S., Wang L., Huang W.G., Xu C.S., Meng X.L., Wang P. Semi-mechanism-based pharmacodynamic model for the anti-inflammatory effect of baicalein in LPS-stimulated REW 264.7 macrophages. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00793. article 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueqin Z., Recio M.C., Máñez S., Giner R.M., Cerdá-Nicolás M., Ríos J.L. Isolation of two triterpenoids and a biflavanone with anti-inflammatory activity from Schinus molle fruits. Planta Med. 2003;69:893–898. doi: 10.1055/s-2003-45096. [DOI] [PubMed] [Google Scholar]