Abstract
The diabetogenic effects of metals including lead (Pb), mercury (Hg), cadmium (Cd), and molybdenum (Mo) have been reported with poorly identified underlying mechanisms. The current study assessed the effect of metals on the roles of oxidative stress, apoptosis, and inflammation in beta pancreatic cells isolated from CD-1 mice, via different biochemical assays. Data showed that the tested metals were cytotoxic to the isolated cells with impaired glucose stimulated insulin secretion (GSIS). This was associated with increased reactive oxygen species (ROS) production, lipid peroxidation, antioxidant enzymes activities, active proapoptotic caspase-3 (cas-3), inflammatory cytokines interleukin–6 (IL-6) and tumor necrosis factor-alpha (TNF-α) levels in the intoxicated cells. Furthermore, antioxidant-reduced glutathione (GSH-R), cas-3 inhibitor z-VAD-FMK, IL-6 inhibitor bazedoxifene (BZ), and TNF-α inhibitor etanercept (ET) were found to significantly decrease metal-induced cytotoxicity with improved GSIS in metals’ intoxicated cells. In conclusion, oxidative stress, apoptosis, and inflammation can play roles in metals–induced diabetogenic effect.
Keywords: Lead, Mercury, Cadmium, Molybdenum, Inflammation, Oxidative stress, Pancreatic beta cells
1. Introduction
Metals are elements that naturally present in the soil and contaminate the water resources. Some metals play important roles in various enzymatic reactions and are therefore essential to normal physiology (Tchounwou et al., 2012). Other metals pose great health hazards as chronic medical stressors. Metal toxicity is of major concern because most metals have long half-lives in the human body. Strong evidence of toxicity has been found for metals such as arsenic (Ar), lead (Pb), mercury (Hg), cadmium (Cd), and chromium (Cr) (Maret, 2017, Tchounwou et al., 2012, Wang et al., 2016). Recently, studies have indicated the potential toxicity of molybdenum (Mo), which is a common food component (Menke et al., 2016). The mechanisms of metal toxicity have been the targets of different articles. Although the toxicities of different metals may share some common mechanisms, individual signatures have been recorded for certain metals. For example, Pb is known to deplete antioxidant reserves, whereas Cd is known to generate free radicals (Wu et al., 2016).
Diabetes mellitus (DM) comprises a group of metabolic disorders characterized by hyperglycemia with a wide range of symptoms, including polyuria, polyphagia, and increased thirst, and mostly complicated by life-threatening comas, cardiovascular complications, nephropathies, retinopathies, diabetic foot ulcers, and neuropathies (Fasil et al., 2019). Different risk factors have been supposed to play roles in the development of DM, including genetic factors, obesity, and aging (Bellou et al., 2018). Clinical research has indicated that heavy metal cocktails can act as risk factors for obesity, type 2 diabetes, and the development of diabetic complications (Yang et al., 2015a, Wang et al., 2018).
Significant positive correlations have been observed between blood Pb and fasting blood glucose levels, suggesting a possible link between Pb exposure and diabetes (Bener et al., 2001, Kolachi et al., 2011). Several published studies have supported the existence of a biological relationship between Hg or methyl Hg exposure and DM (Chen et al., 2006, Chen et al., 2010, Maqbool et al., 2016, Maqbool et al., 2019). Roy et al. (2016) showed that Hg fulfills five out of Bradford Hill's nine criteria for risk factors for the development of DM. The association between Cd and DM in population-based studies is a matter of controversy (Wu et al., 2017). However, several studies have shown that Cd could affect pancreatic beta cells and glucose metabolism by downregulating glucose transporter-4 (GLUT4) translocation and inducing pancreatic beta cell cytotoxicity with decreased insulin secretion and increased glucose levels (Han et al., 2003). Moreover, experimental studies of rats have shown that Cd increases insulin resistance (Chen et al., 2009, El Muayed et al., 2012, Chang et al., 2013, Treviño et al., 2015, Jacquet et al., 2018, Huang et al., 2019). However, the existing data concerning the relationship between Mo and DM are inconsistent. In a study by Ajibola et al. (2014), diabetic patients had significantly higher serum Mo levels than controls. On the other hand, another study showed that Mo decreased hyperglycemia and glycosuria and corrected the elevation of plasma non-esterified fatty acids in streptozotocin diabetic rats (Ozcelikay et al., 1996).
Oxidative stress is a common mechanism underlying cytotoxicity. This mechanism mainly occurs due to the accumulation of reactive oxygen species (ROS) and the impairment of enzymes and antioxidants that serve to detoxify ROS. This leads to the oxidative damage of cellular proteins, fats, and genetic materials as well as the disruption of cellular signaling, cellular dysfunction, and subsequent death (Costantini, 2019). Apoptosis is another common mechanism of cellular cytotoxicity involving specific cellular morphological changes (Grilo and Mantalaris, 2019). Inflammation is also a mechanism of cytotoxicity and is defined as a biological response of the body’s tissues to harmful irritants that occurs through molecular mediators (Grilo and Mantalaris, 2019).
The present study aimed to further explain the mechanisms of the metal toxicity of Pb, Hg, and Cd compared to excess doses of Mo. Oxidative stress, inflammation, and apoptosis were evaluated as potential underlying mechanisms of metal-induced (Pb, Hg, Cd, and Mo) toxicity to pancreatic beta cell lines. In addition, the effects of the examined metals on the aryl hydrocarbon receptors (AHRs) and glucocorticoid receptors (GRs) of the pancreatic cells were evaluated as potential inducers of cellular stress cascades.
2. Materials and methods
2.1. Chemical and reagents
All chemicals were obtained from Sigma (St. Louis, MO, USA), except where other sources are mentioned. The metal salts used in this study included lead nitrate, mercury chloride, cadmium dichloride, and molybdenum trioxide. Stock solutions of all the metals were made in double-distilled aseptic water. Lactate dehydrogenase (LDH), caspase-3 (cas-3), and ApoAlert caspase fluorescent assay kits were obtained from Clontech Laboratories (Mountain View, CA, USA). An Interleukin-6 (IL-6) Mouse ELISA Kit (ab100712) and a Tumor Necrosis Factor-Alpha (TNF-α) Mouse ELISA Kit (ab100747) were purchased from Abcam (Cambridge, MA, USA). A total RNA isolation kit was purchased from Qiagen (USA). A cDNA reverse transcription kit was ordered from Thermo Fisher Scientific Inc. (USA). A HotStart-IT® FideliTaq™ Polymerase chain reaction (PCR) master mix (2X; catalog no. 71156) was purchased from Affymetrix (USA). A total insulin content ELISA kit was purchased from Crystal Chem (Downers Grove, IL, USA). The following western blotting primary antibodies were ordered: mouse monoclonal cas-3 p17 (Santa Cruz Biotechnology, CA, USA), Bax (Cell Signaling Technology, Danvers, MA, USA), Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-IL-6, anti-TNF-α, anti-SOD1, anti-SOD2 (Cambridge, MA, USA), and total actin (Millipore, USA) antibodies. The western blotting secondary antibodies, including a goat anti-rabbit secondary antibody and a horseradish peroxidase-conjugated anti-mouse antibody, were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). A Pierce enhanced chemiluminescence (ECL) western blotting substrate kit was ordered from Thermo Fisher Scientific Inc. (USA).
2.2. Ethical issues, cell isolation, and culture conditions
The present study was approved by the local bioethics committee of Northern Border University. The experimental male CD-1 mice (30–35 g) were housed in air-conditioned rooms with daily 14-h illumination and free access to water and food. For all experiments, four mice were sacrificed by decapitation under thiopental anesthesia, and their pancreata were collected for further beta cell isolation. Beta cells were isolated and purified according to the method of Smelt et al. (2008), which was based on flow cytometry cell sorting according to the autofluorescence of flavin adenine dinucleotide and nicotinamide-adenine dinucleotide phosphate, which are present in higher levels in the beta cells of islets than in other islet cell types. After the islets were dispersed, the cells were washed, filtered, and pre-incubated in high-glucose (10 mM) modified Eagle’s medium (MEM) for an hour at 37 °C to enhance autofluorescence. Then, dead cells were excluded by Dapi staining, and the remaining cells were sorted by flow cytometry based on the autofluorescent compounds of the beta cells. The purity of the beta cells was evaluated by flow cytometry with Alexa Fluor 647 Mouse anti-insulin and PE Mouse anti-glucagon (BD Biosciences, San Diego, CA), in accordance with the method of Clardy et al. (2015). The purified beta cells were then cultured in Dulbecco’s MEM (DMEM) supplemented with 2 mM L-glutamine, 285 mM 2-mercaptoethanol, 10% fetal calf serum, and 25 mM Hepes.
2.3. The cytotoxic effects of metals on pancreatic beta cell viability, measured by 3-(4, 5-dimethylthiazol, 2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) and lactate dehydrogenase (LDH) assays
The cytotoxic effects of the metals on the beta pancreatic cells were studied using two assays. The first assay was a 3-(4, 5-dimethylthiazol, 2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay, which is experimentally based on the ability of the viable cells to reduce MTT substrate to insoluble formazan based on NADPH oxidoreductase enzyme activity. Second, LDH leakage was used as a marker of cytotoxicity due to the altered cell membrane integrity of the affected cells.
For both assays, the cells were seeded at a density of 5 × 104 cells/well and kept overnight. Then, the cells were treated with lead nitrate, mercury chloride, cadmium dichloride, and molybdenum trioxide at concentrations of 0.1, 1, 10, and 100 μM for 3, 6, 12, 24, and 48 h. The MTT assay was conducted in accordance with the method of Elmorsy et al. (2014), while the LDH assay was conducted in accordance with the manufacturer’s protocol. Formazan and LDH absorbances were measured with an ELISA microplate reader (Dyne MRX, Dyne Technologies, Virginia, USA) at 590 and 450 nm, respectively. Wells containing 2% Triton X-100 were used as positive controls for total cell lysis with lost viability. All assay points were performed in triplicate with each metal concentration represented by three wells in each experiment. Blank wells (without cells) were subtracted from all well readings before further analysis. The inhibitory concentrations 50 (IC50s), which are the concentration required to reduce the viability of the tested cells to its half normal control levels, were estimated for each metal in the tested exposure durations.
For the LDH assays, cytotoxicity was estimated with the following equation:
2.4. Caspase-3 (Cas-3) assay
Cas-3 is a common effector caspase in both the intrinsic and extrinsic pathways of apoptosis. The effects of the metals of interest on the cas-3 activities of the exposed beta cells were evaluated with a commercial kit in accordance with the manufacturer’s protocol. Approximately 5 × 106 cells were seeded in six-well plates and treated with the metals at their MTT-estimated inhibitory concentrations 50 (IC50s; 50 µM, 70 µM, 30 µM, and 120 µM for Pb, Hg, Cd, and Mo, respectively) and 10 µM of each metal for 2 h. This assay was conducted in accordance with the manufacturer’s protocol. Finally, fluorescence was measured in a 96-well plate using excitation/emission wavelengths of 400 and 505 nm, respectively.
2.5. Measurement of oxidative stress markers
The cells were treated with the tested metals in their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM for Pb, Hg, Cd, and Mo, respectively) and 10 µM of each metal for 24 h. ROS levels were assessed according to the method of Elmorsy et al. (2014). NF-E2-related factor 2 (NRf2) was assessed as a biomarker of oxidative stress according to the protocol of Al-Ghafari et al. (2019). Thiobarbituric acid reactive substances (TBARS) were quantified as markers of lipid peroxidation (Armstrong and Browne, 1994). A TBARS assay was performed according to the method of Alam et al. (2013). Regarding the activities of antioxidant enzymes, superoxide dismutase (SOD) activity was measured based on the assay protocol of Beauchamp and Fridovich (1971), which depends on SOD–mediated inhibition of the reduction of nitroblue tetrazolium to blue formazan by superoxide anions and normalized to cellular protein contents. Catalase (CAT) activity was assayed according to the colorimetric assay method of Singh et al. (2008) and the results were read at 620 nm. The activities of CAT were expressed as µmoles of H2O2 consumed/min/mg of the estimated sample protein. Reduced glutathione (GSH) was determined as described by Ullah, (2011).
2.6. Measurement of cytokines (interleukin-6 and tumor necrosis factor-alpha) activities
Cytokines TNF-α and IL-6 can regulate inflammatory reactions to different irritants. The effects of the metals of interest on these cytokines were evaluated with ELISA assay kits according to the manufacturer’s protocols. Briefly, cells were treated with the metals in their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM for Pb, Hg, Cd, and Mo, respectively) and 10 µM concentrations for 24 h. Then, the media was collected and IL-6 and TNF-α levels were evaluated. Plate absorbance was read at 450 nm for both cytokines. Cytokine levels were determined using the corresponding standard curves for IL-6 and TNF-α. Each experiment was carried out in triplicate with at least three wells for each metal concentration for data robustness.
2.7. Reverse transcription- polymerase chain reaction (RT-PCR) assay
The effects of the tested metals on the expression of beta pancreatic cell genes coding for oxidative stress [Cat, SOD1 (Cu-Zn-SOD), and SOD2 (Mn-SOD)], inflammation (TNF-α and IL-6), and apoptosis (Cas-3, Bax, and Bcl2), were evaluated as modulators of cellular stress signaling pathways. The primer sequences used for this analysis are shown in Table 1. Total RNA was isolated according to the manufacturer’s protocol. Then, cDNA was synthesized with a 200-ng RNA by cDNA Reverse Transcription Kit. Quantitative PCR and thermocycling condition adjustments were performed according to the methods of Huang et al. (2020) using a CFX96 Real‑Time System (Bio‑Rad Laboratories, Inc.). Transcript levels were calculated and normalized to the expression of the internal reference gene GAPDH. These experiments were carried out in triplicate.
Table 1.
List of primers used for RT-PCR for cas-3-coding genes, anti-apoptotic Bcl-2 genes, pro-apoptotic Bax genes, CAT genes, SOD1 genes, SOD2 genes, IL-6 coding genes, and TNF-α coding genes. The GAPDH gene was used as a reference gene.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Cas-3 | 5′-GGATGTGGACGCAGCCAA-3′ | 5′-CCTTCATCACCATGGCTTAG-3′ |
| Bax | 5′-CTACAGGGTTTCATCCAG −3′ | 5′-CCAGTTCATCTCCAATTCG −3′ |
| Bcl-2 | 5′-GTGGATGACTGAGTACCT −3′ | 5′-CCAGGAGAAATCAAACAGAG-3′ |
| CAT | 5′-GCAGATACCTGTGAACTGTC-3′ | 5′-GTAGAATGTCCGCACCTGAG-3′ |
| Mn-SOD | 5′-GCACATTAACGCGCAGATCA-3′ | 5′-AGCCTCCAGCAACTCTCCTT-3′ |
| Cu-Zn-SOD (SOD1) | 5′-AAGGCCGTGTGCGTGCTGAA-3′ | 5′-CAGGTCTCCAACATGCCTCT-3′ |
| Mn-SOD (SOD2) | 5′-GCACATTAACGCGCAGATCA-3′ | 5′-AGCCTCCAGCAACTCTCCTT-3′ |
| TNF-α | 5′- GAGGCACTCCCCCAAAAG-3′ | 5′-GGGTCTGGGCCATAGAACTG-3′ |
| IL-6 | 5′- GACAACTTTGGCATTGTGG -3′ | 5′-ATGCAGGGATGATGTTCTG-3′ |
| GAPDH | 5′-TGACGTGCCGCCTGGAGAAA-3′ | 5′-AGTGTAGCCCAAGATGCCCTTCAG-3 |
2.8. Western blot
Cells were treated with the metal salts at their estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM for Pb, Hg, Cd, and Mo, respectively) and 10 µM concentrations for 24 h. Then, proteins were extracted from these cells and quantified. These extracts (50 µg) were separated by SDS–polyacrylamide gels for cas-3, Bax, and Bcl2 proteins and then electrotransferred to nitrocellulose membranes. Then, these membranes were incubated with the following primary antibodies: mouse monoclonal cas-3 p17 (1:1000), Bax (1:400), Bcl-2 (1:400), rabbit polyclonal anti-IL-6, anti-TNF-α, anti-SOD1, anti-SOD2 (1:1000), and total actin (1:50,000) antibodies. A goat anti-rabbit antibody and a horseradish peroxidase-conjugated anti-mouse antibody (1:20,000) were used as secondary antibodies. Protein bands were detected with the Pierce ECL western blotting substrate kit. The density of the bands was quantified and normalized in relation to the total actin bands as a reference protein using GelQuant.NET software provided by biochemlabsolutions.com.
2.9. Effects of metals on insulin secretion
The protocol of Cheng et al. (2012) was followed to study the effects of the tested metals on glucose-stimulated insulin secretion (GSIS). Briefly, cells were incubated with the tested metals at their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM for Pb, Hg, Cd, and Mo, respectively) and 10 µM concentrations for 24 h. Then, the media were removed and the cells were washed with phosphate buffer saline (PBS) and incubated for 15 min in glucose-free DMEM supplemented with L–glutamine (2 mM), Hepes (25 mM, pH 7.4), and glucose (25 mM). Next, the cells were lysed with hydrochloric acid/ethanol for the measurement of total insulin content using an ELISA kit. Absorbance was measured at a wavelength of 450 nm, and GSIS was normalized to the total insulin content of the corresponding well.
2.10. Effects of antioxidant-reduced glutathione (GSH-R), a cas-3 inhibitor, and cytokine inhibitors on metal-induced beta pancreatic cell cytotoxicity and dysfunction
The present study aimed to prove the roles of oxidative stress, apoptosis, and inflammation in metal-induced beta cell cytotoxicity and altered insulin secretion. Therefore, the MTT and GSIS assays were reevaluated in the presence of GSH-R (10 µM), Cas-3 inhibitor z–VAD-FMK (200 µM), IL-6 inhibitor bazedoxifene (BZ; 10 µM), and TNF-α inhibitor etanercept (ET; 150 µg mL−1) to evaluate the functional effects of metal-induced oxidative stress, inflammation, and apoptosis on the GSIS of the pancreatic beta cells.
2.11. Statistical analysis
All statistical analyses were performed using GraphPad Prism software (GraphPad Software, San Diego, CA). IC50s were estimated using nonlinear regression curve fitting statistics and response versus log concentration variable slope response models. For the MTT assay, the bottom and top constraints were adjusted to 0 and 100, assuming a vehicle control viability of 100% and a maximum inhibition viability of 0%. A hill slope constraint of one was used to fit the LDH data. A two-way ANOVA was used to evaluate the effects of concentration and exposure duration on MTT and LDH, and a one-way ANOVA was used with a Tukey or Dunnett multiple comparisons post–test to compare the controls with the metal-exposed groups. Statistical significance was defined as p < 0.05.
3. Results
The cytotoxic effects of several metal salts on the viability of pancreatic beta cells isolated from CD-1 mice were investigated using an MTT assay. The resulting data showed that the tested metals were cytotoxic to the isolated cells. This cytotoxicity correlated significantly and proportionately with the metals’ concentrations at all tested time points (Fig. 1 and Table 2). Apart from Mo, the tested metals had significant cytotoxic effects at a concentration of 10 µM after 3 h of incubation (Table S1–2). Specifically, Cd was the most cytotoxic metal with the lowest estimated IC50s at all time points, while Mo was the safest with the lowest cytotoxic effect and the highest IC50s at all time points (Table 3). A BrdU assay was conducted to confirm that these results were mainly due to the effects of the metals on the cells’ metabolic activity and not their rate of proliferation. This assay showed that the tested metals did not significantly affect the proliferation of the isolated beta pancreatic cells using the same range of concentrations and time points (data not shown). The results of the MTT assay were also confirmed by an LDH assay, which showed that the cytotoxic effects of the metals on the pancreatic cells followed concentration-dependent and exposure-duration-dependent patterns.
Fig. 1.
MTT and LDH assays showed the cytotoxic effects of Pb, Hg, Cd, and Mo on pancreatic beta cells isolated from CD-1 mice. The cytotoxic effects of these metals were tested at concentrations of 0.1, 1, 10, and 100 µM and time points of 3, 6, 12, 24, and 48 h post-exposure. Cell viability was expressed as percent from controls assuming their corresponding controls viability is 100%. Experiments were conducted in triplicates with at least 3 wells for each tested metal’s concentration per experiment. Data are expressed as means ± SEMs.
Table 2.
Correlation statistics for the cytotoxic effects of Pb, Hg, Cd, and Mo on pancreatic beta cells isolated from CD-1 mice at concentrations of 0.1, 1, 10, 100, and 1000 µM and time points of 3, 6, 12, 24, and 48 h post-exposure. The p-values were estimated using Pearson correlation statistics.
| 3 h | 6 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|---|
| Pb | |||||
| Pearson r | −0.96 | −0.9832 | −0.9868 | −0.9949 | −0.9994 |
| p value | 0.0096** | 0.0026** | 0.0018** | 0.0004*** | < 0.0001**** |
| Hg | |||||
| Pearson r | −0.9675 | −0.9909 | −0.9899 | −0.9944 | −0.9972 |
| p value | 0.007** | 0.001** | 0.0012** | 0.0005*** | 0.0002*** |
| Cd | |||||
| Pearson r | −0.9762 | −0.9982 | −0.9958 | −0.9976 | −0.999 |
| p value | 0.0044** | < 0.0001**** | 0.0003*** | 0.0001*** | < 0.0001**** |
| Mo | |||||
| Pearson r | −0.9923 | −0.9876 | −0.9894 | −0.9963 | −0.997 |
| p value | 0.0008*** | 0.0016** | 0.0013** | 0.0003*** | 0.0002*** |
Table 3.
MTT-assay-estimated IC50s of the cytotoxic effects of Pb, Hg, Cd, and Mo on pancreatic beta cells isolated from CD-1 mice. The cytotoxic effects of these metals were tested at concentrations of 0.1, 1, 10, 100, and 1000 µM and time points of 3, 6, 12, 24, and 48 h post–exposure.
| Assay | MTT |
||
|---|---|---|---|
| IC50s | Mean (µM) | 95% Confidence interval |
|
| Lower limit (µM) | Upper limit (µM) | ||
| Pb | |||
| 3 h | 647.9 | 550.3 | 762.9 |
| 6 h | 240.9 | 206.5 | 281 |
| 12 h | 151.5 | 130.6 | 175.7 |
| 24 h | 51.48 | 40.95 | 63.82 |
| 48 h | 31.13 | 26.75 | 36.22 |
| Hg | |||
| 3 h | 1361 | 1057 | 1753 |
| 6 h | 717.3 | 547.2 | 940.3 |
| 12 h | 226 | 181.1 | 282 |
| 24 h | 69.97 | 58.02 | 84.38 |
| 48 h | 21.24 | 17.94 | 25.16 |
| Cd | |||
| 3 h | 378.9 | 323.7 | 443.4 |
| 6 h | 125.2 | 103.9 | 151 |
| 12 h | 59.31 | 50.53 | 69.61 |
| 24 h | 31.5 | 28.28 | 35.28 |
| 48 h | 13.3 | 11.41 | 15.51 |
| Mo | |||
| 3 h | 11,380 | 6386 | 20,270 |
| 6 h | 874.9 | 678.5 | 1128 |
| 12 h | 278.1 | 224.8 | 344.2 |
| 24 h | 118.2 | 90.3 | 129.7 |
| 48 h | 60.08 | 49.88 | 72.37 |
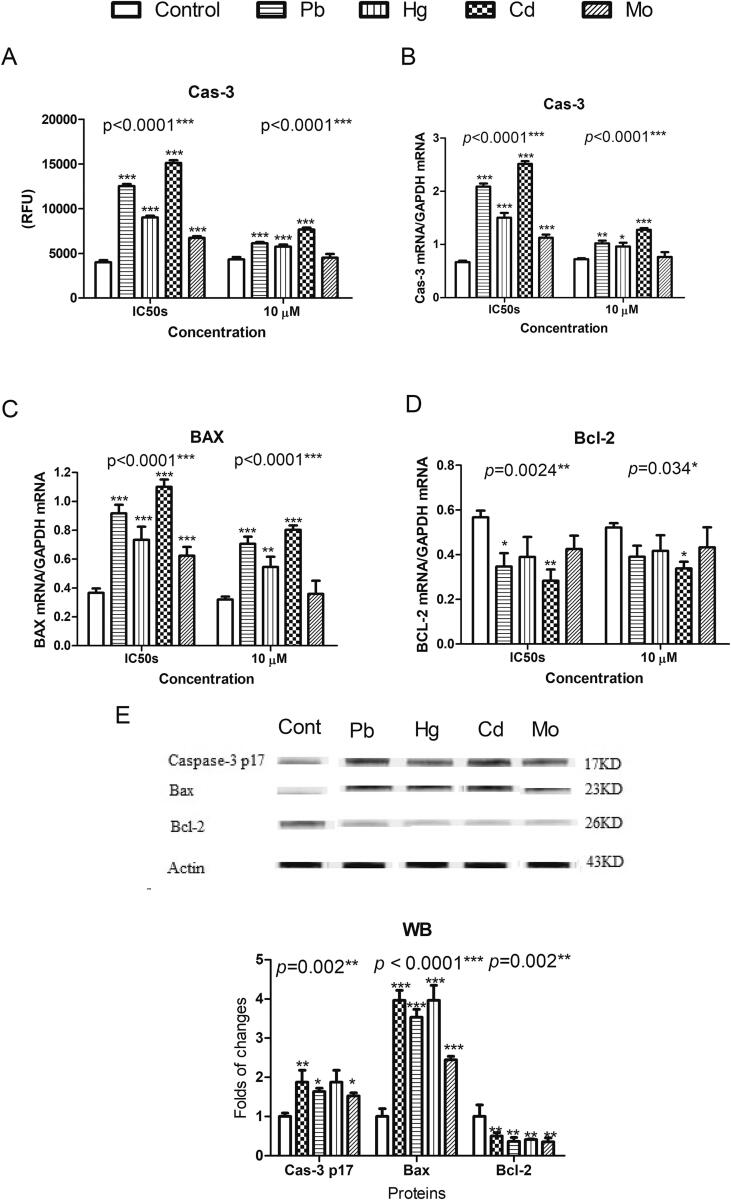
Apoptosis is a major subcellular mechanism of cytotoxicity and is induced via different triggers that stimulate an intrinsic and/or extrinsic pathway, with active cas-3 as an intermediate effector between the two common pathways. The effects of the tested metals on cas-3 activity and cas–3–coding gene expression were investigated in the present study. At their estimated IC50s, the tested metals were found to significantly activate cas-3 activities as well as the expression of genes coding for cas-3. Apart from Mo, the metals retained these effects at a lower concentration (10 µM; Fig. 2A). The effects of the metals on the expression of apoptosis regulatory genes Bax (pro–apoptotic) and Bcl-2 (anti-apoptotic) were investigated as well. Apart from Mo, the metals were found to significantly increase Bax gene expression using both tested concentrations (Fig. 2B). However, only Cd and Pb significantly inhibited Bcl-2 gene expression (Fig. 2C). These data were supported by western blotting, which showed that the tested metals significantly increased the levels of active cas-3 p17 subunit and proteins coded by the Bax gene; western blotting also showed that the tested metals caused a parallel decrease in Bcl-2-coded protein levels (Fig. 2D and E).
Fig. 2.
The effects of Pb, Hg, Cd, and Mo at their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM, respectively) and at a lower concentration of 10 µM on cas-3 activities (2A) and the expression of genes coding for cas-3 (2B), anti-apoptotic Bcl-2 (2C), and pro-apoptotic Bax (2D) in pancreatic beta cells isolated from CD-1 mice. 2E and 2F show the effects of the tested metals at their estimated IC50s on cas-3, anti-apoptotic Bcl-2, and pro-apoptotic Bax protein levels, as measured by western blotting. The density of the bands was quantified and normalized in relation to the total actin bands as a reference protein using GelQuant.NET software provided by biochemlabsolutions.com. All experiments were conducted in triplicates. One-way ANOVA p-values are shown herein. A Dunnett post-test was used to compare the metal-exposed cells to the non-exposed control cells. Data are expressed as means ± SDs. Significance is shown as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
Oxidative stress is another common mechanism of cytotoxicity. Metal-induced oxidative stress was evaluated in beta pancreatic cells isolated from CD-1 mice using different oxidative stress biomarkers. The results showed that the metals of interest significantly increased ROS, TBARS, and Nrf2 expression at their MTT-estimated IC50s. Only Cd and Pb showed significant effects on the same oxidative stress markers at a concentration of 10 µM. However, Hg caused a significant increase in Nrf2 expression at 10 µM, while MO caused nonsignificant increases in ROS, TBARS, and Nrf2 expressions at this concentration (Fig. 3A–C). In parallel, the tested metals significantly decreased the activities of antioxidant enzymes CAT and SOD at their estimated IC50s but showed difference degrees of the inhibitory activities at a lower concentration (Fig. 4A and B). With respect to the effects of the metals on the coding gene levels measured by RT-PCR, the antioxidant enzyme CAT gene was the most affected, while SOD2 was the least affected. In particular, Cd affected the tested gene expressions at both concentrations 45 and 10 µM. On the other hand, Mo did not have any effects on the activities of the antioxidant enzymes and their coding genes at a concentration of 10 µM (Fig. 3C–E). Western blotting showed that the metals significantly lowered the CAT, SOD1, and SOD2 protein levels after 24 h at their MTT-estimated IC50s (Fig. 4F–G).
Fig. 3.
The effects of Pb, Hg, Cd, and Mo at their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM, respectively) and at a concentration of 10 µM on the ROS production (3A), lipid peroxidation (TBARS; 3B), and Nrf2 gene expression (3C) of pancreatic beta cells isolated from CD-1 mice. Each experiment was repeated for at least 3 times. One-way ANOVA p-values are shown herein. A Dunnett post-test was used to compare the metal-exposed cells to the non-exposed control cells. Data are expressed as means ± SDs. Significance is shown as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
Fig. 4.
The effects of Pb, Hg, Cd, and Mo at their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM, respectively) and at a concentration of 10 µM on the CAT activities (4A), SOD activities (4B), and expression of genes coding for CAT (4C), SOD1 (4D), and SOD2 (4E) in pancreatic beta cells isolated from CD-1 mice. 4F and 4G show the effects of the tested metals at their estimated IC50s on CAT, SOD1, and SOD2 protein levels, as measured by western blotting. The density of the bands was quantified and normalized in relation to the total actin bands as a reference protein using GelQuant.NET software provided by biochemlabsolutions.com. All experiments were conducted in triplicates. One-way ANOVA p–values are shown herein. A Dunnett post-test was used to compare the metal-exposed cells to the non-exposed control cells. Data are expressed as means ± SDs. Significance is shown as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
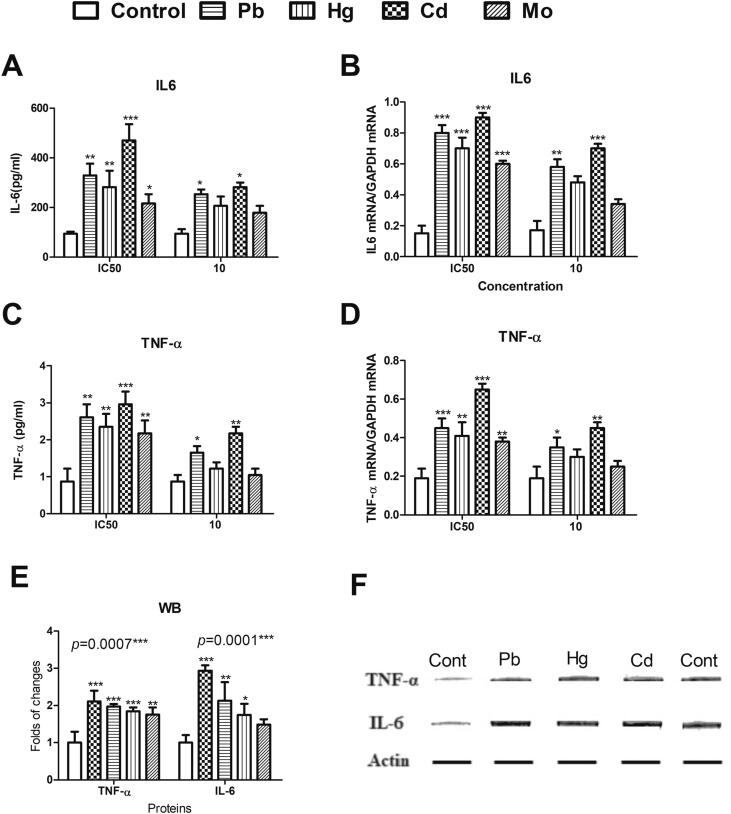
Inflammation was also investigated as an underlying mechanism of the diabetogenic effects of the tested metals. Inflammation occurs as a biological response to pathogens and irritants, including toxicants. The most common regulatory inflammatory cytokines are IL6 and TNF-α. At their MTT-estimated IC50s, the tested metals significantly increased the levels of cytokines IL6 and TNF-α as well as the expressions of their coding genes. However, only Cd and Pb retained this effect at a concentration of 10 µM according to the ELISA assay (Fig. 5).
Fig. 5.
The effects of Pb, Hg, Cd, and Mo at their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM, respectively) and at a concentration of 10 µM on IL-6 (5A), TNF-α (5B), and the expression of genes coding for IL-6 (5C) and TNF-α (5D) in pancreatic beta cells isolated from CD-1 mice. 5E and 5F show the effects of the tested metals at their estimated IC50s on IL-6 and TNF-α protein levels, as measured by western blotting. The density of the bands was quantified and normalized in relation to the total actin bands as a reference protein using GelQuant.NET software provided by biochemlabsolutions.com. All experiments were conducted in triplicates. One-way ANOVA p-values are shown herein. A Dunnett post-test was used to compare the metal-exposed cells to the non-exposed control cells. Data are expressed as means ± SDs. Significance is shown as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
The effects of the tested metals on the GSIS of the pancreatic beta cells were also evaluated. The tested metals were found to significantly decrease insulin secretion at their estimated IC50s and at 10 µM, except for Mo, which showed no significant effect on insulin secretion at the lower concentration (Fig. 6A–D).
Fig. 6.
The effects of Pb, Hg, Cd, and Mo at their MTT-estimated IC50s (50 µM, 70 µM, 30 µM, and 120 µM, respectively) and at a concentration of 10 µM on the GSIS of pancreatic beta cells isolated from CD-1 mice (6A–6D), 24 hrs post exposure. Also, the protective effects of GSH-R (10 µM), cas-3 inhibitor z-VAD-FMK (200 µM), IL–6 inhibitor BZ (10 µM), and TNF-α inhibitor ET (150 µg mL−1) were evaluated. GSH-R, cas-3 inhibitor z-VAD-FMK, IL–6 inhibitor BZ, and TNF-α inhibitor ET were shown to have significant protective effects on the GSIS of metal-treated samples to differing extents (A–D). These compounds also significantly reduced the cytotoxic effects of the tested metals on the isolated beta pancreatic cells (E–H). MTT data were shown after subtraction of the blank’s readings from all wells values. All experiments were conducted in triplicates. One-way ANOVA p-values are shown herein. A Tukey post-test was used to compare the metal-exposed cells to the non-exposed control cells. Data are expressed as means ± SDs. For comparison between the metal-treated samples and other samples treated with metals in association with the protective compounds, significance is shown as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001. For comparison between the non-treated control cells and the experimental groups, significance is shown as $ for p < 0.05, $$ for p < 0.01, and $$$ for p < 0.001.
Interestingly, an antioxidant (reduced GSH), a cas-3 inhibitor (z-VAD-FMK), an IL-6 inhibitor (BZ), and a TNF-α inhibitor (ET) significantly reduced the metal–induced functional disruption of insulin secretion by the treated cells (Fig. 6A–D). In addition, these inhibitors were shown to significantly decrease the cytotoxic effects of the metals of interest in the MTT assay (Fig. 6E–H). According to the GSIS and MTT assays, these inhibitors did not show any significant effects on the insulin secretions and cellular viability of the isolated pancreatic beta cells in their tested concentrations (data not shown).
4. Discussion
Metals are naturally occurring health hazards reported to have diabetogenic effects without any clear underlying mechanisms. The present study was conducted to investigate the effects of oxidative stress, apoptosis, and inflammation as potential underlying mechanisms of the diabetogenic effects of several metals. Due to their widely occurring reported diabetogenic effects, Pb, Hg, Cd, and Mo were selected for investigation in the present study. Beta pancreatic cells isolated from male CD-1 mice were used as a model to test the current study hypothesis as this strain was previously used as a successful in vivo models for antipsychotics (APs) induced DM (Savoy et al., 2010) and there are available commercial cell lines for pancreatic beta cells of this strain. Purified cells were preferred to whole islets, as pure beta cells can provide more robust data concerning the diabetogenic effects of the tested metals by eliminating the concurrent effects of the metals on other types of islet cells. Furthermore, purification eliminated the effects of somatostatin produced by other cells, which have been reported to affect beta pancreatic cells activities (Daunt et al., 2006). Metal salts were tested in a wide range of concentrations covering the references and reported toxic blood and serum levels of the tested metals. The reference levels of the tested metals were reported as 1.5, 0.07, 0.1, and 0.018 µM for Pb, Hg, Cd, and Mo, respectively. The toxic levels of these metals were reported as 7.7, 25.8, 3.7, and 0.08 µM for Pb, Hg, Cd, and Mo, respectively (Berislav, 1999, Menezes Filho et al., 2003, Yıldırım et al., 2012, Chakraborty et al., 2013). In the MTT and LDH assays, the metals were tested in a wide range of concentrations from 0.1 to 1000 µM to estimate their IC50s. Further assays were conducted using the MTT-estimated IC50s, which are of high biological importance. These assays were also conducted using a lower concentration of 10 µM, which is closer to the reported toxic levels in humans, to compare the effects of the different metals at the same lower concentration. Moreover, the lower concentration was also used to compare the current study results with other (independent) toxicological studies. As the tested metals are known to be nondegradable and to accumulate markedly in tissues, higher concentrations were used in the present study to simulate long-term effects within the limited time frames of the in vitro assays.
The results of the present study showed that the tested metals were cytotoxic to the pancreatic beta cells of CD-1 mice, as indicated by a marked decrease in MTT dye and a significant increase in LDH leakage. Of these metals, Cd was the most cytotoxic, while Mo showed the smallest inhibitory effect. These findings were in accordance with those of other studies, which demonstrated that the tested metals were cytotoxic to different extents in different mammalian cell lines (Fischer and Hofmann, 1990, Limaye and Shaikh, 1999, Yedjou et al., 2003, Tchounwou et al., 2004, Silva-Pereira et al., 2005, Hwang et al., 2013, Terpiłowska et al., 2018, Rosli et al., 2018). The IC50s of the metals were estimated based on metal concentrations, exposure durations, cell line types, metabolic activity rates, cellular proliferation rates, and life cycle phases (Schieke et al., 2008). With regard to other types of pancreatic beta cells, Cd was not cytotoxic to the rat insulinoma cell line (INS-1) or the mouse insulinoma cell line (MIN-6) at concentrations of 5 and 0.1 µM, respectively, 24 h post-treatment (El Muayed et al., 2012, Dover et al., 2018). In the present study, Cd was found to be cytotoxic to the isolated pancreatic beta cells even at the lowest concentration of 1 µM. This difference highlights species-specific responses and higher primary cell line responses to Cd cytotoxicity. Regarding the effect of Mo on the beta pancreatic cells, Yang et al. (2016) concluded that Mo altered insulin secretion in the pancreatic β-cell-derived RIN-m5F cells and the isolated mouse islets with induction of apoptosis via activation of mitochondrial apoptotic pathway and activation of caspases cascade. No data were available regarding the cytotoxic effects of Pb nor Hg on the pancreatic beta cell lines.
The cytotoxic effects of metals have mainly been explained by their inhibitory effects on mitochondria and their electron transport chain complexes (Belyaeva et al., 2008, Belyaeva et al., 2011, Belyaeva et al., 2012). Mitochondrial inhibition has been reported to cause the formation of ROS and the activation of apoptosis and pro-inflammatory signaling pathways (López-Armada et al., 2013, Bhola and Letai, 2016, Chen et al., 2019, Vringer and Tait, 2019). Interestingly, the results of the present study showed that the tested metals significantly induced oxidative stress with a significant increase in activated cleaved cas-3, a significant increase in the expression of the Bax gene (pro-apoptotic), a decrease in the Bcl-2 gene (anti-apoptotic), and a marked increase in IL-6 and TNF-α inflammatory cytokine activities. Functionally, the metals were found to impair GSIS in the exposed cells relative to the controls. Interestingly, GSH-R, a cas-3 inhibitor (z-VAD-FMK), and an IL-6 inhibitor (BZ) were found to block the metal–induced inhibitory effects on the beta cells, resulting in marked improvements in their functioning.
The three investigated cytotoxic mechanisms—oxidative stress, apoptosis, and inflammation—are closely related to each other. The inflammatory cytokine TNF has been shown to control inflammation as well as oxidative damage by increased ROS and reactive nitrogen species that are involved in neurodegenerative disease (Fischer and Maier, 2015). Further, IL-6 has been shown to induce oxidative stress and endothelial dysfunction through overexpression of the angiotensin II type 1 receptor in the pathogenesis of atherosclerosis (Wassmann et al., 2004). With respect to the relationship between apoptosis and oxidative stress, high levels of ROS have been shown to activate the pathways of apoptosis (Kaminskyy and Zhivotovsky, 2014, Redza-Dutordoir and Averill-Bates, 2016). On the other hand, apoptosis is mainly induced via a class of death receptors that includes tumor necrosis factor receptor (TNFR)1, TNFR2, and Fas (Yang et al., 2015b). TNFR1 and 2 form complex I, which contains a death domain of different molecules that are essential to initiating and controlling cellular apoptosis (Wertz and Dixit, 2008).
In conclusion, the present study showed that the tested metals were cytotoxic to the pancreatic beta cells of CD-1 mice, resulting in subsequent functional impairment. The results also showed that the tested metals increased ROS production and inhibited antioxidant enzymes. In parallel, these metals increased cas-3 activity, increased pro-apoptotic Bax gene expression, and markedly decreased anti-apoptotic Bcl-2 expression. In addition, the tested metals initiated the inflammatory pathways of the exposed cells. These findings revealed that oxidative stress, apoptosis, and inflammation are underlying mechanisms of the diabetogenic potential of the tested metals.
The current study findings are clinically significant as they highlight the direct toxic effects of the metals on the beta pancreatic cells which proved the previously published association studies between DM and higher levels of the tested metals among the diabetic population in biological samples. According to the current data, diabetic patients should avoid occupations with occupational hazardous exposure to the tested metals. In addition, periodical check of blood glucose levels is advisable among those workers. Furthermore, the study showed the protective roles of antioxidants. Hence, these workers should be advised to high supplementary antioxidants or at least guide them to take higher amounts of the natural dietary antioxidants. On the other hand, although the in vitro studies are widely accepted in studying the cytotoxicity with less unethical violation against the experimental animals, their main limitation is mainly originating from the absence of the normal body environment and the normal cell to cell interaction within the different biological pathways of stress, so the current findings are in need to be further supported by other in-vivo studies.
Funding
The research was funded by Dr. Suhair Hassan Al-Qurashi, President of Dar Al-Hekma University, Kingdom of Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors gratefully acknowledge Dr. Suhair Hassan. Al-Qurashi, President of Dar Al-Hekma University, Kingdom of Saudi Arabia, for funding this research and for her continuous support.
Author’s Contribution
H.A.: Manuscript writing and editing.
E.E.: Experimental work.
A.A.: Protocol/ project development.
J.G. Data analysis.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.10.056.
Contributor Information
Huda Al Doghaither, Email: haldoghaither@kau.edu.sa.
Ekramy Elmorsy, Email: ekramyelmorsy@mans.edu.eg.
Ayat Al-Ghafari, Email: abalghafari@kau.edu.sa.
Jihan Ghulam, Email: jgholam@dah.edu.sa.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ajibola R.S., Ogundahunsi O.A., Soyinka O.O., Ogunyemi E.O., Odewabi A.O. Serum chromium, molybdenum, zinc and magnesium levels in diabetes mellitus patients in Sagamu, South West Nigeria. Asian J. Med. Sci. 2014;6(2):15–19. doi: 10.19026/ajms.6.5350. [DOI] [Google Scholar]
- Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghafari A., Elmorsy E., Fikry E., Alrowaili M., Carter W.G. The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress. PloS One. 2019;14(11) doi: 10.1371/journal.pone.0225341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D., Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv. Exp. Med. Biol. 1994;336:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bener A., Obineche E., Gillett M., Pasha M.A., Bishawi B. Association between blood levels of lead, blood pressure and risk of diabetes and heart disease in workers. Int. Arch. Occup. Environ. Health. 2001;74(5):375–378. doi: 10.1007/s004200100231. [DOI] [PubMed] [Google Scholar]
- Bellou V., Belbasis L., Tzoulaki I., Evangelou E. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PloS One. 2018;13(3) doi: 10.1371/journal.pone.0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva E.A., Dymkowska D., Więckowski M.R., Wojtczak L. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol. Appl. Pharmacol. 2008;231(1):34–42. doi: 10.1016/j.taap.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Belyaeva E.A., Korotkov S.M., Saris N.E. In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: a comparison of copper and mercury with cadmium. J. Trace Elem. Med. Biol. 2011;25(Suppl 1):S63–S73. doi: 10.1016/j.jtemb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Belyaeva E.A., Sokolova T.V., Emelyanova L.V., Zakharova I.O. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper. Sci. World J. 2012;2012 doi: 10.1100/2012/136063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berislav M. A case report of acute human molybdenum toxicity from a dietary molybdenum supplement–A new member of the» lucor metallicum «family. Arhiv za higijenu rada i toksikologiju. 1999;50(3):289–297. https://hrcak.srce.hr/2715 [PubMed] [Google Scholar]
- Bhola P.D., Letai A. Mitochondria—judges and executioners of cell death sentences. Mol. Cell. 2016;61(5):695–704. doi: 10.1016/j.molcel.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S., Dutta A.R., Sural S., Gupta D., Sen S. Ailing bones and failing kidneys: a case of chronic cadmium toxicity. Ann. Clin. Biochem. 2013;50(Pt 5):492–495. doi: 10.1177/0004563213481207. [DOI] [PubMed] [Google Scholar]
- Chang S.Y., Kim D.B., Ryu G.R., Ko S.H., Jeong I.K., Ahn Y.B., Jo Y.H., Kim M.J. Exendin-4 inhibits iNOS expression at the protein level in LPS-stimulated Raw264.7 macrophage by the activation of cAMP/PKA pathway. J. Cell Biochem. 2013;114(4):844–853. doi: 10.1002/jcb.24425. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Huang C.F., Tsai K.S., Yang R.S., Yen C.C., Yang C.Y., Lin-shiau S.Y., Liu S.H. Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem. Res. Toxicol. 2006;19(8):1080–1085. doi: 10.1021/tx0600705. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Huang C.F., Yang C.Y., Yen C.C., Tsai K.S., Liu S.H. Inorganic mercury causes pancreatic β-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol. Appl. Pharmacol. 2010;243(3):323–331. doi: 10.1016/j.taap.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Yang C.Y., Huang C.F., Hung D.Z., Leung Y.M., Liu S.H. Heavy metals, islet function and diabetes development. Islets. 2009;1(3):169–176. doi: 10.4161/isl.1.3.9262. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhou Y., Hu C., Wang Y., Yan Z., Li Z., Wu R. Mitochondria and oxidative stress in ovarian endometriosis. Free Radic. Biol. Med. 2019;136:22–34. doi: 10.1016/j.freeradbiomed.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Cheng K., Delghingaro-Augusto V., Nolan C.J., Turner N., Hallahan N., Andrikopoulo S.S., Gunton J.E. High passage MIN6 cells have impaired insulin secretion with impaired glucose and lipid oxidation. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clardy S.M., Mohan J.F., Vinegoni C., Keliher E.J., Iwamoto Y., Benoist C., Mathis D., Weissleder R. Rapid, high efficiency isolation of pancreatic ß-cells. Sci. Rep. 2015;5:13681. doi: 10.1038/srep13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D. Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. J. Exp. Biol. 2019;222(13):jeb194688. doi: 10.1242/jeb.194688. [DOI] [PubMed] [Google Scholar]
- Daunt M., Dale O., Smith P.A. Somatostatin inhibits oxidative respiration in pancreatic β-cells. Endocrinology. 2006;147(3):1527–1535. doi: 10.1210/en.2005-0873. [DOI] [PubMed] [Google Scholar]
- Dover E.N., Patel N.Y., Stýblo M. Impact of in vitro heavy metal exposure on pancreatic β-cell function. Toxicol. Lett. 2018;299:137–144. doi: 10.1016/j.toxlet.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Muayed M., Raja M.R., Zhang X., MacRenaris K.W., Bhatt S., Chen X., Urbanek M., O’halloran T.V., Lowe W.L., Jr Accumulation of cadmium in insulin-producing β cells. Islets. 2012;4(6):405–416. doi: 10.4161/isl.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmorsy E., Elzalabany L.M., Elsheikha H.M., Smith P.A. Adverse effects of antipsychotics on micro-vascular endothelial cells of the human blood–brain barrier. Brain Res. 2014;1583:255–268. doi: 10.1016/j.brainres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Fasil A., Biadgo B., Abebe M. Glycemic control and diabetes complications among diabetes mellitus patients attending at University of Gondar Hospital, Northwest Ethiopia. Diabetes Metab. Syndr. Obes. 2019;12:75–83. doi: 10.2147/DMSO.S185614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.B., Hofmann J. Studies of cadmium cytotoxicity and genotoxicity—cell cycle effects and cytogenetic findings in cultured mammalian cells. Toxicol. Environ. Chem. 1990;27(1–3):143–152. doi: 10.1080/02772249009357567. [DOI] [Google Scholar]
- Fischer R., Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo A.L., Mantalaris A. Apoptosis: a mammalian cell bioprocessing perspective. Biotechnol. Adv. 2019;37(3):459–475. doi: 10.1016/j.biotechadv.2019.02.012. [DOI] [PubMed] [Google Scholar]
- Han J.C., Park S.Y., Hah B.G., Choi G.H., Kim Y.K., Kwon T.H., Kim E.K., Lachaal M., Jung C.Y., Lee W. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch. Biochem. Biophys. 2003;413(2):213–220. doi: 10.1016/s0003-9861(03)00120-6. [DOI] [PubMed] [Google Scholar]
- Huang Q., Gong Q., Wen T., Feng S., Xu J., Liu J., Han X., Liu Q., Hu J., Zhu L. Loss of LAMTOR1 in pancreatic β–cells increases glucose–stimulated insulin secretion in mice. Int. J. Mol. Med. 2020;45(1):23–32. doi: 10.3892/ijmm.2019.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.C., Kuo C.Y., Yang C.Y., Liu J.M., Hsu R.J., Lee K.I., Su C.C., Wu C.C., Lin C.T., Liu S.H., Huang C.F. Cadmium exposure induces pancreatic β-cell death via a Ca2+-triggered JNK/CHOP-related apoptotic signaling pathway. Toxicol. 2019;425 doi: 10.1016/j.tox.2019.152252. [DOI] [PubMed] [Google Scholar]
- Hwang T.L., Chen H.Y., Changchien T.T., Wang C.C., Wu C.M. The cytotoxicity of mercury chloride to the keratinocytes is associated with metallothionein expression. Biomed. Rep. 2013;1(3):379–382. doi: 10.3892/br.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet A., Cottet-Rousselle C., Arnaud J., Julien Saint Amand K., Ben Messaoud R., Lénon M., Demeilliers C., Moulis J.M. Mitochondrial morphology and function of the pancreatic β-cells INS-1 model upon chronic exposure to sub-lethal cadmium doses. Toxics. 2018;6(2):20. doi: 10.3390/toxics6020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskyy V.O., Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 2014;21(1):86–102. doi: 10.1089/ars.2013.5746. [DOI] [PubMed] [Google Scholar]
- Kolachi N.F., Kazi T.G., Afridi H.I., Kazi N., Khan S., Kandhro G.A., Shah A.Q., Baig J.A., Wadhwa S.K., Shah F., Jamali M.K. Status of toxic metals in biological samples of diabetic mothers and their neonates. Biol. Trace Elem. Res. 2011;143(1):196–212. doi: 10.1007/s12011-010-8879-7. [DOI] [PubMed] [Google Scholar]
- Limaye D.A., Shaikh Z.A. Cytotoxicity of cadmium and characteristics of its transport in cardiomyocytes. Toxicol. Appl. Pharmacol. 1999;154(1):59–66. doi: 10.1006/taap.1998.8575. [DOI] [PubMed] [Google Scholar]
- López-Armada M.J., Riveiro-Naveira R.R., Vaamonde-García C., Valcárcel-Ares M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13(2):106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Maqbool F., Bahadar H., Hassani S., Niaz K., Baeeri M., Rahimifard M., Ghasemi-Niri S.F., Abdollahi M. Biochemical evidence on the potential role of methyl mercury in hepatic glucose metabolism through inflammatory signaling and free radical pathways. J. Cell Biochem. 2019;120(9):16195–16205. doi: 10.1002/jcb.28899. [DOI] [PubMed] [Google Scholar]
- Maqbool F., Bahadar H., Niaz K., Baeeri M., Rahimifard M., Navaei-Nigjeh M., Ghasemi-Niri S.F., Abdollahi M. Effects of methyl mercury on the activity and gene expression of mouse Langerhans islets and glucose metabolism. Food Chem. Toxicol. 2016;93:119–128. doi: 10.1016/j.fct.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Maret W. The bioinorganic chemistry of lead in the context of its toxicity. Met. Ions Life Sci. 2017 doi: 10.1515/9783110434330-001. [DOI] [PubMed] [Google Scholar]
- Menezes Filho J.A., Carvalho W.A.D., Spínola A.G. Assessment of occupational exposure to lead in a metallurgy plant a cross-sectional study. Rev. Soc. Bras. Med. Trop. 2003;28(105–106):63–72. doi: 10.1590/S0303-76572003000100007. [DOI] [Google Scholar]
- Menke A., Guallar E., Cowie C.C. Metals in urine and diabetes in US adults. Diabetes. 2016;65(1):164–171. doi: 10.2337/db15-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcelikay A.T., Becker D.J., Ongemba L.N., Pottier A.M., Henquin J.C., Brichard S.M. Improvement of glucose and lipid metabolism in diabetic rats treated with molybdate. Am. J. Physiol. 1996;270(2 Pt 1):E344–E352. doi: 10.1152/ajpendo.1996.270.2.E344. [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Rosli N.F., Latiff N.M., Sofer Z., Fisher A.C., Pumera M. In vitro cytotoxicity of covalently protected layered molybdenum disulfide. Appl. Mater. Today. 2018;11:200–206. doi: 10.1016/j.apmt.2018.02.001. [DOI] [Google Scholar]
- Roy M.M., Ferguson M.J., McDonald R., Rivard E. Investigation of N-heterocyclic carbene-supported group 12 triflates as pre-catalysts for hydrosilylation/borylation. Chemistry. 2016;22(50):18236–18246. doi: 10.1002/chem.201603704. [DOI] [PubMed] [Google Scholar]
- Savoy Y.E., Ashton M.A., Miller M.W., Nedza F.M., Spracklin D.K., Hawthorn M.H., Rollema H., Matos F.F., Hajos-Korcsok E. Differential effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in the mouse: evidence for the involvement of sympathetic regulation. Schizophr. Bull. 2010;36(2):410–418. doi: 10.1093/schbul/sbn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieke S.M., McCoy J.P., Jr, Finkel T. Coordination of mitochondrial bioenergetics with G1 phase cell cycle progression. Cell Cycle. 2008;7(12):1782–1787. doi: 10.4161/cc.7.12.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Pereira L.C., Cardoso P.C.D.S., Leite D.S., Bahia M.D.O., Bastos W.R., Smith M.D.A.C., Burbano R.R. Cytotoxicity and genotoxicity of low doses of mercury chloride and methylmercury chloride on human lymphocytes in vitro. Braz. J. Med. Biol. Res. 2005;38(6):901–907. doi: 10.1590/S0100-879X2005000600012. [DOI] [PubMed] [Google Scholar]
- Singh R., Wiseman B., Deemagarn T., Jha V., Switala J., Loewen P.C. Comparative study of catalase-peroxidases (KatGs) Arch. Biochem. Biophys. 2008;471(2):207–214. doi: 10.1016/j.abb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Smelt M.J., Faas M.M., De Haan B.J., De Vos P. Pancreatic beta-cell purification by altering FAD and NAD (P) H metabolism. Exp. Diabetes Res. 2008;2008 doi: 10.1155/2008/165360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou P.B., Yedjou C.G., Foxx D.N., Ishaque A.B., Shen E. Lead-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG 2) cells. Mol. Cell. Biochem. 2004;255(1–2):161–170. doi: 10.1023/b:mcbi.0000007272.46923.12. PMID: 11678604. [DOI] [PubMed] [Google Scholar]
- Terpiłowska S., Siwicka-Gieroba D., Siwicki A.K. Cytotoxicity of iron (III), molybdenum (III), and their mixtures in BALB/3T3 and HepG2 cells. J. Vet. Res. 2018;62(4):527–533. doi: 10.2478/jvetres-2018-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treviño S., Waalkes M.P., Flores Hernández J.A., León-Chavez B.A., Aguilar-Alonso P., Brambila E. Chronic cadmium exposure in rats produces pancreatic impairment and insulin resistance in multiple peripheral tissues. Arch. Biochem. Biophys. 2015;583:27–35. doi: 10.1016/j.abb.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Ullah H. Determination of glutathione concentration after its interaction with cadmium nitrate tetrahydrate by using Ellman’s modified method. Gomal Univ. J. Res. 2011;27(2):18–25. http://www.gomal.pk/GUJR/PDF/PDF-December-2011/2-DONE%20Hashmat%201%20full%20and%20final.pdf [Google Scholar]
- Vringer E., Tait S.W. Mitochondria and inflammation: cell death heats up. Front. Cell Dev. Biol. 2019;7:100. doi: 10.3389/fcell.2019.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wang Z., Kou C., Ma Z., Zhao D. Responses of wheat yield, macro- and micro-nutrients, and heavy metals in soil and wheat following the application of manure compost on the North China Plain. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0146453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Mukherjee, B., Park, S.K., 2018. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 121(1), 683–694. doi: 10.1016/j.envint.2018.09.035 [DOI] [PMC free article] [PubMed]
- Wassmann S., Stumpf M., Strehlow K., Schmid A., Schieffer B., Böhm M., Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- Wertz I.E., Dixit V.M. Ubiquitin-mediated regulation of TNFR1 signaling. Cytokine Growth Factor Rev. 2008;19(3–4):313–324. doi: 10.1016/j.cytogfr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Wu X., Cobbina S.J., Mao G., Xu H., Zhang Z., Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016;23(9):8244–8259. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- Wu M., Song J., Zhu C., Wang Y., Yin X., Huang G., Zhao K., Zhu J., Duan Z., Su L. Association between cadmium exposure and diabetes mellitus risk: a prisma-compliant systematic review and meta-analysis. Oncotarget. 2017;8(68):113129–113141. doi: 10.18632/oncotarget.21991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.M., Cheng N., Pu H.Q. Metal exposure and risk of diabetes and prediabetes among Chinese occupational workers. Biomed. Environ. Sci. 2015;28(12):875–883. doi: 10.3967/bes2015.121. [DOI] [PubMed] [Google Scholar]
- Yang Y., Jiang G., Zhang P., Fan J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015;2(1):12. doi: 10.1186/s40779-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.Y., Yen C.C., Lee K.I., Su C.C., Yang C.Y., Wu C.C., Hsieh S.S., Ueng K.C., Huang C.F. Molybdenum induces pancreatic β-cell dysfunction and apoptosis via interdependent of JNK and AMPK activation-regulated mitochondria-dependent and ER stress-triggered pathways. Toxicol. Appl. Pharmacol. 2016;294:54–64. doi: 10.1016/j.taap.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Yedjou C., Haynes L., Dorsey W., McMurray R., Tchounwou P.B. Lead-induced cytotoxicity and oxidative stress in human leukemia (HL-60) cells. Arch. Environ. Contam Toxicol. 2003;44(3):417–420. doi: 10.1007/s00244-002-2023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yıldırım R., Erdem F., Gündoğdu M., Bilen Y., Koca E., Yıllıkoğlu Y., Sahin Y.N. Mercury toxicity: a family case report. Turk. J. Haematol. 2012;29(1):76–79. doi: 10.5152/tjh.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.