Abstract
Candidal vulvovaginitis (CVV), is the second most leading vaginal infection (global prevalence > 75%), caused due to excessive growth of Candida spp., predominantly Candida albicans (>95% cases). The current treatment regimens for CVV are marred with the challenges of fungal resistance & infection recurrence, subsequently leading to the compromised therapeutic efficacy of anti-fungal drugs, prolonged treatment and low patient compliance. The core of the present research was the fabrication & investigation of 2 T-SLN (solid lipid nanoparticles) gel carrying luliconazole for the amelioration of CVV. ‘2T’ symbolizes transvaginal & thermosensitive attributes of the present formulation. SLNs were prepared by a modified melt emulsification-ultra sonication method using a combination of solid lipids (Gelucire 50/13 & Precirol ATO 5), surfactant (Tween 80) and co-surfactant (Kolliphor). Formulation by design (FbD) approach was adopted to obtain appropriately screened and tailored SLNs. The optimized SLNs yielded a particle size, polydispersity index & entrapment efficiency of 62.18 nm, 0.263 & 81.5% respectively. To formulate the 2 T-gel, the final SLNs were loaded into Carbopol 971P-NF and Triethanolamine based gel. The 2 T-SLN gel was found to be easily spreadable and homogenous with mean extrudability (15 ± 0.4 g/cm2), viscosity (696.42 ± 2.34 Pa·s) and %drug content (93.24 ± 0.73%) values.. The pH of the prepared 2 T-SLN gel (4.5 ± 0.5) was in concordance with the vaginal pH (normal conditions). For in-vitro characterization of an optimized 2 T-SLN gel the release kinetics & anticandidal activity were assessed which offers a %cumulative drug release of 62 ± 0.5% in 72 h and 37.3 ± 1.5 mm zone of inhibition in 48 h. The visual appearance & dimensions were determined using fluorescent microscopy (spherical shape) & transmission electron microscopy (90–120 nm) respectively. The optimized 2 T-SLN gel showcases a skin-friendly profile with no significant signs of erythema and oedema and was found to be stable at room temperature for 2 months without any visual non-uniformity/cracking/breaking. In conclusion, the current research serves a new therapeutic perspective in assessing the activity of luliconazole for vaginal drug delivery using a 2 T-SLN gel system.
Keywords: Candida albicans, Solid lipid nanoparticles, Transvaginal, Thermosensitive, Formulation by design
1. Introduction
Candidal vulvovaginitis (CVV) is a widespread fungal infection of the female reproductive tract, mainly, vagina and vulva (tissues present at vaginal orifice). Clinically, it appears as a thick, white, odourless vaginal discharge at the orifice (vaginal) with contributing symptoms such as severe irritations, itching, redness, swelling, pain, rash, burning sensations (in & around the vagina) and painful micturition. In many complicated cases of CVV extensive tears, cracks and sores in the vaginal cavity are observed (Cassone, 2015, Reichman et al., 2015). CVV particularly owes its occurrence to altered and excessive growth of Candida, a commensal fungal species of vaginal flora under a variety of conditions such as pH alterations, non-optimal antibiotic usage, impaired host immune system, douching (vaginal washing with vinegar), clinical conditions (pregnancy, diabetes) and increased estrogen levels (Aguin and Sobel, 2015, Bhagat and Desai, 2015). Candida albicans is the predominantly isolated causative agent with an intermittent presence of C. glabrata and non-albicans in complicated CVV cases (Bitew and Abebaw, 2018).
CVV is reportedly the second most common vaginal infection (>75%) which is often not considered serious enough to warrant clinical intervention and thus the plethora of health issues which entails this practice leads to compromised quality of life (QoL) in patients (Denning et al., 2018). The available treatment modalities are associated with issues like resistance development by the cells of inhabiting fungal species (Ford et al., 2017), enhanced disease recurrence due to low therapeutic efficacy, low retention capacity in vaginal epithelium, low bioavailability, leakage and messiness (creams, ointments), prolong treatment duration and low patient compliance (Hani et al., 2015). False diagnosis, wrong/self-medication, social stigma, and economic burden adds on to the pre-existing therapeutic challenges (Yarmohammadi et al., 2015).
These shortcomings necessitate the fabrication of a tailored vaginal drug delivery system (VDDS) capable of mitigating, treating, and subsequently eliminating CVV in a clinically efficient manner thereby, countering the chances of disease recurrence and persistence (Dovnik et al., 2015). Recent reports have suggested the exploration of the vagina as an efficient platform for drug delivery. The conventional VDDS such as creams, ointments, suppositories have low or insufficient binding capacity to mucosal tissues and the viscoelastic nature of the mucosal lining further impedes the drug penetration (Graziottin and Gambini, 2015). On the contrary, solid lipid nanoparticles (SLN) based gel offers high vaginal mucosal (lipid bilayer) binding, thus, enhancing gel adhesion, drug penetration & retention (Mirza et al., 2016). Taking a cue from their advantages, the current research proposes fabrication & investigation of luliconazole (a highly potent imidazole antifungal agent) (Mishra et al., 2019) based 2 T-SLN gel (Dolatabadi et al., 2015) as an improved therapeutic modality for the amelioration of CVV. The ‘2T’, transvaginal & thermosensitive, approach of the purported SLN gel would ensure an efficient drug delivery at the site of action whilst targeting superficial fungal cells as well as its underlying hyphae, which in many CVV cases extends through the vaginal walls and are mostly responsible for causing inefficient clearance of fungal cells, resistance development and infection recurrence (Fidel et al., 2004, Gonçalves et al., 2016). The proposed locoregional targeting at the large vaginal surface area having rich blood supply would support a prolonged drug release, higher absorption and improved bioavailability. The avoidance/protection from the first-pass metabolism, systemic adverse effects and reactions (oxidative, chemical & enzymatic) could also be achieved (Katz et al., 2015, Machado et al., 2015). Innovatively, as a step-forward to conventional formulations, the 2 T-SLN gel would be non-greasy with prolonged retention at the vaginal temperature and pH consequently leading to an improved patient compliance (Caramella et al., 2015). It would also be unaffected by the action of gravity (leakage), self-cleansing action of the vagina and would circumvent these significant disadvantages of the current treatment regimens (Das and Bahia, 2006).
Despite an excellent antifungal profile and prominent literature support, luliconazole has not been much explored for the treatment of CVV. It has been found most suitable for treatment and management of skin, eye & nail related fungal infections such as jock itch, ringworm, fungal keratitis and onychomycosis. (Hasan, 2018, Khanna and Bharti, 2014, Scher et al., 2014, Todokoro et al., 2019). The recent findings showcasing it’s very low MIC and potent anticandidal activity (MIC range: 0.031–0.13 µg/ml) (Koga et al., 2006, Taghipour et al., 2018), skin safety profile (Kaur et al., 2019), efficacy at low drug concentrations & lipophilic nature (Mishra et al., 2019) has made it an exceptional drug of choice for the present endeavour. Evidently and quite recently, the molecular docking studies of luliconazole has shown high binding affinity & interaction activity with the cellular contents of C. albicans, subsequently, leading to cell disruption & death (Hassan et al., 2019). Keeping in view the disease pathophysiology, high drug potency and route of administration the purported formulation was designed with a minimum API concentration of 0.1% (FDA approved concentration 1%) (DPT-Laboratories, 2013) which would add to its commercial viability.
The success of any formulation heavily relies on the optimum choices of formulation components (API and the excipients). Formulation by design (FbD) approach has recently emerged as a successful tool for developing an optimized formulation commensurate with the most desired attributes. FbD is a data-based concept to predict & estimate the effect of pre-determined variables on formulation performance and to study any possible interactions and synergies among formulation parameters (Bhoop, 2013, Debnath et al., 2018). FbD involves various experimental design depending upon the nature of employment and data required. For the current research, the 2 T-SLN gel was prepared by employing Box Behnken Design (BBD) which was used to predict & study the exact image of dependent responses (particle size, polydispersity index & entrapment efficiency) through pre-selected independent variables (lipid concentration, surfactant concentration & sonication time) to obtain a stable and biopharmaceutically acceptable dosage form (Cunha et al., 2020).
2. Materials and methods
Luliconazole was obtained as a gift sample from Sun Pharmaceuticals, Gurgaon, India. Gelucire 50/13 and Precirol ATO 5 were obtained from Gattefosse, France (ex gratia). Tween 80, Kolliphor, Carbopol (grade 940) and Triethanolamine were obtained from Sigma Aldrich, India. All other chemicals and reagents used were of analytical grade and were used without further purifications.
2.1. Preformulation studies
Development of an analytical method for drug estimation in SVF: UV spectral analysis.
The solubility & stability of the drug was determined in SVF (simulated vaginal fluids; pH = 4.5) to assess the effect of the vaginal pH & conditions on the drug (Tietz and Klein, 2018). The pH of SVF was adjusted using a 0.1 M HCl solution. The preparation of stock solution (10 ml) was done by dissolving a known quantity of the drug, 1 mg, in a 1:1 ratio of methanol & freshly prepared SVF. The serial dilutions were prepared using SVF in the concentration range of 5–25 μg/ml and are analysed using UV Spectrophotometer at λmax, 296.5 nm (Chaudhari et al., 2018, Shaikh et al., 2020). Stability of drug solution was observed over one week to assess the effect of environmental factor such as light, temperature, etc. (Sowjanya and Mohana, 2019). The experiment was repeated in triplicate and results were represented as mean value (µg/ml) ± SD.
2.2. Screening of excipients
The preliminary screening of excipients (solid lipids and surfactants) was done based on their drug solubilisation capacity (Kathe et al., 2014).
The solid lipids available for screening are Campritol 888 ATO, Precirol ATO 5, Stearic Acid, Gelucire 50/13, Gelucire 43/01 and Tefose 63. To each melted lipid, 5 ml (heated at 5 °C above their melting point), an accurately weighed amount of drug (1 mg) was added incrementally with continuous stirring on a magnetic stirrer, 100 rpm (Remi Instrument Ltd., Mumbai, India) till the point of saturation i.e. no further drug solubilisation in molten lipid phase (Cassano et al., 2016). The amount of drug dissolved was noted and the lipids in which the maximum amount of drug was soluble were selected for further studies. The experiment was conducted in triplicate.
The surfactants available for screening are Tween 20, Tween 40, Tween 60, Tween 80, PEG, Triethanolamine and Kolliphor. In an Eppendorf, an equal quantity of drug (excess) was dissolved in a 2 ml of surfactant. The Eppendorf's were carefully covered & closed, and were agitated for 72 h at 25 °C in a mechanical shaker/incubator to reach equilibrium. After that, the Eppendorf's were centrifuged (High-Speed Centrifuge, 3 K30, SIGMA, Germany) at 3000 rpm for 30 min at 25 °C to obtain a supernatant. The supernatant was dissolved in methanol and analysed spectrophotometrically at λmax, 296 nm, to estimate the amount of drug dissolved (Cassano et al., 2016). The surfactant in which the least amount of drug is dissolved were discarded and the rest were tested for their %transmittance. The results were obtained in triplicate.
2.3. Formulation studies
2.3.1. Preparation of luliconazole based SLN Gel: Thermosensitive & Transvaginal (2 T)
The preparation of 2 T-SLN gel was divided into two steps. For the primary step, a luliconazole loaded SLNs were prepared by a modified melt emulsification-ultrasonication method (Garse et al., 2015). For the preparation of the lipid phase, a known quantity of solids lipids (mixture) was melted in a predetermined ratio of 3:1. To this lipid mixture, the drug was dissolved aided by magnetic stirrer at 1000 rpm. The aqueous phase was prepared by dissolving the surfactant and co-surfactant (4:1) in double-distilled water. The hot aq. phase was then added dropwise to the molten lipid phase and was maintained on continuous stirring for 3 h to obtain a pre-emulsion. Further, to nanosize the emulsion sonication was done for 1–3 min. The nanoformulation was then cooled at room temperature to get SLNs.
Followed by the above, the second step of 2 T-SLN gel preparation was done using Carbopol (grade 940). In the aqueous carbopol solution (1.5%) the prepared formulation was added dropwise with continuous stirring at 600 rpm. A few drops of triethanolamine (TEA) were also added to improve the gel formation, viscosity and to adjust the pH of a prepared gel (Anurova et al., 2015).
2.4. Optimization of prepared formulations using BBD
2.4.1. Selection of dependent and independent variables
Independent variables are those process parameters (Table 1) which may affect formulation design and characteristics such as lipid concentration, surfactant concentration & sonication time. The dependent variables/responses are formulation attributes such as particle size (nm), polydispersity index (PDI) and entrapment efficiency (%) (Cunha et al., 2020). The statistical analysis used for current research is BBD (Design-Expert 10.0.6.), a computer-based application to assess the exact image of dependent responses through pre-selected independent variables, thus, contributing to an optimized and biopharmaceutically stable formulation.
Table 1.
BBD formulation variables for the preparation of a 2 T-SLN gel.
| Independent Variables | Units | Levels |
|
|---|---|---|---|
| Low | High | ||
| Lipid (mixture of solid lipid & co-lipid) | % | 2 | 3 |
| Surfactant (mixture of surfactant & co-surfactant) | % | 2 | 3 |
| Sonication time | sec | 60 | 180 |
2.4.2. Particle size & PDI
Dynamic Light Scattering (DLS) technique using a computerised inspection system, Zetasizer (Nano ZS90, Malvern Instruments Ltd, Worcestershire, UK) with DTS software was used for the measurement of particle size & PDI of prepared SLNs. A suitably diluted suspension formulation (lyophilized SLNs) was used for the analysis (Esposito, 2015). The experiment was performed in triplicate.
2.4.3. Entrapment efficiency (%EE)
To calculate the %EE of SLNs a High-Speed Centrifuge (Sigma- 3K30, Sigma Laboratory Centrifuges, Germany) was used at 10,000 rpm for 45 min. The supernatant obtained was appropriately diluted and analysed spectrophotometrically at λmax, 296 nm to measure the amount of drug present (Esposito, 2015). The experiment was performed in triplicate and the %EE was calculated using the following equation:
Where,
Dt - Total amount of drug present in SLNs/formulation Ds - Amount of drug present entrapped in the supernatant.
2.5. Characterization of an optimized formulation
2.5.1. Rheological assessment
The rheological parameters assessed for the characterization of an optimized 2 T-SLN gel were spreadability, homogeneity, pH, mean extrudability, viscosity and %drug content. Further, the optimized 2 T-SLN gel was visually observed for stability at room temperature over 2 months for any signs of breaking, non-homogeneity i.e. presence of aggregates & unwanted changes in colour, odour, pH and viscosity. For determination of viscosity & pH a Brook-field viscometer and pH meter were used. Further, the drug content analysis of 2 T-SLN gel was also done using UV spectrophotometer at λmax, 296 nm (Lippacher et al., 2004).
2.5.2. TEM (Transmission electron microscopy) analysis
The surface morphology and dimensions of an optimized SLNs were visualized under TEM (Morgagni 268D, Fei Electron Optics). A suitably diluted sample solution was placed on the carbon-coated grid at room temperature before analysis. After drying a drop of phosphotungstic acid, 1% aq. solution, (negative staining) is added to the sample and was further analysed under TEM (Shah et al., 2015).
2.5.3. Fluorescent microscopy
The technique used to determine the physical characteristics (vesicle formation) of an SLN was fluorescent microscopy. The optimized formulation was appropriately diluted (10 times) with water and subjected to staining using Rhodamine B (water-soluble fluorescent dye). The prepared sample was then analysed under a fluorescent microscope (Shah et al., 2015).
2.5.4. In-vitro drug release study
The dissolution method using a dialysis membrane was performed to quantify and determine the IVR of a drug through an optimized 2 T-SLN gel. Prior to analysis the dialysis membrane (Sigma Aldrich, Merck) was activated by treating with 0.3% w/v sodium sulphide solution in water at 80 °C for 1 min to remove sulphur compounds. The treated membrane was washed with hot water at 60 °C for 2 min, acidified with 0.2% v/v H2SO4 in distilled water, rinsed to remove excess acid and immersed in dissolution medium (SVF) for overnight. For IVR analysis, an accurately weighed amount of 2 T-SLN gel formulation (10 ml) was placed in a pre-activated dialysis membrane and immersed in a freshly prepared SVF (200 ml) with constant stirring, 400 rpm at 37 °C ± 0.5 °C. An aliquot was withdrawn from dissolution medium at regular time intervals and replaced with fresh SVF to maintain sink conditions. The aliquots were suitably diluted and analysed spectrophotometrically at λmax, 296 nm, for estimation of drug released (Şenyiğit et al., 2014). The experiment was performed in triplicate.
2.5.5. Skin irritation study
The skin irritation potential of an optimized 2 T-SLN gel was determined using Wistar strain rats (180–200 g). A small amount of gel formulation was applied to the hairless (properly shaven) abdominal skin (2 cm2) of rats and maintained for a period of 24 h. After 24 h the skin of rats was visually scrutinized for any changes or irritations such as erythema and oedema on the scale of 1–5 (Doktorovová et al., 2016, Ganesan and Narayanasamy, 2017).
2.5.6. Anticandidal activity
The anti-candida potential (in-vitro) of an optimized 2 T-SLN gel was determined by zone of inhibition diameter using an agar cup method (Şenyiğit et al., 2014). An agar medium (30 ml) was prepared, sterilized (autoclaving) and inoculated with the standard C. albicans strain (ATCC 90028). The concentration of inoculum was adjusted with 0.5 McFarlands standard which is equivalent to 1.5 × 108 C. albicans colony forming units, CFU/mL (Hassan et al., 2019). The prepared medium was allowed to solidify and incubated at 35 °C for 48 h. After, incubation period a well (10 mm diameter) was prepared in a solidified medium using a sterile borer. A suspension formulation of optimized SLNs, 0.5 ml, was introduced into the fungal well aseptically and the ZoI diameter was recorded at incubation period of 12, 24 & 48 h (35 °C) using Antibiotic Zone Reader (HICON, New Delhi) (Doktorovová et al., 2016, Ganesan and Narayanasamy, 2017).
3. Results and discussion
3.1. Preformulation studies
3.1.1. UV spectral analysis
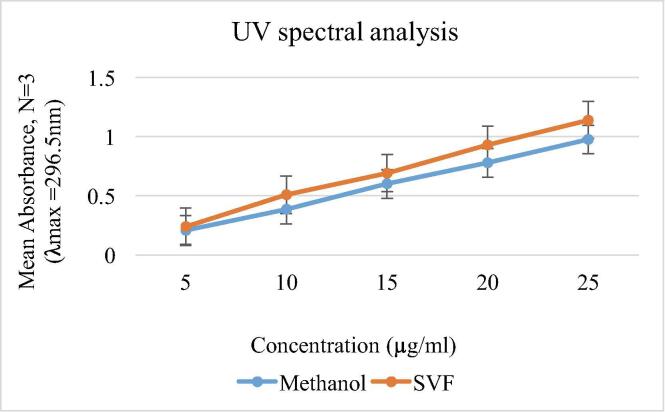
The solubility of luliconazole in SVF & methanol was estimated by UV analysis and have yielded satisfactory and concordant results (Fig. 1). The stock solution was found to be stable for a period of one week, thus, indicating its stability from light, temperature, etc. The acceptable solubility & stability of luliconazole further extend its use for formulation development.
Fig. 1.
The UV analysis of luliconazole in methanol and SVF.
3.2. Screening of excipients
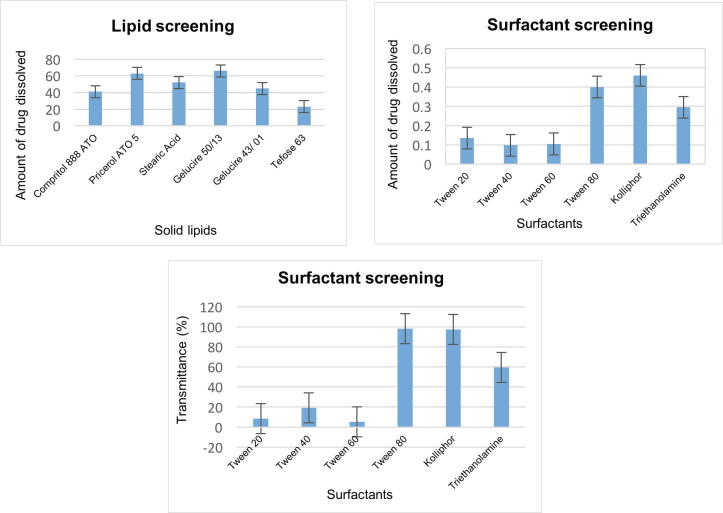
The maximum drug solubilisation capacity was shown by solid lipids (Gelucire 50/13 & Precirol ATO 5) and surfactants (Tween 80 & Kolliphor) (Fig. 2). As luliconazole is highly lipophilic in nature, its interaction with all the selected lipids was exceptionally high, however, the choice of Gelucire 50/13 & Precirol ATO 5 was mainly attributable to their well-suited property for formulating lipid-based systems such as SLN, emulsions (self-emulsifying & self-micro emulsifying), and micellar solutions. Also, the quality, high binding capacity, flexible process, close melting points & modified-release further compliment their use for present research (Hazzah et al., 2015, Notario-Pérez et al., 2019).
Fig. 2.
Preliminary screening of excipients on the basis of drug solubilisation capacity & %Transmittance.
The selection of surfactants, Tween 80 & Kolliphor, primarily depends on drug solubilisation capacity and %transmittance i.e. sample quality to transmit (pass) the light energy either by absorption, reflection & scattering (Fig. 2). Higher the transmittance higher will be the dispersion & low globule size of a solution. Also, the non-ionic nature of both the surfactants imparts electrostatic repulsion & stearic stabilization negating particles agglomeration (Gurpreet and Singh, 2018).
3.3. Formulation development & optimization: Box Behnken Design
BBD, a specially designed response surface methodology to predict the exact image of dependent responses (particle size, polydispersity index & entrapment efficiency) through independent variables (lipid, surfactant & sonication time). The estimated interactions were represented as 3D-response surface quadratic graphs. In the present study, BBD presented a total of 17 formulation designs (Table 2) to be subjected to optimization.
Table 2.
BBD formulation designs for optimization of a 2T-SLN gel.
| Formulation Code |
Factor A Lipid (%w/v) |
Factor B Surfactant (%v/v) |
Factor C Sonication Time (sec) |
Response 1 Average Size (nm) |
Response 2 PDI |
Response 3 Entrapment Efficiency (%) |
|---|---|---|---|---|---|---|
| F1 | 2.5 | 2 | 180 | 96.19 | 0.328 | 75.2 |
| F2 | 3 | 2.5 | 60 | 141.9 | 0.7 | 80 |
| F3 | 2.5 | 2 | 60 | 107 | 0.697 | 78.2 |
| F4 | 2.5 | 2.5 | 120 | 93.14 | 0.218 | 77.6 |
| F5 | 3 | 2.5 | 180 | 160 | 0.282 | 76.5 |
| F6 | 2 | 3 | 120 | 61.52 | 0.208 | 74 |
| F7 | 2.5 | 2.5 | 120 | 93.14 | 0.218 | 77.6 |
| F8 | 2.5 | 2.5 | 120 | 93.14 | 0.218 | 77.6 |
| F9 | 2.5 | 3 | 60 | 62.18 | 0.263 | 81.5 |
| F10 | 3 | 2 | 120 | 167.4 | 0.597 | 78.9 |
| F11 | 2 | 2 | 120 | 71.79 | 0.272 | 72.5 |
| F12 | 3 | 3 | 120 | 123.2 | 0.301 | 80.5 |
| F13 | 2.5 | 3 | 180 | 79.79 | 0.250 | 74.6 |
| F14 | 2.5 | 2.5 | 120 | 93.14 | 0.218 | 77.6 |
| F15 | 2.5 | 2.5 | 120 | 93.14 | 0.218 | 77.6 |
| F16 | 2 | 2.5 | 60 | 69.54 | 0.374 | 75 |
| F17 | 2 | 2.5 | 180 | 64.14 | 0.286 | 70.54 |
3.3.1. Response 1: Particle size
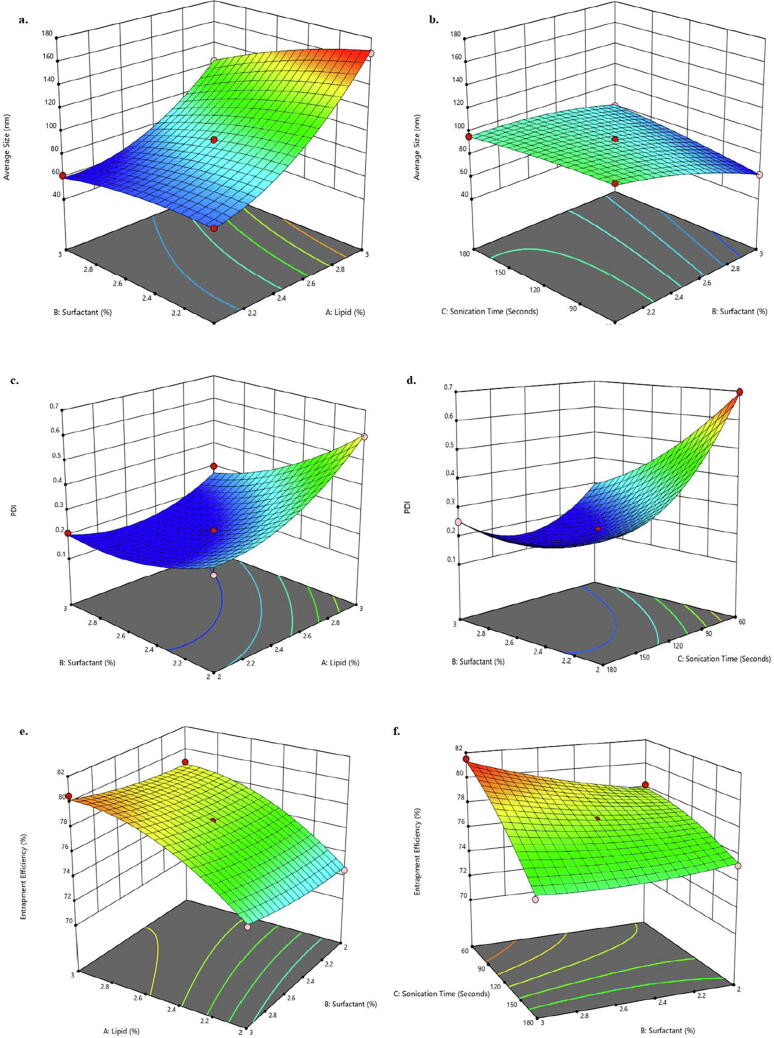
In the majority of CVV cases, the hyphae of fungal cells (C. albicans) penetrates the mucus lining of a vagina thus, making infection recurrent (Fidel et al., 2004, Gonçalves et al., 2016). The particle size (nano) of 2 T-SLN gel plays an important role in mucus permeation, retention and bioadhesion (Qais et al., 2019). In addition, the purported formulation follows transvaginal (through the vagina) delivery thereby, targeting the active concentration of formulation in the vaginal region only. As highlighted in 3D-response surface graphs (Fig. 3a) the particle size increases with an increase in total lipid concentration. The is mainly attributable to the increasing chain length of lipids which sufficiently enhance particles adhesion and gradually decrease monodispersity of a formulation. The size of SLN also depends on the viscosity of lipids which increases with an increase in chain length of lipids. Higher the viscosity, larger will be the size of lipid droplets and untimely bigger size of SLNs (Shi et al., 2011). However, to support the transvaginal administration a nanosized SLN is desired. Henceforth, surfactant and co-surfactants were added to improve stabilization of the smaller lipid droplets. The application of sonication technique also reduces or prevents coalescing of lipids into larger droplets. Thereby, with increase in sonication time & surfactants concentration the particle size decreases (Fig. 3b) (Shah et al., 2017).
Fig. 3.
BBD graphs representing particle size (a, b); PDI (c, d); entrapment efficiency (e, f).
3.3.2. Response 2: PDI
PDI refers to the size distribution of particles in a formulation (2 T-SLN gel) which affects sample uniformity (homogeneous & heterogeneous), drug distribution and dose. A high PDI (>0.3) generally signifies particles agglomeration whereas, low PDI (<0.2) signifies particles disintegration leading to drug expulsion (El-Hammadi and Arias, 2015). As presented in 3D quadratic graphs, PDI increases with an increase in total lipid concentration due to the increase in chain length of lipids (Fig. 3c) (Shi et al., 2011). However, with an increase in sonication time, PDI decreases due to less agglomeration among particles (Fig. 3d). Therefore, to obtain the desired PDI an optimum balance has to be strike among the total lipid concentration and sonication time (Table 2) (Madani et al., 2018). The concentration of surfactants has a slight or negligible effect on PDI.
3.3.3. Response 3: Entrapment efficiency
Entrapment Efficiency (%EE), a quantitative measurement to estimate the amount of drug entrapped in a lipid matrix (2 T-SLN gel) (Esposito, 2015). As represented in BBD graphs %EE increases with an increase in total lipid concentration (Fig. 3e) as higher the amount of core matrix higher will be the drug entrapped. This could be mainly attributable to the high solubility of luliconazole in Gelucire 50/13 and Precirol ATO 5. On contrary, with an increase in sonication time %EE decreases (Fig. 3f) due to SLNs disintegration/breaking down leading to drug expulsion (ud Din et al., 2015). The concentration of surfactants has no significant effect on %EE.
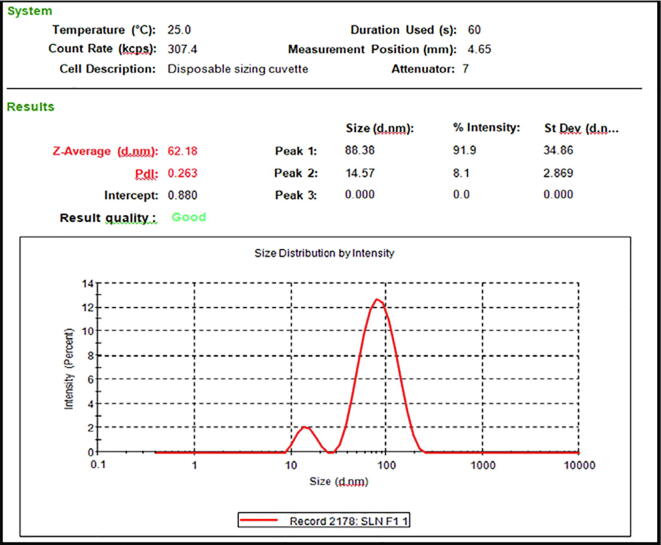
As per BBD, the present formulation (2 T-SLN gel) numbered as F9 (Table 2) with independent variables, lipids conc. (2.5%), surfactant conc. (3%) & sonication time (60 sec) has provided the optimum responses (Fig. 4) i.e. particle size (62.18 nm), PDI (0.263) and %EE (81.5%).The optimized formulation, F9 was further subjected to the following characterization parameters.
Fig. 4.
The particle size & PDI of an optimized formulation, F9.
3.4. Rheological parameters
The various rheological parameters for assessment of a 2 T-SLN gel has yielded satisfactory results with mean extrudability, viscosity and %drug content of 15 ± 0.4 g/cm2, 696.42 ± 2.34 Pa·s and 93.24 ± 0.73% respectively. The gel was found to be easily spreadable and homogenous with an optimum pH of 4.5 ± 0.5, which was in concordance with the vaginal pH (normal conditions). The rheological assessment of 2 T-SLN gel generally determines the effect of application & therapy outcome at the administration site. They also serve as a potential pre-requisite to sustain formulation. The attribute of extrudability & viscosity, mainly, classifies a low force expulsion of a gel from pharmaceutical packaging, and also its easy applicability at the targeted site. Viscosity also contributes to gel spreadability, thus, enhancing patient compliance. Henceforth, it can be said that rheological parameters are an equally important consideration in fabricating a 2 T-SLN gel. Further, the 2 T-SLN gel was found to be stable at room temperature for 2 months without any visual signs of non-uniformity/cracking/breaking, thus, suggesting suitable blending of formulation components. Higher the stability of a formulation higher will be its chances in terms of specificity & preference for use. This is also considered as a prominent step for effective designing of a drug delivery product.
3.5. Surface morphology analysis
3.5.1. TEM & fluorescent microscopy
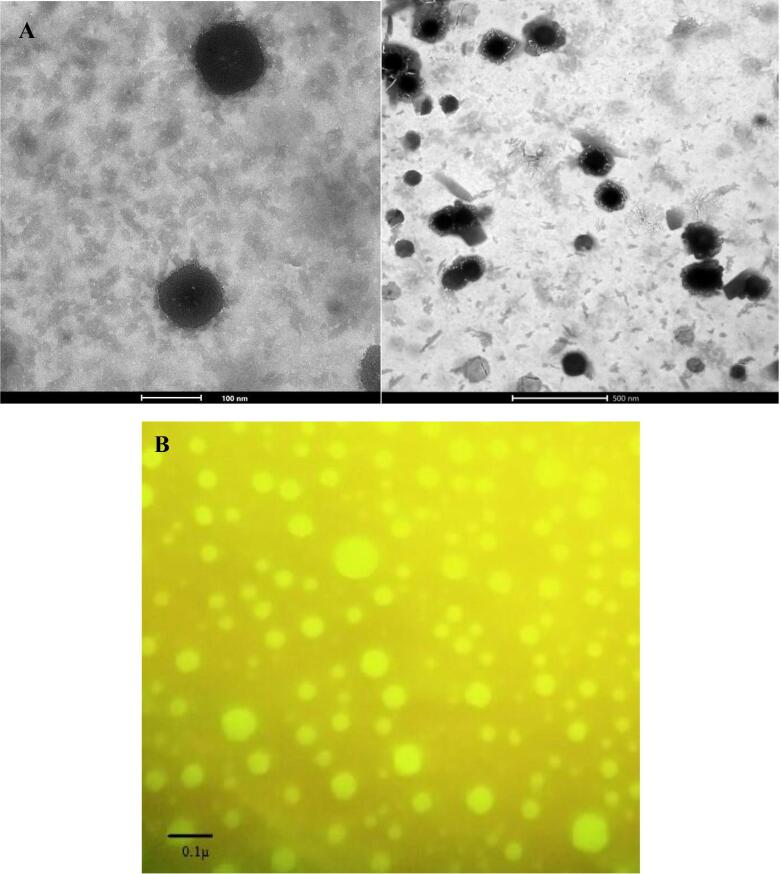
The surface morphology & dimensions of an optimized formulation (F9) was found to be slightly spherical with a size range between 60 and 100 nm (Fig. 5a). The obtained size dimensions were well corroborated with the results of particle size (Malvern Zetasizer). Further, the presence of spherically shaped vesicles was confirmed by fluorescent microscopy as presented in Fig. 5b. The obtained results of both the analysis compliments each other and the optimized formulation was found to be acceptable in the context of shape & size for efficient vaginal delivery and permeation (Rathod and Mehta, 2015).
Fig. 5.
(A) The TEM analysis of an optimized formulation. (B) Fluorescent microscopy showing the presence of spherically shaped vesicles in an optimized formulation.
3.6. In-vitro drug release study
The %cumulative drug release of an optimized 2 T-SLN gel was found to be 62 ± 0.5% in 72 h (Fig. 6). Initially, a burst release of 10 ± 0.6% was observed within 10 min. After which an incremental release of 48 ± 1.1% was observed in 40 h followed by a sustained release up till 72 h. It can be deduced that the incorporation of selected lipids (Gelucire 50/13 and Precirol ATO 5) has mitigated a modified release of an API in a sustained/controlled manner (Hazzah et al., 2015, Notario-Pérez et al., 2019). Since the API is active in lower concentration as well (MIC range against C. albicans: 0.031–0.13 µg/ml) the burst or incremental release may essentially provide a drug pool or depot in the vaginal milieu for effective and continuous targeting of the fungal cells. The sustained release of an API from 2 T-SLN also justifies the incorporation of a low API concentration. The obtained results further suggest high drug retention & bioavailability in in-vivo conditions as well (Attama and Umeyor, 2015).
Fig. 6.
The in-vitro release pattern of an optimized 2 T-SLN gel.
3.7. Skin irritation study
The optimized 2 T-SLN gel was found to be non-irritant and skin-friendly with no significant visual signs of erythema and oedema (Table 3). The obtained results are only preliminary and could be further employed for in-vivo studies.
Table 3.
The skin safety analysis of an optimized formulation.
| Group (n=3) | Erythemaa | Edemab |
|---|---|---|
| Formulation (with API) | 1 | 1 |
| Control (without API) | 0 | 0 |
aErythema scale: 0 = none, 1 = slight, 2 = well defined, 3 = moderate, 4 = scar formation.
bEdema scale: 0 = none, 1 = slight, 2 = well defined, 3 = moderate, 4 = severe.
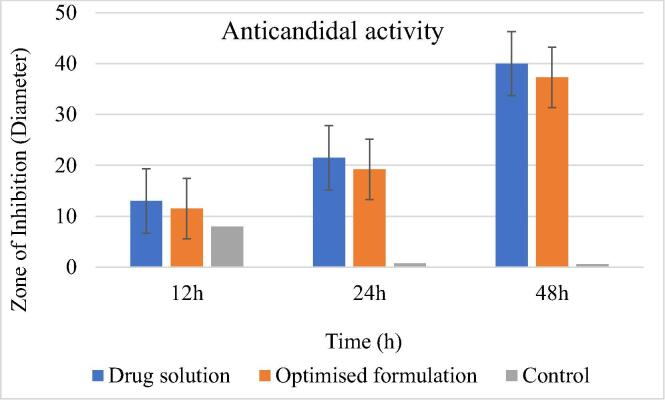
3.8. Anticandidal activity
The optimized formulation offers a 37.3 ± 1.5 mm zone of inhibition (diameter) thus, signifying the high anticandidal potential of luliconazole (MIC range: 0.031–0.13 µg/ml) (Koga et al., 2006) against the strains of C. albicans (Fig. 7). The results obtained are in concordance with the ZoI diameter of a drug solution i.e. 40.2 ± 0.5 mm thus, it can be inferred that encapsulation of an API in a lipid matrix does not affect its anticandidal potential.
Fig. 7.
The anticandidal potential of an optimized formulation.
4. Conclusion
CVV although not associated with severe disease outcomes like mortality or debilitating morbidity but remains a cause of concern for women. Its chronic nature, frequent recurrence due to resistance development in fungal cells, associated stigma, non-availability of appropriate therapeutic modalities and poor patient compliance adds to the health care burden.
Furthermore, the limited success of available treatment tools triggers the need for the development of a tailored 2 T-SLN gel with pharmacotechnical attributes of locoregional targeting, thermosensitive application & transvaginal delivery. The 2 T-SLN gel encompasses an active blend of solid lipids, surfactant and co-surfactant along with the API (luliconazole) in a core enriched aqueous colloidal dispersion, which would be able to provide improved bioadhesion, intimate contact, permeability, bioavailability and release within vaginal mucosa. In conclusion, the 2 T-SLN gel carrying luliconazole offers potential result to be exploited for wider clinical implications and therapeutic advancements.
Acknowledgements
The authors would like to thank Indian Council of Medical Research, Government of India for providing Senior Research Fellowship. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/24), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aguin T.J., Sobel J.D. Vulvovaginal candidiasis in pregnancy. Current Infectious Disease Reports. 2015;17:30. doi: 10.1007/s11908-015-0462-0. [DOI] [PubMed] [Google Scholar]
- Anurova M.N., Bakhrushina E.O., Demina N.B. Review of contemporary gel-forming agents in the technology of dosage forms. Pharm. Chem. J. 2015;49:627–634. [Google Scholar]
- Attama A.A., Umeyor C.E. The use of solid lipid nanoparticles for sustained drug release. Therapeutic delivery. 2015;6:669–684. doi: 10.4155/tde.15.23. [DOI] [PubMed] [Google Scholar]
- Bhagat B., Desai P. Vulvovaginal candidiasis-an opportunistic infection in childbearing age group women, its isolation, identification and antibiotic profile. Int. J. Sci. Res. 2015;4:1855–1859. [Google Scholar]
- Bhoop B.S. The pharma review; Nov-Des: 2013. An Autobiographical Account on Formulation by Design (FbD) pp. 36–42. [Google Scholar]
- Bitew A., Abebaw Y. Vulvovaginal candidiasis: species distribution of Candida and their antifungal susceptibility pattern. BMC women's health. 2018;18:1–10. doi: 10.1186/s12905-018-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramella C.M., Rossi S., Ferrari F., Bonferoni M.C., Sandri G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015;92:39–52. doi: 10.1016/j.addr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Cassano R., Ferrarelli T., Mauro M.V., Cavalcanti P., Picci N., Trombino S. Preparation, characterization and in vitro activities evaluation of solid lipid nanoparticles based on PEG-40 stearate for antifungal drugs vaginal delivery. Drug Delivery. 2016;23:1037–1046. doi: 10.3109/10717544.2014.932862. [DOI] [PubMed] [Google Scholar]
- Cassone A. Vulvovaginal Candida albicans infections: pathogenesis, immunity and vaccine prospects. BJOG: An Int. J. Obstetrics Gynaecology. 2015;122:785–794. doi: 10.1111/1471-0528.12994. [DOI] [PubMed] [Google Scholar]
- Chaudhari M.J., Chaudhari S.R., Chalikwar S.S., Shirkhedkar A.A. Application of area under curve technique for UV-Spectrophotometric determination of Luliconazole in bulk and pharmaceutical formulation. Asian J. Pharmaceutical Analysis. 2018;8:45–48. [Google Scholar]
- Cunha S., Costa C.P., Moreira J.N., Lobo J.M.S., Silva A.C. Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: a review. Nanomed. Nanotechnol. Biol. Med. 2020;102206 doi: 10.1016/j.nano.2020.102206. [DOI] [PubMed] [Google Scholar]
- Das N., Bahia M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006;318:1–14. doi: 10.1016/j.ijpharm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Debnath S., Aishwarya M.N.L., Babu M.N. Formulation by design: an approach to designing better drug delivery systems. Pharma Times. 2018;50:9–14. [Google Scholar]
- Denning D.W., Kneale M., Sobel J.D., Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect. Dis. 2018;18:e339–e347. doi: 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- Doktorovová S., Kovačević A.B., Garcia M.L., Souto E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016;108:235–252. doi: 10.1016/j.ejpb.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Dolatabadi J.E.N., Valizadeh H., Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv. Pharmaceutical Bulletin. 2015;5:151. doi: 10.15171/apb.2015.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovnik A., Golle A., Novak D., Arko D., Takač I. Treatment of vulvovaginal candidiasis: a review of the literature. Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:5–7. doi: 10.15570/actaapa.2015.2. [DOI] [PubMed] [Google Scholar]
- DPT-Laboratories, 2013. LUZU (luliconazole) Cream, 1% for topical use Drug Label. DPT Laboratories, Ltd., San Antonio, TX.
- El-Hammadi M., Arias J. Nano-sized platforms for vaginal drug delivery. CPD. 2015;21:1633–1644. doi: 10.2174/1381612820666141029150427. [DOI] [PubMed] [Google Scholar]
- Esposito, E., 2015. Production, Physico-Chemical Characterization and Biodistribution Studies of Lipid Nanoparticles. J Nanomed Nanotechnol, 06. Available from https://www.omicsonline.org/open-access/production-physicochemical-characterization-and-biodistribution-studies-of-lipid-nanoparticles-2157-7439.1000256.php?aid=36712 [Accessed 2020/07/30]. DOI 10.4172/2157-7439.1000256.
- Fidel P.L., Barousse M., Espinosa T., Ficarra M., Sturtevant J., Martin D.H., Quayle A.J., Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect. Immun. 2004;72:2939–2946. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford N.D., Patel S.A., Narayan K.M.V. Obesity in low-and middle-income countries: burden, drivers, and emerging challenges. Annu. Rev. Public Health. 2017;38:145–164. doi: 10.1146/annurev-publhealth-031816-044604. [DOI] [PubMed] [Google Scholar]
- Ganesan P., Narayanasamy D. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustainable Chem. Pharm. 2017;6:37–56. [Google Scholar]
- Garse H., Jagtap P., Kadam V. Solid lipid nanoparticles based gel for topical delivery of antifungal agent. Int. J. Pharmaceutical Sci. Research. 2015;6:3571. [Google Scholar]
- Gonçalves B., Ferreira C., Alves C.T., Henriques M., Azeredo J., Silva S. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016;42:905–927. doi: 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- Graziottin A., Gambini D. Anatomy and physiology of genital organs–women handbook of clinical neurology. Elsevier. 2015:39–60. doi: 10.1016/B978-0-444-63247-0.00004-3. [DOI] [PubMed] [Google Scholar]
- Gurpreet K., Singh S.K. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharmaceutical Sci. 2018;80:781–789. [Google Scholar]
- Hani U. Candidiasis: a fungal infection-current challenges and progress in prevention and treatment. Infectious Disorders-Drug Targets (Formerly Current Drug Targets-Infectious Disorders) 2015;15:42–52. doi: 10.2174/1871526515666150320162036. [DOI] [PubMed] [Google Scholar]
- Hasan Design, development and optimization of a transungual duple nail lacquer for onychomycosis therapy. J. Eur. Acad. Dermatol. Venereol. 2018 doi: 10.1111/jdv.14773. [DOI] [PubMed] [Google Scholar]
- Hassan N., Singh M., Sulaiman S., Jain P., Sharma K., Nandy S., Dudeja M., Ali A., Iqbal Z. Molecular docking-guided ungual drug-delivery design for amelioration of onychomycosis. ACS Omega. 2019;4:9583–9592. doi: 10.1021/acsomega.9b00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzah H.A., Farid R.M., Nasra M.M.A., Hazzah W.A., El-Massik M.A., Abdallah O.Y. Gelucire-based nanoparticles for curcumin targeting to oral mucosa: preparation, characterization, and antimicrobial activity assessment. J. Pharm. Sci. 2015;104:3913–3924. doi: 10.1002/jps.24590. [DOI] [PubMed] [Google Scholar]
- Kathe N., Henriksen B., Chauhan H. Physicochemical characterization techniques for solid lipid nanoparticles: principles and limitations. Drug Dev. Ind. Pharm. 2014;40:1565–1575. doi: 10.3109/03639045.2014.909840. [DOI] [PubMed] [Google Scholar]
- Katz D.F., Yuan A., Gao Y. Vaginal drug distribution modeling. Adv. Drug Deliv. Rev. 2015;92:2–13. doi: 10.1016/j.addr.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M., Singh K., Jain S.K. Luliconazole vesicular based gel formulations for its enhanced topical delivery. J. Liposome Res. 2019:1–19. doi: 10.1080/08982104.2019.1682602. [DOI] [PubMed] [Google Scholar]
- Khanna D., Bharti S. Luliconazole for the treatment of fungal infections: an evidence-based review. Core Evid. 2014;9:113. doi: 10.2147/CE.S49629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Tsuji Y., Inoue K., Kanai K., Majima T., Kasai T., Uchida K., Yamaguchi H. In vitro antifungal activity of luliconazole against clinical isolates from patients with dermatomycoses. J. Infection Chemotherapy. 2006;12:163–165. doi: 10.1007/s10156-006-0440-4. [DOI] [PubMed] [Google Scholar]
- Lippacher A., Müller R.H., Mäder K. Liquid and semisolid SLN™ dispersions for topical application: rheological characterization. Eur. J. Pharm. Biopharm. 2004;58:561–567. doi: 10.1016/j.ejpb.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Machado R.M., Palmeira-de-Oliveira A., Gaspar C., Martinez-de-Oliveira J., Palmeira-de-Oliveira R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015;92:14–26. doi: 10.1016/j.addr.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Madani F., Esnaashari S.S., Mujokoro B., Dorkoosh F., Khosravani M., Adabi M. Investigation of effective parameters on size of paclitaxel loaded PLGA nanoparticles. Adv. Pharm. Bull. 2018;8:77. doi: 10.15171/apb.2018.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M.A., Panda A.K., Asif S., Verma D., Talegaonkar S., Manzoor N., Khan A., Ahmed F.J., Dudeja M., Iqbal Z. A vaginal drug delivery model. Drug Deliv. 2016;23:3123–3134. doi: 10.3109/10717544.2016.1153749. [DOI] [PubMed] [Google Scholar]
- Mishra A.K., Kumar A., Singh H., Verma S., Sahu J.K., Mishra A. Chemistry and pharmacology of luliconazole (imidazole derivative): a novel bioactive compound to treat fungal infection-a mini review. Curr. Bioact. Compd. 2019;15:602–609. [Google Scholar]
- Notario-Pérez F., Cazorla-Luna R., Martín-Illana A., Ruiz-Caro R., Peña J., Veiga M.-D. Tenofovir hot-melt granulation using Gelucire® to develop sustained-release vaginal systems for weekly protection against sexual transmission of HIV. Pharmaceutics. 2019;11:137. doi: 10.3390/pharmaceutics11030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qais F.A., Khan M.S.A., Ahmad I., Althubiani A.S. Potential of nanoparticles in combating Candida infections. Lett. Drug Des. Discovery. 2019;16:478–491. doi: 10.2174/1570180815666181015145224. [DOI] [Google Scholar]
- Rathod H.J., Mehta D.P. A review on pharmaceutical gel. Int. J. Pharmaceutical Sci. 2015;1:33–47. [Google Scholar]
- Reichman O., Moyal-Barracco M., Nyirjesy P. Comment on “vulvovaginal candidiasis as a chronic disease: diagnostic criteria and definition”. J. Lower Genital Tract Disease. 2015;19:e23–e24. doi: 10.1097/LGT.0000000000000038. [DOI] [PubMed] [Google Scholar]
- Scher R.K., Nakamura N., Tavakkol A. Luliconazole: a review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses. 2014;57:389–393. doi: 10.1111/myc.12168. [DOI] [PubMed] [Google Scholar]
- Şenyiğit Z.A., Karavana S.Y., Eraç B., Gürsel Ö., Limoncu M.H., Baloğlu E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharmaceutica. 2014;64:139–156. doi: 10.2478/acph-2014-0013. [DOI] [PubMed] [Google Scholar]
- Shah R., Eldridge D., Palombo E., Harding I. Characterization and Stability; Cham: 2015. Lipid Nanoparticles: Production. [Google Scholar]
- Shaikh M.S., Kale M.A., Mahaparle P.R., Rajput H., Karkhele S.M. Development and validation of UV spectrophotometric method for the estimation of luliconazole in bulk, marketed formulations. J. Current Pharma Res. 2020;10:3759–3770. [Google Scholar]
- Shah D., Gupta D., Shah Y. Effect of lipid and surfactant concentration on cefpodoximeproxetilsolid lipid nanoparticle. Eur. J. Biomed. 2017;4:817–823. [Google Scholar]
- Shi L., Li Z., Yu L., Jia H., Zheng L. Effects of surfactants and lipids on the preparation of solid lipid nanoparticles using double emulsion method. J. Dispers. Sci. Technol. 2011;32:254–259. [Google Scholar]
- Sowjanya G., Mohana K. Quantification and stability aspects of Luliconazole in bulk and pharmaceutical dosage forms by UV spectroscopy. J. Drug Delivery Therapeutics. 2019;9:300–306. [Google Scholar]
- Taghipour S., Kiasat N., Shafiei S., Halvaeezadeh M., Rezaei-Matehkolaei A., Mahmoudabadi A.Z. Luliconazole, a new antifungal against Candida species isolated from different sources. J. Mycologie Medicale. 2018;28:374–378. doi: 10.1016/j.mycmed.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Tietz K., Klein S. Simulated genital tract fluids and their applicability in drug release/dissolution testing of vaginal dosage forms. Dissolut. Technol. 2018;25:40–51. [Google Scholar]
- Todokoro D., Suzuki T., Tamura T., Makimura K., Yamaguchi H., Inagaki K., Akiyama H. Efficacy of luliconazole against broad-range filamentous fungi including Fusarium solani species complex causing fungal keratitis. Cornea. 2019;38:238. doi: 10.1097/ICO.0000000000001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ud Din F. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur. J. Pharm. Biopharm. 2015;94:64–72. doi: 10.1016/j.ejpb.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Yarmohammadi S., Taheri G., Mousavi S.S., Sheikhehpour M., Paykoub M.H., Hashemian A.H. The effect of education on knowledge, attitude and practice of patients with vaginitis. Adv. Biol. Res. 2015;9:196–200. [Google Scholar]