Abstract
Attention prioritizes stimuli previously associated with reward or punishment. The present study examined whether this attentional bias, widely considered to be involuntary and automatic, could be suppressed with sufficient motivation. Participants performed visual search for a shape-defined target. One color-singleton distractor predicted the possibility of receiving a reward and another an electric shock, with each outcome occurring infrequently. Participants were informed that the likelihood to earn a reward or avert punishment depended on fast and accurate performance, thus providing strong motivation to resist distraction by reward-and shock-related stimuli. Results revealed a reduction in the magnitude of attentional capture by reward- and threat-associated distractors, relative to neutral distractors, that persisted into extinction. In a second experiment, we replicated the suppression of value-modulated attentional capture in the absence of the shock condition, thus confirming that the suppression did not result from the presence of threat. Finally, in a third experiment, we replicated the typical pattern of attentional capture by reward cues using a more conventional procedure in which the motivation to suppress valent stimuli was low (the likelihood to be rewarded was high and not contingent on fast performance). This study demonstrates that signals for reward and threat can be actively suppressed with sufficient motivation.
Keywords: associative learning, attentional capture, reward, signal suppression, threat
Attention determines which aspects of sensory input are selected for further analysis. Traditional accounts (e.g., Corbetta & Shulman, 2002; Yantis, 2000) differentiate between voluntary attention guided by contextually appropriate goals and intentions (goal-directed attention) and involuntary attention captured by physically salient stimuli (stimulus-driven attention). However, recent research has evidenced a new facet of attention, labeled selection history, that is independent of current goals or perceptual salience (Awh, Belopolsky, & Theeuwes, 2012; Failing & Theeuwes, 2018). Selection history refers to the tendency to prioritize information that has previously been attended in a given context. Convincingly illustrating this bias, numerous studies have revealed that attention can be influenced by prior experience of the relationship between stimuli and motivationally significant outcomes: reward and punishment (see Watson, Pearson, Wiers, & Le Pelley, 2019, for a review).
Attentional biases toward reward-related stimuli are typically observed in visual search tasks. For example, when participants are given monetary rewards for orienting to color-defined targets during a training phase, the color associated with high reward impairs visual search performance (Anderson & Halpern, 2017; Anderson, Laurent, & Yantis, 2011, 2014) and draws more frequent eye movements (Anderson & Kim, 2019a, 2019b; Anderson & Yantis, 2012; Theeuwes & Belopolsky, 2012) when presented as a task-irrelevant distractor in a subsequent test phase. This value-driven attentional prioritization persists durably without further learning of the stimulus-reward association (Anderson et al., 2011, Experiment 3; Anderson & Yantis, 2013). Reward-related stimuli can also grab attention in situations where looking at these stimuli is explicitly counterproductive (Le Pelley, Pearson, Griffiths, & Beesley, 2015; Pearson, Donkin, Tran, Most, & Le Pelley, 2015), suggesting that the attentional priority afforded to reward cues is irrepressible and automatic (Anderson, 2016a).
Threat-related stimuli produce relatively similar effects to reward-related stimuli in visual search tasks. For instance, when presented as a distractor, a stimulus (e.g., a blue diamond) previously conditioned with aversive electrical shock impairs performance compared with a neutral stimulus (e.g., an orange diamond never associated with shock), independently of perceptual salience (Schmidt, Belopolsky, & Theeuwes, 2015a). Distraction by threat-associated cues was also observed after conditioning with white noise (e.g., Koster, Crombez, Van Damme, Verschuere, & De Houwer, 2004; Smith, Most, Newsome, & Zald, 2006), monetary loss (e.g., Wentura, Müller, & Rothermund, 2014), or negative social feedback (Anderson, 2017a; Anderson & Kim, 2018). Furthermore, oculomotor capture by threat-related stimuli was demonstrated in eye-tracking studies (Mulckhuyse & Dalmaijer, 2016; Schmidt, Belopolsky, & Theeuwes, 2015b). Nissens, Failing, and Theeuwes (2017) reported that threat-modulated attentional capture occurred even though fixating threat-related cues increased the probability of receiving punishment (see also Anderson & Britton, 2020). Consequently, stimuli previously associated with reward or punishment alter search performance in a comparable manner, acting as powerful attractors of attention, possibly in an automatic way (Anderson, 2016a; Watson et al., 2019).
Value- and threat-modulated attentional capture seem both resistant to extinction and relatively immune to suppression. Using a variant of the additional singleton paradigm (Theeuwes, 1992) devised by Gaspelin, Leonard, and Luck (2015, 2017), Pearson, Watson, Cheng, and Le Pelley (2020) recently revealed that value-modulated attentional capture was evident even under conditions that foster suppression of attentional capture via top-down control. Although physically salient distractors associated with low value were suppressed when searching for a specific target feature (i.e., feature-search mode, Bacon & Egeth, 1994), replicating the signal suppression evident in Gaspelin et al. (2015, 2017), attentional capture by high-value distractors was not affected by feature-search mode and remained robust (Pearson et al., 2020). A similar failure to suppress attention to physically salient reward cues when suppression of salient signals normally occurs has been observed in the context of spatially focused attention (Munneke, Belopolsky, & Theeuwes, 2016; Munneke, Hoppenbrouwers, & Theeuwes, 2015) and center-surround inhibition (Wang, Duan, Theeuwes, & Zhou, 2014; Wang et al., 2015). In the domain of response selection, other studies have produced comparable results, demonstrating a failure to inhibit responses evoked by previously high-value stimuli when inhibition is otherwise evident for value-neutral or low-value stimuli (e.g., Anderson, Folk, Garrison, & Rogers, 2016; Kim & Anderson, 2019). These findings suggest that stimuli associated with high reward are particularly resistant to suppression. Although some situations have been identified in which reward-related stimuli fail to capture attention (e.g., Feldmann-Wüstefeld, Brandhofer, & Schubö, 2016; Wang, Yu, & Zhou, 2013), to our knowledge, there have been no demonstrations of reduced interference by a reward-associated or punishment-associated stimulus compared with a neutral distractor consistent with active signal suppression (although see Moher, Anderson, & Song, 2015, for reduced interference in motor behavior in a goal-directed reaching task, which was evidence for both physically salient and high-value stimuli).
The question of whether value- and threat-modulated attentional biases can be suppressed has important implications for how we characterize their adaptiveness. On the one hand, these biases can be thought of as subserving a cognitive function that is sufficiently important for survival that they are implemented in a manner that is fully automatic and robust to the modulatory influence of task goals and contingencies. That is, the cost of orienting is sufficiently small and the cost of failing to orient and recognize a threat or opportunity to obtain a reward sufficiently great that the orienting response triggered by such stimuli is essentially obligatory. Although this issue is seldom addressed explicitly, both early (Anderson, 2013) and subsequent accounts of value-driven attention (Anderson, 2016a, 2017b, 2018, 2019) tend to favor a position along these lines, consistent with the evidence described in the preceding paragraph. On the other hand, value- and threat-modulated attentional biases can be considered as an adaptive response to the task contingencies under which they are typically studied, and only automatic under conditions that are sufficiently favorable to them. That is, organisms monitor for reward- and threat-signals by default when the cost of doing so is low, reflecting a bias toward information-seeking (Gottlieb & Oudeyer, 2018), but are able to resist and perhaps even leverage such associations to facilitate improved ignoring when the benefit of ignoring is high (in terms of the ability to obtain reward or the ability to escape punishment—the same drivers of the bias itself). By this latter formulation, reward and punishment can also come to drive an ignoring bias when the amount of reward or ability to escape punishment is sufficiently reinforcing to itself shape perceptual processing.
The current study examined whether value- and threat-modulated attentional capture could be suppressed with sufficient motivation in a visual search task using eye tracking. The experiment design was modeled after studies probing value-modulated (e.g., Le Pelley et al., 2015) and threat-modulated (e.g., Nissens et al., 2017) attentional capture by physically salient stimuli in which the valent stimuli always appear as task-irrelevant distractors. Participants first performed a training phase which involved fixating a shape-defined target. One color-singleton distractor predicted the possibility of receiving a reward and another an electric shock, each outcome occurring with a reinforcement ratio of 33%. The likelihood of earning a reward or averting a punishment was directly related to the speed with which the target was fixated in the presence of the corresponding distractor, thus providing strong motivation to resist distraction by reward- and shock-related stimuli. For reward, the reinforcement ratio was substantially lower than in previous studies of value-modulated attentional capture (in which rewards are delivered on the vast majority of trials), using an otherwise similar paradigm (e.g., Le Pelley et al., 2015; Pearson et al., 2015; Wang, Li, Zhou, & Theeuwes, 2018), and the frequency of withholding punishment was also somewhat lower than in prior work using this type of paradigm (e.g., Anderson & Britton, 2020; Nissens et al., 2017). Participants were informed of the general relationship between performance and outcomes but were not informed of any relationship between color and outcomes, which needed to be learned from experience in the task.
In a subsequent test phase, a similar task was carried out with no reward or shock to evaluate the generalization of learning. Attentional capture by signals of reward and threat would be indicated by an increased frequency of fixations on such stimuli and an increase in time to fixate the target, relative to an equally salient distractor never paired with either outcome (neutral distractor), whereas learned suppression of signals associated with reward and punishment would predict the opposite. The inclusion of a neutral distractor condition is important because it allows for comparisons in which the physical salience of the distractors and its influence on attention is equated. We also added a two-distractor condition in the test phase, which included one color associated with reward and another associated with shock, to assess whether signals for punishment are prioritized over signals for reward, as might be predicted from a prior attentional capture study (Wang et al., 2013), greater sensitivity to loss (Tom, Fox, Trepel, & Poldrack, 2007; Tversky & Kahneman, 1992), and the particular relevance of signals related to fear and pain for survival (Öhman & Mineka, 2001).
Experiment 1
Method
Participants.
Forty participants, between the ages of 18 and 35 inclusive, were recruited from the Texas A&M University community. All participants were English-speaking and reported normal or corrected-to-normal visual acuity and normal color vision. Data from two participants were removed because of an inability to reliably track eye position (resulting in a failure to register a target fixation on over 20% of trials), leading to a final sample of 38 participants (24 females), with a mean age of 21.05 years (SD = 3.33). All procedures were approved by the Texas A&M University Institutional Review Board and were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant. The sample size was based on two previous publications employing a paradigm analogous to the one used in the present study. The power analysis thus considered the power to detect value-driven attentional capture and the power to detect threat-driven attentional capture. Anderson and Kim (2019b) reported an effect size of dz = 0.55 for attentional capture by a high-value distractor. Schmidt et al. (2015a) also reported an effect size of dz = 0.55 for attentional capture by a threat-related distractor. At α = .05, a sample size of at least 28 participants would provide β > 0.80 to detect each of the two effects (computed using G*Power 3.1).
Apparatus.
A Dell OptiPlex 7040 (Dell, Round Rock, TX) equipped with Matlab software (Mathworks, Natick, MA) and Psychophysics Toolbox extensions (Brainard, 1997) was used to present the stimuli on a Dell P2717H monitor. The participants viewed the monitor from a distance of approximately 70 cm in a dimly lit room. Paired electrodes (EL500, BioPac Systems, Inc., Goleta, CA) were attached to the left forearm of each participant, and 2-ms mild electric shocks were delivered through an isolated linear stimulator under the constant current setting (STMISOLA, BioPac Systems), which was controlled by custom Matlab scripts. Eye-tracking was conducted using the EyeLink 1000 Plus system (SR Research Ltd., Ottawa, Ontario, Canada), and head position was maintained using a manufacturer-provided chin rest (SR Research Ltd.).
Stimuli.
In the training phase, each trial started with a gaze-contingent fixation display followed by a search array and a 1,000-ms blank intertrial interval (see Figure 1). The fixation display consisted of a cross (0.8° × 0.8° visual angle) at the center of the screen. The search array comprised six filled shapes: five circles and one diamond or five diamonds and one circle. Each circle had a 4.5° visual angle diameter and each diamond was 4.1° visual angle in width and 3.7° visual angle in height. The six shapes’ centers were equally distributed on an imaginary circle (10.2° radius) around the center of the screen. The unique shape was defined as the target (and so, the distractors corresponded to the nontarget shapes). On distractor-present trials, one of the distractors was rendered in red (CIE: u’ = 0.420, v’ = 0.530, cd/m2 = 44.2), green (CIE: u’ = 0.137, v’ = 0.565, cd/m2 = 44.2) or blue (CIE: u’ = 0.152, v’ = 0.306, cd/m2 = 44.2) whereas the rest were gray (CIE: u’ = 0.201, v’ = 0.460, cd/m2 = 44.2). On distractor-absent trials,1 all six shapes were gray. All shape stimuli were equiluminant. We used color singleton distractors and shape-singleton targets, rather than vice versa, both with respect to prior precedent (Anderson & Britton, 2020; Le Pelley et al., 2015; Nissens et al., 2017; Pearson et al., 2015; Wang et al., 2018) and to ensure robust processing of distractors (Theeuwes, 1992; see also Wang et al., 2013), which may be important for both the learning of the task contingencies and creating a situation in which suppression of the distractors would be beneficial. All possible combinations between the position of the target and each colored distractor were presented an equal number of times. In distractor-absent trials, the target was also presented in all positions of the search array an equal number of times. When participants obtained a reward, a feedback display was presented consisting of the amount of monetary reward earned on the current trial (+15¢) and the total reward accumulated across all trials. If the target was not fixated within the timeout limit (1000 ms), the word “miss” appeared at the center of the screen.
Figure 1.
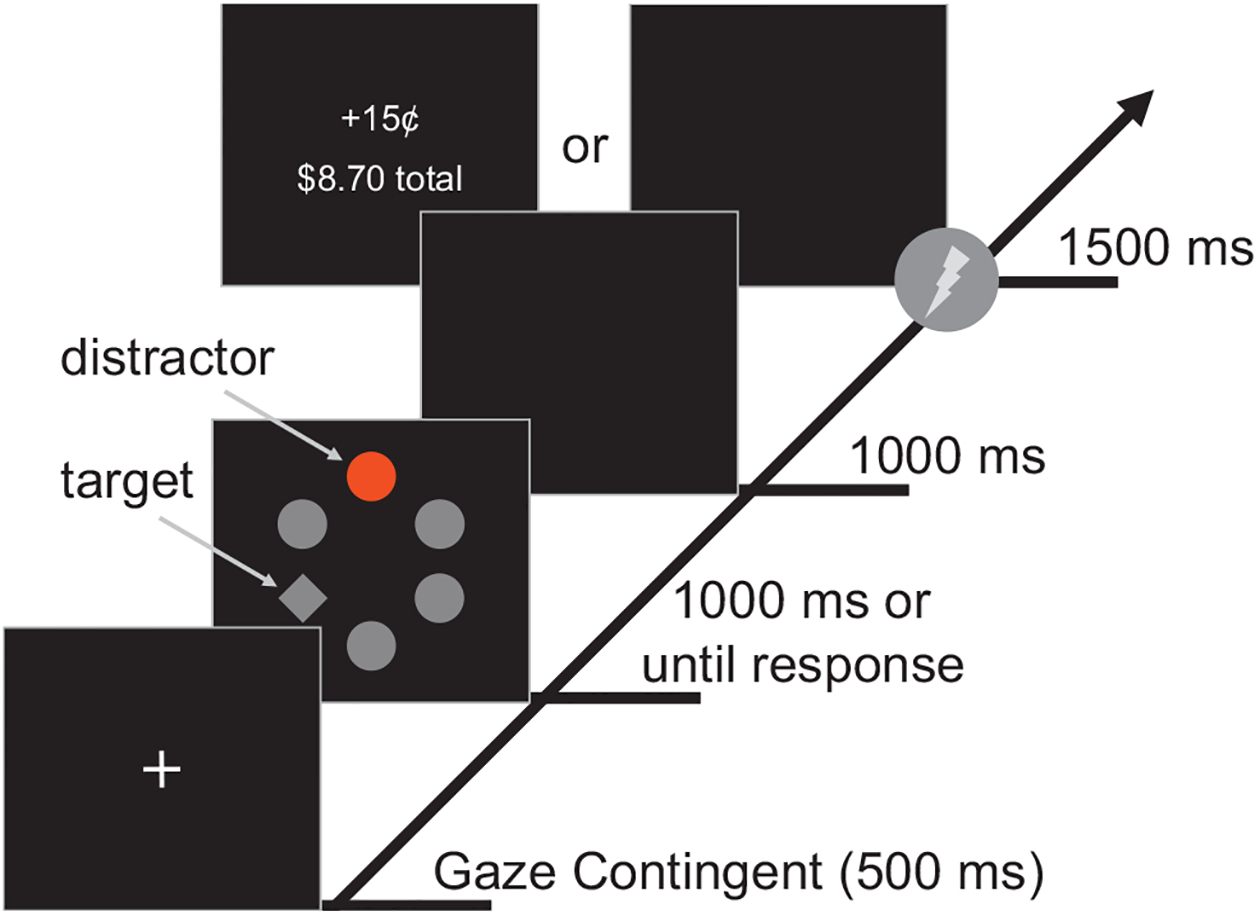
Sequence of trial events in training and test phases of Experiments 1 and 2 (no reward or shock was delivered during the test phases). Each trial began with the presentation of a fixation cross at the center of the screen. After the participant fixated the cross for 500 ms, the search display was presented for 1,000 ms or until the participant fixated the unique shape (i.e., the target) for 100 ms. A 1,000-ms blank screen followed the search display. When a reward (Experiments 1 and 2) or a shock (Experiment 1) was delivered during the training phase, a 1,500-ms delay was added before the start of the next trial. The shock was administered 1,000 ms after the search display in 33% of the threat-related trials. Correct responses were followed by the delivery of monetary reward feedback in 33% of the reward-related trials. Note that the distractor color associated with the reward was different from the distractor color associated with the shock. See the online article for the color version of this figure.
In the test phase, stimuli were similar to those of the training phase, with one additional search array including two colored distractors (each one in a different color) in red, blue or green. The two colors were consistent throughout the test phase. The two colored distractors were never presented side-by-side leading to 72 possible search arrays for each target shape (i.e., 144 possible search arrays in total). No reward or shock was delivered during the test phase.
Throughout the experiment, the background of the screen was black while the fixation cross and feedback appeared in white. Written information was presented in 40-point Arial font.
Design.
The training phase was split into five runs, with each run consisting of 120 trials (600 trials in total). Each run comprised 36 reward-related trials, 36 threat-related trials, 36 neutral trials and 12 distractor-absent trials, presented in a random order. In each run, a monetary reward (15¢) was delivered in 33% of the reward-related trials and a shock was administered in 33% of the threat-related trials. No reward or shock was delivered in the neutral or distractor-absent trials. Each distractor-present condition (i.e., reward, shock and neutral) was related to a specific color (red, green or blue). Color-condition associations were counterbalanced across participants. In each condition, the target and the colored distractor appeared in each stimulus position equally often. Furthermore, in each condition, the target was equally often a diamond among circles and a circle among diamonds.
The test phase was split into two runs, with each run consisting of 120 trials (240 total trials). Each run comprised 36 trials with two colored distractors, 24 reward-related trials, 24 threat-related trials, 24 neutral trials, and 12 distractor-absent trials, presented in a random order. In the two-distractor condition, one color corresponded to the reward-related color in the training phase and one color corresponded to the threat-related color. All the 72 possible search arrays relative to the position of the target and distractors were presented (the two colored distractors were never side-by-side), but the shape target was pseudorandom, with the same number of circle and diamond targets for each participant. For the reward, shock and neutral conditions, the target and the distractor appeared four times in each stimulus position for each target shape. In the distractor-absent condition, the target appeared once in each stimulus position for each target shape. The order of trials in each run of each phase was randomized.
Procedure.
Prior to the experiment, the participant was connected to the isolated linear stimulator and a shock calibration procedure was conducted to achieve a level that was “unpleasant, but not painful” (Grégoire & Greening, 2019, 2020; Murty, LaBar, & Adcock, 2012; Schmidt et al., 2015a; Schmidt, Belopolsky, & Theeuwes, 2017). A practice phase (with no reward and no shock) comprising 10 trials with no time limit followed by 34 trials with a time limit was then performed before the training phase.
Each trial began with the presentation of a fixation cross that remained on screen until eye position was registered within 1.1° of the center of the cross for a continuous period of 500 ms. Then, the search array was displayed for 1,000 ms or until the target was fixated. A 1000-ms blank screen followed the search display. When a reward or a shock was delivered, a 1,500-ms blank screen (for shock) or reward feedback display (for reward) appeared before the start of the next trial. The shock was administered 1,000 ms after the search display (at the same time that the 1,500-ms blank screen appeared). If the target was not fixated within the timeout limit (1,000 ms), a 1,000-ms feedback display (“miss”) was added in the sequence of trial events just after the search display. Participants were required to look at the unique shape as fast and accurately as possible. Before beginning the training phase, participants were also informed that they were more likely to be rewarded if their response was fast and accurate and that they were more likely to be shocked if their response was slow. Note that it was possible to fixate nontarget stimuli before fixating the target within the timeout limit and still receive the reward (on reward-related trials) or avoid the shock (on threat-related trials), although the reinforcement contingencies used made this unlikely.
Participants received a shock if they responded too slowly (i.e., above the latency limit for shock) or missed the target on the threat-related trials, with a limit of 12 shocks per run. The latency limit for shock was based on the 66th percentile of all the correct response times (i.e., times taken to fixate the target for 100 ms) of the threat-related trials from the previous run (or the 34 trials with a time limit from the practice phase for the first run). Participants received a reward if they fixated the target below the latency limit for reward (i.e., the 33rd percentile of all the correct response times of the reward-related trials from the previous run) on the reward-related trials, with a limit of 12 rewards per run. To ensure that the number of rewards and shocks delivered in each run was comparable, 12 trials were preconfigured to produce a shock (on the threat-related trials) regardless of speed or accuracy, and 12 trials were preconfigured to deliver a reward (on the reward-related trials) as long as participants fixated the target within the timeout limit. The first five threat- and reward-related trials were not preconfigured to deliver a shock or a reward, respectively. If the participant received a shock on a nonpreconfigured trial before the first preconfigured trial, then the first preconfigured trial became a regular (threat-related) trial. The same logic was applied for all the following preconfigured trials. An analogous rationale was employed in the reward condition. Furthermore, if the participant failed to fixate the target within the timeout limit on a preconfigured reward-related trial, the next nonpreconfigured reward-related trial became a preconfigured trial. The last reward-related trial of a run was never preconfigured in advance (before the beginning of a run). Thus, if the participant was too slow and did not receive the reward on the penultimate reward-related trial of a run, when this trial was preconfigured, the last reward-related trial became preconfigured. If the participant missed again the reward on this trial, the first reward-related trial of the following run became a preconfigured trial (but this situation never occurred; all the participants received 12 rewards per run). Materials for this experiment can be found at https://osf.io/bc3qf/.
In the test phase, the sequence of trial events was similar to the training phase but no reward or shock was delivered. Participants were informed about this change before beginning the test phase. However, shock-electrodes were kept attached until the end of the experiment to not eliminate the physical possibility to receive electrical stimulations. This manipulation aimed to prevent that the absence of predictability of shock affects reactions to threat-related stimuli (Sevenster, Beckers, & Kindt, 2012). Again, participants were instructed to look at the unique shape as fast and accurately as possible.
After the test phase, participants provided self-report evaluations of their contingency awareness about the relationship between each distractor-present condition of the training phase (i.e., reward, shock and neutral) and each of the two following consequences: reward and shock. Four search arrays of each distractor-present condition were presented, in a random order, for reward and shock (24 trials in total). The position of the target and distractors, as well as the target shape, was random. Participants were asked to indicate how likely each trial was to result in reward or shock (0 meant reward or shock was impossible and 100 meant reward or shock was guaranteed) by clicking on a continuous scale ranging from 0 to 100. Then, participants reported how they felt about each trial outcome (i.e., reward and shock) on a continuous scale ranging from −10 = very negative to 10 = very positive. Finally, participants indicated how motivated they were to earn a reward or avoid a shock on a continuous scale from −10 = not motivated at all to 10 = extremely motivated. Scripts for this questionnaire can be found at https://osf.io/jvktr/.
Head position was maintained throughout the experiment using an adjustable chin rest that included a bar upon which to rest the forehead (SR Research). Participants were provided a short break between each run of the task in which they were allowed to reposition their head to maintain comfort. Eye position was calibrated prior to each block of trials using 9-point calibration (Anderson & Yantis, 2012), and was manually drift corrected by the experimenter as necessary. During the presentation of the search array, the x and y positions of the eyes was continuously monitored in real time with respect to the six stimulus positions, such that fixations were coded on line (Anderson & Kim, 2019a, 2019b; Le Pelley et al., 2015). At the end of the experiment, participants were paid the total monetary reward obtained during the training phase.
Data analysis.
We measured which of the six shape stimuli was initially fixated on each trial, as well as whether the target was fixated before the timeout limit along with the time required to fixate the target (i.e., response time [RT]). Fixation of a stimulus was registered if the eye position remained within a region extending 0.7° around the stimulus for a continuous period of at least 50 ms (100 ms on the target triggered the termination of the stimulus array; see, e.g., Anderson & Kim, 2019a, 2019b; Le Pelley et al., 2015). Percentages of initial fixations on a distractor were taken over all trials within the respective condition. On distractor-absent trials, to quantify the probability of initially fixating a distractor for the sake of comparison, one of the nontargets was dummy-coded as the critical distractor on each trial using the same parameters that were used to define the position of the critical distractors on distractor-present trials (Anderson & Kim, 2019a, 2019b). RT was measured from the onset of the display until a valid target fixation was registered, from which 100 ms was subtracted to yield the time at which eye position first entered into the region of the target. RTs in fixating the target that exceeded three standard deviations of the mean for a given condition for a given participant were trimmed (Anderson & Kim, 2019b; Anderson & Yantis, 2012).
Repeated-measures analyses of variance (ANOVA) were conducted for each dependent variable (i.e., RT and proportion of first saccades to the distractor) of the training and test phases. Subsequent t tests were performed; for completeness, all pairwise comparisons are performed, although the comparisons of each of the valent distractor conditions to the neutral condition (to establish suppression or capture) and to each other on two-distractor trials (to address the question of relative strength of bias) in the test phase were of primary interest and determined a priori. Note that we calculated Cohen’s dz using the formula dz = t/sqrt(n) for all significant (or marginally significant) paired sample t tests (Lakens, 2013; Rosenthal, 1991). We also performed Bayesian analyses to quantify the evidence in favor of the null hypothesis with respect to the two-distractor condition (and the parallel analysis in Experiment 2) for the proportion of first saccades to the distractor. All Bayesian analyses were computed with JASP, using the JZS Bayes Factor with the scale r on effect size set to the default of 0.707 (http://jasp-stats.org/). The data of the three experiments reported in this article can be found at https://osf.io/9dj57/.
Results
Training phase.
A fixation on the target was registered within the timeout limit on 95.14% of all trials. A 4 × 5 ANOVA conducted on mean RTs with distractor condition (reward, shock, neutral, no distractor) and run (1, 2, 3, 4, 5) as within-subject variables revealed a significant main effect of condition, F(3, 111) = 24.58, p < .001, . Subsequent t tests indicated that RTs in the distractor-present conditions were significantly slower than in the distractor-absent condition (all ps < 0.001). Furthermore, RTs in the neutral condition were marginally slower than in the reward condition, t(37) = 1.94, p = .061, dz = 0.32. No difference was observed between the shock condition and neutral or reward conditions (all ps > 0.10). The ANOVA also showed a significant main effect of run, F(4, 148) = 31.76, p < .001, , with an overall decrease of RTs across runs (the linear trend was significant, F(1, 37) = 52.56, p < .001, ), and a significant interaction between condition and run, F(12, 444) = 2.38, p = .005, . Post hoc analyses revealed that the RT difference between the shock and the neutral condition followed a significant quadratic trend, with an increase from run 1 to run 3 and a decrease from run 3 to run 5, F(1, 37) = 12.36, p = .001, . RTs were also significantly faster in the shock condition than in the neutral condition in run 5, at the end of the training, t(37) = 2.17, p = .037, dz = 0.35. The partial interaction between condition (reward, neutral) and run (1, 2, 3, 4, 5) was not significant, F(4, 148) = 0.20, p = .936 (Figure 2A).
Figure 2.
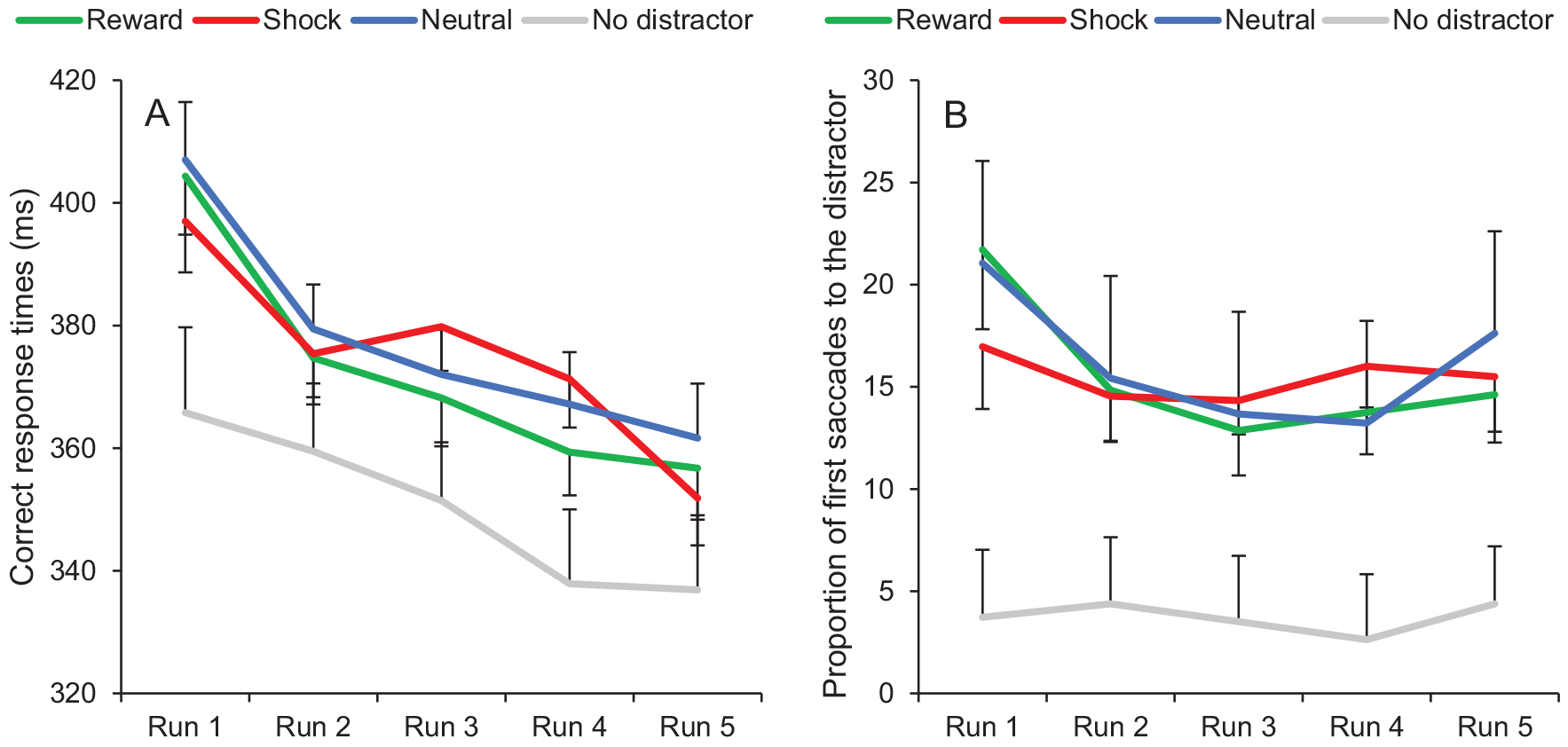
Training phase of Experiment 1. (A) Correct response times and (B) proportion of first saccades to the distractor as a function of condition (reward, shock, neutral, no distractor) and run (1, 2, 3, 4, 5). Error bars depict within-subjects 95% confidence intervals calculated using the Cousineau method (Cousineau, 2005) with a Morey correction (Morey, 2008). See the online article for the color version of this figure.
The same ANOVA conducted on oculomotor capture revealed a significant main effect of condition, F(3, 111) = 31.42, p < .001, , resulting from a greater proportion of first saccades to the distractor in the distractor-present conditions than in the distractor-absent condition (all ps < 0.001). We also observed a significant main effect of run, F(4, 148) = 8.60, p < .001, , with an overall decrease of RTs across runs (the linear trend was significant, F(1, 37) = 5.11, p = .030, ), and a significant interaction between condition and run, F(12, 444) = 3.53, p < .001, . Post hoc analyses indicated that the difference between the shock and the neutral condition followed a significant quadratic trend, with an increase from run 1 to run 4 and a decrease from run 4 to run 5, F(1, 37) = 6.62, p = .014, (the linear trend was also marginally significant, F(1, 37) = 3.41, p = .073, ). In run 5, the proportion of first saccades to the distractor was numerically reduced in the shock condition compared to the neutral condition, but the difference was not significant, t(37) = 0.96, p = .342. The partial interaction between condition (reward, neutral) and run (1, 2, 3, 4, 5) was not significant, F(4, 148) = 1.26, p = .289 (Figure 2B).
Test phase.
A fixation on the target was registered within the timeout limit on 96.07% of all trials. A 5 × 2 repeated-measures ANOVA conducted on mean RTs with distractor condition (two distractors, reward, shock, neutral, no distractor) and run (1, 2) as within-subject variables revealed a significant main effect of condition, F(4, 148) = 19.05, p < .001, , no main effect of run, F(1, 37) = 0.46, p = .503, and no interaction between condition and run, F(4, 148) = 0.88, p = .476. Subsequent t tests indicated that RTs in the distractor-present conditions were significantly slower than in the distractor-absent condition (all ps < 0.001). Furthermore, RTs were significantly slower in the neutral condition than in the reward and shock conditions, t(37) = 2.20, p = .034, dz = 0.36, and t(37) = 2.09, p = .044, dz = 0.34, respectively. No difference was observed between the shock and reward conditions, t(37) = 0.16, p = .877. RTs in the two-distractor condition were lower than in the neutral condition, t(37) = 2.85, p = .007, dz = 0.46, but did not differ from the reward and shock conditions, t(37) = 0.51, p = .611, and t(37) = 0.66, p = .515, respectively (Figure 3A).
Figure 3.
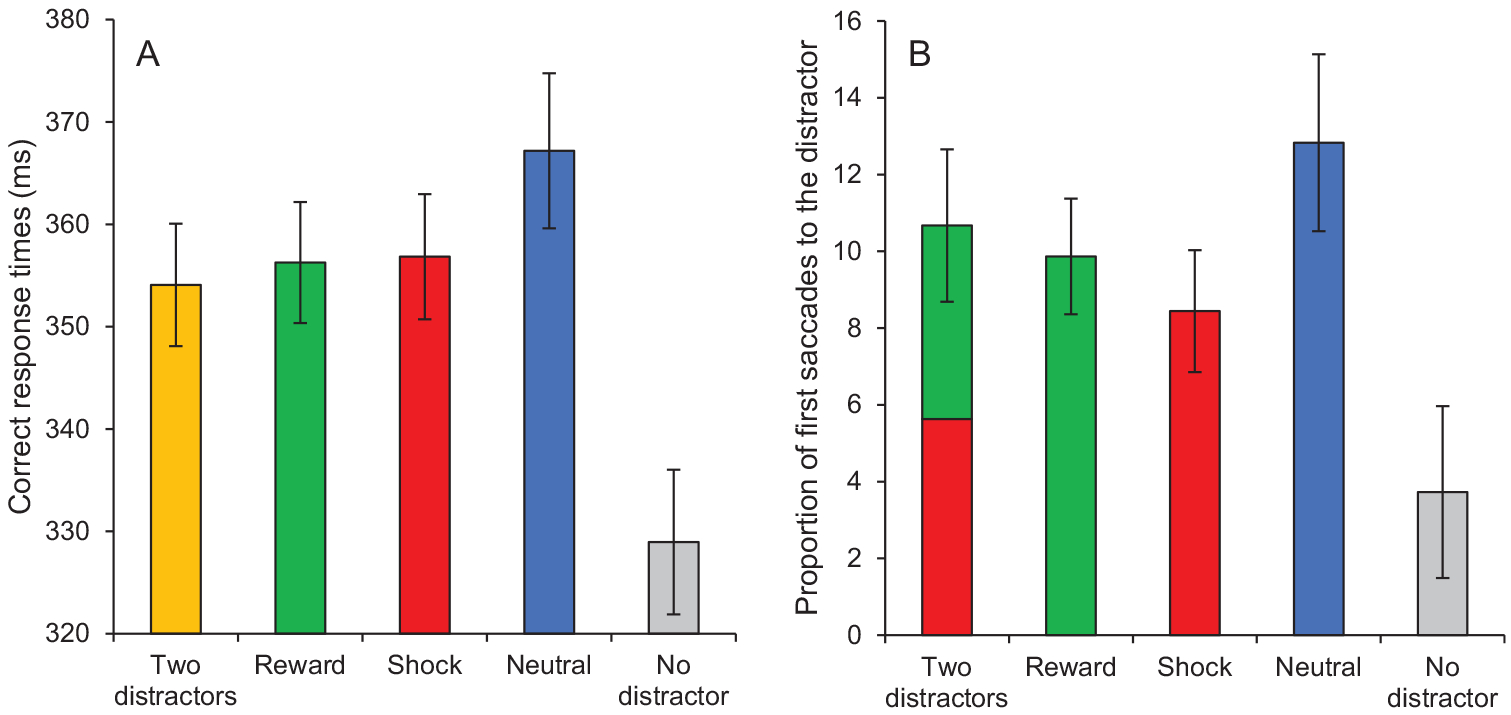
Test phase of Experiment 1. (A) Correct response times and (B) proportion of first saccades to the distractor as a function of condition (two distractors, reward, shock, neutral, no distractor). Error bars depict within-subjects 95% confidence intervals calculated using the Cousineau method (Cousineau, 2005) with a Morey correction (Morey, 2008). See the online article for the color version of this figure.
The same ANOVA conducted on oculomotor capture revealed a significant main effect of condition, F(4, 148) = 12.45, p < .001, , no main effect of run, F(1, 37) = 0.04, p = .841, and no interaction between condition and run, F(4, 148) = 1.29, p = .276. Subsequent t tests indicated a significantly greater proportion of first saccades to the distractor in the distractor-present conditions than in the (dummy-coded) distractor-absent condition (all ps < 0.001). The proportion of first saccades to the distractor was also significantly greater in the neutral condition than in the reward and shock conditions, t(37) = 2.17, p = .037, dz = 0.35, and t(37) = 3.42, p = .002, dz = 0.56, respectively. No difference was observed between the shock and reward conditions, t(37) = 1.26, p = .215. Oculomotor capture in the two-distractor condition was marginally greater than in the shock condition, t(37) = 1.80, p = .079, dz = 0.29, but did not differ from the reward and neutral conditions, t(37) = 0.76, p = .452, and t(37) = 1.36, p = .184, respectively. In the two-distractor condition, the proportion of first saccades to the reward-related distractor did not differ from the proportion of first saccades to the shock-related distractor, t(37) = 0.52, p = .604, JZS Bayes Factor = 5.04 in favor of the null hypothesis, indicating that the data are 5.04 times more likely to have occurred under the null hypothesis than under the alternative hypothesis, a “substantial” Bayes factor according to the coarse category scheme proposed by Jeffreys (1961; Figure 3B).
Questionnaires.
Participants self-reported that the likelihood of receiving a reward on reward-related trials (M = 57.70%, SD = 21.44) did not differ significantly from the likelihood of receiving a reward on threat-related and neutral trials (M = 55.58%, SD = 21.32), t(37) = 1.04, p = .305. Similarly, the likelihood of receiving a shock on threat-related trials (M = 45.21%, SD = 17.51) did not differ significantly from the likelihood of receiving a shock on reward-related and neutral trials (M = 43.05%, SD = 17.28), t(37) = 0.82, p = .417.
We also evaluated participants’ subjective feelings about each trial outcome (i.e., reward and shock). We compared the absolute value reported for reward to the absolute value reported for shock. Scores were significantly greater for reward (M = 6.97, SD = 2.96) than for shock (M = 5.04, SD = 2.90), t(37) = 3.69, p = .001, dz = 0.60. Finally, self-reported motivation to earn a reward (M = 7.90, SD = 2.35) was significantly greater than self-reported motivation to avoid a shock (M = 1.15, SD = 6.02), t(37) = 6.66, p < .001, dz = 1.08.
Discussion
Results of Experiment 1 evidenced suppression of the value-and threat-modulated attentional capture. Stimuli previously associated with reward or threat were less likely to capture attention than neutral stimuli in the test phase (in which no reward or shock was delivered). RTs to fixate a shape-defined target were also faster when one color-singleton distractor was related to reward or threat, relative to search displays with one neutral color-singleton distractor. Thus, signals for reward and threat can be actively suppressed after training arousing strong motivation to avoid these cues.
Somewhat surprisingly, a corresponding effect was not shown to be significant during training, although an RT benefit for the reward-associated compared to the neutral distractor approached significance and interactions between the shock-associated and neutral distractors evidenced a reduction in attentional capture by the shock-associated distractors toward the end of training, with a significantly faster RT in the last run for the shock condition. It is noteworthy that there was no evidence for increased attentional capture by reward- and punishment-associated distractors compared with neutral distractors in either phase, which is the frequently replicated result using different reinforcement contingencies (e.g., Anderson & Britton, 2020; Le Pelley et al., 2015; Nissens et al., 2017). We also note that it is possible that participants only learned to suppress the valent distractors toward the end of training, and the test phase is designed to assess the influence of learning divorced from current reward and punishment considerations (see Anderson & Halpern, 2017). The learned signal suppression we observed in the test phase was largely implicit, as shown by a test asking participants to indicate the probability of different outcomes when presented with stimulus displays from the experiment.
It was also the case that reward-associated and shock-associated distractors produced similar patterns of performance in the test phase, similarly capturing attention less robustly than neutral distractors. This was even true when the two distractors directly competed with each other for selection in the two-distractor display, suggesting that learning from reward and punishment had a comparable influence on the attention system when equated for the frequency of stimulus-outcome pairings, at least under the sort conditions tested in our experiment. This conclusion was supported by Bayesian analysis, which provided evidence in favor of no difference between the two conditions. We therefore found no evidence for a threat-bias in our task.
Participants self-reported greater motivation to obtain reward than avoid shock, although this was not reflected in differential attentional capture or suppression by associated stimuli. There are several possible reasons for this disconnect. First, self-reported motivation was probed by a single question at the end of the experiment, and how self-reported motivation might translate to how participants approached and performed the experiment task is unclear, as is the scaling and anchoring that participants may have applied to their responses. We therefore restrict our conclusions to task-specific motivation rather than subjective motivation experienced by the participant, which also fits with the fact that participants were not explicitly aware of the stimulus-outcome contingencies (and so it is perhaps unsurprising that conscious, explicit motivation might be less related to the observed behavioral effects). Participants were aware of the relationship between the need to respond quickly to the target and the outcomes they experienced, although as a group they never became aware of the stimulus-outcome contingencies, and so it appears that the global motivational state brought about by the manipulation of performance-dependent outcomes influenced the manner in which implicit learning shaped stimulus processing. However, monetary reward feedback and electric shock are fundamentally different sensory experiences that may differentially promote learning, and so even if explicit self-reported motivation did have a direct influence on performance, it is possible that outcome-specific considerations to some degree offset this effect (e.g., the salience of the shock events served as a more effective teaching signal, which counteracted relatively lower motivation to avoid shock than obtain reward).
Experiment 2
Experiment 1 demonstrates a reduced magnitude of attentional capture by signals for reward and punishment, relative to a neutral distractor, consistent with signal suppression. However, a recent study showed that value-modulated attentional capture was blunted by the experience of threat (Kim & Anderson, 2020). Specifically, the threat of electrical shock (randomly delivered) during a visual search task reduced attentional capture by reward-associated stimuli. To determine whether the suppression of value-modulated attentional capture observed in Experiment 1 resulted, at least partially, from the threat of electrical shock, we replicated this experiment, replacing the shock condition by a neutral condition (to keep the same proportion of rewarded trials in the training phase). Given the sharp contrast between our findings and (a) prior demonstrations of a failure to suppress attention to reward cues (e.g., Anderson et al., 2016; Munneke et al., 2015, 2016; Pearson et al., 2015, 2020; Wang et al., 2014, 2015, 2018) and (b) prior studies using a conceptually similar paradigm (e.g., Le Pelley et al., 2015; Nissens et al., 2017), we also wanted to provide a simpler replication of the pattern of reduced attentional capture evident in Experiment 1.
Method
Participants.
Thirty-eight new participants, between the ages of 18 and 35 inclusive, were recruited from the Texas A&M University community. All participants were English-speaking and reported normal or corrected-to-normal visual acuity and normal color vision. Data from two participants were removed because of an inability to reliably track eye position (resulting in a failure to register a target fixation on more than 20% of trials). Two additional participants were removed as outliers. Specifically, in the test phase, their difference between the reward condition and the collapsed neutral condition (see the Data Analysis section) for the proportion of first saccades to the distractor exceeded 2.5 standard deviations of the participants’ mean. The final sample included 34 participants (26 females) with a mean age of 22.12 years (SD = 3.97). All procedures were approved by the Texas A&M University Institutional Review Board and were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant.
Stimuli and procedure.
Experiment 2 was similar to Experiment 1 except that no shock was delivered during training, resulting in a second neutral color. This condition was called neutral 1 to avoid confusion with the neutral 2 condition (which was strictly similar to the neutral condition of Experiment 1). Note that the neutral 1 color was also used in the two-distractor trials during the test phase. Materials for this experiment can be found at https://osf.io/6evsw/. After the test phase, participants provided self-report evaluations of their contingency awareness about the relationship between each condition of the training phase (i.e., reward, neutral 1, neutral 2 and no distractor) and each of the two following consequences: reward and no reward. Six search arrays of each condition were presented, in a random order, for reward and no reward (48 trials in total). The target was presented once in each position of the search array for each condition. The position of distractors was random. Participants were asked to indicate how likely each trial was to result in reward or no reward (0 meant reward or no reward was impossible and 100 meant reward or no reward was guaranteed). Then, participants reported how they felt about each trial outcome (i.e., reward and no reward) on a continuous scale ranging from −10 = very negative to 10 = very positive. Finally, participants indicated how motivated they were to earn a reward on a continuous scale from −10 = not motivated at all to 10 = extremely motivated. Scripts for this questionnaire can be found at https://osf.io/v67nc/.
Data analysis.
Data were analyzed in the same manner as in Experiment 1, with the exception that each ANOVA included one fewer condition. The two neutral conditions (i.e., neutral 1 and neutral 2) were combined into one unique condition because no difference was observed between the neutral 1 and neutral 2 conditions for each measure (i.e., RT and proportion of first saccades to the distractor) in both the training and test phases (all ps < 0.10).
Results
Training phase.
A fixation on the target was registered within the timeout limit on 95.05% of all trials. A 3 × 5 ANOVA conducted on mean RTs with distractor condition (reward, neutral, no distractor) and run (1, 2, 3, 4, 5) as within-subject variables revealed a significant main effect of condition, F(2, 66) = 41.28, p < .001, . Subsequent t tests indicated that RTs in the distractor-present conditions were significantly slower than in the distractor-absent condition (all ps < 0.001). No significant difference was observed between the reward condition and the neutral condition, t(33) = 0.50, p = .619. The ANOVA also showed a significant main effect of run, F(4, 132) = 40.87, p < .001, , with an overall decrease of RTs across runs (the linear trend was significant, F(1, 33) = 65.32, p < .001, ), and a significant interaction between condition and run, F(8, 264) = 2.57, p = .010, . Post hoc analyses revealed that the RT difference between the reward and the neutral condition followed a significant quadratic trend, with a decrease from run 1 to run 4 and an increase from run 4 to run 5, F(1, 33) = 5.44, p = .026, (Figure 4A).
Figure 4.
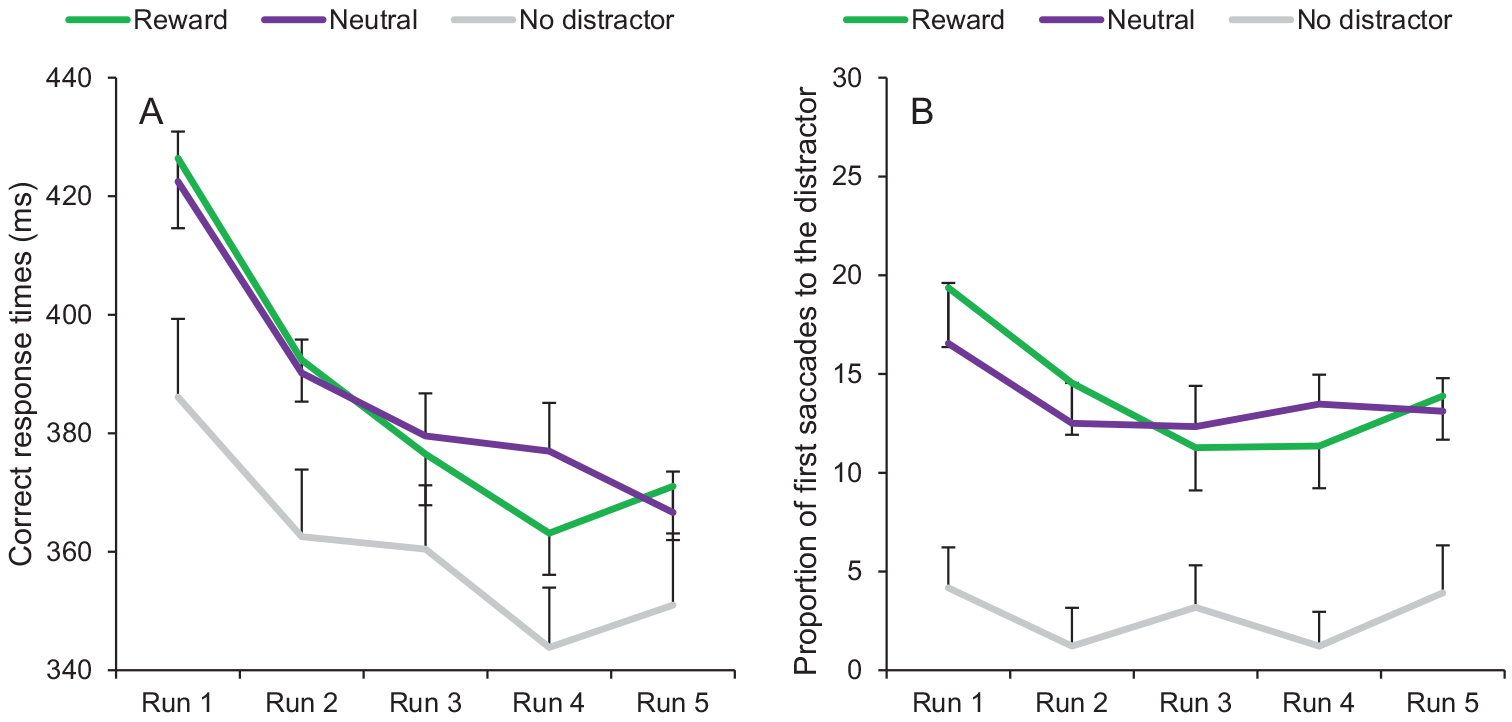
Training phase of Experiment 2. (A) Correct response times and (B) proportion of first saccades to the distractor as a function of condition (reward, neutral, no distractor) and run (1, 2, 3, 4, 5). Error bars depict within-subjects 95% confidence intervals calculated using the Cousineau method (Cousineau, 2005) with a Morey correction (Morey, 2008). See the online article for the color version of this figure.
The same ANOVA conducted on oculomotor capture revealed a significant main effect of condition, F(2, 66) = 78.01, p < .001, , resulting from a greater proportion of first saccades to the distractor in the distractor-present conditions than in the distractor-absent condition (all ps < 0.001). We also observed a significant main effect of run F(4, 132) = 7.67, p < .001, , with an overall decrease of the proportion of first saccades to the distractor across runs (the linear trend was significant, F(1, 33) = 5.88, p = .021, ), and a significant interaction between condition and run, F(8, 264) = 3.46, p = .001, . Post hoc analyses indicated that the difference between the reward and the neutral condition followed a significant quadratic trend, with a decrease from run 1 to run 4 and an increase from run 4 to run 5, F(1, 33) = 4.67, p = .038, . The linear trend was also significant, F(1, 33) = 5.61, p = .024, (Figure 4B).
Test phase.
A fixation on the target was registered within the timeout limit on 95.64% of all trials. A 4 × 2 repeated-measures ANOVA conducted on mean RTs with distractor condition (two distractors, reward, neutral, no distractor) and run (1, 2) as within-subject variables revealed a significant main effect of condition, F(3, 99) = 16.04, p < .001, , a significant main effect of run, F(1, 33) = 9.30, p = .004, , with longer RTs for run 1 than for run 2, and no interaction between condition and run, F(3, 99) = 0.99, p = .403. Subsequent t tests indicated that RTs in the distractor-present conditions were significantly slower than in the distractor-absent condition (all ps < 0.01). RTs were also significantly slower in the neutral condition than in the reward condition, t(33) = 2.42, p = .021, dz = 0.42. No difference was observed between the two-distractor condition and the reward and the neutral conditions, t(33) = 1.45, p = .148, and t(33) = 1.23, p = .228, respectively (Figure 5A).
Figure 5.
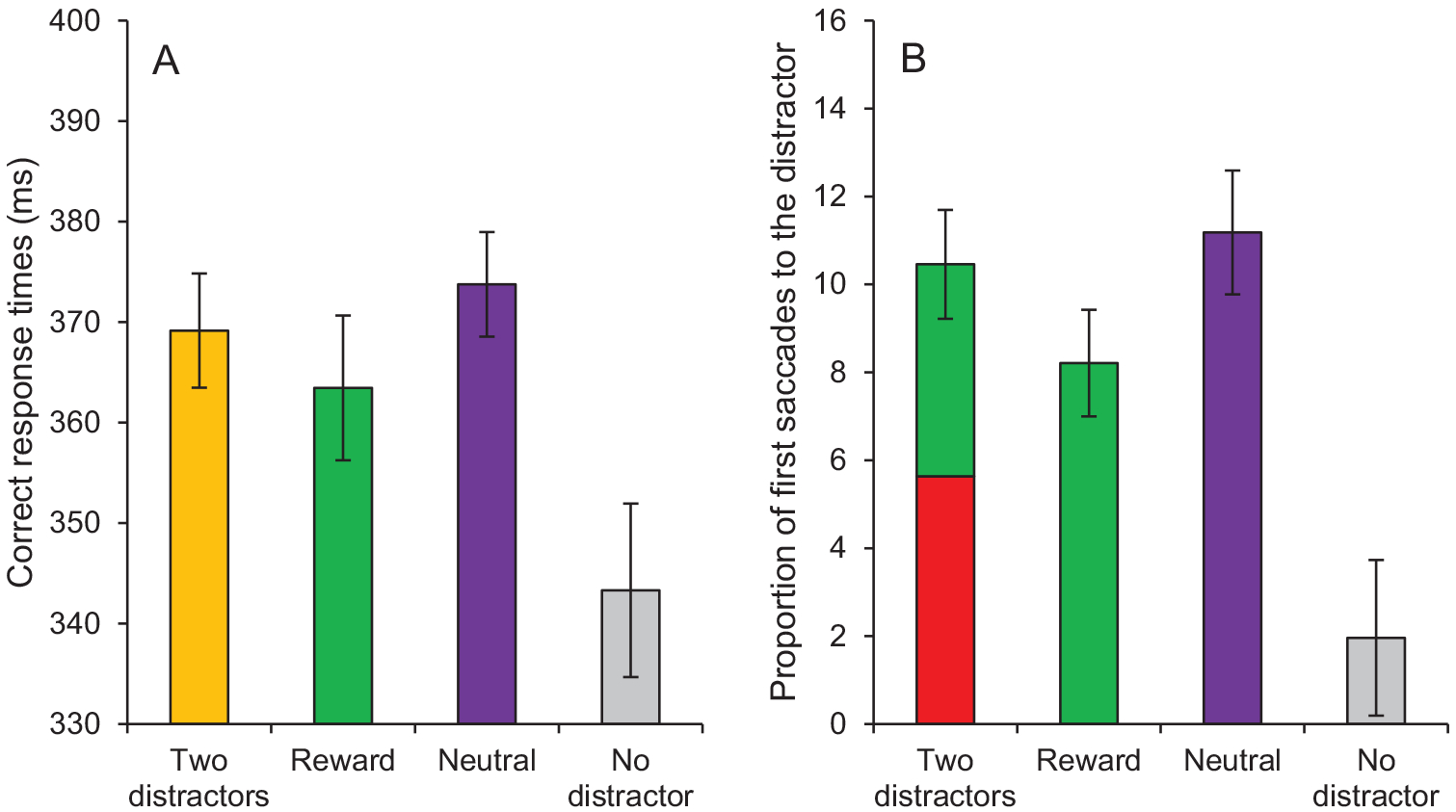
Test phase of Experiment 2. (A) Correct response times and (B) proportion of first saccades to the distractor as a function of condition (two distractors, reward, neutral, no distractor). Note that the two-distractors condition in the panel B includes the reward-related distractor (in green) and the neutral distractor (i.e., the distractor of the neutral 1 condition; in red). Error bars depict within-subjects 95% confidence intervals calculated using the Cousineau method (Cousineau, 2005) with a Morey correction (Morey, 2008). See the online article for the color version of this figure.
The same ANOVA conducted on oculomotor capture revealed a significant main effect of condition, F(3, 99) = 35.78, p < .001, , no main effect of run, F(1, 33) = 0.03, p = .870, and no interaction between condition and run, F(3, 99) = 0.88, p = .455. Subsequent t tests indicated a significantly greater proportion of first saccades to the distractor in the distractor-present condi tions than in the (dummy-coded) distractor-absent condition (all ps < 0.001). The proportion of first saccades to the distractor was also significantly greater in the neutral condition than in the reward condition, t(33) = 3.38, p = .002, dz = 0.58. The proportion of first saccades to the distractor in the two-distractor condition was greater than in the reward condition, t(33) = 2.99, p = .005, dz = 0.51, but did not differ from the neutral condition, t(33) = 0.81, p = .425. In the two-distractor condition, the proportion of first saccades to the reward-related distractor did not differ statistically from the proportion of first saccades to the neutral distractor, t(33) = 0.90, p = .375, JZS Bayes Factor = 3.75 in favor of the null hypothesis (Figure 5B).
Questionnaires.
Participants self-reported that the likelihood of receiving a reward on reward-related trials (M = 59.27%, SD = 23.38) did not differ significantly from the likelihood of receiving a reward on neutral trials (M = 58.22%, SD = 23.55), t(33) = 0.47, p = .642. Similarly, the likelihood of receiving no reward on neutral trials (M = 55.21%, SD = 21.26) did not differ significantly from the likelihood of receiving no reward on reward-related trials (M = 59.15%, SD = 23.38), t(33) = 1.55, p = .130.
We also evaluated participants’ subjective feelings about each trial outcome (i.e., reward and no reward). We compared the absolute value reported for reward to the absolute value reported for no reward. Unsurprisingly, scores were significantly greater for reward (M = 6.50, SD = 2.93) than for no reward (M = 4.59, SD = 3.67), t(33) = 2.83, p = .008, dz = 0.49. Finally, self-reported motivation to earn a reward (M = 6.91, SD = 2.65) was significantly greater than zero, t(33) = 15.20, p < .001, dz = 2.61.
Discussion
The results of Experiment 2 largely mirror the results for the reward condition of Experiment 1. Significant interactions in which attentional capture by reward-associated distractors decreased in magnitude relative to attentional capture by neutral distractors over block were evident for both RT and eye movement measures in the training phase. Importantly, the same reduction in RT and oculomotor capture for a reward-associated distractor relative to a neutral distractor was replicated in the test phase, in stark contrast to the typical pattern of increased attentional capture observed in this paradigm when a much higher rate of reinforcement is used (e.g., Le Pelley et al., 2015). Once again, participants showed no evidence for awareness of the reward contingencies, suggesting that the learning was implicit.
Experiment 3
In Experiment 2, the reward was delivered only for fast and correct responses (in the reward condition of the training phase), with a low reinforcement ratio. This manipulation could have substantially accentuated the motivation of participants to suppress reward-associated signals insofar as the likelihood of receiving a reward was related to the efficiency to suppress valent distractors. To ensure that motivation to suppress attention to reward cues affected the pattern of results, we conducted a replication of Experiment 2 in which a monetary reward was delivered for each correct response (i.e., when participants looked at the unique shape before the 1-s timeout limit) when the reward-associated color was present in the training phase. We hypothesized that this procedure would lead to value-modulated attentional capture as previously observed in the literature (e.g., Le Pelley et al., 2015; Pearson et al., 2015), or at least to an absence of value-based attentional suppression, because, in this situation, the efficiency to suppress reward cues does not markedly affect the likelihood to be rewarded. Thus, the motivation to resist distraction by reward-related stimuli should be substantially attenuated relative to Experiment 2.
Method
Participants.
Thirty new participants, between the ages of 18 and 35 inclusive, were recruited from the Texas A&M University community. All participants were English-speaking and reported normal or corrected-to-normal visual acuity and normal color vision. Data from two participants were removed because of an inability to reliably track eye position (resulting in a failure to register a target fixation on over 20% of trials). The final sample included 28 participants (18 females) with a mean age of 21.71 years (SD = 4.59). All procedures were approved by the Texas A&M University Institutional Review Board and were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant.
Stimuli and procedure.
Experiment 3 was otherwise identical to Experiment 2 except that a monetary reward was delivered for each correct response (i.e., when participants looked at the unique shape before the 1s time-out) of the reward condition in the training phase. Materials for this experiment can be found at https://osf.io/jhvn4/. After the test phase, participants performed the same questionnaire as in Experiment 2.
Data analysis.
Data were analyzed in the same manner as in Experiment 2, with the addition of between-experiment comparisons.
Results
Training phase.
A fixation on the target was registered within the timeout limit on 95.37% of all trials. A 3 × 5 ANOVA conducted on mean RTs with distractor condition (reward, neutral, no distractor) and run (1, 2, 3, 4, 5) as within-subject variables revealed a significant main effect of condition, F(2, 54) = 50.67, p < .001, , a significant main effect of run, F(4, 108) = 9.60, p < .001, , with an overall decrease of RTs across runs (the linear trend was significant, F(1, 27) = 25.32, p < .001, ), and no significant interaction between condition and run, F(8, 216) = 0.54, p = .825 (Figure 6A). Subsequent t tests indicated that RTs in the distractor-present conditions were significantly slower than in the distractor-absent condition (all ps < 0.001). More importantly, RTs in the reward condition were significantly greater than in the neutral condition, t(27) = 2.89, p = .007, dz = 0.55. This effect (reward—neutral) was significantly greater in Experiment 3 than in Experiment 2, t(60) = 2.59, p = .012, d = 0.66.
Figure 6.
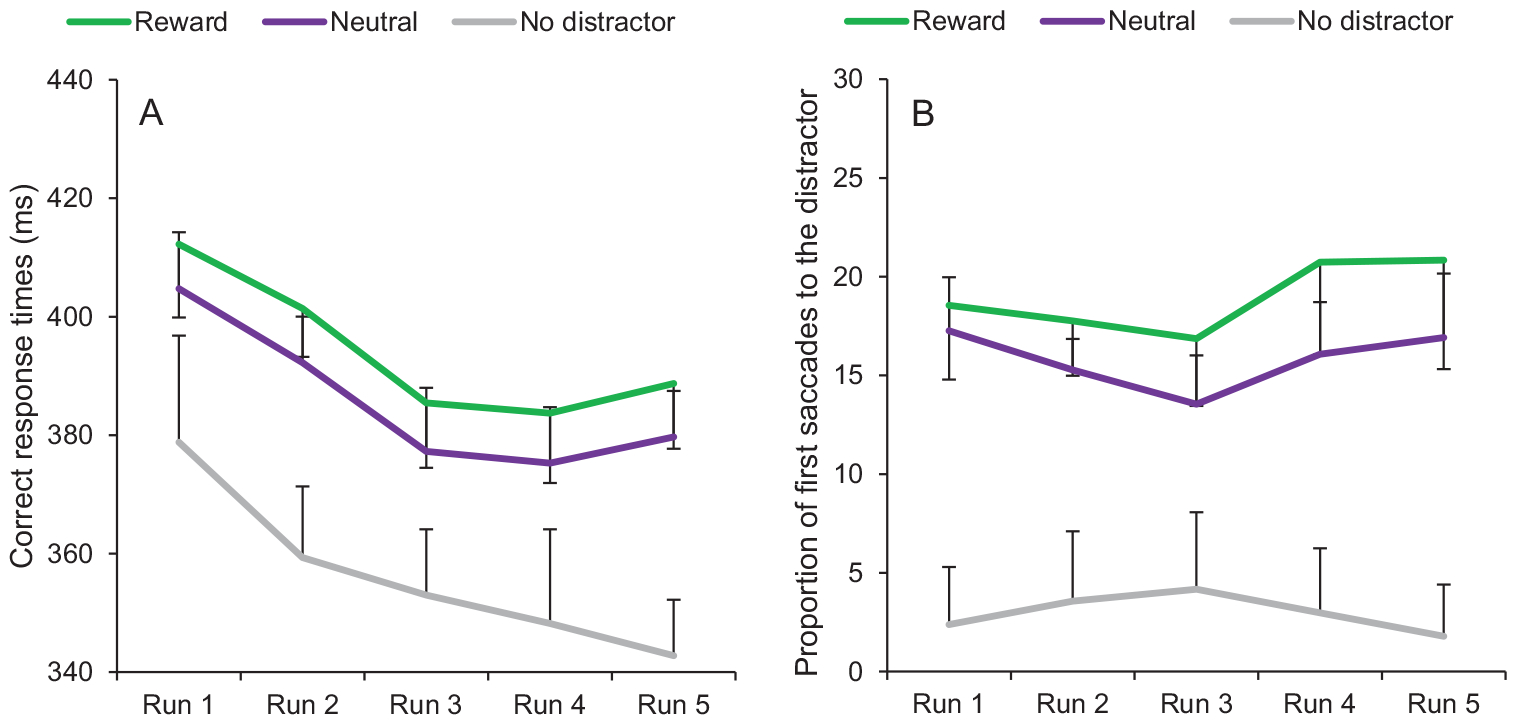
Training phase of Experiment 3. (A) Correct response times and (B) proportion of first saccades to the distractor as a function of condition (reward, neutral, no distractor) and run (1, 2, 3, 4, 5). Error bars depict within-subjects 95% confidence intervals calculated using the Cousineau method (Cousineau, 2005) with a Morey correction (Morey, 2008). See the online article for the color version of this figure.
The same ANOVA conducted on oculomotor capture revealed a significant main effect of condition, F(2, 54) = 54.06, p < .001, , no significant main effect of run F(4, 108) = 0.57, p = .686, and no significant interaction between condition and run, F(8, 216) = 1.34, p = .226 (Figure 6B). Subsequent t tests indicated that the proportion of first saccades to the distractor was significantly greater in the distractor-present conditions than in the distractor-absent condition (all ps < 0.001). Oculomotor capture was also significantly greater in the reward condition than in the neutral condition, t(27) = 3.13, p = .004, dz = 0.59. This effect (reward–neutral) was significantly greater in Experiment 3 than in Experiment 2, t(60) = 2.19, p = .032, d = 0.56.
Test phase.
A fixation on the target was registered within the timeout limit on 94.21% of all trials. A 4 × 2 repeated-measures ANOVA conducted on mean RTs with distractor condition (two distractors, reward, neutral, no distractor) and run (1, 2) as within-subject variables revealed a significant main effect of condition, F(3, 81) = 13.66, p < .001, , no significant main effect of run, F(1, 27) = 0.56, p = .460, and no interaction between condition and run, F(3, 81) = 1.02, p = .390. Subsequent t tests indicated that RTs in the distractor-present conditions were significantly slower than in the distractor-absent condition (all ps < 0.001). RTs were numerically greater in the reward condition than in the neutral condition, but the effect was not significant, t(27) = 1.12, p = .274 (Figure 7A). However, this difference (reward—neutral) was significantly larger in Experiment 3 than in Experiment 2, t(60) = 2.40, p = .020, d = 0.61. RTs in the two-distractor condition was significantly greater than in the neutral condition, t(27) = 2.41, p = .023, dz = 0.46, but did not differ statistically from the reward condition, t(27) = 1.07, p = .296.
Figure 7.
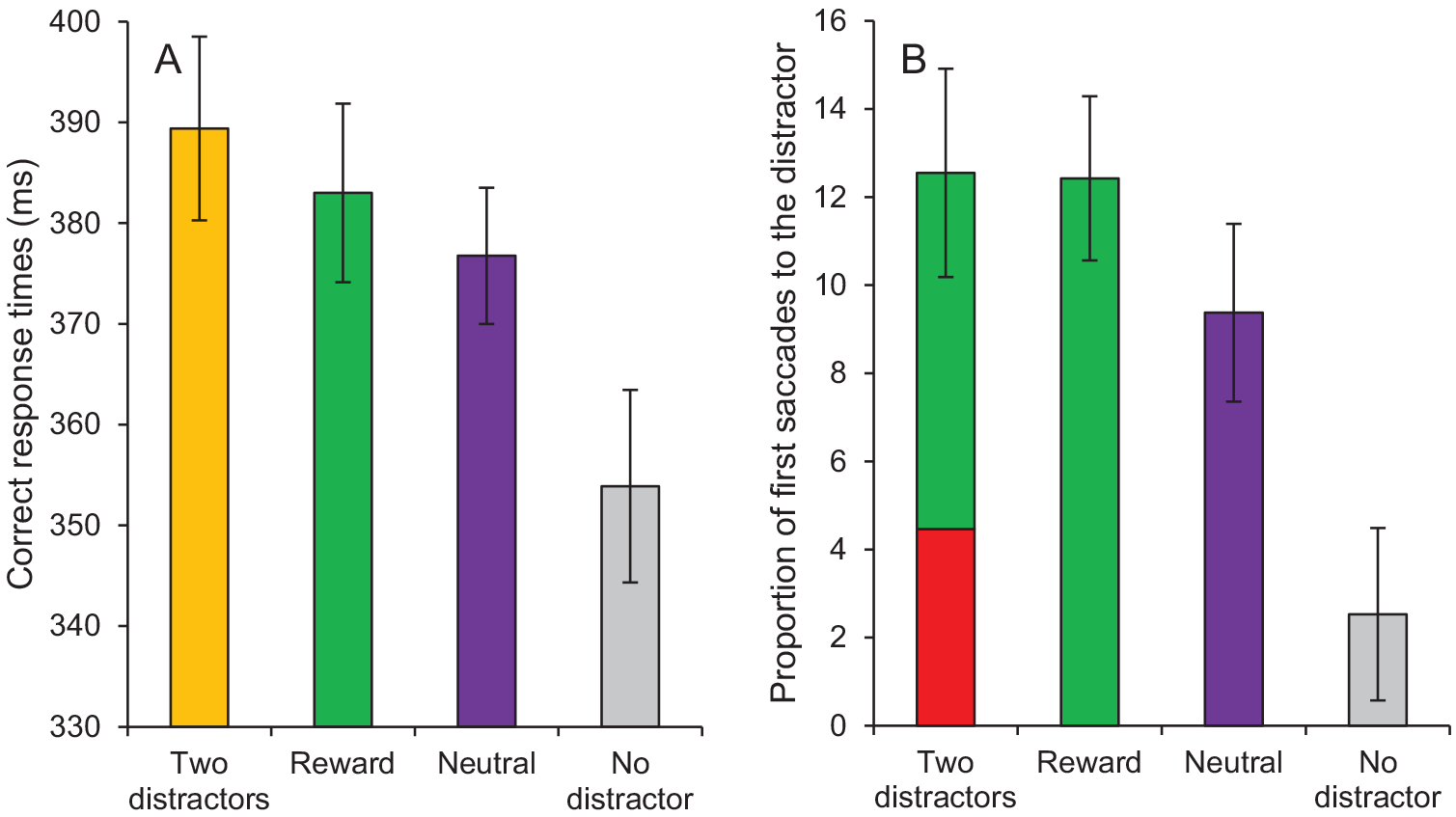
Test phase of Experiment 3. (A) Correct response times and (B) proportion of first saccades to the distractor as a function of condition (two distractors, reward, neutral, no distractor). Note that the two-distractors condition in the panel B includes the reward-related distractor (in green) and the neutral distractor (i.e., the distractor of the neutral 1 condition; in red). Error bars depict within-subjects 95% confidence intervals calculated using the Cousineau method (Cousineau, 2005) with a Morey correction (Morey, 2008). See the online article for the color version of this figure.
The same ANOVA conducted on oculomotor capture revealed a significant main effect of condition, F(3, 81) = 21.90, p < .001, , a significant main effect of run, F(1, 27) = 4.84, p = .044, , with a greater proportion of first saccades to the distractor in run 2 than in run 1, and no interaction between condition and run, F(3, 81) = 0.99, p = .404 (Figure 7B). Subsequent t tests indicated a significantly greater proportion of first saccades to the distractor in the distractor-present conditions than in the (dummy-coded) distractor-absent condition (all ps < 0.001). The proportion of first saccades to the distractor was also significantly greater in the reward condition than in the neutral condition, t(27) = 2.11, p = .044, dz = 0.40. This effect (reward–neutral) was significantly greater in Experiment 3 than in Experiment 2, t(60) = 3.70, p < .001, d = 0.93. The proportion of first saccades to the distractor in the two-distractor condition was significantly greater than in the neutral condition, t(27) = 2.07, p = .048, dz = 0.39, but did not differ statistically from the reward condition, t(27) = 0.09, p = .929. In the two-distractor condition, the proportion of first saccades to the reward-related distractor was significantly greater than the proportion of first saccades to the neutral distractor, t(27) = 2.96, p = .006, dz = 0.56. This effect was also significantly greater in Experiment 3 than in Experiment 2, t(60) = 2.97, p = .004, d = 0.75.
Questionnaires.
Participants self-reported that the likelihood of receiving a reward on reward-related trials (M = 64.65%, SD = 21.05) did not differ significantly from the likelihood of receiving a reward on neutral trials (M = 57.48%, SD = 22.62), t(27) = 1.36, p = .185. Similarly, the likelihood of receiving no reward on neutral trials (M = 48.67%, SD = 19.31) did not differ significantly from the likelihood of receiving no reward on reward-related trials (M = 52.36%, SD = 20.58), t(27) = 0.72, p = .479.
We also evaluated participants’ subjective feelings about each trial outcome (i.e., reward and no reward). We compared the absolute value reported for reward to the absolute value reported for no reward. Scores were numerically greater for reward (M = 6.35, SD = 3.41) than for no reward (M = 5.30, SD = 3.35), but the difference did not reach significance, t(27) = 1.63, p = .115. Finally, self-reported motivation to earn a reward (M = 7.65, SD = 2.79) was significantly greater than zero, t(27) = 14.50, p < .001, dz = 2.74.
Discussion
The results of Experiment 3 clearly demonstrate value-modulated attentional capture. Reward-related distractors impaired performance, measured by RT and oculomotor capture, relative to neutral distractors in the training phase, and these effects persisted into extinction (though nonsignificantly for RT). Crucially, the difference between the reward condition and the neutral condition was significantly greater in Experiment 3 than in Experiment 2, for each measure (RT and the proportion of first saccades to the distractor), in the training and the test phase. Thus, using a high reinforcement ratio (one hundred percent for each correct response of the reward condition), we replicated the typical pattern of accentuated attentional capture by reward cues observed with this paradigm (e.g., Le Pelley et al., 2015; Pearson et al., 2015). As in Experiment 2, participants showed no evidence for awareness of the reward contingencies, suggesting that the learning was implicit. Together with Experiments 1 and 2, Experiment 3 demonstrates the task-specific motivation to suppression attentional capture in order to maximize trial-specific outcomes has a profound influence on value-modulated attention.
General Discussion
The goal of the present study was to examine whether value- and threat-modulated attentional capture could be suppressed with sufficient motivation. In Experiment 1, participants were first trained to look at a shape-defined target in a visual search task. One color-singleton distractor predicted the possibility of receiving a reward and another an electric shock, each outcome occurring with a reinforcement ratio of 33%. Participants were informed that the likelihood to earn a reward or avert punishment depended on fast and accurate performance, thus providing strong motivation to resist distraction by reward- and shock-related stimuli. Results revealed suppression of value- and threat-modulated attentional capture in a subsequent test phase (in which no reward and no shock was delivered). Specifically, eye movements were less likely to be directed toward the color-singleton distractors related to reward or shock than toward the neutral color-singleton distractors. Unsurprisingly, given that reorienting attention from the salient distractor takes time, the same pattern of results was observed with RTs: Participants were faster to fixate the target when one color-singleton distractor previously associated with reward or threat was presented, relative to the neutral color-singleton distractor. These results demonstrate that signals for reward and threat can be actively suppressed with sufficient motivation.
Postexperiment measures suggest that participants were not explicitly aware of the stimulus–outcome contingencies, although they were informed of the relationship between the speed of performance and the likelihood of obtaining reward and avoiding shock. It therefore seems that the global motivational state brought about by the performance-dependent nature of the outcomes influenced the manner in which implicit learning shaped stimulus processing. In this sense, we interpret the observed suppression as a different form of learning-dependent automatic responding rather than the consequence of a conscious, deliberate strategy to maximize the quality of outcomes. Note that the three colors used for singleton distractors were equiluminant (and the assignment of color to condition counterbalanced across participants), so effects observed between the three conditions (i.e., reward, shock and neutral) could not be explained by perceptual salience.
The test phase of Experiment 1 also included a condition in which two distractors (among five) were colored: one color was previously associated with reward and the other one with shock. The proportion of first saccades was not more frequently directed toward the threat-related distractor than toward the reward-related distractor, with substantial evidence for the null hypothesis, indicating that signals for threat were not stronger competitors for attention than signals for reward. This result contrasts with the outcome reported by Wang et al. (2013). These authors showed that electrical stimulation was more likely to produce attentional capture than monetary reward, suggesting that threat-related stimuli are potentially stronger competitors for attention than reward-related stimuli. However, in the present study, the suppression of value- and threat-modulated attentional capture ensuing from motivational effects could have attenuated the probability of observing a difference between reward and threat stimuli in the two-distractor condition. It is also possible that presenting the two outcomes in the same experiment prevented global differences in task motivation from producing differential learning effects for valent stimuli that could have led to a difference in subsequent capture. We caution generalization of our findings with respect to the differential impact of reward and punishment on attention beyond the specific conditions probed in our study.
Experiment 2 was conducted to test whether the suppression of value-modulated attentional capture evidenced in Experiment 1 resulted from the presence of threat. Indeed, Kim and Anderson (2020) showed that the experience of threat reduced attentional capture by reward-associated stimuli. Therefore, we replicated Experiment 1 replacing the shock condition by a neutral condition (to keep the same proportion of rewarded trials). Results observed in the test phase were very consistent with Experiment 1. Oculomotor capture by distractors previously associated with reward was less frequent than capture by neutral distractors (i.e., never paired with reward during the training). RTs to fixate the target were also faster when a reward-associated distractor was present in the search display. It is also worth pointing out that, during the training phase, the difference between the reward and the neutral condition for both the percentage of first saccades to the distractor and RTs linearly decreased from run 1 to run 4 (F(1, 33) = 14.50, p = .001, , and F(1, 33) = 12.27, p = .001, , respectively). Although this decline did not increase in run 5, as revealed by the quadratic trends for the two measures (the linear trend for oculomotor capture was however still significant when analyzed from run 1 to run 5), these data indicated that attentional capture by the reward-associated distractor tended to be suppressed over learning. Thus, Experiment 2 confirms that the suppression of value-modulated attentional capture observed in Experiment 1 did not result from the presence of threat. Note also that no RT difference between the two-distractor and the neutral condition was evidenced in the test phase of Experiment 2, which is inconsistent with the results of Experiment 1. However, in Experiment 1, both distractors of the two-distractor condition were presumably suppressed, whereas in Experiment 2, only one dis-tractor (i.e., the reward-related distractor) was suppressed and the other essentially served as a neutral distractor. The suppression of the two valent distractors in Experiment 1 could therefore explain the greater RT difference between the two-distractor and the neutral condition, relative to Experiment 2.
Experiment 3 replicated the typical pattern of attentional capture by reward cues using a high reinforcement ratio. Specifically, a monetary reward was delivered for each correct response (i.e., when participants looked at the unique shape before the 1-s timeout limit) of the reward condition; the likelihood to be rewarded was therefore not contingent on fast performance, thus reducing the motivation of participants to suppress attentional capture by reward-associated stimuli relative to Experiment 2. Results showed that the reward effect (reward—neutral) was greater in Experiment 3 than in Experiment 2 for both RT and proportion of first saccades to distractor, in the training and the test phase. Data also revealed that, in the test phase, RT and proportion of first saccades to distractor were greater in the two-distractor condition than in the neutral condition. Furthermore, in the two-distractor condition, the proportion of first saccades to the reward-related distractor was significantly higher than the proportion of first saccades to the neutral distractor. That a difference between the two-distractor condition and the neutral condition was evident in this experiment but not Experiment 2 may stem from the fact that reward learning increased attentional capture in Experiment 3, making the reward-related distractor the strongest competitor for attention in the two-distractor display, whereas in Experiment 2 the strongest competitor was the neutral distractor, which also appeared in the neutral distractor only condition. Altogether, our data provide direct evidence for the idea that task-specific motivation, as manipulated through the link between trial-specific outcomes and the need to quickly fixate the target (in the face of possible distraction), is responsible for the suppression of valent stimuli observed in the present study.
Our results from Experiments 1 and 2 diverge from previous studies which reported that reward- or threat-associated stimuli captured attention even when looking at these stimuli was counterproductive (e.g., Le Pelley et al., 2015; Nissens et al., 2017; Pearson et al., 2015; Wang et al., 2018), but methodological differences could explain these discrepancies. For instance, in the study of Le Pelley et al. (2015), participants received a reward for each correct response under the latency limit, but errors resulted in a monetary loss (Experiments 1 and 2) and oculomotor capture by reward-related distractors lead to the omission of reward (Experiment 3). These reward-omitting outcomes occurred infrequently, however, with reward being delivered on over 80% of trials (e.g., Le Pelley et al., 2015; Pearson et al., 2015; Wang et al., 2018). In the present study, participants earned a reward in only 33% of reward-related trials (in the training phase), for accurate and fast performance in Experiments 1 and 2, whereas reward was minimally dependent on the speed of performance in Experiment 3. The low frequency of rewarded trials in Experiments 1 and 2 could have markedly increased the motivation of participants to selectively suppress attention to reward cues. Nissens et al. (2017) observed oculomotor capture by threat-related stimuli with a lower reinforcement ratio (12.5% of shock maximum in threat-related trials), meaning that shock was more easily avoidable than in the present study (see also Anderson & Britton, 2020), which could have also resulted in reduced motivation to resist capture compared to the present study. It is also worth noting that participants were informed that the presence of a specific color among distractors predicted the possibility of receiving a shock in Nissens et al. (2017); this could have affected how participants performed the task, for instance, inciting them to actively monitor signals for threat or engage in ironical attentional processing (Moher & Egeth, 2012).
In contrast to several prior reports suggesting that value-associated stimuli are particularly resistant to suppression (e.g., Anderson et al., 2016; Le Pelley et al., 2015; Munneke et al., 2015, 2016; Pearson et al., 2015, 2020; Wang et al., 2014, 2015, 2018), we provide evidence that when task contingencies strongly encourage participants to resist attentional capture, the selective suppression of stimuli that need to be ignored to secure a positive outcome (reward or the avoidance of shock) is possible even though these stimuli serve as a predictive cue for such outcomes. Thus, attentional capture by reward- and threat-associated stimuli is not obligatory and may be less robustly automatic than previously assumed. This finding fits with an adaptive, ecological account of value- and threat-modulated attention in which organisms monitor for reward- and threat-signals when the cost of doing so is low, reflecting a bias toward information-seeking (Gottlieb & Oudeyer, 2018), but are able to not only resist but even leverage such associations to facilitate improved ignoring when the benefit of ignoring is high. It remains an open question whether the sort of contingencies employed in the present study produce an exception to the rule, or whether typical design elements used in studies of the value- and threat-modulated control of attention have inflated estimates of the robustness of capture by providing low motivation to resist such capture. The findings of the present study also have important translational implications in that they provide a proof-of-concept that training with reward and/or punishment can be used to train selective ignoring, which could potentially be leveraged to improve visual search performance under distracting conditions or mitigate unwanted attention to stimuli such as drug cues (Anderson, 2016b).
Acknowledgments
This research was supported by the Brain and Behavior Research Foundation (NARSAD Young Investigator Grant 26008) and the National Institute on Drug Abuse (R01-DA046410).
Footnotes
Distractor-present and distractor-absent trials refer to search displays with or without a colored distractor, respectively.
References
- Anderson BA (2013). A value-driven mechanism of attentional selection. Journal of Vision, 13(3), 7. 10.1167/13.3.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2016a). The attention habit: How reward learning shapes attentional selection. In Kingstone A & Miller MB (Eds.), Year in cognitive neuroscience (Vol. 1369, pp. 24–39). New York: New York Academy of Sciences. 10.1111/nyas.12957 [DOI] [PubMed] [Google Scholar]
- Anderson BA (2016b). What is abnormal about addiction-related attentional biases? Drug and Alcohol Dependence, 167, 8–14. 10.1016/j.drugalcdep.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2017a). Counterintuitive effects of negative social feedback on attention. Cognition and Emotion, 31, 590–597. 10.1080/02699931.2015.1122576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2017b). Going for it: The economics of automaticity in perception and action. Current Directions in Psychological Science, 26, 140–145. 10.1177/0963721416686181 [DOI] [Google Scholar]
- Anderson BA (2018). Controlled information processing, automaticity, and the burden of proof. Psychonomic Bulletin & Review, 25, 1814–1823. 10.3758/s13423-017-1412-7 [DOI] [PubMed] [Google Scholar]
- Anderson BA (2019). Neurobiology of value-driven attention. Current Opinion in Psychology, 29, 27–33. 10.1016/j.copsyc.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Anderson BA, & Britton MK (2020). On the automaticity of attentional orienting to threatening stimuli. Emotion. Advance online publication. 10.1037/emo0000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Folk CL, Garrison R, & Rogers L (2016). Mechanisms of habitual approach: Failure to suppress irrelevant responses evoked by previously reward-associated stimuli. Journal of Experimental Psychology: General, 145, 796–805. 10.1037/xge0000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Halpern M (2017). On the value-dependence of value-driven attentional capture. Attention, Perception, & Psychophysics, 79, 1001–1011. 10.3758/s13414-017-1289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2018). Relating attentional biases for stimuli associated with social reward and punishment to autistic traits. Collabra. Psychology, 4, 10. 10.1525/collabra.119 [DOI] [Google Scholar]
- Anderson BA, & Kim H (2019a). On the relationship between value-driven and stimulus-driven attentional capture. Attention, Perception, & Psychophysics, 81, 607–613. 10.3758/s13414-019-01670-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2019b). Test-retest reliability of value-driven attentional capture. Behavior Research Methods, 51, 720–726. 10.3758/s13428-018-1079-7 [DOI] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2011). Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America, 108, 10367–10371. 10.1073/pnas.1104047108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2014). Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Research, 1587, 88–96. 10.1016/j.brainres.2014.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Yantis S (2012). Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Attention, Perception, & Psychophysics, 74, 1644–1653. 10.3758/s13414-012-0348-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Yantis S (2013). Persistence of value-driven attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 39, 6–9. 10.1037/a0030860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, & Theeuwes J (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16, 437–443. 10.1016/j.tics.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon WF, & Egeth HE (1994). Overriding stimulus-driven attentional capture. Perception & Psychophysics, 55, 485–496. 10.3758/BF03205306 [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cousineau D (2005). Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology, 1, 42–45. 10.20982/tqmp.01.1.p042 [DOI] [Google Scholar]
- Failing M, & Theeuwes J (2018). Selection history: How reward modulates selectivity of visual attention. Psychonomic Bulletin & Review, 25, 514–538. 10.3758/s13423-017-1380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Brandhofer R, & Schubö A (2016). Rewarded visual items capture attention only in heterogeneous contexts. Psychophysiology, 53, 1063–1073. 10.1111/psyp.12641 [DOI] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, & Luck SJ (2015). Direct evidence for active suppression of salient-but-irrelevant sensory inputs. Psychological Science, 26, 1740–1750. 10.1177/0956797615597913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, & Luck SJ (2017). Suppression of overt attentional capture by salient-but-irrelevant color singletons. Attention, Perception, & Psychophysics, 79, 45–62. 10.3758/s13414-016-1209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J, & Oudeyer PY (2018). Towards a neuroscience of active sampling and curiosity. Nature Reviews Neuroscience, 19, 758–770. 10.1038/s41583-018-0078-0 [DOI] [PubMed] [Google Scholar]
- Grégoire L, & Greening SG (2020). Fear of the known: Semantic generalisation of fear conditioning across languages in bilinguals. Cognition and Emotion, 34, 352–358. 10.1080/02699931.2019.1604319 [DOI] [PubMed] [Google Scholar]
- Grégoire L, & Greening SG (2019). Opening the reconsolidation window using the mind’s eye: Extinction training during reconsolidation disrupts fear memory expression following mental imagery reactivation. Cognition, 183, 277–281. 10.1016/j.cognition.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Jeffreys H (1961). Theory of probability. Oxford, UK: Oxford University Press. [Google Scholar]
- Kim AJ, & Anderson BA (2020). Threat reduces value-driven but not salience-driven attentional capture. Emotion. Advance online publication. 10.1037/emo0000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, & Anderson BA (2019). Neural evidence for automatic value-modulated approach behaviour. NeuroImage, 189, 150–158. 10.1016/j.neuroimage.2018.12.050 [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, & De Houwer J (2004). Does imminent threat capture and hold attention? Emotion, 4, 312–317. 10.1037/1528-3542.4.3.312 [DOI] [PubMed] [Google Scholar]
- Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pelley ME, Pearson D, Griffiths O, & Beesley T (2015). When goals conflict with values: Counterproductive attentional and oculomotor capture by reward-related stimuli. Journal of Experimental Psychology: General, 144, 158–171. 10.1037/xge0000037 [DOI] [PubMed] [Google Scholar]
- Moher J, Anderson BA, & Song JH (2015). Dissociable effects of salience on attention and goal-directed action. Current Biology, 25, 2040–2046. 10.1016/j.cub.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher J, & Egeth HE (2012). The ignoring paradox: Cueing distractor features leads first to selection, then to inhibition of to-be-ignored items. Attention, Perception, & Psychophysics, 74, 1590–1605. 10.3758/s13414-012-0358-0 [DOI] [PubMed] [Google Scholar]
- Morey RD (2008). Confidence intervals from normalized data: A correction to Cousineau (2005). Tutorials in Quantitative Methods for Psychology, 4, 61–64. 10.20982/tqmp.04.2.p061 [DOI] [Google Scholar]
- Mulckhuyse M, & Dalmaijer ES (2016). Distracted by danger: Temporal and spatial dynamics of visual selection in the presence of threat. Cognitive, Affective & Behavioral Neuroscience, 16, 315–324. 10.3758/s13415-015-0391-2 [DOI] [PubMed] [Google Scholar]
- Munneke J, Belopolsky AV, & Theeuwes J (2016). Distractors associated with reward break through the focus of attention. Attention, Perception, & Psychophysics, 78, 2213–2225. 10.3758/s13414-016-1075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munneke J, Hoppenbrouwers SS, & Theeuwes J (2015). Reward can modulate attentional capture, independent of top-down set. Attention, Perception, & Psychophysics, 77, 2540–2548. 10.3758/s13414-015-0958-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Labar KS, & Adcock RA (2012). Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. The Journal of Neuroscience, 32, 8969–8976. 10.1523/JNEUROSCI.0094-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissens T, Failing M, & Theeuwes J (2017). People look at the object they fear: Oculomotor capture by stimuli that signal threat. Cognition and Emotion, 31, 1707–1714. 10.1080/02699931.2016.1248905 [DOI] [PubMed] [Google Scholar]
- Öhman A, & Mineka S (2001). Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review, 108, 483–522. 10.1037/0033-295X.108.3.483 [DOI] [PubMed] [Google Scholar]
- Pearson D, Donkin C, Tran SC, Most SB, & Le Pelley ME (2015). Cognitive control and counterproductive oculomotor capture by reward-related stimuli. Visual Cognition, 23, 41–66. 10.1080/13506285.2014.994252 [DOI] [Google Scholar]
- Pearson D, Watson P, Cheng P, & Le Pelley ME (2020). Overt attentional capture by reward-related stimuli overcomes inhibitory suppression. Journal of Experimental Psychology: Human Perception and Performance, 46, 489–501. 10.31234/osf.io/db9hg [DOI] [PubMed] [Google Scholar]
- Rosenthal R (1991). Meta-analytic procedures for social research (2nd ed.). Newbury Park, CA: Sage. 10.4135/9781412984997 [DOI] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015a). Attentional capture by signals of threat. Cognition and Emotion, 29, 687–694. 10.1080/02699931.2014.924484 [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015b). Potential threat attracts attention and interferes with voluntary saccades. Emotion, 15, 329–338. 10.1037/emo0000041 [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2017). The time course of attentional bias to cues of threat and safety. Cognition and Emotion, 31, 845–857. 10.1080/02699931.2016.1169998 [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, & Kindt M (2012). Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiology of Learning and Memory, 97, 338–345. 10.1016/j.nlm.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome LA, & Zald DH (2006). An emotion-induced attentional blink elicited by aversively conditioned stimuli. Emotion, 6, 523–527. 10.1037/1528-3542.6.3.523 [DOI] [PubMed] [Google Scholar]
- Theeuwes J (1992). Perceptual selectivity for color and form. Perception & Psychophysics, 51, 599–606. 10.3758/BF03211656 [DOI] [PubMed] [Google Scholar]
- Theeuwes J, & Belopolsky AV (2012). Reward grabs the eye: Oculomotor capture by rewarding stimuli. Vision Research, 74, 80–85. 10.1016/j.visres.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, & Poldrack RA (2007). The neural basis of loss aversion in decision-making under risk. Science, 315, 515–518. 10.1126/science.1134239 [DOI] [PubMed] [Google Scholar]
- Tversky A, & Kahneman D (1992). Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty, 5, 297–323. 10.1007/BF00122574 [DOI] [Google Scholar]
- Wang L, Duan Y, Theeuwes J, & Zhou X (2014). Reward breaks through the inhibitory region around attentional focus. Journal of Vision, 14(6), 2. 10.1167/14.12.2 [DOI] [PubMed] [Google Scholar]
- Wang L, Li S, Zhou X, & Theeuwes J (2018). Stimuli that signal the availability of reward break into attentional focus. Vision Research, 144, 20–28. 10.1016/j.visres.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Wang L, Yu H, Hu J, Theeuwes J, Gong X, Xiang Y, … Zhou X (2015). Reward breaks through center-surround inhibition via anterior insula. Human Brain Mapping, 36, 5233–5251. 10.1002/hbm.23004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu H, & Zhou X (2013). Interaction between value and perceptual salience in value-driven attentional capture. Journal of Vision, 13(3), 5. 10.1167/13.3.5 [DOI] [PubMed] [Google Scholar]
- Watson P, Pearson D, Wiers RW, & Le Pelley ME (2019). Prioritizing pleasure and pain: Attentional capture by reward-related and punishment-related stimuli. Current Opinion in Behavioral Sciences, 26, 107–113. 10.1016/j.cobeha.2018.12.002 [DOI] [Google Scholar]
- Wentura D, Müller P, & Rothermund K (2014). Attentional capture by evaluative stimuli: Gain- and loss-connoting colors boost the additional-singleton effect. Psychonomic Bulletin & Review, 21, 701–707. 10.3758/s13423-013-0531-z [DOI] [PubMed] [Google Scholar]
- Yantis S (2000). Goal-directed and stimulus-driven determinants of attentional control. In Monsell S & Driver J (Eds.), Attention and performance XVIII (pp. 73–103). Cambridge, MA: MIT Press. [Google Scholar]


