Abstract
Cu/Zn Superoxide Dismutase (Sod1) catalyzes the disproportionation of cytotoxic superoxide radicals (O2•−) into oxygen (O2) and hydrogen peroxide (H2O2), a key signaling molecule. In Saccharomyces cerevisiae, we previously discovered that Sod1 participates in an H2O2-mediated redox signaling circuit that links nutrient availability to the control of energy metabolism. In response to glucose and O2, Sod1-derived H2O2 stabilizes a pair of conserved plasma membrane kinases - yeast casein kinase 1 and 2 (Yck1/2) - that signal glycolytic growth and the repression of respiration. The Yck1/2 homolog in humans, casein kinase 1-γ (CK1γ), is an integral component of the Wingless and Int-1 (Wnt) signaling pathway, which is essential for regulating cell fate and proliferation in early development and adult tissue and is dysregulated in many cancers. Herein, we establish the conservation of the SOD1/YCK1 redox signaling axis in humans by finding that SOD1 regulates CK1γ expression in human embryonic kidney 293 (HEK293) cells and is required for canonical Wnt signaling and Wnt-dependent cell proliferation.
Keywords: Cu/Zn Superoxide Dismutase, redox signaling, superoxide, hydrogen peroxide, reactive oxygen species, Wnt signaling, cancer
Introduction.
Cu/Zn superoxide dismutase (Sod1) is a highly conserved antioxidant enzyme that detoxifies superoxide radicals (O2•−) by catalyzing its disproportionation into hydrogen peroxide (H2O2) and molecular oxygen (O2). Sod1 is amongst the most abundant soluble proteins, accounts for > 80% of intracellular SOD activity [1], and its importance in oxidative stress protection is underscored by reduced proliferation, decreased lifespan, and metabolic defects when SOD1 is deleted in various cell lines and organisms [2-14]. Most interestingly, using Baker’s yeast as a model unicellular eukaryote, we found that only a small fraction (< 1%) of the total Sod1 pool is required to protect cells against cell-wide damage from O2•− [15,16]. Rather, we found that much larger quantities of Sod1 are required to produce H2O2 for the redox regulation of a pair of highly conserved plasma membrane yeast casein kinases, Yck1 and Yck2, that regulates nutrient-sensing [15].
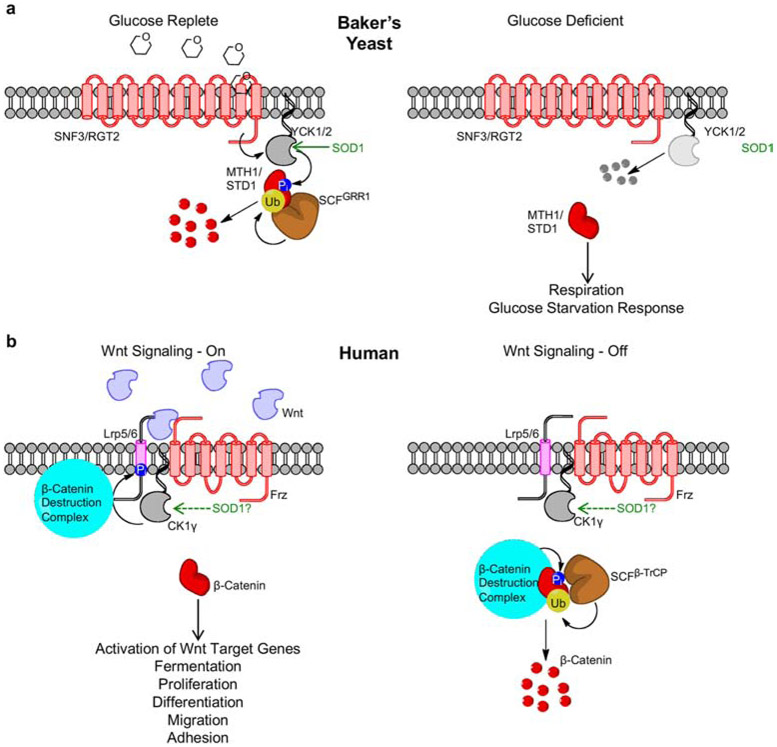
Yck1 and Yck2 link extracellular glucose availability to repression of respiration and fermentative growth [16] (Figure 1a). Glucose binding to transmembrane glucose receptors, Snf3 and Rgt2, activates Yck1 and Yck2, which in turn phosphorylates transcriptional effectors Mth1 and Std1 [17] and signals their ubiquitination and proteasomal degradation by the Skp-Cullin-F-box (SCF) E3 ubiquitin ligase (UbL) Grr1. When glucose is limiting, Mth1 and Std1 are stable and activate the expression of genes required for the metabolic adaptation to low glucose and enhance respiratory metabolism. Sod1-derived H2O2 stabilizes Yck1/2, which leads to glucose-mediated repression of respiration and fermentative growth. When Sod1 or O2•− is diminished, Yck1/2 is degraded and respiratory metabolism is enhanced. Sod1 regulates Yck1 and Yck2 stability via the peroxide-dependent regulation of a still unknown post-translational modification (PTM) on conserved lysine (K) residues at the C-terminus of Yck1 and Yck2.
Figure 1.
Comparison of (a) glucose sensing in Baker’s yeast (Saccharomyces cerivisaie) and (b) Wnt signaling in humans, with an emphasis on the respective roles of Sod1 and plasma membrane casein kinase homologs, Yck1/Yck2 (yeast) and CK1γ (humans). See text for a detailed description of the two pathways.
The Yck1/2 homolog in humans, casein kinase 1-γ (CK1γ), is an integral component of the Wnt signaling pathway, which is essential for regulating cell fate and proliferation in early development and adult tissue and is dysregulated in many cancers [18]. In the canonical Wnt pathway, the transcriptional effector β-catenin activates the expression of Wnt target genes in control of cell proliferation, differentiation, migration, adhesion, and energy metabolism. Just as yeast Yck1/2 link extracellular glucose availability to energy metabolism (Figure 1a), human CK1γ links extracellular signals from a family of Wnt proteins to control metabolism and physiology (Figure 1b). Wnt ligands bind to a heterodimeric receptor complex consisting of Frizzled (Frz) and low-density lipoprotein receptor-related proteins LRP5 and LRP6. This binding event activates CK1γ, which then phosphorylates LRP5/6, ultimately leading to the sequestration of the β-catenin destruction complex. With the destruction complex unavailable, β-catenin is able to translocate to the nucleus where it can activate Wnt-target genes (Figure 1b, left). In the absence of Wnt ligands, the β-catenin destruction complex phosphorylates β-catenin, which in turn leads to its ubiquitination and proteasomal degradation by the SCF UbL β-Transducin Repeat-Containing Protein (β-TrCP) (Figure 1b, right).
Having demonstrated previously that Sod1 regulates Yck1/2 expression and glucose signaling in yeast [15,16], we sought to determine if Sod1 regulates CK1γ expression and Wnt signaling in mammalian cells. Herein, using RNA interference to silence SOD1 in human embryonic kidney cell line (HEK293), we report that SOD1 regulates CK1γ expression and Wnt signaling in mammalian cells.
Materials and Methods.
Chemicals, media components, and immunological reagents.
Dihydroethidium (Cat. # 50-850-563) was purchased from Thermo Fisher Scientific. Paraquat dichloride (Cat # 856177-1G) was purchased from Sigma-Aldrich. The mammalian nuclear isolation kit was purchased from Thermo Fisher Scientific (Cat. #78833). Rabbit polyclonal antibodies against GAPDH (VWR; Cat. # 89348-232), PGK1 (Thermo Fisher; Cat. # PA528612), LRP6 (Cell Signaling; Catalog # 2560), phospho-β-catenin S33/S37/T41 (Cell Signaling; Catalog # 9561), β-catenin (Abcam; Cat # 6302), Phospho-LRP-6 T1479 (Abnova; Cat. # PAB12632) [19], Lamin A/C (Cell Signaling; Cat. # 2032S), CK1γ3 (Thermo Fisher; Cat # PA5-99838) [20], Prdx1 (Millipore Sigma; Cat. # HPA007730-100UL), Trx1 (Cell Signaling; Cat. # 2429), Gpx1 (Abcam; Cat. # ab22604), and Prdx-SO3 (Abcam; Cat. # Ab16830), and mouse monoclonal antibodies against Porin (Invitrogen; Cat. #459500) and Prdx2: PRDX2 (Fisher Scientific; Cat. # LFMA0144) were purchased from commercial sources as indicated. A previously described rabbit polyclonal antibody against Sod1 was obtained from the laboratory of Valeria Culotta (Johns Hopkins University) [16]. Goat α-rabbit (VWR; Cat # 89138-520) or α-mouse (VWR; Cat # 89138-516) secondary antibodies conjugated to a 680-nm emitting fluorophore were obtained from Biotium through VWR.
Cell culture, growth, gene silencing, Wnt stimulation, and nuclear fractionation.
Human embryonic kidney (HEK293) cells were obtained from (American Type Culture Collections. ATCC, Cat. # CRL-1573). Cells were cultured in Dulbecco’s Minimal Essential Medium/ DMEM media (Thermo Fisher Scientific (Cat. # 3105328) containing Heat Inactivated Fetal Bovine Serum (HIFBS, 10 %, VWR Cat # 89510-188), supplemented with L-glutamine in 5% CO2 and 37°C with 1% antibiotics (Penicillin-Streptomycin). Cells were plated in 6 well plates for immunoblotting or qPCR experiments (0.25 - 0.3 million cells/ well), or on 13 mm coverslips in 24 well plates for immunostaining. SOD1 siRNA (Cat. # 4390824) and Control siRNA (Cat. # 4390843) were purchased from (Thermo Fisher Scientific). SOD1 silencing was accomplished in reduced serum medium (Opti-MEM, Cat. # 11058021, Thermo Fischer Scientific) by transfection of SOD1 siRNA or scrambled control siRNA (for 72 hours) into 60-70% confluent HEK cellsusing Lipofectamine-2000 transfection reagent according to Manufacturer's protocols (Cat. # 11668019, Thermo Fischer Scientific).
Wnt conditioned media was produced by culturing L-Wnt-3A cells (ATCC, Cat. # CRL-2647) according to AtCC protocol, and media from L Cells (ATCC, Cat. # CRL-2648) was used as the corresponding negative control. Wnt stimulation was achieved by treating HEK293 cells with DMEM: Wnt3a conditioned media (1:1 v/v) for 3 hours.
Immunoblotting and SOD activity.
Immunoblotting was performed exactly as described previously with cells from 6-well dishes being harvested, washed in ice-cold Milli-Q water, and lysed in two pellet volumes of lysis buffer (10 mM sodium phosphate, 50 mM sodium chloride, 5 mM EDTA, 1.0% Triton X-100, 1 mM PMSF and a protease and phosphatase inhibitor cocktail (GBiosciences) [21]. For assessing peroxiredoxin oxidation, cell cultures were quenched with 10% trichloroacetic acid (TCA), followed by TCA-precipitating lysate protein and washing it in cold acetone. The dried pellet was resuspended in degassed resuspension buffer (6M urea, 10mM EDTA, 20mM Tris, 0.5%SDS, 10μM neocuproine, pH 8.5) containing 1mM PMSF and a protease inhibitor cocktail (GBiosciences) in an anaerobic chamber (Coy laboratories). Lysate protein concentrations were determined by the Pierce™ BCA protein assay kit (Thermo Scientific). SOD activity was measured using an in-gel assay based on native PAGE and nitroblue tetrazolium staining as described previously [16,22-24].
Immunofluorescence.
HEK293 cells plated on coverslips and transfected with control or Sod1 siRNA (72 h), were fixed using 4% paraformaldehyde in phosphate buffered saline (PBS) for 30 min and blocked with 10% FBS for 1 hour. The coverslips were then incubated with the β-catenin antibody for 2 h, followed by Cy3-conjugated secondary antibody for 1 hour. 4′, 6-diamidino-2-phenylindole (DAPI,1:10,000 dilution, Sigma) was used for nuclear staining. The mounted coverslips were imaged using Zeiss LSM510 confocal microscopy to assess cytoplasmic versus nuclear localization of β-catenin. Results were expressed as percent localization relative to the total number of DAPI positive cells. At least 10 fields were imaged from each condition from 3 independent experiments.
qRT-PCR.
RNA was isolated from HEK293 cells using the RNAeasy kit (Qiagen). cDNA was synthesized from 1 ug of total RNA using iscript cDNA synthesis kit (Biorad). qRT-PCR was performed using SYBR Green PCR Master Mix (Biorad) and 7500 Real-Time PCR System (Applied Biosystems) using primers for β-catenin, Cyclin D1, c-myc, GAPDH, and SOD1.
Results.
Sod1 regulates the canonical Wnt signaling pathway.
In order to probe the role of Sod1 in the Wnt signaling pathway, we determined the effect of Sod1 silencing on CK1γ expression and its impact on Wnt pathway activation in HEK293 cells upon stimulation with the Wnt3a ligand (Figure 1b). There are three plasma membrane tethered CK1γ isoforms in humans: CK1γ1, CK1γ2, and CK1γ3. We chose to focus our efforts on Sod1-mediated regulation of CK1γ3 for the following reasons:
in HEK293 cells, CK1γ3 protein levels are ~4-fold higher than CK1γ2 and > 10-fold higher than CK1γ1, which is undetectable [25];
CK1γ3 (and CK1γ2) contains a conserved C-terminal K implicated in Sod1 and peroxide regulation of Yck1/Yck2 - K383 in Yck1 and K355 in CK1γ3 [16];
Sod1 regulates bovine CK1γ3 heterologously expressed in yeast [16].
SOD1 expression and activity is consistently reduced by ~60% using siRNA against SOD1 in HEK293 cells after 72 hours (Figure 2a and 2b). This degree of SOD1 silencing results in a ~40% decrease in CK1γ3 expression (Figure 2a and 2c), as measured using a CK1γ3-specific antibody. These results are consistent with our previous studies in Baker’s yeast that found that sod1Δ cells exhibited a marked loss in Yck1/2 expression and heterologously expressed bovine CK1γ3. It is important to note that SOD1 silencing does not affect the expression of other cytosolic peroxide metabolizing enzymes, including peroxiredoxins 1 and 2 (Figure S1a-d), thioredoxin 1 (Figure S1e and S1f), and glutathione peroxidase 1 (Figure S1e and S1g), thereby indicating that other antioxidant enzymes are not compensating for the loss of Sod1 function. Moreover, silencing of SOD1 does not significantly impact steady-state peroxide levels and oxidative stress as indicated by the lack of significant changes to peroxiredoxin oxidation (Figure S1h-j).
Figure 2.
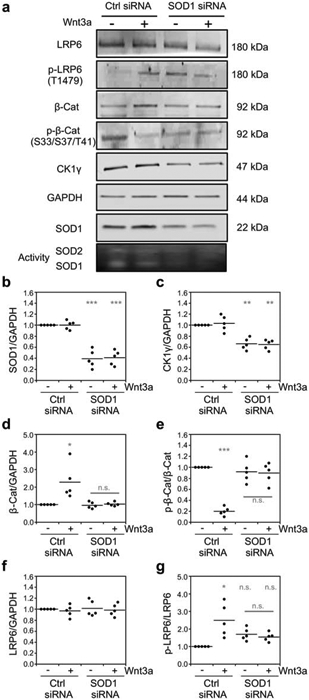
Sod1 is required for the Wnt3a-dependent activation of the canonical Wnt signaling pathway. (a) Representative immunoblots and SOD activity gel of Wnt signaling pathway markers in response to Wnt3a activation and/or silencing of Sod1 with siRNA. (b-g) Quantification of the normalized levels of (b) Sod1, (c) CK1γ3, (d) β-catenin (β-Cat), (e) phosphorylated β-catenin at residues S33, S37, and T41 p-β-Cat), (f) LRP6, and (g) phosphorylated LRP6 at residue T1479 (p-LRP6) from five independent trials. p-LRP6 and p-β-Cat is normalized to total LRP6 or β-Cat, respectively. The statistical significance relative to – Wnt3a/Ctrl RNAi cells or for the indicated pairwise comparison is denoted by grey asterisks and determined by ordinary one-way ANOVA with the Bonferroni multiple-comparison post-hoc test. * P < 0.01, ** P < 0.001, *** P < 0.0001, n.s. = not significant.
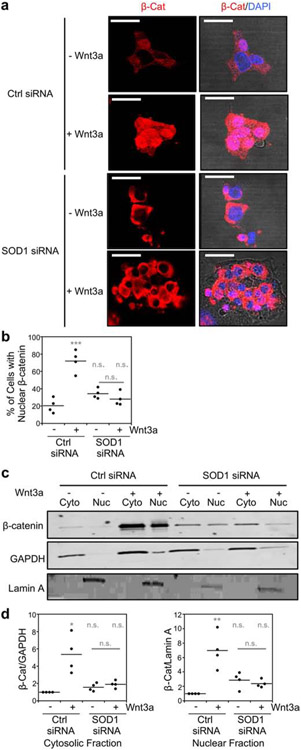
SOD1 silencing and the concomitant decrease in CK1γ3 expression resulted in a significant decrease in multiple markers of Wnt pathway activation (Figure 1b). Wnt3a binding to its cell surface receptor activates CK1γ which in turn phosphorylates LRP6 at T1479, ultimately leading to sequestration of the β-catenin destruction complex [18]. As a consequence, cytosolic β-catenin cannot be phosphorylated at residues S33, S37, and T41, allowing it to escape proteasomal degradation and translocate to the nucleus to regulate gene expression. Accordingly, we find that Wnt3a stimulation results in an increase in LRP6 phosphorylation (Figure 2a and 2g), a decrease in β-catenin phosphorylation (Figure 2a and 2e), an increase in β-catenin stability (Figure 2a and 2d), and increased nuclear β-catenin as measured by immunofluorescence (Figure 3a and 3b) and subcellular fractionation (Figure 3c and 3d). In contrast, Wnt3a stimulation in SOD1 silenced cells does not increase LRP6 phosphorylation (Figure 2a and 2g), decrease β-catenin phosphorylation (Figure 2a and 2e), increase β-catenin stability (Figure 2a and 2d), or increase nuclear localization of β-catenin (Figure 3). Thus, in total, Sod1 is required for Wnt-dependent activation of the canonical Wnt pathway.
Figure 3.
Sod1 is required for the Wnt3a-dependent nuclear localization of β-catenin. (a) Representative immunofluorescence of β-catenin localization relative to the nuclear marker, DAPI, in response to Wnt3a activation and/or silencing of Sod1 with siRNA. Scale bar is 20 μm. (b) Quantification of β-catenin nuclear localization from four independent trials. ~200 cells were counted for each condition in each trial. (c) Representative immunoblots of β-catenin expression in cytosolic and nuclear fractions of HEK293 cells. (d) Quantification of the normalized levels of cytosolic and nuclear β-catenin (β-Cat) from four independent trials. Cytosolic β-Cat is normalized to the cytosolic marker GAPDH and nuclear β-Cat is normalized to the nuclear marker Lamin A. The statistical significance relative to – Wnt3a/Ctrl RNAi cells or for the indicated pairwise comparison is denoted by grey asterisks and determined by ordinary one-way ANOVA with the Bonferroni multiple-comparison post-hoc test. * P < 0.01, ** P < 0.001, *** P < 0.0001, n.s. = not significant.
Sod1 regulates the Wnt-dependent activation of Wnt target genes and cell proliferation.
We next determined if Sod1 impacts the expression of Wnt target genes and Wnt-dependent cell proliferation. Once in the nucleus, β-catenin associates with transcription factors from the T cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors and drives transcription of hundreds of Wnt/β-catenin target genes. Using qRT-PCR, we determined if silencing SOD1 in Wnt stimulated cells altered the expression of Wnt/β-catenin target genes β-catenin, c-Myc, and Cyclin D1. Silencing SOD1 resulted in a 50% decrease in SOD1 mRNA (Figure 4d), consistent with the decrease in Sod1 polypeptide (Figure 2a and 2b). Moreover, silencing SOD1 in Wnt3a stimulated cells reversed the Wnt-induced increase in expression of β-catenin (Figure 4a), c-Myc (Figure 4b), and Cyclin D1 (Figure 4c) mRNA as expected.
Figure 4.
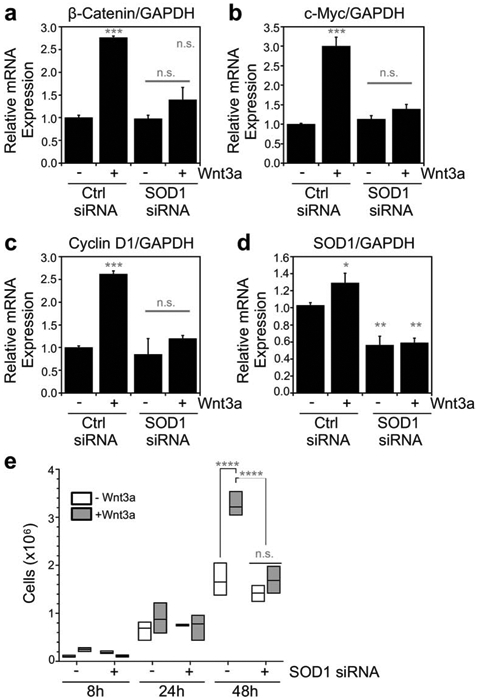
Sod1 regulates Wnt3a-dependent gene expression and HEK293 cell proliferation. (a-d) Normalized mRNA expression of (a) β-catenin, (b) c-Myc, (c) Cyclin D1, and (d) SOD1 relative to GAPDH as measured by qRT-PCR in response to Wnt3a activation and/or silencing of Sod1 with siRNA. The indicated values represent the mean ± s.d. from triplicate cultures. (e) Cell proliferation in response to Wnt3a activation and/or silencing of Sod1 with siRNA. The statistical significance relative to – Wnt3a/Ctrl RNAi cells or for the indicated pairwise comparison is denoted by grey asterisks and determined by ordinary one-way ANOVA with the Bonferroni multiple-comparison post-hoc test. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, n.s. = not significant.
Given that Wnt/β-catenin target genes are associated with proliferative metabolism, we assessed if SOD1 silencing reversed Wnt-induced cell proliferation. Wnt stimulation results in a pronounced enhancement in cell proliferation by 48 hours (Figure 4e). However, in SOD1 silenced cells, the Wnt-mediated stimulation in proliferation at 48 hours is completely reversed (Figure 4e). Notably, simply silencing SOD1 in the absence of Wnt3a stimulation does not cause growth defects at any time point, indicating that the ~50% depletion in SOD1 does not result in overt oxidative stress. Altogether, our data support a role for SOD1 in regulating the Wnt signaling pathway.
Discussion.
Wnt signaling regulates cell fate, proliferation, motility, and differentiation during development and in adult tissues. Dysregulation of the Wnt pathway is associated with many diseases, including various cancers and neurodegenerative disorders [26]. The plasma membrane casein kinases, Yck1/2 in yeast and CK1γ in humans, integrate extracellular ligand binding events to an intracellular signaling cascade that triggers changes in gene expression and cell physiology (Figure 1). Herein, we report that SOD1 regulates CK1γ expression in human embryonic kidney 293 (HEK293) cells and is required for canonical Wnt signaling and Wnt-dependent gene expression and cell proliferation. Our findings have a number of implications for the redox regulation of the Wnt pathway, redox signaling, and Sod1 biology.
H2O2 can both activate and inhibit the Wnt pathway [27,28]. Supplementation with high doses of exogenous peroxide (~100μM) inhibits Wnt signaling in HEK293 cells [29]. In contrast, low doses of H2O2 rapidly stabilize β-catenin and increases the expression of Wnt target genes [30]. The mechanism involves the H2O2 dependent regulation of nucleoredoxin (Nrx) [28], an inhibitor of Dishevelled (Dvl), which inhibits the GSK3β mediated phosphorylation of β-catenin that leads to its proteasomal degradation [31] (Figure 1b). Thus, physiological redox signaling at multiple points in the Wnt pathway and pathological oxidative stress have the potential to modulate Wnt signaling.
A number of cancers are associated with hyperactive Wnt signaling and elevated Sod1 and CK1γ expression [32-39]. Our results suggest that inhibiting Sod1 in such cancers may be a selective therapeutic strategy for suppressing growth in proliferative cells. Indeed, we found that silencing SOD1 only inhibited cell proliferation in Wnt-stimulated HEK293 cells (Figure 4e). These results are consistent with a prior study that found that SOD1 is essential for oncogene-driven mammary tumor formation but dispensable for normal development and proliferation [34]. Altogether, our results provide a strong foundation for exploring the role of SOD1 in regulating redox gradients in control of Wnt signaling in health and disease and anti-SOD1 based cancer therapeutics.
Supplementary Material
Figure S1. Sod1 silencing does not affect the expression of other cytosolic peroxide metabolizing enzymes or peroxiredoxin oxidation. (a) Immunoblots of peroxiredoxins Prdx1 and Prdx2 and Sod1 in response to silencing of SOD1 with siRNA. (b-d) Quantification of the normalized levels of (b) Sod1, (c) Prdx1, and (d) Prdx2. (e) Immunoblots of glutathione peroxidase Gpx1 and thioredoxin Trx1 in response to silencing of SOD1 with siRNA. (f-g) Quantification of the normalized levels of (f) Trx1 and (g) Gpx1. (h) Immunoblots of sulfenic acid oxidized peroxiredoxin (Prdx-SO3) and Sod1 in response to silencing of SOD1 with siRNA. (i-j) Quantification of the normalized levels of (i) Sod1 and (j) Prdx-SO3. Expression of the indicated proteins is normalized to total protein as assessed by the Revert stain from Licor. The statistical significance is assessed using an unpaired student's t-test, where: *p < 0.05, ***p < 0.001, and n.s. = not significant.
Highlights.
Cu/Zn Superoxide Dismutase (Sod1) is a well conserved and abundant antioxidant enzyme.
Sod1 plays important roles in redox signaling due to its production of H2O2.
Sod1 regulates the canonical Wnt signaling pathway.
Acknowledgements.
We acknowledge support from the U.S. National Institutes of Health (GM118744 to A.R.R.), the Blanchard Professorship (to A.R.R.) and start-up funding from the Georgia Institute of Technology (to A.R.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors do not have any conflicts of interest to declare.
REFERENCES
- 1.Reddi AR; Culotta VC Regulation of Manganese Antioxidants by Nutrient Sensing Pathways in Saccharomyces cerevisiae. Genetics 2011, 189, 1261–1270, doi:genetics.111.134007 [pii] 10.1534/genetics.111.134007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elchuri S; Oberley TD; Qi W; Eisenstein RS; Jackson Roberts L; Van Remmen H; Epstein CJ; Huang TT CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 2005, 24, 367–380. [DOI] [PubMed] [Google Scholar]
- 3.Jackson MJ Lack of CuZnSOD activity: a pointer to the mechanisms underlying age-related loss of muscle function, a commentary on "absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy". Free. Radic. Biol. Med 2006, 40, 1900–1902. [DOI] [PubMed] [Google Scholar]
- 4.Muller FL; Song W; Liu Y; Chaudhuri A; Pieke-Dahl S; Strong R; Huang TT; Epstein CJ; Roberts LJ 2nd; Csete M, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med 2006, 40, 1993–2004. [DOI] [PubMed] [Google Scholar]
- 5.Jang YC; Lustgarten MS; Liu Y; Muller FL; Bhattacharya A; Liang H; Salmon AB; Brooks SV; Larkin L; Hayworth CR, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 2010, 24, 1376–1390, doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sentman ML; Granstrom M; Jakobson H; Reaume A; Basu S; Marklund SL Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem 2006, 281, 6904–6909. [DOI] [PubMed] [Google Scholar]
- 7.Reaume AG; Elliott JL; Hoffman EK; Kowall NW; Ferrante RJ; Siwek DF; Wilcox HM; Flood DG; Beal MF; R.H.B. Jr., et al. Motor neurons in Cu/Zn-SOD superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet 1996, 13, 43–47. [DOI] [PubMed] [Google Scholar]
- 8.Phillips J; Campbell S; Michard D; Charbonneau M; Hilliker A Null mutations of copper/zinc superoxide in Drosophila confer hypersensitivity to paraquat and reduced longevity. Proc. Natl. Acad. Sci. U.S.A 1989, 83, 3820–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang E; Kosman D O2-dependent methionine auxotrophy in Cu,Zn Superoxide dismutase deficient mutants of Saccharomyces cerevisiae. J. Bacteriol 1990, 172, 1840–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slekar KH; Kosman D; Culotta VC The yeast copper/zinc superoxide dismutase and the pentose phosphate pathway play overlapping roles in oxidative stress protection. J. Biol. Chem 1996, 271, 28831–28836. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez RJ; Srinivasan C; Munroe WH; Wallace MA; Martins J; Kao TY; Le K; Gralla EB; Valentine JS Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J Biol Inorg Chem 2005, 10, 913–923, doi: 10.1007/s00775-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 12.Bilinski T; Krawiec Z; Liczmanski L; Litwinska J Is hydroxyl radical generated by the fenton reaction in vivo? Biochem. Biophys. Res. Comm 1985, 130, 533–539. [DOI] [PubMed] [Google Scholar]
- 13.Freitas JMD; Liba A; Meneghini R; Valentine JS; Gralla EB Yeast Lacking Cu-Zn Superoxide Dismutase Show Altered Iron Homeostasis. Role of oxidative stress in iron metabolism. J. Biol. Chem 2000, 275, 11645–11649. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan SC; Liba A; Imlay JA; Valentine JS; Gralla EB Yeast Lacking Superoxide Dismutase(s) Show Elevated Levels of "Free Iron" as Measured by Whole Cell Electron Paramagnetic Resonance. J. Biol. Chem 2000, 275, 29187–29192. [DOI] [PubMed] [Google Scholar]
- 15.Montllor-Albalate C; Colin AE; Chandrasekharan B; Bolaji N; Andersen JL; Wayne Outten F; Reddi AR Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae. Redox Biol 2019, 21, 101064, doi: 10.1016/j.redox.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddi AR; Culotta VC SOD1 integrates signals from oxygen and glucose to repress respiration. Cell 2013, 152, 224–235, doi: 10.1016/j.cell.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriya H; Johnston M Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 1572–1577, doi: 10.1073/pnas.0305901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson G; Wu W; Shen J; Bilic J; Fenger U; Stannek P; Glinka A; Niehrs C Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 2005, 438, 867–872, doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 19.Del Valle-Perez B; Arques O; Vinyoles M; de Herreros AG; Dunach M Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol. Cell. Biol 2011, 31, 2877–2888, doi: 10.1128/MCB.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SY; Kim H; Li CM; Kang J; Najafov A; Jung M; Kang S; Wang S; Yuan J; Jung YK Casein kinase-1gamma1 and 3 stimulate tumor necrosis factor-induced necroptosis through RIPK3. Cell Death Dis 2019, 10, 923, doi: 10.1038/s41419-019-2146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna DA; Harvey RM; Martinez-Guzman O; Yuan X; Chandrasekharan B; Raju G; Outten FW; Hamza I; Reddi AR Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc Natl Acad Sci U S A 2016, 113, 7539–7544, doi: 10.1073/pnas.1523802113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luk E; Carroll M; Baker M; Culotta VC Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc. Natl. Acad. Sci. USA 2003, 100, 10353–10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flohe L; Otting F Superoxide dismutase assays In Methods in enzymology: oxygen radicals in biological systems, Packer L, Ed. Academic press: New York, 1984; Vol. 105, pp. 93–104. [DOI] [PubMed] [Google Scholar]
- 24.Montllor-Albalate C; Colin AE; Chandrasekharan B; Bolaji N; Andersen JL; Outten FW; Reddi AR Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae. Redox biology 2019, 21, 101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger T; Wehner A; Schaab C; Cox J; Mann M Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol Cell Proteomics 2012, 11, M111 014050, doi: 10.1074/mcp.M111.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y; Guan Y; Liu H; Wu X; Yu L; Wang S; Zhao C; Du H; Wang X Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun 2012, 420, 397–403, doi: 10.1016/j.bbrc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Caliceti C; Nigro P; Rizzo P; Ferrari R ROS, Notch, and Wnt signaling pathways: crosstalk between three major regulators of cardiovascular biology. Biomed Res Int 2014, 2014, 318714, doi: 10.1155/2014/318714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korswagen HC Regulation of the Wnt/beta-catenin pathway by redox signaling. Dev Cell 2006, 10, 687–688, doi: 10.1016/j.devcel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Shin SY; Kim CG; Jho EH; Rho MS; Kim YS; Kim YH; Lee YH Hydrogen peroxide negatively modulates Wnt signaling through downregulation of beta-catenin. Cancer Lett 2004, 212, 225–231, doi: 10.1016/j.canlet.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Funato Y; Michiue T; Asashima M; Miki H The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol 2006, 8, 501–508, doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 31.Li L; Yuan H; Weaver CD; Mao J; Farr GH 3rd; Sussman DJ; Jonkers J; Kimelman D; Wu D Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J 1999, 18, 4233–4240, doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulalamba W; Janvilisri T Nasopharyngeal carcinoma signaling pathway: an update on molecular biomarkers. Int J Cell Biol 2012, 2012, 594681, doi: 10.1155/2012/594681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S; Fu L; Tian T; Deng L; Li H; Xia W; Gong Q Disrupting SOD1 activity inhibits cell growth and enhances lipid accumulation in nasopharyngeal carcinoma. Cell Commun Signal 2018, 16, 28, doi: 10.1186/s12964-018-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez ML; Shah N; Kenny TC; Jenkins EC Jr.; Germain D SOD1 is essential for oncogene-driven mammary tumor formation but dispensable for normal development and proliferation. Oncogene 2019, 38, 5751–5765, doi: 10.1038/s41388-019-0839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somwar R; Erdjument-Bromage H; Larsson E; Shum D; Lockwood WW; Yang G; Sander C; Ouerfelli O; Tempst PJ; Djaballah H, et al. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc Natl Acad Sci U S A 2011, 108, 16375–16380, doi: 10.1073/pnas.1113554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart DJ; Chang DW; Ye Y; Spitz M; Lu C; Shu X; Wampfler JA; Marks RS; Garces YI; Yang P, et al. Wnt signaling pathway pharmacogenetics in non-small cell lung cancer. Pharmacogenomics J 2014, 14, 509–522, doi: 10.1038/tpj.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart DJ Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst 2014, 106, djt356, doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 38.Bansal N; Mims J; Kuremsky JG; Olex AL; Zhao W; Yin L; Wani R; Qian J; Center B; Marrs GS, et al. Broad phenotypic changes associated with gain of radiation resistance in head and neck squamous cell cancer. Antioxid Redox Signal 2014, 21, 221–236, doi: 10.1089/ars.2013.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X; Waddell DS; Wang W; Wang Z; Liberati NT; Yong S; Liu X; Wang XF Ligand-dependent ubiquitination of Smad3 is regulated by casein kinase 1 gamma 2, an inhibitor of TGF-beta signaling. Oncogene 2008, 27, 7235–7247, doi: 10.1038/onc.2008.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sod1 silencing does not affect the expression of other cytosolic peroxide metabolizing enzymes or peroxiredoxin oxidation. (a) Immunoblots of peroxiredoxins Prdx1 and Prdx2 and Sod1 in response to silencing of SOD1 with siRNA. (b-d) Quantification of the normalized levels of (b) Sod1, (c) Prdx1, and (d) Prdx2. (e) Immunoblots of glutathione peroxidase Gpx1 and thioredoxin Trx1 in response to silencing of SOD1 with siRNA. (f-g) Quantification of the normalized levels of (f) Trx1 and (g) Gpx1. (h) Immunoblots of sulfenic acid oxidized peroxiredoxin (Prdx-SO3) and Sod1 in response to silencing of SOD1 with siRNA. (i-j) Quantification of the normalized levels of (i) Sod1 and (j) Prdx-SO3. Expression of the indicated proteins is normalized to total protein as assessed by the Revert stain from Licor. The statistical significance is assessed using an unpaired student's t-test, where: *p < 0.05, ***p < 0.001, and n.s. = not significant.