Abstract
Lewy body disease (LBD) is a spectrum of progressive neurodegenerative disorders characterized by the wide distribution of Lewy bodies and neurites in the central and peripheral nervous system (CNS, PNS). Clinical diagnoses include Parkinson’s disease (PD), dementia with Lewy bodies, or pure autonomic failure. All types of LBD are accompanied by non-motor symptoms (NMSs) including gastrointestinal dysfunctions such as constipation. Its relationship to Lewy body-related α-synucleinopathy (Lewy pathology) of the enteric nervous system (ENS) is attracting attention because it can precede the motor symptoms. To clarify the role of ENS Lewy pathology in disease progression, we performed a clinicopathological study using the Brain Bank for Aging Research in Japan. Five-hundred and eighteen cases were enrolled in the study. Lewy pathology of the CNS and PNS, including the lower esophagus as a representative of the ENS, was examined via autopsy findings. Results showed that one-third of older people (178 cases, 34%) exhibited Lewy pathology, of which 78 cases (43.8%) exhibited the pathology in the esophagus. In the esophageal wall, Auerbach’s plexus (41.6%) was most susceptible to the pathology, followed by the adventitia (33.1%) and Meissner’s plexus (14.6%). Lewy pathology of the esophagus was significantly associated with autonomic failures such as constipation (p < 0.0001) and among PNS regions, correlated the most with LBD progression (r = 0.95, p < 0.05). These findings suggest that the propagation of esophageal Lewy pathology is a predictive factor of LBD.
Electronic supplementary material
The online version of this article (10.1007/s00401-020-02233-8) contains supplementary material, which is available to authorized users.
Keywords: Lewy body disease, Parkinson’s disease, α-Synuclein, Esophagus, Enteric nervous system, Peripheral nervous system
Introduction
Lewy body disease (LBD) is a neurodegenerative disorder in which Lewy bodies (LBs) and neurites appear in the central and peripheral nervous systems (CNS, PNS). It is clinically diagnosed as Parkinson’s disease (PD), dementia with Lewy bodies (DLB), or pure autonomic failure (PAF) [36, 44–47, 51–53, 86]. The main component of LBs has proven to be phosphorylated α-synuclein [3, 28, 79], thus allowing Lewy pathology—the accumulation and distribution of phosphorylated α-synuclein—to be examined in the CNS and PNS of people with LBD [4, 42, 70, 71].
In addition to well-known motor symptoms, patients with PD might also exhibit non-motor symptoms (NMSs) such as autonomic failure, psychological symptoms such as depression, apathy, and psychosis, cognitive decline, or rapid eye movement sleep-behavior disorder [65]. Some NMSs have been shown to precede the motor symptoms or clinical diagnosis of PD [1, 34, 43, 60, 66, 75]. Indeed, gastrointestinal dysfunction—a primary NMS in PD—has recently been attracting attention because Lewy pathology in the enteric nervous system (ENS) can be a predictive marker for PD/DLB [40, 80, 81, 87].
The way that Lewy pathology propagates was first advanced by Braak and colleagues [11, 12]. The pathology of the PNS is noteworthy in that α-synuclein accumulation can be seen in numerous organs and tissues during the pre-symptomatic or symptomatic phase [4, 21, 88], including sympathetic ganglia [20, 26, 83], the heart [27, 41, 56, 59, 61–63], adrenal gland [20, 29], skin [22, 23, 25, 39, 58, 78, 91], olfactory mucosa [30], olfactory epithelium [73], olfactory bulb [6, 11, 16, 19, 38, 64, 76], posterior pituitary gland [37], spinal cord [14], dorsal root ganglia [82], submandibular gland [18], upper aerodigestive tract [57], gallbladder [40], and genitourinary tract [4]. Lewy pathology of the ENS was first reported in patients who had PD with dysphagia [68] and then in those who had PD with megacolon [48]. Subsequently, LBs have been shown to be most frequent in the lower esophagus [4, 31, 89, 90]. Autopsy studies have estimated the frequency of α-synuclein deposition in the ENS to be between 50–100% in PD/DLB, 14–100% in incidental LBD, and 0–52% in controls [2, 4, 5, 7, 10, 17, 31, 33, 57]. Further, analyses of surgical specimens or biopsies of the ENS have revealed this pathology up to 20 years before the onset of the disease with a positive rate of 13–100% [40, 74, 77, 80, 81]. Therefore, the ENS is vulnerable to Lewy pathology in LBD. However, the precise prevalence of ENS pathology in older people or in those who have pre-symptomatic LBD, and the specificity during LBD development, remain unclear.
To clarify the role of Lewy pathology of the ENS in the LBD progression, we performed a clinicopathological study using community-based, autopsy-confirmed cohorts of older Japanese people from the Brain Bank for Aging Research (BBAR).
Materials and methods
Tissue source
Tissue samples were obtained from autopsy cases that took place at the Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology (TMGHIG). TMGHIG is located in urban Tokyo and provides community-based general and emergency services to the older (+ 65 years) population, including patients with dementia or neurodegenerative diseases. TMGHIG performed 738 autopsies between October 2008 and June 2018 and obtained consent to register them to the BBAR, which is approved by the institutional ethics review committee. Among these cases, 202 excluded from this study because they lacked consent for craniotomy. Among the remaining 536 cases available for this study, we excluded 8 that had undergone brain autopsy (7 cases of Creutzfeldt–Jakob disease and one case of PD), 6 that exhibited multiple system atrophy (another type of α-synucleinopathy), and 4 that showed total brain necrosis (immunocytochemistry not applicable). This left 518 cases for analysis in this study.
Histology
We examined the brain, spinal cord, olfactory bulb and tract, and the PNS, including the sympathetic ganglia [82], anterior wall of the left ventricle of the heart [56], lower esophagus (as a representative of the ENS) [4, 31, 89, 90], adrenal gland [29], and skin of the upper arm and thigh [39]. The CNS was examined as previously reported [30, 70, 71, 76]. Briefly, the cerebral hemisphere, cerebellum, and brain stem were first dissected in the sagittal plane during the autopsy to separate the hemispheres: one to be frozen and the other for fixation. The frozen side of the brain was then cut in several 8-mm slices: cerebral hemisphere in the coronal plane, cerebellum in the sagittal plane, and brain stem in the horizontal plane. The PNS was examined as follows. Six sympathetic ganglia at the level of the heart and adrenal glands with periadrenal adipose tissue were sliced in the maximum sections. Transmural myocardial tissue was obtained from the anterior wall of the left ventricle of the heart. The lower esophagus and skin were dissected in a 2-cm length with the mucosa to adventitia or the epidermis to subcutaneous fat tissue in two sections. The PNS and some parts of the frozen brain sample, including the frontal and temporal pole, parietal lobe (intraparietal sulcus), primary visual cortex, posterior hippocampus, amygdala, cerebellum including the dentate nucleus, substantia nigra, and the olfactory bulb were dissected in a block less than 2 cm × 1.5 cm before rapid freezing and fixed with 4% paraformaldehyde (PFA) for 48 h. The remaining parts of the frozen sample were preserved at − 80 °C for further biochemical and molecular analyses. The other half of the brain was fixed with 20% buffered formalin (WAKO, Japan) for 7–13 days and cut in 5-mm slices as described above. The representative areas were embedded in paraffin and 6-μm-thick sections were prepared for hematoxylin and eosin staining (H&E), the Klüver-Barrera method, and immunohistochemistry. Further, selected slides were stained with Gallyas-Braak and modified methenamine-silver staining for evaluation of changes related to senility.
Immunohistochemistry and evaluation
Sections were immunostained with Ventana BenchMark GX autostainer (Roche, Switzerland) and iView DAB Detection Kit (Roche, Switzerland) according to the manufacturers’ instructions. The antibodies used in this study were mouse monoclonal anti-phosphorylated-α-synuclein antibody (pSyn#64, 1:20,000, a gift from Dr. Iwatsubo [70]; now available for purchase from FUJIFILM Wako Pure Chemical Corporation, Japan), mouse monoclonal anti-amyloid-β antibody (clone 12B2, 1:50, IBL, Japan), rabbit monoclonal anti-phosphorylated-tau antibody (clone AT8, 1:1000, Fujirebio, Japan), and mouse monoclonal anti-phosphorylated-TAR DNA-binding protein 43 (TDP-43) antibody (pS409/410, 1:10,000, a gift from Dr. Hasegawa; now available for purchase from Cosmo Bio Co Ltd, Japan [85]). Two anti-phosphorylated-α-synuclein antibodies—rabbit polyclonal antibody (PSer129, 1:100, a gift from Dr. Iwatsubo [28] and Dr. Akiyama [4]) and rabbit monoclonal antibody (MJF-R13, ab168381, 1:80,000, Abcam, UK)—and anti-non-phosphorylated-α-synuclein antibody (LB509, 1:100, gift from Dr. Iwatsubo [3]; now available for purchase as ab27766, Abcam, UK or as SIG-39725, Biolegend, USA) were employed to confirm positive immunoreactivity in some select cases after protease treatment (Supplementary Fig. 1, online resource).
Histological and immunohistochemical evaluations were performed via light microscopic observation (Eclipse Ni, Nikon, Japan). For clinicopathological staging of the Lewy pathology, we evaluated the BBAR LB stage (Table 1; modified from Saito et al. 2003 [70] and Funabe et al. 2013 [30]), DLB Consensus Guidelines [51–53], and Braak LB stage [11, 12]. Semi-quantitative analyses for the CNS and PNS Lewy pathology were performed using the grading system from the third report of the DLB consortium [52]. Lewy pathology distribution in the esophageal wall was analyzed with the following subdivisions: mucosa, muscularis mucosa, submucosa including Meissner’s plexus, muscularis propria including Auerbach’s plexus, and adventitia.
Table 1.
BBAR LB stagea
| Stage | pSyn-IR | LB | Loss of pigmentation of the LC/SN | Parkinsonism | Dementiab | LB scorec | Diagnosis |
|---|---|---|---|---|---|---|---|
| 0 | − | − | − | − | − | 0 | |
| 0.5 | + | − | − | − | − | 0 | Earliest LBD |
| 1 | + | + | − | − | − | 0–10 | Preclinical LBD |
| 2 | + | + | + | − | − | 0–10 | Prodromal LBD |
| 3 | + | + | + | + | − | 0–10 | PD |
| 4 | + | + | + | + / − | + | 3–6 | PDD/DLBT |
| 5 | + | + | + | + / − | + | 7–10 | PDD/DLBN |
BBAR brain bank for aging research, pSyn-IR phosphorylated α-synuclein immunoreactivity, LB Lewy body, LC locus coeruleus, SN substantia nigra, LBD Lewy body disease, PD Parkinson’s disease, PDD Parkinson’s disease with dementia, DLBT dementia with Lewy bodies, transitional form, DLBN dementia with Lewy bodies, neocortical form; + presence; − absence
aRef. Funabe S et al. [30]
bNIA-AA criteria [54]
cDLB consensus guideline 1996 [53]
For staging of amyloid β and phosphorylated tau, we evaluated the Braak senile plaque stage [9], CERAD score [55], Thal senile plaque phase [84], Braak neurofibrillary tangle stage [8, 9], and Saito argyrophilic grain stage [72]. For pTDP-43, we scored the medial temporal lobe (amygdala and anterior hippocampus), medulla oblongata, and lumbar spinal cord as previously reported [85].
Clinicopathological and genetic information
Information including age, sex, presence or absence of autonomic failures (such as severe constipation, orthostatic hypotension, or urinary dysfunction), Parkinsonism, and dementia was obtained from the BBAR database and extracted from medical records by neurologists. The Mini-Mental State Examination, the Revised Hasegawa’s dementia scale, and the Clinical Dementia Rating were used for evaluating dementia. The interval between the last assessment upon admission and time to death was less than 2 months in most of the individuals. Brain weights, neuropathological diagnoses, and apolipoprotein (APOE) status were based on the BBAR database and reviewed by pathologists.
Statistical analysis
Quantitative and semi-quantitative data were statistically analyzed by the χ2 test, Student’s t test, Mann–Whitney U test, and Spearman's rank correlation coefficient using GraphPad Prism 6 (GraphPad Software, San Diego, CA).
Results
Prevalence of Lewy pathology in older individuals
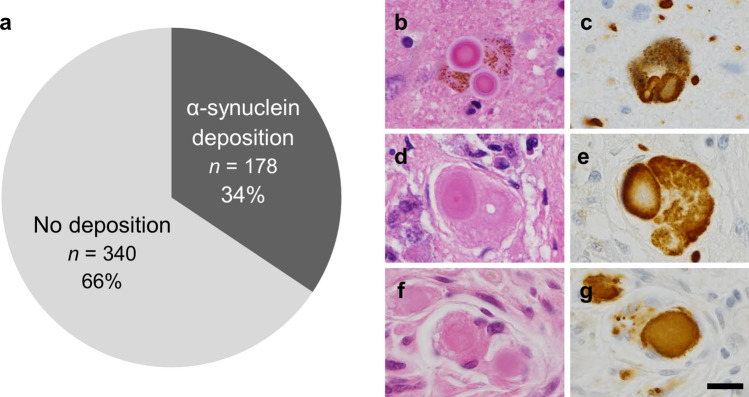
Among the 518 autopsy cases, 73% of which died from respiratory diseases, malignant neoplasm, or cardiovascular disease (Supplementary Fig. 2, Supplementary Table 1, online resource), approximately one-third of older individuals exhibited Lewy pathology either in the CNS and/or the PNS (178/518: 34%, Fig. 1; Supplementary Fig. 3, online resource). In most of these cases, the pathology was observed in both the CNS and PNS (121/178: 68%). PNS-only pathology was found in 9 cases (5%), including 5 cases in which it was limited to the sympathetic ganglia (Supplementary Table 3, online resource). CNS-only pathology was observed in 48 cases (27%), of which 23 were amygdala-predominant, 14 were brainstem-predominant, 8 were limbic, and 3 were olfactory-bulb-only according to the LB type pathology by DLB Consensus Guidelines [51–53]. Fifteen cases were neuropathologically diagnosed as Alzheimer’s disease (AD). None exhibited Lewy pathology in the esophagus only or esophageal α-synuclein accumulation without CNS pathology.
Fig. 1.
Prevalence of Lewy pathology in the 518 BBAR cases. a One-third of older individuals exhibited α-synuclein accumulation. b–g LB in the locus coeruleus (b, c), adrenal gland (d, e), and esophagus (f, g). H&E (b, d, f) and pSyn#64 (c, e, g). Scale bar = 10 μm. BBAR the Brain Bank for Aging Research
The clinicopathological characteristics are summarized in Table 2. The positivity of Lewy pathology was significantly associated with age (male: positive cases, mean age at death = 81.1 ± 9.5 years; negative cases, 77.9 ± 11.8 years; female: positive cases, 86.4 ± 8.5 years; negative cases, 82.3 ± 11.0 years; p < 0.05) and higher pathological stages of neurofibrillary tangles (p < 0.0001) or senile plaques (p < 0.05), but not with sex, APOE status, or stages of argyrophilic grains or pTDP-43 (data not shown).
Table 2.
Characteristics of the 518 BBAR cases
| Lewy pathology | P value | ||
|---|---|---|---|
| Positive | Negative | ||
| Clinical and genetic characteristics | |||
| Gender (n) | |||
| Male | 98 (18.9%) | 205 (39.6%) | 0.26 |
| Female | 80 (15.4%) | 135 (26.1%) | |
| Mean Age at death (y) (SD, range) | |||
| Male | 81.1 (9.5, 49–99) | 77.9 (11.8, 25–99) | 0.04 |
| Female | 86.4 (8.5, 61–104) | 82.3 (11.0, 24–111) | 0.004 |
| APOE (n) | |||
| ε2/ε3 | 11 (2.1%) | 32 (6.2%) | 0.7143 |
| ε3/ε3 | 130 (25.1%) | 224 (43.2%) | |
| ε2/ε4 | 1 (0.2%) | 2 (0.4%) | |
| ε3/ε4 | 31 (6.0%) | 58 (11.2%) | |
| ε4/ε4 | 3 (0.6%) | 5 (1.0%) | |
| Neuropathological factors | |||
| Brain weight (g, mean) | 1208 | 1234 | 0.051 |
| (SD, range) | (132, 758–1666) | (145, 730–1937) | |
| Braak NFT stage (n) | |||
| 0–II | 92 (17.8%) | 249 (48.1%) | < 0.0001 |
| III–VI | 85 (16.4%) | 91 (17.6%) | |
| Braak SP stage (n) | |||
| 0–A | 109 (21.0%) | 257 (49.6%) | 0.0011 |
| B–C | 68 (13.1%) | 83 (16.0%) | |
| CERAD score (n) | |||
| 0–A | 91 (17.6%) | 237 (45.8%) | 0.0008 |
| B–C | 77 (14.9%) | 103 (19.9%) | |
| Thal phase (mean) | 2.5 | 2.1 | 0.0021 |
BBAR the Brain Bank for Aging Research, CERAD the Consortium to establish a registry for Alzheimer’s disease, NFT neurofibrillary tangle, SD standard deviation, SP senile plaque
Lewy pathology of the PNS
To clarify the specificity of esophageal Lewy pathology in the PNS, we compared the positivity in the esophagus, sympathetic ganglia, heart, adrenal gland, and skin with the BBAR LB stage.
Among 178 cases positive for Lewy pathology, the mean percentages of α-synuclein deposition were as follows: sympathetic ganglia, 70.2% (125 cases); heart, 55.1% (98); esophagus, 43.8% (78); adrenal gland, 33.7% (60); skin, 18.0% (32). The incidence increased with the progression of BBAR LB stage, with the percentages in the esophagus correlating the most (r = 0.95), followed by the sympathetic ganglia (r = 0.85), the heart (r = 0.87), adrenal gland (r = 0.81), and skin (r = 0.71, Spearman's rank correlation coefficient, all p < 0.05, Fig. 2; Supplementary Table 2, online resource). Although the percentage of α-synuclein in the esophagus was almost the same as that in the heart and adrenal gland, the esophageal positivity gradually increased and reached 94.7% (18/19) by stage 5. In contrast, the incidence in the heart and adrenal gland decreased with the stage of progression (heart, stage 4–5: 94.7% (18/19) to 84.2% (16/19); adrenal gland, stage 3–5: 75.0% (6/8) to 57.9% (11/19)). The sympathetic ganglia exhibited the highest incidence and Lewy pathology was observed in all cases that were at least stage 3.
Fig. 2.
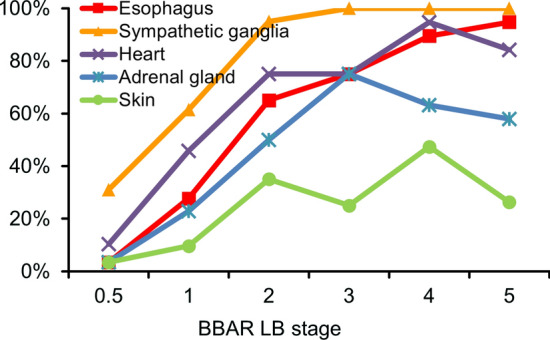
Lewy pathology of the PNS at different BBAR LB stages. The positive rate of Lewy PNS pathology increased with the BBAR LB stage. Among PNS regions, the correlation with the BBAR LB stage was highest in the esophagus (r = 0.95), followed by the sympathetic ganglia (r = 0.85), heart (r = 0.87), adrenal gland (r = 0.81), and skin (r = 0.71; Spearman’s rank correlation coefficient, all p < 0.05). Positivity in the esophagus gradually increased with stage, while that in the heart and adrenal gland decreased at the last stage. The sympathetic ganglia exhibited the highest rate of pathology among the regions in the PNS. Details are summarized in Supplementary Table 2, online resource. BBAR the Brain Bank for Aging Research, LB Lewy body, PNS peripheral nervous system
Lewy pathology of the esophagus and clinicopathological characteristics
To examine the characteristics of the esophageal Lewy pathology, we compared the presence or absence of the pathology with clinical, genetic, and neuropathological factors (Table 3). Analysis showed that the pathology was significantly associated with autonomic failures, Parkinsonism, and the stage of LB pathology as determined by the 4th DLB Consensus Guidelines, the BBAR LB stage [70], and the Braak LB stage (p < 0.0001) [11, 12], but not with age, sex, or brain weight. Lewy pathology tended to be associated with dementia, although the tendency did not reach statistical significance (p = 0.0562).
Table 3.
Esophageal Lewy pathology and clinicopathological factors among 178 older people who showed Lewy pathology in their nervous systems
| Esophageal Lewy pathology | P value | |||
|---|---|---|---|---|
| Positive | Negative | |||
| Clinical and genetic characteristics | ||||
| Age at death (y, mean) | 84.3 | 82.8 | 0.675 | |
| Gender (n) | ||||
| Male | 40 (22.5%) | 58 (32.6%) | 0.4479 | |
| Female | 38 (21.3%) | 42 (23.6%) | ||
| APOE (n) | ||||
| ε2/ε3 | 6 (3.4%) | 5 (2.8%) | ND | |
| ε3/ε3 | 55 (30.9%) | 75 (42.1%) | ||
| ε2/ε4 | 1 (0.6%) | 0 (0%) | ||
| ε3/ε4 | 13 (7.3%) | 18 (10.1%) | ||
| ε4/ε4 | 2 (1.1%) | 1 (0.6%) | ||
| Constipation (n) | ||||
| Positive | 23 (12.9%) | 5 (2.8%) | < 0.0001 | |
| Negative | 55 (30.9%) | 95 (53.4%) | ||
| Other autonomic failures (n) | ||||
| Positive | 18 (10.1%) | 5 (2.8%) | 0.0005 | |
| Negative | 60 (33.7%) | 95 (53.4%) | ||
| Parkinsonism (n) | ||||
| Positive | 31 (17.4%) | 9 (5.1%) | < 0.0001 | |
| Negative | 47 (26.4%) | 91 (51.1%) | ||
| Dementia (n) | ||||
| Positive | 48 (27.0%) | 46 (25.8%) | 0.0562 | |
| Negative | 30 (16.9%) | 54 (30.3%) | ||
| Neuropathological factors | ||||
| Brain weight (g, mean) | 1212 | 1205 | 0.7406 | |
| DLB 4th consensus report (n) | ||||
| Diffuse neocortical | 44 (24.7%) | 5 (2.8%) | < 0.0001 | |
| Limbic | 22 (12.4%) | 23 (12.9%) | ||
| Brainstem-predominant | 11 (6.2%) | 32 (18.0%) | ||
| Amygdala-predominant | 1 (0.6%) | 28 (15.7%) | ||
| BBAR LB stage (n) | ||||
| 0–2 | 37 (20.8%) | 95 (53.4%) | < 0.0001 | |
| 3–5 | 41 (23.0%) | 5 (2.8%) | ||
| Braak LB stage (n) | ||||
| 0–3 | 21 (11.8%) | 85 (47.8%) | < 0.0001 | |
| 4–6 | 57 (32.0%) | 15 (8.4%) | ||
BBAR the Brain Bank for Aging Research, DLB dementia with Lewy bodies, LB Lewy body, ND not determined
Lewy pathology in the esophageal wall
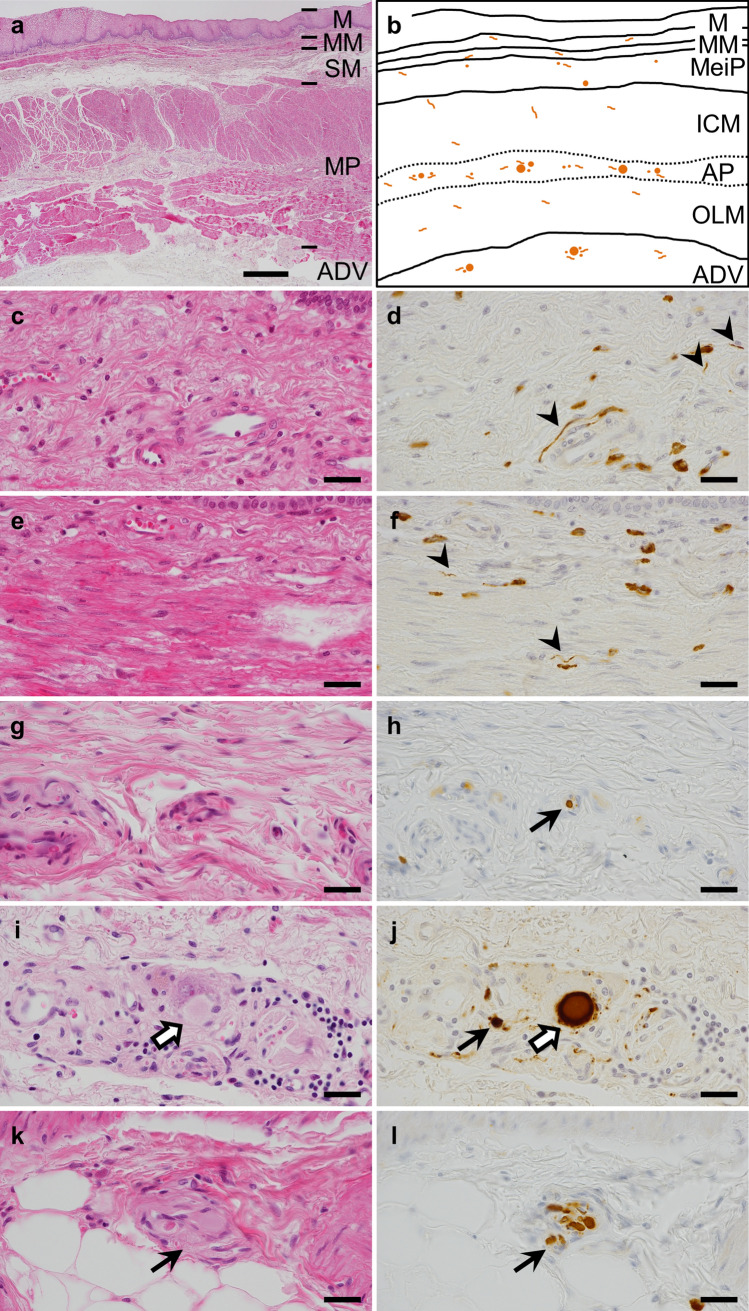
To examine the locus of Lewy pathology in the esophageal wall, we semi-quantified the density of the α-synuclein accumulation in separate regions of the wall (Fig. 3a, b) and compared each density with the BBAR LB stage.
Fig. 3.
Lewy pathology in the esophageal wall. a Low magnification view of the esophageal wall and its anatomy. b Schema of Lewy pathology, shown in orange spherical or neuritic structures in the esophageal wall. c–l High magnification view of the mucosa, especially lamina propria (c, d), muscularis mucosa (e, f), submucosa (g, h), muscularis propria (i, j) and adventitia (k, l). LB were observed in Auerbach’s plexus in the MP (white arrows, i, j) and ADV. Lewy neurites or pSyn#64-immunoreactive (IR) aggregates were found in Meissner’s plexus of the SM, Auerbach’s plexus, and the ADV (arrows, h, j, k, l). Only pSyn#64-IR neurites were observed in the M and MM (arrowheads, d, f). Non-specific staining of stromal cell cytoplasm—most were mast cell granules—was observed in the background (d, f). H&E (a, c, e, g, i, k) and pSyn#64 (d, f, h, j, l). Scale bar = 500 µm (a), 20 µm (c–l). LB Lewy body, M mucosa, MM muscularis mucosa, SM submucosa, MeiP Meissner’s plexus, MP muscularis propria, ICM inner circular muscle layer of MP, OLM outer longitudinal muscle layer of MP, AP Auerbach’s plexus, ADV adventitia
LBs were observed in Auerbach’s plexus and the adventitia (ADV) as homogenous and spherical cytoplasmic inclusions with clear halos via H&E and round inclusions via anti-α-synuclein immunohistochemistry (Fig. 3i, j). Additionally, Lewy neurites or pSyn#64-immunoreactive (IR) aggregates (round or elongated cytoplasmic or neuronal process inclusions) were found in Meissner’s plexus, Auerbach’s plexus, and the ADV (Fig. 3h, j–l). Only pSyn#64-IR neurites (thread-like structures) were observed in the mucosa (M) and muscularis mucosa (MM) (Fig. 3d, f). Mean percentages of the pathology were as follows: muscularis propria (MP), 41.6% (74/178); ADV, 33.1% (59/178); submucosa (SM), 14.6% (26/178); MM, 8.4% (15/178); M, 4.5% (8/178). The incidence increased with both BBAR and Braak LB-stage progression. MP and ADV exhibited the highest rates, reaching 89.5% (17/19) by BBAR stage 5, and 92.9% (26/28) and 78.6% (22/28) for MP and ADV, respectively, by Braak stage 6. In contrast, Lewy pathology was observed less in the M and MM (Fig. 4a, b; Table 4a, b).
Fig. 4.
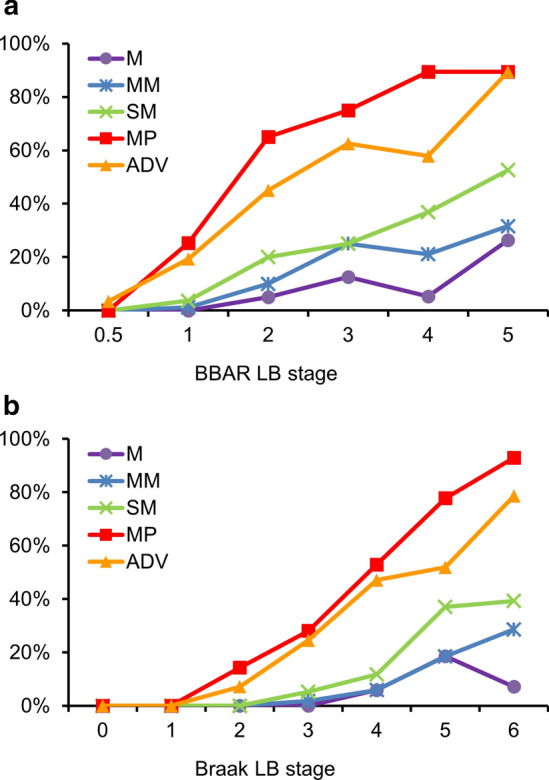
Lewy pathology in the esophageal wall at different BBAR and Braak LB stages. a BBAR LB stages; The positive rate of Lewy pathology increased with BBAR LB stage. The highest rates were found in the MP and ADV (mean rates of 41.6% and 33.1% respectively), reaching 89.5% at stage 5. Lewy pathology was observed less in the M and MM. b Braak LB stages; The positivity of Lewy pathology also increased with Braak LB stage. The highest rates were found in the MP, reaching 92.9% at stage 6. Lewy pathology was observed less in the M and MM. Details are summarized in Table 4a, b. BBAR the Brain Bank for Aging Research, LB Lewy body, M mucosa, MM muscularis mucosa, SM submucosa, MP muscularis propria, ADV adventitia
Table 4.
Incidence of Lewy pathology in the esophageal wall (a) BBAR LB stage, (b) Braak LB stage
| Number of cases (Mean age at death) | M n (%) |
MM n (%) |
SM n (%) |
MP n (%) |
ADV n (%) |
|
|---|---|---|---|---|---|---|
| (a) BBAR LB stage | ||||||
| 0.5 | 29 (81.2, SD 9.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.4) |
| 1 | 83 (82.9, SD 10.1) | 0 (0) | 1 (1.2) | 3 (3.6) | 21 (25.3) | 16 (19.3) |
| 2 | 20 (83.8, SD 9.5) | 1 (5.0) | 2 (10.0) | 4 (20.0) | 13 (65.0) | 9 (45.0) |
| 3 | 8 (84.0, SD 6.8) | 1 (12.5) | 2 (25.0) | 2 (25.0) | 6 (75.0) | 5 (62.5) |
| 4 | 19 (85.2, SD 8.2) | 1 (5.3) | 4 (21.1) | 7 (36.8) | 17 (89.5) | 11 (57.9) |
| 5 | 19 (86.9, SD 7.6) | 5 (26.3) | 6 (31.6) | 10 (52.6) | 17 (89.5) | 17 (89.5) |
| Total | 178 | 8 (4.5) | 15 (8.4) | 26 (14.6) | 74 (41.6) | 59 (33.1) |
| (b) Braak LB stage | ||||||
| 0 | 12 (81.2, SD 12.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 23 (84.5, SD 7.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 14 (79.8, SD 10.9) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 1 (7.1) |
| 3 | 57 (82.6, SD 9.8) | 0 (0) | 1 (1.8) | 3 (5.3) | 16 (28.1) | 14 (24.6) |
| 4 | 17 (85.2, SD 10.2) | 1 (5.9) | 1 (5.9) | 2 (11.8) | 9 (52.9) | 8 (47.1) |
| 5 | 27 (83.4, SD 9.9) | 5 (18.5) | 5 (18.5) | 10 (37.0) | 21 (77.8) | 14 (51.9) |
| 6 | 28 (86.0, SD 6.1) | 2 (7.1) | 8 (28.6) | 11 (39.3) | 26 (92.9) | 22 (78.6) |
| Total | 178 | 8 (4.5) | 15 (8.4) | 26 (14.6) | 74 (41.6) | 59 (33.1) |
BBAR the Brain Bank for Aging Research, LB Lewy body, SD standard deviation, M mucosa, MM muscularis mucosa, SM submucosa, MP muscularis propria, ADV adventitia
Discussion
The present study demonstrates the following: (1) one-third of older people exhibited Lewy pathology in the CNS and/or PNS (178/518: 34%); and (2) the incidence of Lewy pathology in the esophagus was 43.8% in LBD (78/178): 92.1% in PDD (Parkinson disease with dementia)/DLB (35/38, BBAR LB stage 4–5), 75% in PD (Parkinson disease without dementia) (6/8, stage 3), 35% in preclinical/prodromal LBD (36/103, stage 1–2), 3.4% in the earliest stage of LBD (1/29, stage 0.5), and zero in controls (0/340, stage 0), which correlated with the progression of the BBAR LB stage.
Clinicopathological study based on autopsy-confirmed cohorts of older individuals
Since esophageal LBs were first reported in PD [68], more than 40 articles investigating the incidence of Lewy pathology of the human ENS have been published (summarized in Supplementary Table 4, online resource). The significance of this study is that our target is a geriatric cohort, including PDD/DLB, PD without dementia, preclinical/prodromal PD, and controls. This differs most of the previous research, which only analyzed patients with PD. Further, this study evaluated multiple locations in the PNS based on autopsy data from numerous older cohorts and was quality-controlled by board-certified general pathologists and a specialist in gastrointestinal systems. Brain Banks in Japan are based on general autopsies and have a stronger impact on the study of Lewy pathology than do specimens of surgical pathology because autopsy can both confirm the neuropathological diagnosis and reveal its distribution throughout the whole body.
Although it is not a population-based cohort, the BBAR is more generalizable to the public than research cohorts are. The pathological causes of death for the 518 BBAR individuals had incidences as follows: respiratory disease, 27% (140 cases); malignant neoplasm, 27% (138); cardiovascular disease, 19% (100); cerebrovascular disease, 6% (33). The clinical causes of death for older people in Japan from 2010 to 2017 had incidence percentages as follows: malignant neoplasm, 27–28%; cardiovascular disease, 16–17%; respiratory disease, 9–13%; cerebrovascular disease, 8–11%; senile decay, 4–8% (Portal Site of Official Statistics of Japan, https://www.e-stat.go.jp/). The proportions are similar to those of the BBAR, except that the BBAR had a higher percentage of respiratory disease because it was a hospital cohort, while the overall statistics for Japanese include senile decay. Furthermore, in Japan, more than 75% of people die in the hospital rather than in their own houses. For example, in 2017, among the 1.3 million people who died, 1.0 million died in the hospital. Thus, the Japanese hospital-based cohort might reflect the Japanese population.
The PNS in LBD
The way in which Lewy pathology propagates has been advanced by Braak and colleagues [11–13] as well as our research group [73, 76]. Two entries/pathways are hypothesized: (1) a brainstem-ascending pathway propagating from the ENS to the brainstem, limbic, and neocortex, and (2) an olfactory-amygdala pathway propagating from the olfactory epithelium and olfactory bulb to the amygdala [35]. Although Braak’s hypothesis proposed the ENS to be the entry zone of Lewy pathology [10], whether the gastrointestinal system is the origin of PD remains up for discussion [50]. Thus, we required further study.
In the current study, none of the cases exhibited Lewy pathology in the esophagus but not in the CNS. Our results support multiple foci of Lewy pathology in the PNS [4, 49] and reconfirmed its strong connection with the brain stem [10–13]. The incidence of Lewy pathology was higher in the brainstem (133/178: 74.7% in the dorsal motor nucleus of the vagus and locus coeruleus) and the olfactory bulb (146/174: 83.9%) than in the esophagus (43.8%). When the pathology existed in the brainstem, the positive rate for the esophagus was 58.6% (78/133). In the PNS, when the pathology was observed in the heart, adrenal gland, or skin, 15–37% lacked deposition in the esophagus. Therefore, we can say that the distribution of Lewy pathology throughout the PNS is varied. However, how sampling approaches to PNS tissues could influence on the positivity of Lewy pathology is recently discussed on in vivo skin biopsies [15, 32]. Previous studies described the rostral-caudal or proximal-distal gradient of the pathology [24, 25] and mismatch of positivity in a patient [39]. We did not perform serial sections of PNS tissues in this study. Thus, sampling bias might have some influence on the results.
Our results also show that Lewy pathology of the esophagus is a predictive factor for LBD. The incidence and severity of the esophageal pathology correlated with the BBAR LB stage: from 3.4% in the earliest stage (stage 0.5, 1/29) to 94.7% in the latest stage (stage 5; PDD/DLB, neocortical form, 18/19). We suspect that Lewy pathology of the esophagogastric junction (EGJ) could contribute to gastroesophageal reflux. Thus, our results partially explain the frequent complications of aspiration pneumonia in the late stage of LBD. We also show that the incidence of Lewy pathology in the autonomic nerves of the anterior wall of the heart decreased with progression from stage 4 (94.7%, 18/19) to 5 (84.2%, 16/19). We speculate that this could be related to the decrease in nerve fibers as reported previously [27, 62, 63]. However, the decrease might only reflect the small numbers of high-stage groups. The autonomic ganglions, including the segment innervating the heart, show 100% positivity after stage 2. In the esophagus, the myenteric plexus has been reported not to show loss or degeneration of ganglion cells in PD [2, 68, 90]. Further systemic analyses are required.
Lewy pathology of the esophagus was influenced by the type of LBD as described in the 4th DLB Consensus Guidelines. It was high in diffuse neocortical LBD (44/49, 89.7%) and low in amygdala-predominant LBD (1/29, 3.4%). The amygdala variant is thought to be secondary to AD [67, 69], and we use the term “olfactory-amygdala” because this type usually involves olfaction. AD with LB exhibits rare α-synuclein deposition in the ENS [4, 31, 33]. However, our study showed a case with Lewy pathology in the esophagus and one AD case with amygdala-predominant LBD and α-synuclein deposition in sympathetic ganglia. A few neuritic structures were observed via α-synuclein immunohistochemistry, although the amount of staining was very small or faint compared with the diffuse DLB cases. Because AD does not involve the PNS, further research may be required to determine whether or not the olfactory-amygdala variant is secondary to AD.
Limitation of this study
This study has some limitations. (1) The examination of the entire ENS was practically impossible. (2) Each type of PNS tissue, including the esophagus, was evaluated using single slides. However, questionable cases were reexamined by other anti-phosphorylated and non-phosphorylated α-synuclein antibodies. (3) Autonomic symptoms were retrospectively studied in medical charts and not prospectively.
Conclusion
We found that one-third of older individuals exhibited Lewy pathology, 43.8% of whom in the esophagus. Further, among the different regions of the PNS positive for the pathology, the highest correlation with disease progression (BBAR LB stage) was observed in the esophagus. Therefore, this study provides a pathological basis for the development of digestive dysfunctions in LBD and suggests that esophageal Lewy pathology is a predictive factor of LBD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. T. Iwatsubo (Department of Neuropathology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan), Dr. M. Hasegawa (Department of Dementia and Higher Brain Function, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), and Dr. H. Akiyama (Yokohama Brain and Spine Center, Kanagawa, Japan) for the antibodies. We also thank Ms. Mieko Harada, Ms. Nobuko Naoi, Ms. Sachiko Imai, Ms. Kyoko Okamoto, Ms. Shiho Ushiki, and Mr. Yutaka Koga for their technical support, as well as Dr. Kinuko Suzuki and Dr. Maho Morishima for fruitful discussion. We also thank Adam Phillips, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Z.T., Y.S., S.I., T.M., A.M., M.Y., Y.S., I.K., M.I., S.T., R.S., T.A., and S.M. The first draft of the manuscript was written by Z.T. and revised by Z.T., Y.S., S.T., R.S., and S.M., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number JP 16H06277 (Y.S. and S.M.), JP 16K09736 (R.S.), AMED under Grant Number JP18dm0107103 (Y.S. and S.M.), Intramural Research Grant (30-8) for Neurological and Psychiatric Disorders of NCNP (Y.S. and S.M.), and Geriatric Sciences from NCGG (S.M.).
Compliance with ethical standards
Conflict of interest
The authors declare that they had no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the institutional ethics review committee of the Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology.
Informed consent
Informed consent was obtained from all individual participants or their relatives.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124:665–680. doi: 10.1007/s00401-012-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 4.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach TG, Corbille AG, Letournel F, Kordower JH, Kremer T, Munoz DG, et al. Multicenter assessment of immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson’s disease and control subjects. J Parkinsons Dis. 2016;6:761–770. doi: 10.3233/jpd-160888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach TG, White CL, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009;117:169–174. doi: 10.1007/s00401-008-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/bf00308809. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 15.Chahine LM, Beach TG, Brumm MC, Adler CH, Coffey CS, Mosovsky S, et al. In vivo distribution of alpha-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology. 2020;95:e1267–e1284. doi: 10.1212/WNL.0000000000010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson’s disease by olfactory bulb pathology. Lancet. 1992;340:186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- 17.Del Tredici K, Duda JE. Peripheral Lewy body pathology in Parkinson’s disease and incidental Lewy body disease: four cases. J Neurol Sci. 2011;310:100–106. doi: 10.1016/j.jns.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 19.Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 20.Jager WDH, Bethlem J. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry. 1960;23:283–290. doi: 10.1136/jnnp.23.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S1–5. doi: 10.1016/S1353-8020(09)70769-2. [DOI] [PubMed] [Google Scholar]
- 22.Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, et al. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology. 2014;82:1362–1369. doi: 10.1212/wnl.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 23.Donadio V, Incensi A, Rizzo G, Capellari S, Pantieri R, Stanzani Maserati M, et al. A new potential biomarker for dementia with Lewy bodies: skin nerve alpha-synuclein deposits. Neurology. 2017;89:318–326. doi: 10.1212/wnl.0000000000004146. [DOI] [PubMed] [Google Scholar]
- 24.Donadio V, Incensi A, Rizzo G, Scaglione C, Capellari S, Fileccia E, et al. Spine topographical distribution of skin α-synuclein deposits in idiopathic Parkinson disease. J Neuropathol Exp Neurol. 2017;76:384–389. doi: 10.1093/jnen/nlx021. [DOI] [PubMed] [Google Scholar]
- 25.Doppler K, Ebert S, Uceyler N, Trenkwalder C, Ebentheuer J, Volkmann J, et al. Cutaneous neuropathy in Parkinson’s disease: a window into brain pathology. Acta Neuropathol. 2014;128:99–109. doi: 10.1007/s00401-014-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forno LS, Norville RL. Ultrastructure of Lewy bodies in the stellate ganglion. Acta Neuropathol. 1976;34:183–197. doi: 10.1007/bf00688674. [DOI] [PubMed] [Google Scholar]
- 27.Fujishiro H, Frigerio R, Burnett M, Klos KJ, Josephs KA, Delledonne A, et al. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord. 2008;23:1085–1092. doi: 10.1002/mds.21989. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 29.Fumimura Y, Ikemura M, Saito Y, Sengoku R, Kanemaru K, Sawabe M, et al. Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in lewy body disease. J Neuropathol Exp Neurol. 2007;66:354–362. doi: 10.1097/nen.0b013e3180517454. [DOI] [PubMed] [Google Scholar]
- 30.Funabe S, Takao M, Saito Y, Hatsuta H, Sugiyama M, Ito S, et al. Neuropathologic analysis of Lewy-related alpha-synucleinopathy in olfactory mucosa. Neuropathology. 2013;33:47–58. doi: 10.1111/j.1440-1789.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- 31.Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014;29:1010–1018. doi: 10.1002/mds.25776. [DOI] [PubMed] [Google Scholar]
- 32.Gibbons CH, Wang N, Kim JY, Campagnolo M, Freeman R. Skin biopsy in evaluation of autonomic disorders. Continuum (Minneap Minn) 2020;26:200–212. doi: 10.1212/con.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 33.Gold A, Turkalp ZT, Munoz DG. Enteric alpha-synuclein expression is increased in Parkinson’s disease but not Alzheimer’s disease. Mov Disord. 2013;28:237–240. doi: 10.1002/mds.25298. [DOI] [PubMed] [Google Scholar]
- 34.Goldman JG, Postuma R. Premotor and nonmotor features of Parkinson’s disease. Curr Opin Neurol. 2014;27:434–441. doi: 10.1097/wco.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hishikawa N, Hashizume Y, Yoshida M, Sobue G. Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol. 2003;105:341–350. doi: 10.1007/s00401-002-0651-4. [DOI] [PubMed] [Google Scholar]
- 37.Homma T, Mochizuki Y, Mizutani T. Phosphorylated α-synuclein immunoreactivity in the posterior pituitary lobe. Neuropathology. 2012;32:385–389. doi: 10.1111/j.1440-1789.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 38.Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat. 2007;211:117–124. doi: 10.1111/j.1469-7580.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikemura M, Saito Y, Sengoku R, Sakiyama Y, Hatsuta H, Kanemaru K, et al. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67:945–953. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- 40.Ito S, Takao M, Hatsuta H, Kanemaru K, Arai T, Saito Y, et al. Alpha-synuclein immunohistochemistry of gastrointestinal and biliary surgical specimens for diagnosis of Lewy body disease. Int J Clin Exp Pathol. 2014;7:1714–1723. [PMC free article] [PubMed] [Google Scholar]
- 41.Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, et al. Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology. 1999;52:1269–1271. doi: 10.1212/wnl.52.6.1269. [DOI] [PubMed] [Google Scholar]
- 42.Jellinger KA. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm (Vienna) 2004;111:1219–1235. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- 43.Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nat Rev Neurol. 2015;11:625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- 44.Kon T, Tomiyama M, Wakabayashi K. Neuropathology of Lewy body disease: clinicopathological crosstalk between typical and atypical cases. Neuropathology. 2020;40:30–39. doi: 10.1111/neup.12597. [DOI] [PubMed] [Google Scholar]
- 45.Kosaka K. A clinicopathological study of Lewy body disease. Psychiatr Neurol Japan. 1980;83:292–311. [PubMed] [Google Scholar]
- 46.Kosaka K. Lewy body disease and dementia with Lewy bodies. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90:301–306. doi: 10.2183/pjab.90.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree—a new disease? Clin Neuropathol. 1984;3:185–192. [PubMed] [Google Scholar]
- 48.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253–1255. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 49.Leclair-Visonneau L, Magy L, Volteau C, Clairembault T, Le Dily S, Preterre C, et al. Heterogeneous pattern of autonomic dysfunction in Parkinson’s disease. J Neurol. 2018;265:933–941. doi: 10.1007/s00415-018-8789-8. [DOI] [PubMed] [Google Scholar]
- 50.Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH, et al. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018;135:1–12. doi: 10.1007/s00401-017-1777-8. [DOI] [PubMed] [Google Scholar]
- 51.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/wnl.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 53.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 54.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 56.Mitsui J, Saito Y, Momose T, Shimizu J, Arai N, Shibahara J, et al. Pathology of the sympathetic nervous system corresponding to the decreased cardiac uptake in 123I-metaiodobenzylguanidine (MIBG) scintigraphy in a patient with Parkinson disease. J Neurol Sci. 2006;243:101–104. doi: 10.1016/j.jns.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, et al. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013;72:614–623. doi: 10.1097/NEN.0b013e3182965886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarro-Otano J, Casanova-Molla J, Morales M, Valls-Sole J, Tolosa E. Cutaneous autonomic denervation in Parkinson’s disease. J Neural Transm (Vienna) 2015;122:1149–1155. doi: 10.1007/s00702-014-1355-3. [DOI] [PubMed] [Google Scholar]
- 59.Navarro-Otano J, Gelpi E, Mestres CA, Quintana E, Rauek S, Ribalta T, et al. Alpha-synuclein aggregates in epicardial fat tissue in living subjects without parkinsonism. Parkinsonism Relat Disord. 2013;19:27–31. doi: 10.1016/j.parkreldis.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orimo S, Ghebremedhin E, Gelpi E. Peripheral and central autonomic nervous system: does the sympathetic or parasympathetic nervous system bear the brunt of the pathology during the course of sporadic PD? Cell Tissue Res. 2018;373:267–286. doi: 10.1007/s00441-018-2851-9. [DOI] [PubMed] [Google Scholar]
- 62.Orimo S, Takahashi A, Uchihara T, Mori F, Kakita A, Wakabayashi K, et al. Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol. 2007;17:24–30. doi: 10.1111/j.1750-3639.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131:642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 64.Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson’s disease. Mov Disord. 1995;10:283–287. doi: 10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- 65.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 66.Pont-Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study) Mov Disord. 2015;30:229–237. doi: 10.1002/mds.26077. [DOI] [PubMed] [Google Scholar]
- 67.Popescu A, Lippa CF, Lee VM, Trojanowski JQ. Lewy bodies in the amygdala: increase of alpha-synuclein aggregates in neurodegenerative diseases with tau-based inclusions. Arch Neurol. 2004;61:1915–1919. doi: 10.1001/archneur.61.12.1915. [DOI] [PubMed] [Google Scholar]
- 68.Qualman SJ, Haupt HM, Yang P, Hamilton SR. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87:848–856. doi: 10.1016/0016-5085(84)90079-9. [DOI] [PubMed] [Google Scholar]
- 69.Raunio A, Kaivola K, Tuimala J, Kero M, Oinas M, Polvikoski T, et al. Lewy-related pathology exhibits two anatomically and genetically distinct progression patterns: a population-based study of Finns aged 85. Acta Neuropathol. 2019;138:771–782. doi: 10.1007/s00401-019-02071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- 71.Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, et al. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–749. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 72.Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, et al. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol. 2004;63:911–918. doi: 10.1093/jnen/63.9.911. [DOI] [PubMed] [Google Scholar]
- 73.Saito Y, Shioya A, Sano T, Sumikura H, Murata M, Murayama S. Lewy body pathology involves the olfactory cells in Parkinson’s disease and related disorders. Mov Disord. 2016;31:135–138. doi: 10.1002/mds.26463. [DOI] [PubMed] [Google Scholar]
- 74.Sanchez-Ferro A, Rabano A, Catalan MJ, Rodriguez-Valcarcel FC, Fernandez Diez S, Herreros-Rodriguez J, et al. In vivo gastric detection of alpha-synuclein inclusions in Parkinson’s disease. Mov Disord. 2015;30:517–524. doi: 10.1002/mds.25988. [DOI] [PubMed] [Google Scholar]
- 75.Savica R, Boeve BF, Mielke MM. When do alpha-synucleinopathies start? An epidemiological timeline: a review. JAMA Neurol. 2018;75:503–509. doi: 10.1001/jamaneurol.2017.4243. [DOI] [PubMed] [Google Scholar]
- 76.Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, et al. Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol. 2008;67:1072–1083. doi: 10.1097/NEN.0b013e31818b4126. [DOI] [PubMed] [Google Scholar]
- 77.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 78.Shishido T, Ikemura M, Obi T, Yamazaki K, Terada T, Sugiura A, et al. alpha-Synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology. 2010;74:608–610. doi: 10.1212/WNL.0b013e3181cff6d5. [DOI] [PubMed] [Google Scholar]
- 79.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 80.Sprenger FS, Stefanova N, Gelpi E, Seppi K, Navarro-Otano J, Offner F, et al. Enteric nervous system alpha-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology. 2015;85:1761–1768. doi: 10.1212/wnl.0000000000002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79:940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 82.Sumikura H, Takao M, Hatsuta H, Ito S, Nakano Y, Uchino A, et al. Distribution of alpha-synuclein in the spinal cord and dorsal root ganglia in an autopsy cohort of elderly persons. Acta Neuropathol Commun. 2015;3:57. doi: 10.1186/s40478-015-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeda S, Yamazaki K, Miyakawa T, Arai H. Parkinson’s disease with involvement of the parasympathetic ganglia. Acta Neuropathol. 1993;86:397–398. doi: 10.1007/bf00369454. [DOI] [PubMed] [Google Scholar]
- 84.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 85.Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, et al. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun. 2015;3:35. doi: 10.1186/s40478-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wakabayashi K, Miki Y. Multi-organ distribution of alpha-synuclein pathology in dementia with Lewy bodies. Brain Nerve. 2018;70:489–500. doi: 10.11477/mf.1416201031. [DOI] [PubMed] [Google Scholar]
- 87.Wakabayashi K, Mori F, Tanji K, Orimo S, Takahashi H. Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol. 2010;120:1–12. doi: 10.1007/s00401-010-0706-x. [DOI] [PubMed] [Google Scholar]
- 88.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 89.Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F. Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol. 1993;60:609–612. [PubMed] [Google Scholar]
- 90.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/bf00687767. [DOI] [PubMed] [Google Scholar]
- 91.Wang N, Gibbons CH, Lafo J, Freeman R. alpha-Synuclein in cutaneous autonomic nerves. Neurology. 2013;81:1604–1610. doi: 10.1212/WNL.0b013e3182a9f449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.