Abstract
Purpose:
Radiation pneumonitis (RP) is a common and potentially life-threatening toxicity from lung cancer radiotherapy. Data sets reporting RP rates after post-operative radiation therapy (PORT) have historically been small and with predominantly outdated field designs and radiation techniques. We examined a large cohort of patients in this context to assess the incidence and causes of RP in the modern era.
Materials and Methods:
We reviewed 285 patients with non-small cell lung cancer (NSCLC) treated with PORT at our institution from 5/2004 to 1/2017. Complete dosimetric data and clinical records were reviewed and analyzed with grade 2 or higher RP as the endpoint (RP2+) (CTCAE v4.0). Patients were a median of 67 yo (range 28-87), and most had pathologic stage III NSCLC (91%) and received trimodality therapy (90%). Systematic dosimetric analyses using Dx increments of 5% and Vx increments of 2Gy were performed to robustly evaluate dosimetric variables. Lung V5 was also evaluated.
Results:
The incidence of RP2+ after PORT was 12.6%. Dosimetric factors most associated with RP2+ were total lungV4 (HR 1.04, p<0.001) and heart V16 (HR 1.03, p=0.001). On univariate analysis, the clinical factors of age (HR 1.05, P=0.006) and carboplatin chemotherapy (HR 2.32, p=0.012) were correlated with RP2+. On step-up multivariate analysis, only bivariate models remained significant, including lungV5 (HR 1.037, p<0.001) and age (HR 1.052, p=0.011).
Conclusions:
The incidence of RP after PORT is consistent with the literature. Factors correlated with RP include lung and heart doses, age and carboplatin chemotherapy. These data also suggest that elderly patients may be more susceptible to lower doses of radiation to the lung. Based on these data, dose constraints to limit the risk of RP2+ to <5% in the setting of PORT include lungV5 ≤65% in patients <65 years old and lungV5 ≤36% in patients 65 years or older.
Summary:
In a large, modern cohort of patients receiving post-operative radiation therapy (PORT), radiation doses to the lungs and heart and advanced patient age correlated with risk of radiation pneumonitis. The PORT patient population appears to be sensitive to low doses to the lungs, and the lung total V5 constraint should be prioritized, particularly in patients aged 65 years or older.
Introduction:
For patients who undergo surgical resection for locally advanced non-small cell lung cancer (NSCLC), post-operative radiation therapy (PORT) can lower recurrence rates and improve survival 1-5. Radiation pneumonitis (RP) is a treatment-related toxicity that has been reported to occur in approximately 10-40% of patients receiving radiation therapy (RT) to the lung 6. This toxicity can significantly impact quality of life by causing symptoms such as shortness of breath and cough and can be severe enough to result in supplemental oxygen requirement, hospitalization and, rarely, death. RP has been shown to be associated with decreased overall survival in patients treated with PORT 7.
There are multiple clinical and dosimetric factors that have been previously reported to be associated with RP. Clinical features such as increasing tumor size, lower lobe tumor location, interstitial lung disease, patient functional status, age ≥60 years, female gender, lack of marriage, lack of smoking history, pretreatment pulmonary function (FEV1 <2.0L), and use of adjuvant chemotherapy with carboplatin/paclitaxel have been suggested to increase the risk of RP in definitive RT 8-13. Multiple dosimetric variables have been associated with the risk of RP 9,10,14,15. Standard dosimetric constraints used during radiation planning to reduce the risk of RP include the mean lung dose (MLD) and volume of lung receiving 20 Gy (V20)16.
The 2020 National Comprehensive Cancer Network Guidelines indicate that, “After surgery, lung tolerance to RT is much less than for patients with intact lungs; therefore, more conservative constraints should be used for postoperative RT 16.” Although radiation doses prescribed for PORT are lower compared to definitive radiation therapy, patients may be at increased risk for RP due to the post-operative nature of treatment, the additive inflammation from RT following inflammation from both surgery and chemotherapy, and the decreased lung volume after surgery. The risk of RP among PORT patients compared to definitive RT patients has been investigated, but the results are variable. In one study from China, a higher incidence of RP was found in patients treated with PORT compared to definitive chemoradiation despite lower V20, median lung dose (MLD) and mean heart dose (MHD) in the PORT patients 17. Another study from Duke University, however, found no statistically significant difference in the incidence of RP in patients undergoing PORT vs. definitive RT 18.
Due to the results of the PORT meta-analysis published in 1998, there are concerns regarding the toxicity of PORT19. The patients in this analysis, however, were treated with old techniques, large fields, and altered dose fractionation regimens20. There is less known about the impact of PORT in the modern era with CT-based planning, intensity modulated radiation therapy (IMRT) and conventional fractionation. Three recent studies have investigated clinical and dosimetric factors predicting RP in patients undergoing PORT and found that low radiation doses to the lung (V5, V10) in addition to standard lung dosimetric constraints (MLD, V20) are predictive of RP21-23.
The purpose of this analysis was to further identify and validate clinical and dosimetric factors associated with RP in patients treated with PORT. In contrast to the previously published data, this current analysis uses (1) a very large cohort of patients, (2) patients treated with modern radiation techniques and (3) robust and systematic dosimetric modelling techniques.
Methods and Materials:
We reviewed the clinical records and dosimetry of all consecutive patients with NSCLC treated with PORT and CT-based treatment plans at our institution between 5/2004 and 1/2017 to allow adequate time for follow-up. Detailed dose distributions of 285 patients were available for analysis. This study was completed under an institutional review board approved protocol.
Radiation Therapy:
All patients were treated with 6 MV photons with 3-dimensional conformal radiation therapy (3DCRT) or sliding-window intensity-modulated radiation therapy (IMRT). Patients were treated with the arms immobilized in a custom-made mold above their head. Starting in 2008, all patients were simulated using a 4D scan and ITV approach. Treatment plans from prior to mid-2014 had been generated with an in-house planning system and recalculated with the Analytical Anisotropic Algorithm (AAA) algorithm in the Eclipse planning system (Varian Medical Systems, Palo Alto, CA) for this study. Eclipse with AAA was used for all planning after mid-2014. The treatment plans were designed to deliver a uniform dose within the PTV, with less than a 110% hot-spot.
Dosimetric Constraints:
Standard dosimetric constraints for locally advanced NSCLC treatment were applied to our patient population undergoing PORT, such as mean lung dose ≤ 20 Gy, lung V20 ≤37% and heart V30 ≤ 50%, throughout the period in our study. In late 2015, our lung V20 planning constraint for PORT was reduced to lung V20 ≤30% and a constraint of lung V5 ≤65% conditional on not compromising target coverage was implemented for all conventionally fractionated lung treatments.
Definition of Target and Anatomic Volumes:
PORT target volumes have evolved over time from targeting the whole mediastinum towards a more selective approach. As per our current institutional standard, in our patient population receiving comprehensive nodal dissection, the Clinical Target Volume (CTV) includes the involved nodal stations, bronchial stump, as well as ipsilateral hilum extending into the ipsilateral lower paratracheal and subcarinal spaces, at the treating physician’s discretion. The involved nodal stations are typically contoured with guidance from preoperative imaging, surgical pathology report, surgical operative report, thoracic surgeon input as needed and the mediastinal lymph node atlas published by Chapet et al24. Other than the elective coverage of the ipsilateral hilum into the ipsilateral lower paratracheal and subcarinal spaces, elective nodal irradiation is not typically performed. For patients with pathological findings requiring a boost, such as microscopic positive margins or extranodal extension, a boost volume is contoured. An Internal Target Volume (ITV) approach is used for patients who undergo a 4DCT to account for respiratory motion. In PORT cases, there can be some motion, particularly in the ipsilateral hilum and bronchial stump. In general, a 5 mm margin is added to the ITV to create the Planning Target Volume (PTV). Prior to 4DCT, a 1-1.5 cm margin on the CTV was generally used to create the PTV. Two example PORT cases depicting the CTV, ITV and PTV are illustrated in Figure S1. Lungs were contoured using lung windows on the free-breathing scan. Hearts were retrospectively re-contoured according to the RTOG 1106 OAR Atlas. Dose volume histograms for the heart, total lungs, and individual lungs (considered as either ipsilateral or contralateral or left or right) were generated. Our practice is to define the total lung volume as the total lung-GTV. Because the majority of PORT cases do not contain a GTV, the total lung volume was used for dosimetric analysis.
Endpoint definition.
The endpoint of interest was Common Terminology Criteria for Adverse Events (CTCAE) 4.0 ≥ Grade 2 radiation pneumonitis (RP2+). Patients treated with steroids for RP within the first year of completing PORT were considered to have grade 2 RP.
Statistical Analysis.
Dosimetric and clinical variables were correlated with RP2+ using uni- and multivariate Cox proportional hazards models with significance defined as p < 0.05. The cumulative incidence of RP and overall survival were calculated from the start of radiation therapy. For lungs and heart, the dosimetric variables tested were Dx (minimum dose to the hottest x % volume with volumes ranging from 0-100% in increments of 5%,); Vx, (percent volume that received at least dose x with doses ranging from 2-60 Gy in increments of 2 Gy). For purposes of direct comparison with other papers, we also tested total lung V5. The maximum dose (Dmax), minimum dose (Dmin), mean dose (Dmean), and total volume were tested for each anatomic volume. Finally, the asymmetries between dosimetric variables for the left and right lungs (defined as [MetricR-MetricL]/[MetricR+MetricL]) were tested. Clinical variables tested were age, sex, KPS, stage, smoking status, chemotherapy agent and timing (preoperative vs. post-operative), surgical resection type, surgical margin status, radiotherapy technique (IMRT vs 3D) and radiation prescription dose. Clinical variables found to be significant (p<0.05) on univariate analysis were used in step-up multivariate analysis along with the most significant of each type (Vx, Dx etc.) of dosimetric variable for each organ. Additional competitive models were investigated when variables in the final model were strongly confounded with variables from another organ or clinical variable. Modeling of the dosimetric variables with RP2+, step-up multivariable analyses and RP2+ risk modeling was performed and figures were generated to depict results as performed in prior studies25-29.
Results:
Patient, Treatment and Dosimetric Characteristics:
We analyzed 285 patients. The patient demographics and treatment factors are listed in Table 1. Most patients had clinical Stage III disease (62.7%). At the time of surgery, most patients had pathologic AJCC 7th edition Stage III disease (91.2%). Most patients received either pre-operative or adjuvant chemotherapy (92.3%). No patients received concurrent chemotherapy. No patients had clinically apparent interstitial lung disease. A wide variety of chemotherapy regimens were used. Cisplatin-based chemotherapy was administered most commonly (63.1%). The most commonly used carboplatin-based regimens included carboplatin/pemetrexed (59.8%) and carboplatin/taxane (paclitaxel or docetaxel, 18.6%). Most patients underwent a lobectomy (81.3%), and the rates of R0, R1 and R2 resection were 80.6%, 19.4% and 0% respectively.
Table 1:
Patient and treatment characteristics and their univariate correlation (HR [95% confidence interval (CI)] and p-value) with RP2+
| Variable | N (%) | HR [95% CI] | p-value |
|---|---|---|---|
| Age, median (range), yrs | 67 (28-87) | 1.0547 [1.0963-1.0148] | 0.0058 |
| Sex | |||
| Female | 170 (59.6%) | 1 | |
| Male | 115 (40.4%) | 1.79 [0.931-3.45] | 0.081 |
| KPS, median (range) | 90 (70-100) | 0.989 [0.952-1.03] | 0.63 |
| Clinical Stage (prior to surgery) | |||
| I-II | 106 (37.3%) | 1 | |
| III | 179 (62.7%) | 1.36 [0.381-1.42] | 0.36 |
| Smoking Status | |||
| Never (a) | 50 (17.6%) | 1 | |
| Former (b) | 212 (74.3%) | 0.710 [0.327-1.539] | 0.37 |
| Current (c) | 23 (8.1%) | 0.236 [0.029-1.935] | 0.17 |
| Smokers (b&c), Pack Years, median (range) | 30 (0-165) | 0.994 [0.978-1.01] | 0.48 |
| Smokers (b), Years since quitting, median (range) | 11 (0-55) | 1.02 [0.995-1.05] | 0.12 |
| Chemotherapy Use | 263 (92.3%) | ||
| Preoperative | 150 (57.0%) | 1 | |
| Postoperative | 113 (43.0%) | 1.5 [0.75-3.01] | 0.25 |
| Type of Chemotherapy | |||
| Cisplatin-based | 166 (63.1%) | 1 | |
| Carboplatin-based | 97 (36.9%) | 2.319 [1.189-4.522] | 0.012 |
| Type of Surgery | |||
| Wedge Resection | 29 (10.2%) | 1 | |
| Segment Resection | 5 (1.8%) | 1.031 [0.119-8.943] | 0.98 |
| Lobectomy | 232 (81.3%) | 0.559 [0.227-1.375] | 0.20 |
| Pneumonectomy | 19 (6.7%) | 0.231 [0.027-2.005] | 0.18 |
| Surgical Resection Margin Status | |||
| R0 | 230 (80.6%) | 1 | |
| R1 | 55 (19.4%) | 0.952 [0.621-1.46] | 0.82 |
| R2 | 0 (0%) | ||
| Radiation Technique | |||
| IMRT | 201 (70.4%) | 1 | |
| 3DCRT | 84 (29.6%) | 1.5 [0.682-3.29] | 0.31 |
| Radiation Prescription Dose, median (range), Gy | 54 (45-70) | 0.955 [0.864-1.055] | 0.35 |
| 45-50 Gy | 40 (14%) | ||
| 50-54 Gy | 202 (71%) | ||
| 56-66 Gy | 42 (15%) | ||
| 70 Gy | 1 (0%) |
The median follow-up time was 23 months (range: 1-117 months). Median overall survival was 5.4 years. The crude rate of RP2+ was 12.6% (Grade 2: 32 cases, Grade 3: 3 cases, Grade 4: 1 case, Grade 5: 0 cases). RP2+ was diagnosed at a median of 2.6 months from start of treatment (range: 1.4-8.0).
The median prescription dose was 54 Gy (range: 45-70 Gy) in 1.8 or 2 Gy fractions. Only 10% of patients received 60 Gy or above, typically for positive margins. One patient with an R1 resection, extranodal extension, and surgical concern received 70 Gy, although this dose is not our standard practice. The majority (70.4%) of patients underwent treatment with IMRT, and the remainder were treated with static-field 3DCRT.
Correlation of RP2+ with clinical variables
Of the clinical variables tested, only age (p=0.006) and use of carboplatin (p=0.012) were significantly correlated with RP2+. Results of the univariate analyses including p-values, hazard ratios, and 95% uncertainties of their univariate correlation with RP2+ for the clinical variables are demonstrated in Table 1. Sex, KPS, stage, smoking status, chemotherapy timing (preoperative vs. post-operative), surgical resection type, surgical margin status, radiotherapy technique (IMRT vs 3D), and radiation prescription dose were not statistically significant.
Correlation of RP2+ with dosimetric variables
Table 2 lists the median and range of doses to the lung and the heart as well as the results of the univariate analyses including p-values, hazard ratios and 95% uncertainties of their univariate correlation with RP2+. The mean, minimum, and maximum dose of anatomic structures and the structure volumes of the lungs and heart were tested by Cox proportional hazards models for RP2+ (Table 2). The asymmetries of these variables between the left and right lungs were also evaluated. The maximum dose and structure volume for all structures, as well as the asymmetry of dose to the left and right lungs were not statistically significant (p=0.22, mean asymmetry). Mean and minimum doses to the total lungs and heart, the mean dose to the ipsilateral lung, as well as the minimum dose to the left lung were significant in predicting for RP2+ (Table 2).
Table 2:
Dosimetric variables and their univariate correlation (HR [95% confidence interval (CI)] and p-value) with RP2+
| Variable (Units) | Median value (range) |
HR (per Gy or per %) [95% CI] | p-value |
|---|---|---|---|
| Ipsilateral-lung mean dose (Gy) | 21.3 (3.0-45.2) | 1.070 [1.014-1.113] | 0.012 |
| Ipsilateral-lung min dose (Gy) | 0.6 (<0.01-5.3) | 1.438 [0.964-2.145] | 0.07 |
| Contralateral-lung mean dose (Gy) | 6.1 (0.4-19.8) | 1.007 [0.992-1.021] | 0.33 |
| Contralateral-lung min dose (Gy) | 0.46 (<0.01-0.46) | 1.009 [0.974-1.045] | 0.61 |
| Right lung mean dose (Gy) | 17.3 (2.3-33.5) | 1.006 [0.964 - 1.050] | 0.77 |
| Right lung min dose (Gy) | 0.49 (<0.01-3.85) | 1.597 [0.921-2.768] | 0.089 |
| Left lung mean dose (Gy) | 6.78 (0.36-45.2) | 1.0340 [1.000-1.069] | 0.0460 |
| Left lung min dose (Gy) | 0.37 (<0.01-5.27) | 1.502 [1.021-1.210] | 0.0350 |
| Lungs mean dose (Gy) | 11.9 (1.8-19.5) | 1.189 [1.064-1.328] | 0.0018 |
| Lungs min dose (Gy) | 0.30 (< 0.01 - 1.39) | 5.366 [1.889-15.241] | 0.0012 |
| Lungs V20 (%) | 21.3 (1.7-44.8) | 1.090 [1.035- 1.148] | 0.0008 |
| Heart mean dose (Gy) | 11.2 (9.4-33.4) | 1.0656 [1.0213-1.1117] | 0.0027 |
| Heart min dose (Gy) | 0.55 (<0.01-8.11) | 1.284 [1.055-1.563] | 0.0110 |
| Lung D75 (Gy) | 1.85 (0.25-8.25) | 1.369 [1.148-1.633] | 0.0004 |
| Lung V4 (%) | 54.3 (5.4-92.1) | 1.040 [1.018-1.063] | 0.0003 |
| Lung V5 (%) | 50.2 (4.7-88) | 1.039 [1.016-1.062] | 0.0005 |
| Heart D60 (Gy) | 2.35 (0.15-41.1) | 1.068 [1.026-1.110] | 0.0009 |
| Heart V16 (%) | 23.0 (<0.1-90.5) | 1.025 [1.009-1.040] | 0.0011 |
Modeling of dosimetric variables with RP2+
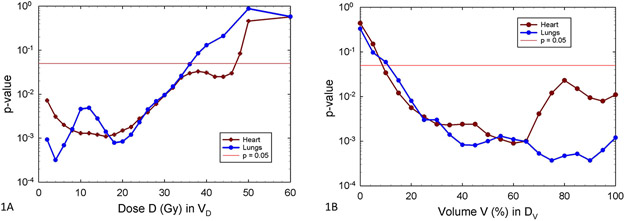
Many lung and heart dosimetric variables correlated with RP2+. The strongest univariate correlations for lung and heart dosimetric variables were for total lung V4 (p=0.00032) and heart V16 (p = 0.0011). For comparison with other studies, we also examined equivalent models based on total lung V5 (p = 0.00048)22,30,31. For heart VD, in the range of 8-20 Gy, there was a broad minimum in the p-values where they are ≤1.5 X 10−3. The univariate correlation of RP2+ with DVH variables for total lungs and heart are shown in Figure 1A and 1B, with p-value on the y-axis and dosimetric parameters on the x-axis. Figure 1A shows significant correlation of VD for both heart and total lungs over a wide range of low doses, up to approximately 30 Gy.
Figure 1:
Univariate correlation of Cox proportional hazards models for RP2+ with DVH variables, for total lung (blue) and heart (maroon). The p-values are shown versus either VD (1A, left), or DV (1B, right).
Significant univariate correlation with DV of heart and total lungs was seen for a wide range of percent volumes (Figure 1B) with the strongest for total lung D75 (p = 0.00037) and heart D60 (p=0.0009). These results demonstrated that RP2+ correlated with low doses to a large volume of heart and total lung, consistent with the VD findings above.
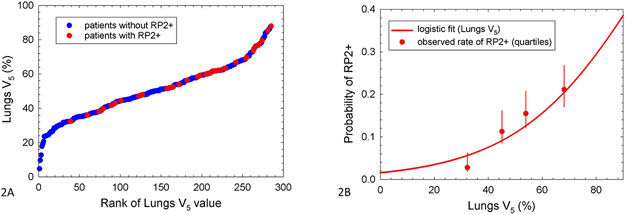
The ranked patient distribution of V5 values, showing RP2+ cases as red dots, is shown in Figure 2A. Patients without RP2+ are indicated in blue dots. Figure 2B depicts a logistic model of the rate of RP2+ by V5, overlaid with observed RP2+ rates in quartiles of V5.
Figure 2:
Association between total lung volume exposed to at least 5 Gy and RP2+. Ranked patient distribution of lungs V5 showing RP2+ cases (2A, left), and logistic model of RP2+ based on lungs V5 (2B, right). In 2A, lungs V5 values for patients with and without RP2+ are shown by red and blue dots, respectively. In 2B, uncertainties are 68% confidence intervals, and observations are plotted at the median value of lungs V5 within the quartiles.
Univariate hazard ratios for the most significant VD and DV variables (total lung V4 and D75; heart V16 and D60), along with those for total lung V5 are given at the bottom of Table 2. These variables had superior correlation with RP2+ compared to the Dmean, Dmin, and Dmax variables, as demonstrated by the lower p-values.
Multivariable analyses and RP Risk Modeling
On step-up multivariable analysis, total lung V5 (HR: 1.037 [1.015-1.060], p=0.0009) and age (HR: 1.052 [1.011-1.095], p=0.011) remained significant. The use of carboplatin was no longer significant, likely because age and carboplatin were themselves strongly correlated on logistic regression (p<10−7). Due to better tolerance, carboplatin is often preferred in frail or elderly patients. Additionally, total lung V5 and heart V16 strongly correlated on logistic regression (p<10−7). Other bivariate models, such as heart V16 (HR: 1.024 [1.009-1.039], p=0.0013) and age (HR: 1.053 [1.014-1.093], p=0.0063) were also significant. There were no significant tri-variate models.
Figures 3 and 4 demonstrate RP risk models that incorporate dosimetric risk factors (total lung V5 and heart V16) with a clinical risk factor (age). Figure 3 show differences in actuarial freedom from RP2+ for patients with Cox model multivariate metrics above and below the median values of “M”. Metrics are based on either total lung V5 (Figure 3A, M= 0.0364*lung V5 + 0.0510*age), or heart V16 (Figure 3B, M= 0.0238* heart V16 + 0.0515*age) in %, and age in years. For patients above and below the median values, actuarial rates of RP2+ at 1 year are 21.1 vs 5.1% (p=0.000078) (Figure 3A) and 18.9 vs 7.3% (p=0.0029) (Figure 3B), respectively.
Figure 3.
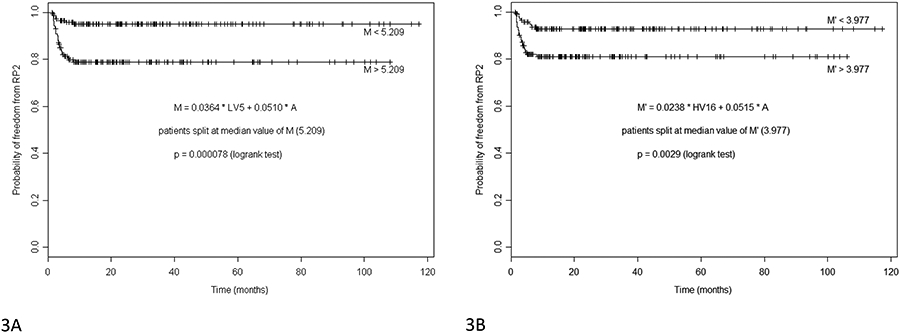
Difference in actuarial freedom from RP2+ for patients with Cox model multivariate metrics (Table 2) above and below the median values. Metrics are based on either Lungs V5 (3A, left), or Heart V16 (3B, right) in %, and age in years. For patients above and below the median values, actuarial rates of RP2+ at 1 year are 21.1 vs 5.1 % (left) and 18.9 vs 7.3 % (right) respectively.
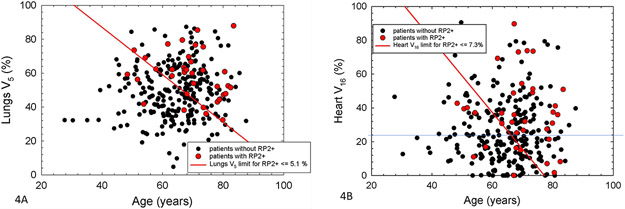
Figure 4:
Scatter plots of patient age and either lungs V5 (4A, left) or heart V16 (4B, right). Values for patients with or without RP2+ are shown by red or black dots, respectively. The red lines show the median value of the multivariate metrics for the relevant Cox models. For patients below and to the left of the red lines, actuarial rates of RP2+ at 1 year are ≤ 5.1 and 7.3 % in 4A and 4B, respectively.
Figure 4 shows the patient distribution of age and total lung V5 (Figure 4A) or heart V16 (Figure 4B) values by RP2+ status. The red circles are patients with RP2+, and the black dots are patients without RP2+. The red lines represent the median value of the appropriate multivariate metric (M), as noted by the equations in Figure 3 and above. In both cases, the patients below and left of the red lines have actuarial rates of RP2+ < 10% at 1 year (5.1% and 7.3% for the models based on age and either total lung V5 or Heart V16, respectively).
Using these risk models, patients <65 years old with a total lung V5 ≤65%, have a risk of RP2+ <5%. For patients 65 years or older, however, the risk RP2+ is less than 5% when the total lung V5 is ≤ 36%. For these older patients, the risk of RP2+ is >15% when the V5 is >65%. Supplemental Figure S2 depicts RP2+ by total lung V5 in patients older or younger than 65 years old to further illustrate this. In our entire patient population, only 21% of patients achieved a total lung V5 ≤36%. These patients had beam arrangements that heavily weighted dose in the anterior/posterior (AP/PA) directions, but many of these patients also had very limited target volumes or underwent prior pneumonectomy.
Discussion:
In this analysis of a large cohort of NSCLC patients treated with modern radiation therapy techniques for PORT, our pertinent findings are as follows: First, dosimetric factors, especially high percentages of low dose volumes to the total lung (V5) and heart (V16), are risk factors for the development of RP. Second, clinical factors, such as age and carboplatin use correlate with the development of RP. Finally, we propose a RP risk model that incorporates dosimetric risk factors (lung V5) with clinical risk factors (age) to help tailor the risk of RP in individual patients.
Our study reported a raw rate of RP2+ of 12.6% and RP3+ of 1.4%. These rates are consistent with the literature from smaller, modern series. A recent publication from Boonyawan et al., which also evaluated risk factors for RP2+ in 199 patients undergoing PORT, reported rates of RP2+ of 15% and RP3+ of 3% 21. In an earlier publication looking at risk factors for RP in 90 patients undergoing PORT, Zhao et al., reported a rate of RP2+ of 10%23. Tang et al. also recently published their data on the predictors of RP in 109 patients undergoing PORT in China and reported rates of RP3+ of 23.9%, RP4+ of 7.3% and RP5 of 1.8%22. These higher rates may possibly be explained by the relatively high rate (25.7%) of concurrent chemotherapy used with PORT in that analysis, which the investigators identified as a risk factor for pneumonitis. In our study, no patients received concurrent chemotherapy.
The significant dosimetric factors seen in our analysis, particularly total lung V5 and heart V16, indicate the importance of minimizing low doses of radiation to the lungs and heart. While a range of doses to the lung and heart (V2-V30) were highly significant for predicting for RP2+, lung V4 and heart V16 demonstrated the strongest correlation with the lowest p-value. Older studies evaluating dosimetric predictors of RP after PORT, which used 3DCRT, demonstrated a correlation with lung V20-V40 and RP23,32. The correlation between low lung dose and radiation pneumonitis has been established in patients with esophageal cancer undergoing trimodality therapy33,34. In patients with NSCLC undergoing trimodality therapy, the correlation of low lung doses with RP has been seen in more recent studies21,22. In these studies, many (49-90%) patients were treated with IMRT 21,22. In our study, 71% of patients were treated with IMRT. However, IMRT as an independent variable did not demonstrate a correlation with RP2+ in our study or in the studies from Tang et al. or Boonyawan et al 21,22. The extent to which IMRT increases the low dose to the lungs and heart is influenced by the angles and extent of arcs chosen, which may explain the lack of correlation between IMRT and RP2+.
Both Boonyawan et al. and Tang et al. also found that low doses to the lungs correlate with RP. Boonyawan et al. found that lung V10 and lung V20 were the best predictors of RP with the best cut points as V10<30% and V20<20%. They defined patients as high-risk if the lung DVH was above both cut points, intermediate-risk if it was above one cut point and low-risk if it was below both cut points. The RP2+ rates in the high-risk, intermediate-risk and low-risk groups were 33%, 23% and 6%, respectively. The authors did not analyze heart dose. Tang et al. found that total lung mean dose (>10.8 Gy), ipsilateral lung V5 (>64.9%) and concurrent chemotherapy predicted for severe acute RP and created a nomogram using these factors to estimate risk of pneumonitis. The authors analyzed heart dose (V10, V20, V30, V40, V50) but did not find any significant heart dosimetric factors.
Our analysis is novel in identifying low dose to the heart (V2-V30, but the strongest correlation with V16) as a significant predictor of RP2+ in patients undergoing PORT. Heart dose has been associated with RP in patients undergoing definitive radiation in NSCLC and in mesothelioma 35,36. Bradley et al. found that higher heart V5 was associated with decreased overall survival in patients with locally advanced NSCLC undergoing definitive concurrent chemoradiation on RTOG 061735. In a retrospective analysis of 209 NSCLC patients treated with definitive radiation therapy using 3DCRT, Huang et al. found significant correlation between RP and a range of heart doses, including heart V10 36. In an analysis of 103 mesothelioma patients treated with IMRT, Yorke et al. found significant correlation between RP and heart V35- V47, as well as mean heart dose27. Both heart dose and the development of RP has been correlated with overall survival7,35. Although low dose to the heart did correlate with RP 2+ in our analysis, because there was also a strong correlation between lung dose and heart dose, our analysis is underpowered to determine if that heart dose is an independent predictor of RP in PORT patients. Understanding how radiation dose to the heart increases the risk for RP is challenging. In addition to potential immunological mechanisms, because the correlation between heart dose and lung dose is strong, it is possible that heart dose is a surrogate for lung dose, particularly dose to the lower lobes of the lung, which has been shown to increase the risk of RP9.
Significant clinical risk factors in our analysis were increasing age and carboplatin chemotherapy. Due to the large variety of chemotherapy combinations and the low number of patients who received carboplatin/paclitaxel, we did not further analyze specific platinum doublets. In a metanalysis of 836 patients undergoing definitive concurrent chemoradiation, elderly patients undergoing concurrent chemoradiation with carboplatin/paclitaxel were at the highest risk for developing RP9. This study created a recursive partitioning analysis incorporating the type of chemotherapy and age with dosimetric factors (lung V20 and mean lung dose) to create groups that are high risk, intermediate risk and low risk groups for RP. There is less reported to date in the PORT literature about the role of clinical risk factors. Boonyawan et al., in contrast, found that carboplatin/paclitaxel chemotherapy decreased the risk for RP2+ on univariate analysis, and that age was not significant. The authors found performance status to be predictive on univariate analysis, but our analysis did not demonstrate this. On their multivariable analyses, however, all clinical risk factors were no longer significant21. Tang et al. did not analyze the type of chemotherapy but found that concurrent chemotherapy increased the risk of severe RP. Other clinical risk factors analyzed, including age and performance status, were not significant on their univariate analysis22. Zhao et al., found that adjuvant chemotherapy was the only clinical risk factor that increased the risk of RP. The type of adjuvant chemotherapy was not predictive23. These three PORT studies analyzed smaller numbers of patients, which may explain the lack of significance seen on multivariable analysis. Additionally, as seen in our analysis with age and carboplatin use, some of the risk factors may correlate with each other and, therefore, result in a lack of significance. In our analysis, among patients who received chemotherapy, the choice of carboplatin was strongly correlated with age (p <10−7). Age was more strongly correlated (lower p-value) with RP2+ than use of carboplatin, and carboplatin was thus eliminated in the multivariate analysis.
Our analysis is novel in that we propose a RP risk model that incorporates dosimetric risk factors (total lung V5 and heart V16) with a clinical risk factor (age) to help tailor the risk of RP in individual patients. Given the findings of this study, as well as the recently published literature, which indicate that the PORT patient population may be more sensitive to low doses to the lungs, we recommend a tighter lung V5 constraint in our PORT patients, particularly for those patients aged 65 years or older. Based on our model (Figure S2), to minimize the risk of RP2+ to <5%, treatment plans should satisfy dosimetric constraints of:
Lung V5 ≤65% in patients <65 years old
Lung V5 ≤36% in patients 65 years or older
Limiting the lung V5 to ≤36% would be challenging in the average PORT patient. Therefore, it is important for clinicians to counsel patients 65 years or older about the increased risk of RP2+. For these older patients, the risk of RP2+ is >15% when the V5 is >65%. Because the prescription dose for PORT is close to the spinal cord constraint (maximum dose of 45-50 Gy), we recommend that planners weight the AP/PA beams heavily and limit the beam angles and arcs for IMRT cases, while still respecting cardiac constraints. Proton therapy is another modality that can be considered to lower the lung V5 as clinical reports have demonstrated lower lung V5 values in NSCLC patients treated with proton therapy for PORT37. Due to the strong correlation between lung dose and heart dose resulting in uncertainty of heart dose as an independent predictor of RP2+ in PORT patients, we do not have separate heart constraint recommendations. However, incorporating the updated NCCN guidelines constraint of mean heart dose ≤ 20 Gy for all conventionally fractionated lung cancer patients will help to limit unnecessary dose to the heart16.
The pathophysiology of RP is complex and involves cytotoxic damage to type II pneumocytes and vascular endothelial cells, which give rise to an initial inflammatory response and dysregulation of a cytokine signal transduction cascade.38 A hypothesis for the trends seen in our study and other post-operative studies in lung and esophageal cancer is that post-surgical inflammation may increase the sensitivity of the lung to low doses of radiation. Elderly patients are at increased risk for post-surgical morbidity and mortality, likely due to higher rates of medical comorbidities, which may also predispose them to slower rates of recovery from the radiation-induced inflammatory response.39,40
The limitations of our study include the heterogeneity of patients and treatment paradigms, as well as its retrospective nature, which can result in confounding and underreporting of toxicities. Additionally, although no patients were diagnosed with clinically apparent interstitial lung disease, CT imaging was not retrospectively reviewed by our radiologists to evaluate for subclinical interstitial lung changes, which is a risk factor for radiation pneumonitis13. Robust statistical analyses were performed to overcome these limitations as much as possible. Strengths of our study include the largest number of patients used to evaluate predictors of RP in patients undergoing PORT for NSCLC. Additionally, the systematic dosimetric analysis using Dx increments of 5% and Vx increments of 2 Gy demonstrates a thorough and robust strategy for analyzing dosimetric predictors. To our knowledge, our study is the first to find heart dose as a risk factor for RP in the PORT setting. Additionally, the study is novel as we present RP risk models and propose constraints that incorporate both dosimetric and clinical risk factors to predict the risk of RP in the PORT setting. The data presented have helped to identify risk factors for RP so that treatment plans and estimations of risk can be personalized for each patient’s case, depending on their age and dosimetric parameters. Further work validating these findings are necessary.
Conclusion:
Radiation pneumonitis is a toxicity that can develop in patients receiving thoracic radiation therapy. Patients receiving PORT for NSCLC may have unique risk factors and sensitivities to radiation therapy compared to NSCLC patients receiving definitive RT. Given the findings of this study as well as the recently published literature, which indicate that the PORT patient population may be more sensitive to low radiation doses to the lung, the total lung V5 constraint should be prioritized in the PORT patient population, particularly in patients aged 65 years or older. Based on our data, dosimetric constraints to limit the risk of RP2+ to <5% in the setting of PORT include total lung V5 ≤65% in patients <65 years old and total lung V5 ≤36% in patients 65 years or older.
Supplementary Material
Supplemental Figure S1: Example PORT Cases demonstrating the target volumes contours: CTV (blue), ITV (pink) and PTV (red). Figure S1A is a case of a 56 yo male with a clinical T2N2M0 squamous cell carcinoma of the right upper lobe who received neoadjuvant chemotherapy followed by a lobectomy and mediastinal lymph node dissection revealing ypT2N2M0 squamous cell carcinoma with negative margins, positive levels 2R and 4R and negative levels 7, 10, 11 and 12. Figure S1B is a case of a 73 yo female with a pT1bN2M0 adenocarcinoma of the left upper lobe who underwent a lobectomy and mediastinal lymph node dissection with negative margins and positive levels 5 and 7 and negative levels 9, 10, and 11.
Supplemental Figure S2: Radiation Pneumonitis rate by Lung V5 in patients older vs. younger than 65 years old
Acknowledgments
Grants:
MSKCC Cancer Center Support Grant
Principal Investigator (Thompson)
Agency: NIH/NCI
5 P30 CA008748-54
Period: 1/1/19 - 12/31/23
Disclosures:
Travel Reimbursement and Consulting: ASCO
Disclosures: none
Disclosures: none
Disclosures: none
Disclosures: none
Disclosures:
Consulting: PharmaMar, Novartis, Targeted Oncology
Travel: Bristol-Myers Squibb, Merck Sharp & Dohme
Disclosures: Advisory Board and Research Grant Support from BMS, AZ, Genentech, Merck
Disclosures: none
Disclosures:
Consulting: AstraZeneca, Varian Medical Systems, Merck, Cybrexa, MoreHealth
Research Grants: AstraZeneca, Varian Medical Systems, Boehringer Ingelheim, Pfizer, Merck
Travel Reimbursement: Philips/Elekta
Disclosures:
Research grants from CivaTech Oncology
Consulting for AstraZeneca
Honoraria (travel grant) from AlphaTau Medical
Disclosures: none
Disclosures: Research Funding from Novartis
Disclosures: Varian Medical Systems honorarium
Disclosures: none
Disclosures: none
Disclosures: none
Footnotes
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Annemarie Shepherd, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Michelle Iocolano, Stony Brook University School of Medicine, Stony Brook, NY.
Jonathan Leeman, Department of Radiation Oncology, Dana Farber Cancer Institute/Brigham and Women’s Hospital.
Brandon S. Imber, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Aaron T. Wild, Southeast Radiation Oncology Group, Charlotte, NC, United States.
Michael Offin, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, United States..
Jamie E. Chaft, Thoracic Oncology Service, Division of Solid Tumor Oncology, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, United States..
James Huang, Thoracic Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY.
Andreas Rimner, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Abraham J. Wu, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Daphna Y. Gelblum, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Narek Shaverdian, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Charles B. Simone, II, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Daniel R. Gomez, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center NYC, NY.
Ellen D. Yorke, Department of Medical Physics, Memorial Sloan Kettering Cancer Center NYC, NY.
Andrew Jackson, Department of Medical Physics, Memorial Sloan Kettering Cancer Center NYC, NY.
References:
- 1.Bradley JD, Paulus R, Graham MV, et al. : Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: promising long-term results of the Radiation Therapy Oncology Group--RTOG 9705. J Clin Oncol 23:3480–7, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Lally BE, Zelterman D, Colasanto JM, et al. : Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 24:2998–3006, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Rosell R, De Lena M, et al. : Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys 72:695–701, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Robinson CG, Patel AP, Bradley JD, et al. : Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol 33:870–6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herskovic A, Mauer E, Christos P, et al. : Role of Postoperative Radiotherapy in Pathologic Stage IIIA (N2) Non-Small Cell Lung Cancer in a Prospective Nationwide Oncology Outcomes Database. J Thorac Oncol 12:302–313, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues G, Lock M, D'Souza D, et al. : Prediction of radiation pneumonitis by dose - volume histogram parameters in lung cancer--a systematic review. Radiother Oncol 71:127–38, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Butof R, Kirchner K, Appold S, et al. : Potential clinical predictors of outcome after postoperative radiotherapy of non-small cell lung cancer. Strahlenther Onkol 190:263–9, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Robnett TJ, Machtay M, Vines EF, et al. : Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 48:89–94, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Palma DA, Senan S, Tsujino K, et al. : Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 85:444–50, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogelius IR, Bentzen SM: A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol 51:975–83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simone CB 2nd: Thoracic Radiation Normal Tissue Injury. Semin Radiat Oncol 27:370–377, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Glick D, Lyen S, Kandel S, et al. : Impact of Pretreatment Interstitial Lung Disease on Radiation Pneumonitis and Survival in Patients Treated With Lung Stereotactic Body Radiation Therapy (SBRT). Clin Lung Cancer 19:e219–e226, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Lee YH, Kim YS, Lee SN, et al. : Interstitial Lung Change in Pre-radiation Therapy Computed Tomography Is a Risk Factor for Severe Radiation Pneumonitis. Cancer Res Treat 47:676–86, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schallenkamp JM, Miller RC, Brinkmann DH, et al. : Incidence of radiation pneumonitis after thoracic irradiation: Dose-volume correlates. Int J Radiat Oncol Biol Phys 67:410–6, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Luna JM, Chao HH, Diffenderfer ES, et al. : Predicting radiation pneumonitis in locally advanced stage II-III non-small cell lung cancer using machine learning. Radiother Oncol 133:106–112, 2019 [DOI] [PubMed] [Google Scholar]
- 16.NCCN Guidelines Version 2.2020 Non-Small Cell Lung Cancer; https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf, 2020 [Google Scholar]
- 17.Dang J, Li G, Zang S, et al. : Comparison of risk and predictors for early radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with radiotherapy with or without surgery. Lung Cancer 86:329–33, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Kocak Z, Yu X, Zhou SM, et al. : The impact of pre-radiotherapy surgery on radiation-induced lung injury. Clin Oncol (R Coll Radiol) 17:210–6, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet 352:257–63, 1998 [PubMed] [Google Scholar]
- 20.Shepherd AF: Proton therapy for post-operative radiation therapy of non-small cell lung cancer. Transl Lung Cancer Res 7:205–209, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boonyawan K, Gomez DR, Komaki R, et al. : Clinical and Dosimetric Factors Predicting Grade >/=2 Radiation Pneumonitis After Postoperative Radiotherapy for Patients With Non-Small Cell Lung Carcinoma. Int J Radiat Oncol Biol Phys 101:919–926, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Li Y, Tian X, et al. : Predicting severe acute radiation pneumonitis in patients with non-small cell lung cancer receiving postoperative radiotherapy: Development and internal validation of a nomogram based on the clinical and dose-volume histogram parameters. Radiother Oncol 132:197–203, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Ji W, Ou G, et al. : Risk factors for radiation-induced lung toxicity in patients with non-small cell lung cancer who received postoperative radiation therapy. Lung Cancer 77:326–30, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Chapet O, Kong FM, Quint LE, et al. : CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys 63:170–8, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Din SU, Williams EL, Jackson A, et al. : Impact of Fractionation and Dose in a Multivariate Model for Radiation-Induced Chest Wall Pain. Int J Radiat Oncol Biol Phys 93:418–24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai CJ, Jackson A, Setton J, et al. : Modeling Dose Response for Late Dysphagia in Patients With Head and Neck Cancer in the Modern Era of Definitive Chemoradiation. JCO Clin Cancer Inform 1:1–7, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yorke ED, Jackson A, Kuo LC, et al. : Heart Dosimetry is Correlated With Risk of Radiation Pneumonitis After Lung-Sparing Hemithoracic Pleural Intensity Modulated Radiation Therapy for Malignant Pleural Mesothelioma. Int J Radiat Oncol Biol Phys 99:61–69, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yorke ED, Jackson A, Rosenzweig KE, et al. : Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys 63:672–82, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Yorke ED, Jackson A, Rosenzweig KE, et al. : Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 54:329–39, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Khalil AA, Hoffmann L, Moeller DS, et al. : New dose constraint reduces radiation-induced fatal pneumonitis in locally advanced non-small cell lung cancer patients treated with intensity-modulated radiotherapy. Acta Oncol 54:1343–9, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Zhang XJ, Sun JG, Sun J, et al. : Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol 138:2103–16, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Claude L, Perol D, Ginestet C, et al. : A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol 71:175–81, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Shaikh T, Churilla TM, Monpara P, et al. : Risk of radiation pneumonitis in patients receiving taxane-based trimodality therapy for locally advanced esophageal cancer. Pract Radiat Oncol 6:388–394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Chen L, Zhang S, et al. : Predictive factors for acute radiation pneumonitis in postoperative intensity modulated radiation therapy and volumetric modulated arc therapy of esophageal cancer. Thorac Cancer 6:49–57, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley JD, Paulus R, Komaki R, et al. : Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187–99, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang EX, Hope AJ, Lindsay PE, et al. : Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol 50:51–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remick JS, Schonewolf C, Gabriel P, et al. : First Clinical Report of Proton Beam Therapy for Postoperative Radiotherapy for Non-Small-Cell Lung Cancer. Clin Lung Cancer 18:364–371, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Bledsoe TJ, Nath SK, Decker RH: Radiation Pneumonitis. Clin Chest Med 38:201–208, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Miura N, Kohno M, Ito K, et al. : Lung cancer surgery in patients aged 80 years or older: an analysis of risk factors, morbidity, and mortality. Gen Thorac Cardiovasc Surg 63:401–5, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Stamenovic D, Messerschmidt A, Schneider T: Surgery for lung tumors in the elderly: A retrospective cohort study on the influence of advanced age (over 80 years) on the development of complications by using a multivariate risk model. Int J Surg 52:141–148, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Example PORT Cases demonstrating the target volumes contours: CTV (blue), ITV (pink) and PTV (red). Figure S1A is a case of a 56 yo male with a clinical T2N2M0 squamous cell carcinoma of the right upper lobe who received neoadjuvant chemotherapy followed by a lobectomy and mediastinal lymph node dissection revealing ypT2N2M0 squamous cell carcinoma with negative margins, positive levels 2R and 4R and negative levels 7, 10, 11 and 12. Figure S1B is a case of a 73 yo female with a pT1bN2M0 adenocarcinoma of the left upper lobe who underwent a lobectomy and mediastinal lymph node dissection with negative margins and positive levels 5 and 7 and negative levels 9, 10, and 11.
Supplemental Figure S2: Radiation Pneumonitis rate by Lung V5 in patients older vs. younger than 65 years old