Abstract
Background and Aims
Nonalcoholic fatty liver disease (NAFLD) is becoming the most common indication for liver transplantation. The growing prevalence of NAFLD not only increases the demand for liver transplantation, it also limits the supply of available organs since steatosis predisposes grafts to ischemia-reperfusion (IR) injury and many steatotic grafts are discarded. We have shown that monoacylglycerol acyltransferase 1 (MGAT1), an enzyme that converts monoacylglycerol to diacylglycerol, is highly induced in animal models and patients with NAFLD and is an important mediator in NAFLD-related insulin resistance. Herein, we sought to determine whether Mogat1 (gene encoding MGAT1) knockdown in mice with hepatic steatosis would reduce liver injury and improve liver regeneration following experimental IR injury.
Approach and Results
Antisense oligonucleotides (ASO) were used to knockdown expression of Mogat1 in a mouse model of NAFLD. Mice then underwent surgery to induce IR injury. We found that Mogat1 knockdown reduced hepatic triacylglycerol (TAG) accumulation, but unexpectedly exacerbated liver injury and mortality following experimental IR surgery in mice on a high fat diet. The increased liver injury was associated with robust effects on the hepatic transcriptome following IR injury including enhanced expression of proinflammatory cytokines and chemokines and suppression of enzymes involved in intermediary metabolism. These transcriptional changes were accompanied by increased signs of oxidative stress and an impaired regenerative response.
Conclusions
We have shown that Mogat1 knockdown in a mouse model of NAFLD exacerbates IR injury and inflammation and prolongs injury resolution, suggesting that Mogat1 may be necessary for liver regeneration following IR injury and targeting this metabolic enzyme will not be an effective treatment to reduce steatosis-associated graft dysfunction or failure.
Keywords: fatty liver, liver transplantation, graft injury, NAFLD, Mogat1
Introduction
Obesity and obesity-associated nonalcoholic fatty liver disease (NAFLD) are increasingly prevalent worldwide (1). With the growing obesity pandemic and the advent of curative treatments for hepatitis C, end stage liver disease secondary to NAFLD has emerged as one of the most common indications for liver transplantation in the United States (2–4). Moreover, the increasing prevalence of NAFLD also places limitations on the supply of donor livers suitable for transplantation. Because steatotic livers are thought to be more susceptible to ischemia reperfusion (IR) injury with resulting poor graft function or primary non-function, many steatotic livers are deemed unsuitable for transplant and discarded (5–9). With the widening imbalance between supply and demand, there is great interest in the use of expanded criteria donors including older donor age, donation after cardiac death, and steatotic donor organs (9).
IR injury is largely unavoidable in most liver related surgeries, yet there are currently no pharmacological interventions available for prevention or treatment of IR injury (10). IR injury occurs as a result of oxygen and ATP depletion followed by inflammation, mitochondrial dysfunction, endoplasmic reticulum stress and subsequent cell death and organ dysfunction (11). Many clinical and preclinical studies have demonstrated increased susceptibility to IR injury in the setting of steatosis (5, 12–16), but the mechanism by which this occurs is not well understood (10, 11, 17). It has been postulated that alterations in lipid metabolism in the steatotic liver are a contributing factor to insulin resistance and hepatic IR injury (18, 19), but the processes connecting steatosis and insulin resistance to hepatic IR injury are not well defined.
The accumulation of lipid, mainly in the form of triacylglycerol (TAG), within the liver parenchyma is one of the hallmarks of NAFLD. Monoacylglycerol acyltransferases (MGAT) transfer a fatty acyl group to monoacylglycerol (MAG) to form diacylglycerol (DAG), which is then converted to TAG by diacylglycerol acyltransferase (DGAT) (20). Though the MGAT pathway has primarily been studied in intestinal enterocytes, our lab recently demonstrated increased expression of Mogat1 (gene encoding MGAT1) in mouse models of NAFLD and patients with NAFLD (21, 22). Furthermore, antisense oligonucleotides (ASO) mediated knockdown of Mogat1 improved insulin sensitivity and several other metabolic parameters that are deranged in the insulin resistant state (22, 23). These data collectively highlight the importance of the MGAT pathway in hepatic insulin resistance and NAFLD. However, the role of Mogat1 in hepatic IR injury has not been investigated.
In this study, we sought to characterize the effects of Mogat1 knockdown mediated by ASO on IR injury in a mouse model of diet-induced NAFLD that we have previously demonstrated to increase liver injury (24). We hypothesized that enhanced insulin sensitivity and metabolic parameters produced by Mogat1 knockdown would improve outcomes following IR injury. However, Mogat1 knockdown increased hepatic inflammation and oxidative stress, altered lipid accumulation, reduced hepatic regenerative capacity, and was associated with increased late mortality after IR injury compared to control mice. RNA-seq profiling demonstrated that Mogat1 knockdown and IR injury resulted in unique gene expression profiles with significant upregulation of genes in multiple inflammatory pathways and reduced expression of genes in several metabolic pathways. These data suggest that loss of Mogat1 in the liver of mice with diet-induced NAFLD exacerbates IR injury and metabolic dysregulation and blunts regenerative capacity. Moreover, it is likely that targeting Mogat1 in translational studies to alleviate the exacerbated IR injury associated with hepatic steatosis will not be beneficial.
Materials and Methods
Animal study design
Male 5 week-old C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). They acclimated for one week on standard chow diet followed by a high saturated fat “western” diet (Envigo TD 88137, Indianapolis, IN) or low fat control diet (Envigo TD 08485, Indianapolis, IN). Body weight was assessed weekly. Mice were maintained on diet for eleven weeks. Antisense oligonucleotide (ASO) injections were performed as previously described (22, 23, 25). Briefly, after 8 weeks on low fat (LFD) or high fat diet (HFD), mice received intraperitoneal injections of ASO directed against Mogat1 (5’-GGGCGGCCAACTGTGGAAAA-3’) or a scrambled control ASO (IONIS pharmaceuticals, Inc., Carlsbad, CA) 25 mg/kg body weight twice a week for 3 additional weeks. The day following the last injection, mice were then subjected to hepatic IR or sham surgery. All animal studies were approved by the Institutional Animal Use and Care Committee of Washington University School of Medicine and comply with the Guide for the Care and Use of Laboratory Animals as outlined by the National Academy of Sciences.
Hepatic ischemia-reperfusion surgery
Hepatic ischemia was induced using a 70% ischemia model as previously described (26). Briefly, mice were anesthetized using isoflurane inhalation. Lidocaine and sustained release buprenorphine were used for analgesia. Midline laparotomy was performed to expose abdominal contents. Hepatic ischemia was induced by cross-clamping the hepatic artery, portal vein, and bile duct distal to the branch point to the right lateral lobe to induce ischemia to the median and left lobes. The clamp was released after 60 m followed by 4, 24, or 72 h of reperfusion. Mice undergoing sham surgery underwent anesthesia and midline laparotomy for 60 m without vascular clamping. At the specified reperfusion time points, mice were euthanized and plasma and liver samples were collected. A portion of the left lobe of the liver was preserved in 10% neutral buffered formalin for histological evaluation. The remaining liver samples were flash frozen in liquid nitrogen and then stored at −80°C for future analysis. The median and left lobes of the liver were used for all liver analyses. Blood was collected from the inferior vena cava at the time of sacrifice, centrifuged, and plasma was flash frozen in liquid nitrogen and then stored at −80°C for future analysis.
Triglyceride and total cholesterol assay
Liver samples were homogenized in normal saline followed by addition of 1% sodium deoxycholate to solubilize lipids. Hepatic triglyceride and total cholesterol content were measured using commercially available colorimetric kits (Thermo Fisher Scientific, Waltham, MA).
Plasma parameters
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using commercially available colorimetric kinetic assays (Teco Diagnostics, Anaheim, CA) according to the manufacturer’s instructions. Plasma non-esterified fatty acids, total cholesterol and triglycerides were measured using commercially available colorimetric kits (Wako Diagnostics, Mountain View, CA and Thermo Fisher Scientific, Waltham, MA). Plasma cytokine levels were determined, per manufacturer’s protocol, with a commercially available magnetic bead suspension assay (MilliporeSigma, Billerica, MA) using the Luminex 200 analyzer (Luminex Corp., Austin, TX). Plasma total bilirubin levels were measured using a commercially available kit (Pointe Scientific, Canton, MI) according to manufacturer’s instructions.
Histopathology and immunohistochemistry
A portion of the left lateral lobe was harvested at time of sacrifice and placed in 10% neutral buffered formalin followed by 70% ethanol. Formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin-eosin (H&E) stain. Formalin-fixed and paraffin-embedded tissues were also sectioned and stained for PCNA (1:200, Santa Cruz, SC7907), Ki67 (1:500, Thermo Scientific Lab Vision, RM91068S0), and F4/80 (1:200, Abcam, AB6640) by the Advanced Imaging and Tissue Analysis Core at Washington University School of Medicine using DAB immune-peroxidase staining. An experienced liver pathologist (M.H. or P.S.) blinded to the conditions assessed the presence of necrosis and quantified F4/80, PCNA, and Ki67 staining in the liver specimens.
Western blot analysis
Liver protein extracts were obtained from the liver using a buffer containing 15 mM NaCl, 20 mM trishydroxymethylaminomethane, 1 mM ethylene diamine tetraacetic aid, 0.2% nonidet P-40 (NP-40), and 10% glycerol. Liver lysates were normalized to protein concentration, denatured, and run on Criterion precast polyacrylamide gel electrophoresis gels (Bio-Rad, Hercules, CA) and transferred onto polyvinylidene fluoride membranes. For Western blot analysis of MGAT1 expression, membranes were isolated from whole liver tissue using the following lysis buffer: 20 mM Tris pH 7.5, 250 mM sucrose, 2 mM MgCl2, 1x protease inhibitor. The homogenates were then cleared by centrifugation at 2,000 x g for 10 minutes at 4°C. The supernatant was centrifuged at 100,000 x g for 45 minutes at 4°C. The pellets were resuspended in a solution containing 50 mM Tris-HCl, pH 8.0, 80 mM NaCl, 2 mM CalCl2, 1% Triton-X100, 1x protease inhibitor. Antibodies (1:1000 PCNA, Cell signaling #2586; 1:1000 Cyclin D1 #2978, Cell signaling; 1:1000 β-ACTIN, Cell signaling #4967; 1:1000 MGAT1, Proteintech; 1:1000 Calnexin, Enzo Life Sciences #ADI-SPA-860) were used according to manufacturers’ instructions. Membranes were probed with an anti-rabbit IRDye 800CW (1:10,000; LI-COR, Lincoln, Nebraska) secondary antibody or anti-mouse IRDye 700CW secondary antibody (1:10,000 LI-COR, Lincoln, Nebraska). Blots were developed on an infrared Odyssey developer (LI-COR).
RNA isolation and gene expression analysis
Total liver RNA was extracted with RNA Bee (Isotex Diagnostics, Friendswood, TX) based on manufacturer’s instructions. Complementary DNA was synthesized using a high capacity reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction was performed with Power SYBR Green with the use of an ABI-Prism 7500 detection system (Applied Biosystems, Foster City, CA). Arbitrary units of mRNA of the genes of interest were corrected to 36B4 expression. Primers were obtained through Integrated DNA Technologies (Coralville, IA). Primer sequences used in this study are available in Supplemental Table 3.
RNA sequencing and analysis
The Genome Technology Access Center in the McDonnell Genome Institute at Washington University School of Medicine performed and analyzed RNA-seq data. RNA integrity was validated using Agilent Bioanalyzer. Ribosomal RNA depletion was performed with RiboErase and samples were prepared according to library kit manufacturer’s protocol, then indexed, pooled, and sequenced using NovaSeq S4 2×150.Detailed materials and methods can be found in supplementary material
Mass spectrometry and lipid quantification
Mass spectrometry studies were performed in the Metabolomics Facility at Washington University. Lipid species were extracted with methanol from homogenized liver tissues and quantified by a liquid chromatography-mass spectrometry (LC/MS) system using deuterated compounds as internal standards and methods. Detailed materials and methods can be found in supplementary material.
Statistical analysis
Statistical comparisons were made using a t-test or analysis of variance where appropriate. All data are presented as mean ± standard error of the mean with statistical significance defined as p ≤ 0.05. Data from Luminex analysis were analyzed via a nonparametric analysis of variance Kruska-Wallis test.
Results
Mogat1 ASO treatment effectively reduces Mogat1 expression
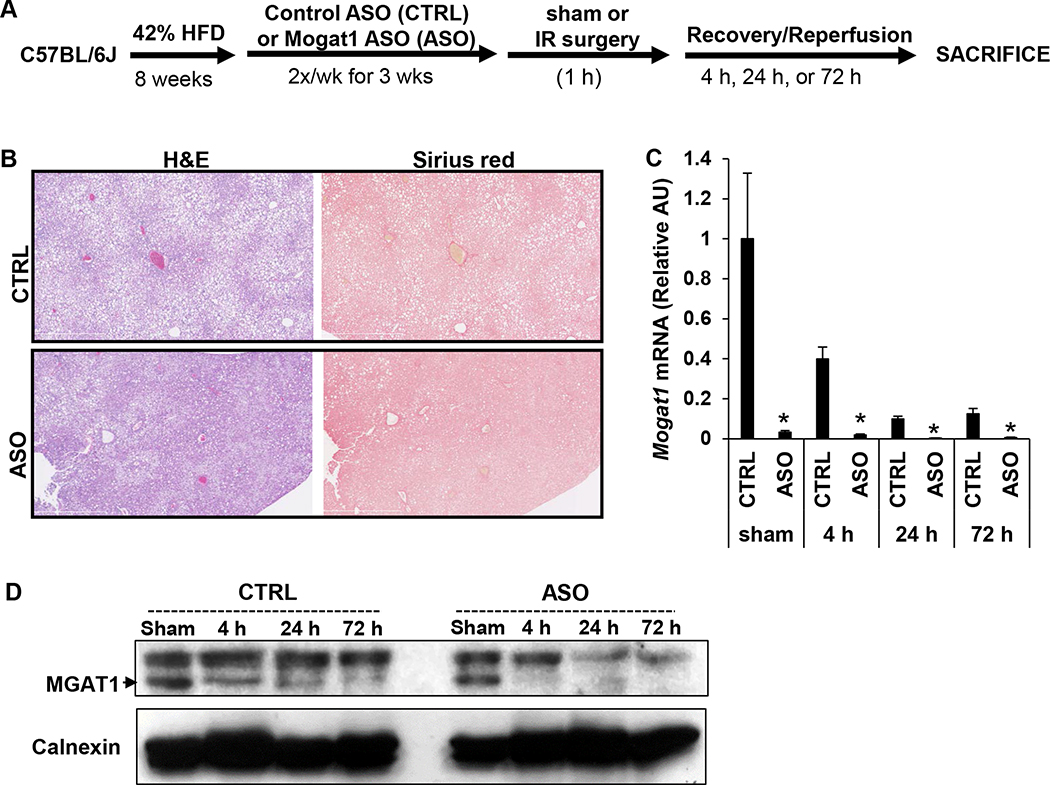
To investigate the role of Mogat1 in IR injury in steatotic livers, mice were fed a HFD for 8 weeks followed by three weeks of treatment with scrambled control ASO (CTRL) or ASO targeted to Mogat1 (ASO) while continuing on HFD for a total of 11 weeks (Fig. 1A). Body weight of mice randomized to receive ASO was not significantly different compared to CTRL (CTRL vs ASO 43.0 +-/ 0.8 g vs 41.8 +/− 0.5 g, p = 0.22) and weight loss was not seen following initiation of intraperitoneal (IP) injections of either CTRL or ASO for three weeks (Supplemental Fig. 1A). Both groups developed moderate to severe hepatic steatosis after eight weeks on high fat diet, defined as >30% steatosis histologically, but minimal fibrosis was seen on Sirius red staining in both CTRL and ASO groups (Fig. 1B). After three weeks of treatment, mice underwent experimental IR surgery or sham surgery as described in the Methods section. As expected, mice treated with ASO had decreased hepatic gene expression of Mogat1 and MGAT1 protein abundance compared to CTRL treated mice after sham surgery and at all reperfusion time points (Fig. 1C, 1D). Interestingly, hepatic Mogat1 gene expression declined by approximately 60% (compared to sham CTRL) following IR surgery in mice treated with CTRL. The decrease was more profound at the 24 h and 72 h time point compared to 4 h, suggesting that IR injury leads to a decrease in Mogat1 expression (Fig. 1C).
Figure 1.
Experimental design utilizing 42% high fat diet and Mogat1 targeted antisense oligonucleotides.
[A] Graphical representation of experimental design. After eight weeks on high fat diet, mice were given IP injections of a scrambled ASO (CTRL) versus Mogat1 ASO (ASO) twice a week for three weeks. [B] Representative H&E and Sirius red stained liver sections from sham operated CTRL and ASO treated mice following eight weeks of high fat diet and 3 weeks of CTRL or Mogat1 ASO injections along with high fat diet. [C] Hepatic gene expression of Mogat1 in CTRL and ASO treated mice following sham or IR surgery and indicated reperfusion times. [D] Representative protein immunoblot showing protein abundance of MGAT1 in livers of CTRL and ASO treated mice in sham or IR surgery and indicated reperfusion times. Values are mean ± standard error of the mean; n = 3–4 mice/sham group; n = 8–10 mice/IR surgery group. *p<0.05 ASO versus CTRL at same reperfusion time point. Abbreviations: ASO, antisense oligonucleotide targeted at Mogat1. AU, arbitrary units. CTRL, control ASO. HFD, high fat diet. IP, intraperitoneal. IR, ischemia reperfusion. H&E, hematoxylin and eosin.
Mogat1 knockdown in mice with diet-induced NAFLD is associated with late mortality following IR injury
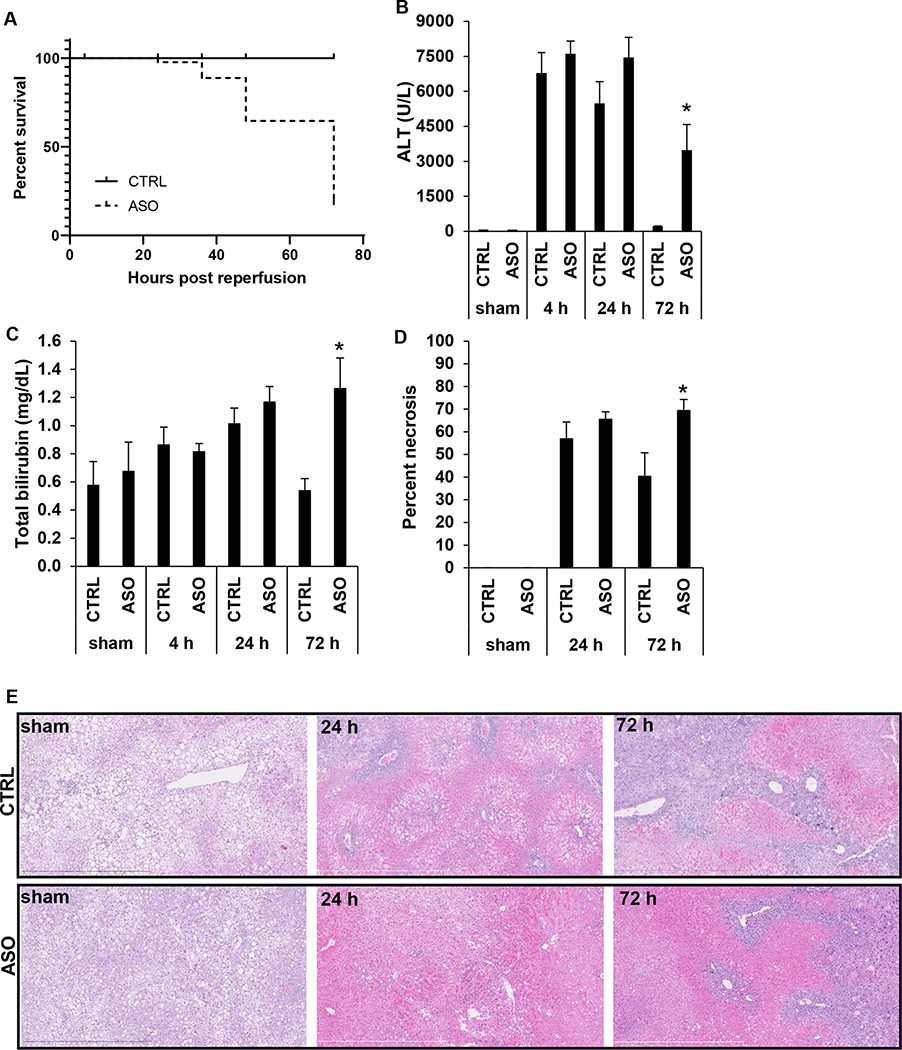
At early reperfusion time points (4 h and 24 h reperfusion), there were no overt differences between CTRL and ASO treated mice. However, at approximately 48 h, when CRTL mice appeared to have recovered from IR surgery, many ASO-treated mice exhibited minimal spontaneous activity, signs of general sickness behavior, and slowed respiratory rate. Some mice perished spontaneously after IR. Other mice that appeared moribund were euthanized as a humane endpoint in accordance with our IACUC protocol, which was also considered a mortality event. Compared to CTRL treated mice, which showed no mortality, mice treated with Mogat1 ASO had poor long-term survival following IR injury with mortality occurring between 24–72 h (Fig. 2A).
Figure 2.
Mogat1 knockdown in mice with diet-induced NAFLD is associated with poorer survival and increased liver injury following IR surgery.
[A] Kaplan-Meier curve demonstrating survival in ASO or CTRL treated mice following IR surgery at specified reperfusion times. [B] Plasma ALT concentrations following sham or IR surgery in mice treated with CTRL or ASO at different reperfusion time points. [C] Plasma total bilirubin concentrations following sham or IR surgery in mice treated with CTRL or ASO at different reperfusion time points. [D] Percentage of liver tissue that was necrotic in sham operated mice and at 24 h and 72 h post reperfusion. [E] Representative H&E stained liver sections from ASO and CTRL treated mice undergoing sham or IR surgery followed by 24 h and 72 h of reperfusion. Values are mean ± standard error of the mean; n = 3–4 mice/sham group; n = 8–10 mice/IR surgery group. *p<0.05 ASO versus CTRL at same reperfusion time point. Abbreviations: ALT, alanine aminotransferase. ASO, antisense oligonucleotide targeted at Mogat1. CTRL, control ASO. H&E, hematoxylin and eosin.
Mogat1 knockdown in mice with diet-induced NAFLD results in increased liver injury following IR injury
There were no differences in plasma alanine aminotransferase (ALT) or aspartate aminotransferase (AST) concentrations between ASO and CTRL mice following sham operation or after 4 h and 24 h reperfusion (Fig. 2B, Supplemental Fig. 2A). However, at 72 h, mice with Mogat1 knockdown had significantly higher plasma ALT and AST concentrations compared to CTRL mice (Fig. 2B, Supplemental Fig. 2A). Similarly, there were no significant differences in plasma total bilirubin concentration between ASO and CTRL mice following sham operation or after 4 h and 24 h reperfusion. However, at 72 h, mice with Mogat1 knockdown had higher plasma total bilirubin concentrations compared to CTRL mice (Fig. 2C). There was minimal histological evidence of necrosis in the liver specimens after 4 h (data not shown), but necrosis was histologically evident at 24 h and 72 h of reperfusion (Fig. 2D, 2E). At 72 h, the ASO treated mice had more extensive areas of necrotic liver tissue compared to CTRL mice (40.3 +/− 10.5 vs 69.4 +/− 4.9 %, p=0.03) (Fig. 2D, 2E). Collectively, these findings suggest that liver injury fails to resolve following Mogat1 knockdown and that this is associated with increased mortality at the later time points following reperfusion. To determine if the effects of Mogat1 knockdown were restricted to steatotic livers, a group of mice fed a low fat control diet was also randomized to CTRL or ASO IP injections for three weeks prior to sham or IR surgery. As expected, mice treated with ASO had decreased hepatic gene expression of Mogat1. In contrast to the HFD group, in the LFD group, there were no overt differences in injury parameters or survival between the CTRL and ASO mice following IR injury (Supplemental Figure 3). Thus, subsequent studies were focused on mice with diet-induced NAFLD to investigate how Mogat1 deficiency alters the response to IR injury in steatotic livers.
Mogat1 knockdown in mice with diet-induced NAFLD elicits a unique transcriptional profile following IR injury
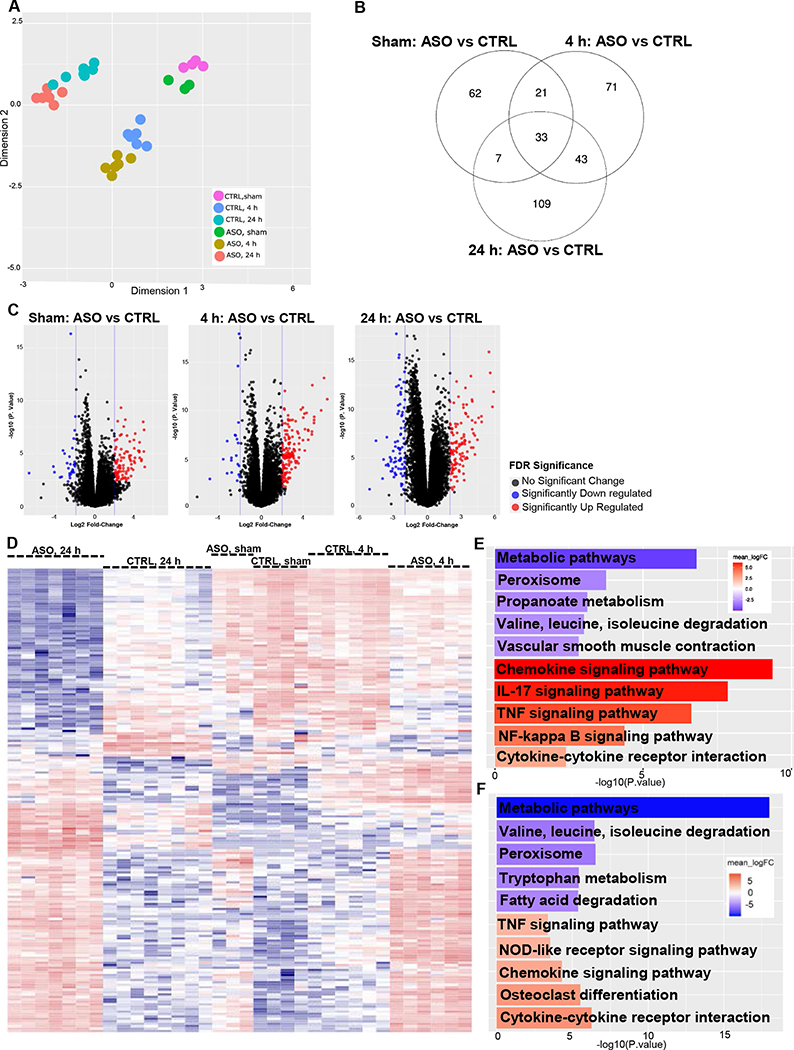
We performed RNA sequencing (RNA-seq) analysis to characterize the hepatic transcriptome and found IR injury was associated with significant transcriptional changes compared to sham operated mice in both CTRL and ASO treated mice. The multidimensional scaling (MDS) plot indicates the greatest source of variation was among the different reperfusion time points and sham operated versus IR surgery groups (Fig. 3A). Specifically, regardless of ASO or CTRL treatment, the sham surgery groups were most similar to each other. This was also the case in the 4 h and 24 h reperfusion groups. However, ASO treated mice formed clusters that were distinct from CTRL treated mice, forming their own unique subgroup within a reperfusion time point. The distinctiveness of each time point is further illustrated in the Venn diagram showing the overlap between the datasets of differentially expressed genes in each comparison and the overlaps between the comparison groups (Fig. 3B). As seen in the Venn diagram, 62 unique genes were differentially expressed between ASO and CTRL in sham operated mice, compared to 71 unique genes at 4 h post reperfusion, and 109 unique genes at 24 h post reperfusion. Only 33 genes were commonly differentially expressed between ASO and CTRL across the three time points (Supplemental Table 1).
Figure 3.
Mogat1 knockdown in mice with diet-induced NAFLD elicits a unique transcriptional profile following IR injury.
[A] Multidimensional scaling plot (MDS) of the transcriptome profiles of sham operated and IR surgery groups in CTRL and ASO treated mice. [B] Venn diagram showing the overlap between the datasets of differentially expressed genes comparing sham operated mice, 4 h reperfusion and 24 h reperfusion. The numbers of significantly expressed transcripts at ≥2 log2 fold change and FDR≤0.05 are depicted in the form of Venn diagrams. The numbers of transcripts shown in overlapping areas illustrate the number of transcripts commonly differentially expressed. [C] Volcano plots comparing CTRL vs ASO treated mice at different reperfusion time points. The volcano plots display pairwise transcript accumulation differences between ASO and CTRL treated mice in sham operated mice and those undergoing 4 h and 24 h of reperfusion. The plots contain all genes expressed greater than 1 count per million. Transcripts whose accumulation differences are significantly upregulated are represented in red and those significantly downregulated are represented in blue (FDR≤0.05). The observed log2 fold change is on the x-axis and the p-value converted to the –log10 scale is on the y-axis. [D] Hierarchical clustering of genes displaying differential expression among individual mice (FDR≤0.01). [E] Bar plot comparing CTRL and ASO treated mice after IR surgery and 4 h reperfusion demonstrates the top 5 significantly upregulated and top 5 significantly downregulated KEGG signaling and metabolism pathways. [F] Bar plot comparing CTRL and ASO treated mice after IR surgery and 24 h reperfusion demonstrates the top 5 significantly upregulated and top 5 significantly downregulated KEGG signaling and metabolism pathways. Abbreviations: ASO, antisense oligonucleotide targeted at Mogat1. CTRL, control ASO. KEGG, Kyoto encyclopedia of genes and genomes. FDR, false discovery rate.
There are substantial differences between ASO and CTRL treated mice within each reperfusion time point, which is demonstrated in the volcano plots showing pairwise transcript accumulation differences between ASO and CTRL treated mice in sham operated mice and those undergoing 4 h and 24 h of reperfusion (Fig. 3C). In the sham operated group, when comparing ASO and CTRL treated mice, there are 94 genes that are upregulated and 29 genes that are downregulated. At 4 h, there are 149 upregulated genes and 20 downregulated genes in ASO versus CTRL treated mice. However, at 24 h post reperfusion, when comparing ASO and CTRL treated mice, there are 113 genes that are upregulated whereas 77 genes are downregulated. Thus, the differences between CTRL and ASO treated mice become more apparent with IR injury over time. After 24 h, differences in transcriptional profiles between CTRL and ASO treated mice form unique clusters. The hierarchical clustering of gene expression generated a heat map in which CTRL and ASO treated mice formed distinct groups at different reperfusion time points (Fig 3D). Further, gene ontology enrichment pathway analysis indicated significant downregulation of multiple pathways involving intermediary metabolism and significant upregulation of several inflammatory pathways including cytokines and chemokines and TNF signaling pathways at both 4 h and 24 h post reperfusion (Fig 3E, 3F).
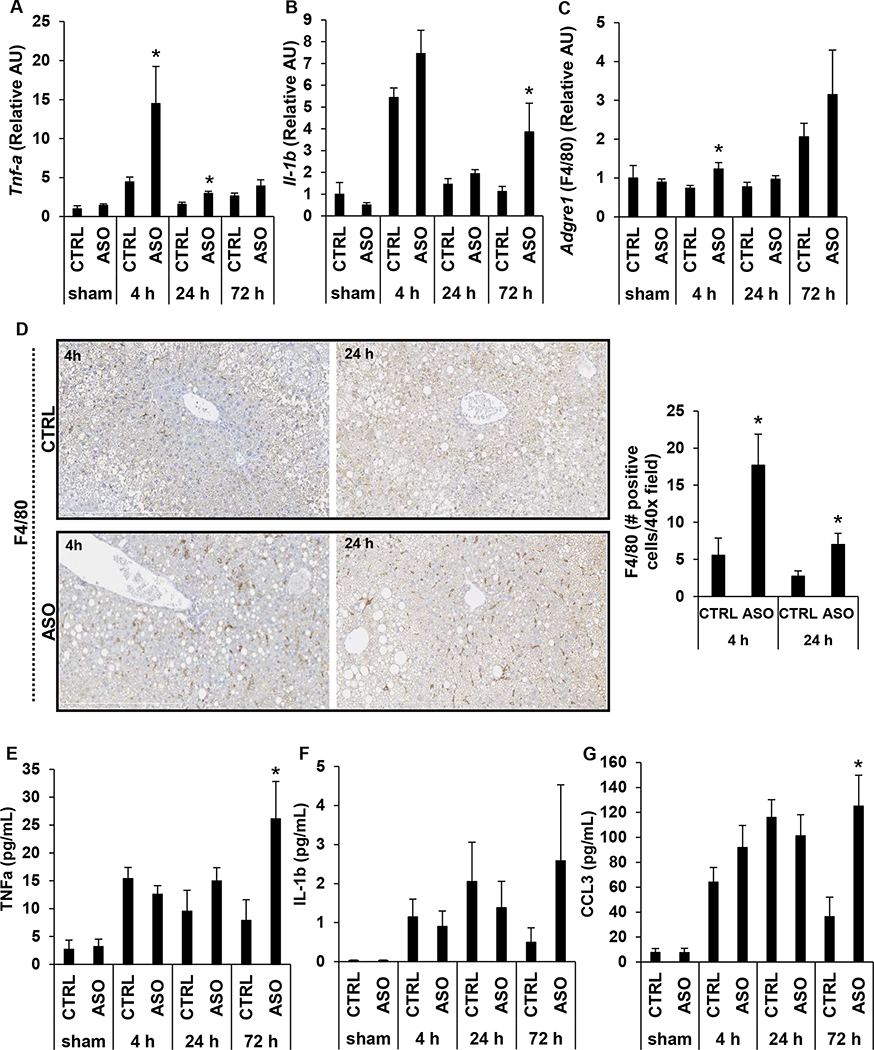
Mogat1 knockdown in mice with diet-induced NAFLD results in increased inflammation following IR injury
RNA-seq analysis suggested that Mogat1 knockdown leads to increased inflammation and expression of leukocyte chemoattractant factors following IR injury (Fig. 3E, 3F; Supplemental Fig. 4A–C). We therefore examined markers of hepatic and systemic inflammation. We found that hepatic expression of Tnfα was significantly higher in ASO treated mice at 4 h and 24 h relative to CTRL mice (Fig. 4A). ASO treated mice demonstrated a trend towards increased hepatic expression of Il-1β at 4 h reperfusion (p=0.11) and was significantly higher at 72 h reperfusion compared to CTRL treated mice (Fig. 4B). Consistent with the increased expression of several genes encoding chemoattractant proteins (Supplemental Fig. 4A–C), hepatic expression of the gene encoding the macrophage marker F4/80 (Adgre1) and immunohistochemical staining for F4/80 qualitatively and quantitatively demonstrated that macrophage infiltration was increased in the liver of ASO treated mice compared to CTRL treated mice following IR injury (Fig. 4C, 4D). Mogat1 knockdown also resulted in increased systemic inflammation following IR injury as measured by plasma cytokines. While there was no difference between CTRL and ASO treated mice in IL-1β plasma concentration at any reperfusion time point, we found that relative to CTRL mice, ASO treated mice had higher levels of TNFα and CCL3 (MIP1-α) after 72 h of reperfusion (Fig. 4E–G). These data are consistent with the RNA-seq data, which indicated significant upregulation of several inflammatory and immune response pathways in ASO treated mice compared to CTRL treated mice following IR injury (Fig. 3E, 3F).
Figure 4.
Mogat1 knockdown in mice with diet-induced NAFLD is associated with increased inflammation following IR injury.
[A, B] Hepatic gene expression of inflammatory cytokines following sham operation or IR injury with specified reperfusion time points. [C] Hepatic gene expression of macrophage marker, Adgre1, following sham operation or IR injury with specified reperfusion time points. [D] Representative F4/80 stained liver sections and quantification of immunohistochemical staining from liver specimens of mice undergoing IR injury followed by the specified reperfusion time points. [E, F, G] Plasma concentration of inflammatory cytokines and chemokines following sham operation or IR injury with specified reperfusion time points. Values are mean ± standard error of the mean; n = 3–4 mice/sham group; n = 8–10 mice/IR surgery group. *p<0.05 ASO versus CTRL at same reperfusion time point. Abbreviations: ASO, antisense oligonucleotide targeted at Mogat1. AU, arbitrary units. CCL, chemokine ligand. CTRL, control ASO. IL, interleukin. IR, ischemia reperfusion. TNF, tumor necrosis factor.
Mogat1 knockdown in mice with diet-induced NAFLD increases oxidative stress following IR injury
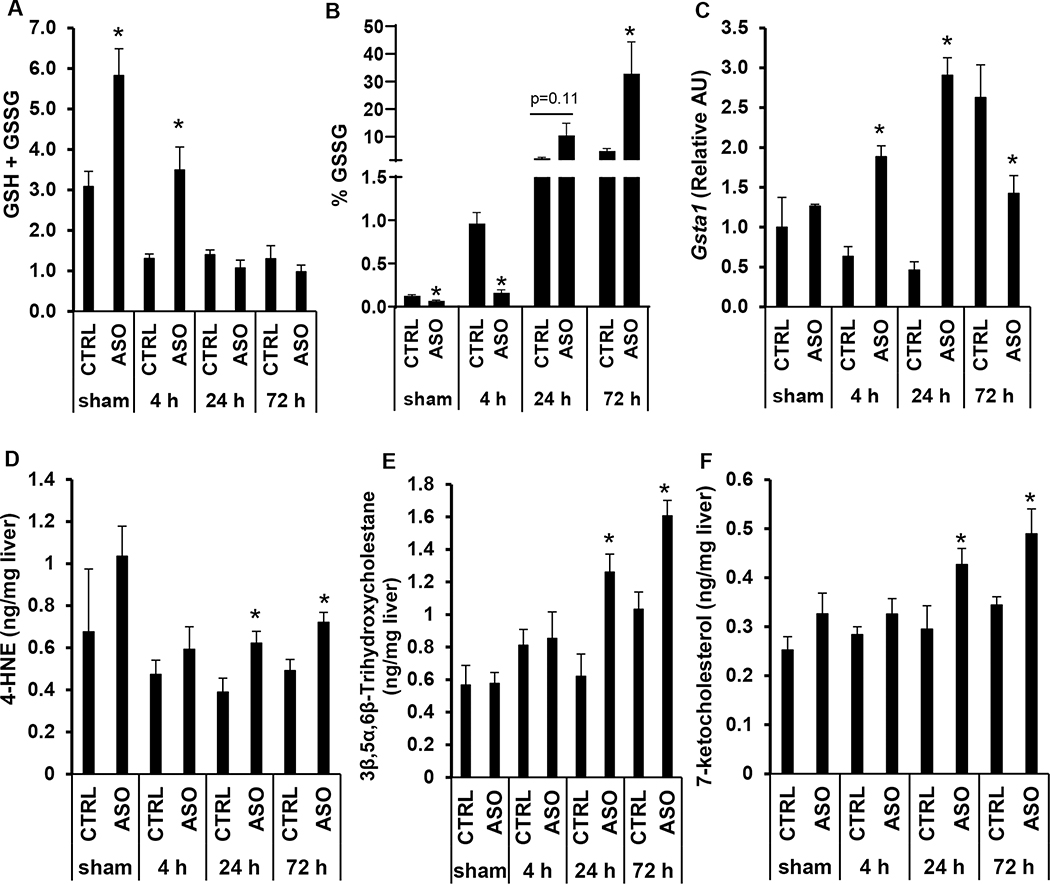
Gene ontology fold-change analysis of RNA-seq data revealed significant downregulation of oxidoreductase activity with alterations in a number of genes involved in oxidative stress (Supplemental Fig. 5A–D). ASO treated mice had higher hepatic content of total glutathione (GSH + GSSG) compared to CTRL mice only after sham surgery or 4 h reperfusion, which was driven mostly by increased total reduced glutathione (GSH) rather than oxidized glutathione (GSSG) (Fig. 5A; Supplemental Fig. 6A, 6B). With increased reperfusion time, oxidized glutathione increased in both CTRL and ASO treated mice. Compared to CTRL treated mice ASO treated mice had lower percentage of GSSG at 4 h, but at 72 h following reperfusion, Mogat1 ASO treated mice had a higher percentage of GSSG compared to CTRL mice indicating greater oxidative stress (32.8 +/− 11.5% vs 4.9 +/− 0.9%, p=0.03) (Fig. 5B). While Gsta1 expression was significantly increased in the ASO treated mice compared to CTRL at the early time points, it was decreased at the 72 h time point and significantly lower compared to CTRL mice at this late time point (Fig. 5C). Liver samples were also analyzed by mass spectrometry for lipid markers of oxidative stress. Compared to CTRL mice, Mogat1 knockdown resulted in elevated levels of oxysterols 7-ketocholesterol and 3β,5α,6β trihydroxycholestane and organic aldehyde hydroxynonenal (4-HNE) following IR injury at 24 h and 72 h of reperfusion indicating increased oxidative stress (Fig. 5D–F). While lipid peroxidation may not be a major contributor to IR induced liver injury (27), these data are consistent with our results for glutathione oxidation and demonstrates an overall increase in oxidative stress.
Figure 5.
Mogat1 knockdown in mice with diet-induced NAFLD is associated with increased oxidative stress following IR injury.
[A] Total glutathione (GSH+GSSG) hepatic content in mice undergoing sham operation or IR injury with specified reperfusion time points. [B] Percentage of oxidized glutathione (GSSG) in the liver following sham operation or IR injury. [C] Hepatic gene expression of Gsta1 in mice undergoing sham operation or IR injury with specified reperfusion time points. [D, E, F] Liver content of toxic lipids and reactive nitrogen products in mice treated with CTRL or Mogat1 ASO followed by sham operation or IR injury followed by specified reperfusion time points. Values are mean ± standard error of the mean; n = 3–4 mice/sham group; n = 8–10 mice/IR surgery group. *p<0.05 ASO versus CTRL at same reperfusion time point. Abbreviations: ASO, antisense oligonucleotide targeted at Mogat1. AU, arbitrary units. CTRL, control ASO. GSH, reduced glutathione. GSSG, oxidized glutathione. Gsta, glutathione S-transferase. HNE, hydroxynonenal.
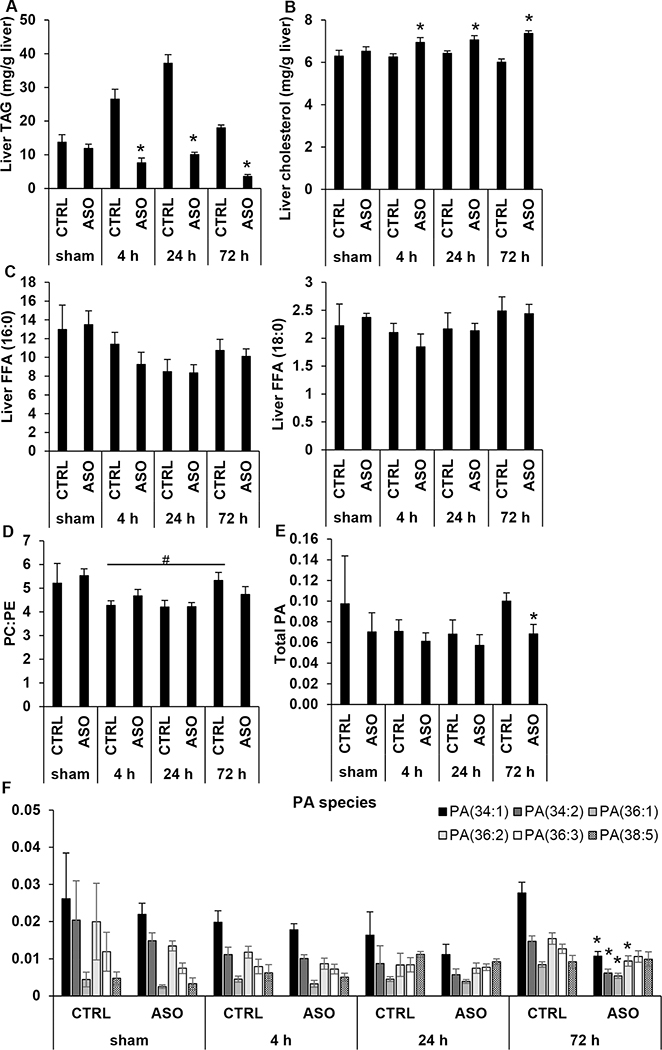
Mogat1 knockdown in mice with diet-induced NAFLD alters hepatic lipid content following IR surgery
RNA-seq data suggested downregulation of multiple pathways involving lipid metabolism and altered expression of several genes that encode enzymes involved in lipid synthesis or remodeling (Supplemental Fig. 7A–B). We therefore sought to further characterize hepatic lipid content following IR injury. We found that IR injury led to a significant increase in hepatic TAG content at 4 h and 24 h followed by a return toward baseline levels at 72 h in CTRL mice (Fig. 6A). Mogat1 ASO treated mice demonstrated no such change in hepatic TAG content. While Mogat1 ASO had no effect on hepatic TAG content in sham mice, liver TAG was reduced compared to CTRL following IR injury at all reperfusion time points (Fig. 6A). Interestingly, Mogat1 ASO treatment led to a concomitant increase in liver cholesterol after IR injury (Fig. 6B). There were no significant differences between CTRL and ASO treated mice in liver content of free fatty acids (FFA) including palmitic acid (16:0) or stearic acid (18:0) following IR surgery at any time point (Fig. 6C). We also measured total plasma cholesterol, TAG, and free fatty acids but found no effect of Mogat1 knockdown in sham operated mice or those subjected to IR injury (Supplemental Table 2). Therefore, despite diminished hepatic steatosis as measured by accumulation of TAG, ASO treated mice were sensitized to liver injury following IR surgery.
Figure 6.
Comparison of hepatic lipid content in ASO or CTRL treated animals with diet-induced NAFLD.
Liver TAG [A], total cholesterol [B], and free fatty acid (FFA 16:0, palmitic acid, FFA 18:0, stearic acid) [C] content in CTRL and ASO treated mice following sham operation or IR injury and indicated reperfusion times. Liver phospholipid content including PC:PE ratio [D] and total PA content [E] in mice undergoing sham operation or IR injury followed by indicated reperfusion times. [F] Hepatic content of individual PA species. Values are mean ± standard error of the mean; n = 3–4 mice/sham group; n = 8–10 mice/IR surgery group. *p<0.05 ASO versus CTRL at same reperfusion time point. #p<0.05 CTRL 4 h vs CTRL 72 h. Abbreviations: ASO, antisense oligonucleotide targeted at Mogat1. CTRL, control ASO. FFA, free fatty acid. PA, phosphatidic acid. PC, phosphatidylcholine. PE, phosphatidylethanolamine. TAG, triacylglycerol.
Phospholipid abundance may be an important factor in liver regeneration (28) and therefore the liver content of several phospholipids were assessed. While there was no difference in total hepatic phosphatidylcholine (PC) content between CTRL or ASO treated mice at any time point, ASO treated mice had significantly higher total phosphatidylethanolamine (PE) content at 72 h (Supplemental Fig. 8A, 8B). Decreased PC:PE ratio has previously been negatively associated with survival following partial hepatectomy in mouse models (29). Though not significant, there was a trend toward increased PC:PE ratio in CTRL treated mice compared to ASO treated mice at 72 h post reperfusion (p=0.24) (Fig. 6D). Furthermore, during reperfusion, CTRL treated mice demonstrated a significant increase in PC:PE ratio over time from 4 h to 72 h while ASO treated mice had no change (Fig. 6D). Phosphatidic acid (PA) is a metabolite that is a potent signaling lipid that may promote liver regeneration in response to acetaminophen toxicity (28). Total hepatic PA did not differ between CTRL and ASO treated mice in sham operated mice or at 4 h or 24 h reperfusion. However, ASO treated mice had significantly lower total PA content than CTRL treated mice at 72 h post reperfusion (Fig. 6E), which was primarily driven by a reduction in four specific PA species, PA 34:1, 34:2, 36:1, and 36:2 (Fig. 6F). Together, these data suggest that hepatic IR injury is associated with alterations in lipid metabolism that are exaggerated in the setting of Mogat1 knockdown.
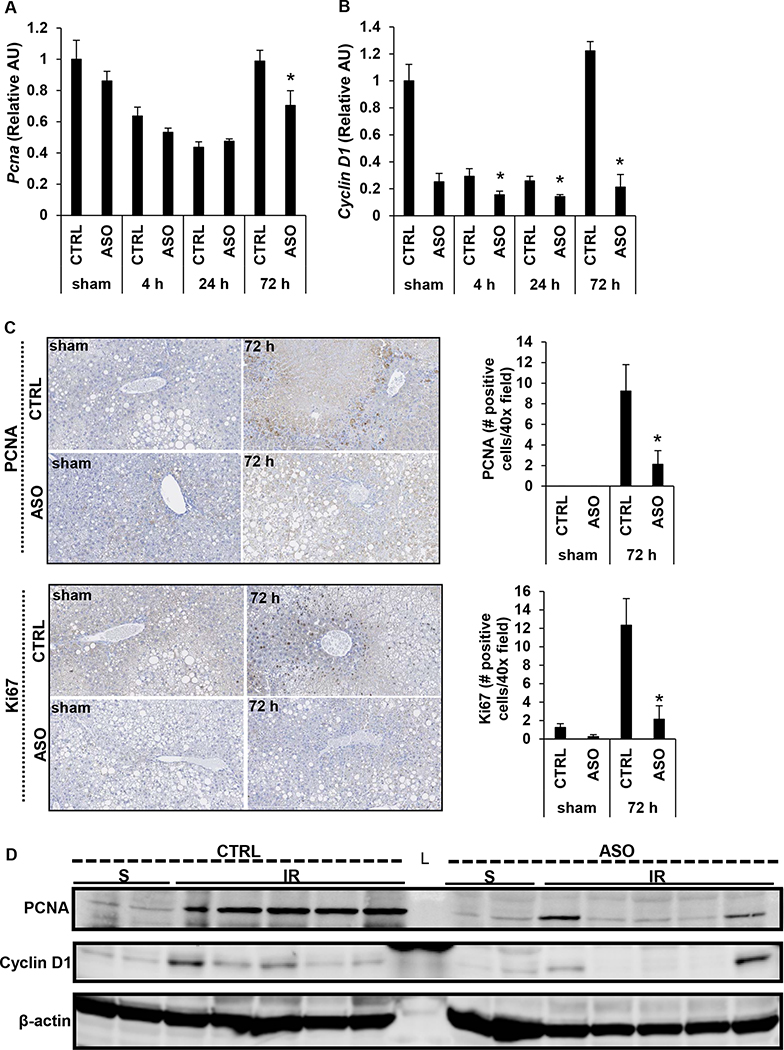
Mogat1 knockdown in mice with diet-induced NAFLD reduces liver regeneration following IR injury
The development of transient steatosis and PA accumulation have been implicated during liver regeneration in models of acute liver injury (28, 30, 31). Therefore, we then investigated whether increased inflammation and oxidative stress and altered lipid content may impact hepatic regeneration following IR injury in the setting of Mogat1 knockdown. ASO treated mice had significantly less hepatic gene expression of proliferating cell nuclear antigen (Pcna) and Cyclin D1 at 72 h post reperfusion compared to CTRL mice (Fig. 7A, 7B). At 72 h, Mogat1 knockdown is associated with decreased hepatic expression of PCNA and Ki67, as measured qualitatively and quantitatively by immunohistochemistry (Fig. 7C). At 72 h of reperfusion, there is also decreased hepatic protein expression of PCNA and Cyclin D1 in ASO treated mice compared to CTRL treated mice as measured by protein immunoblot, which is suggestive of a blunted regenerative response following IR injury in mice with Mogat1 knockdown (Fig. 7D). Collectively, these data suggest that Mogat1 knockdown in mice with diet induced NAFLD is associated with altered hepatic lipid content, increased inflammation, necrosis, and oxidative stress and decreased regenerative capacity that results in increased late mortality following IR injury.
Figure 7.
Mogat1 knockdown in mice with diet-induced NAFLD is associated with decreased expression of markers of hepatic regeneration following IR injury.
Hepatic gene expression of proliferating cell nuclear antigen (Pcna) [A] and Cyclin D1 [B]. [C] Representative images and quantification of immunohistochemical staining of liver sections stained for PCNA and Ki67. [D] Protein immunoblot showing protein abundance of PCNA, Cyclin D1, and β-actin in livers following IR injury and 72 h of reperfusion. Values are mean ± standard error of the mean; n = 3–4 mice/sham group; n = 8–10 mice/IR surgery group. *p<0.05 ASO vs CTRL at same reperfusion time point. Abbreviations: ASO, antisense oligonucleotide targeted at Mogat1. AU, arbitrary units. CTRL, control ASO. PCNA, proliferating cell nuclear antigen. S, sham. IR, ischemia reperfusion. L, ladder.
Discussion
Although IR injury is largely unavoidable in most liver-related surgeries, including liver resections and liver transplantation, there are currently no pharmacological therapies available for its treatment or prevention. Furthermore, hepatic steatosis exacerbates IR injury, but the mechanisms by which liver lipid accumulation worsens IR injury remain poorly defined (10, 17). Many deleterious effects of hepatic steatosis have been attributed to the accumulation of toxic lipid species in the liver, which are linked to inflammation, leukocyte and stellate cell activation, and insulin resistance (32, 33). In the liver and other organs, insulin resistance may exacerbate IR injury by limiting substrate flexibility and inhibiting the pleiotropic signaling effects of this hormone (34–37). Based on our previous demonstration that Mogat1 knockdown improved glucose tolerance in obese mice (22, 23), we hypothesized that inhibition of this enzyme would attenuate IR injury in a mouse model of NAFLD. Herein, we show that Mogat1 knockdown led to increased late mortality following IR injury compared to control mice. This was associated with increased inflammation and oxidative stress and decreased regenerative capacity. These data suggest that lipid metabolism may play an important role in the resolution and regeneration phases that occur after IR injury and that inhibition of Mogat1 is not a viable target for decreasing liver injury in the steatotic liver.
Interestingly, the effect of Mogat1 knockdown was only apparent in mice on HFD as we did not observe any differences in the response to IR injury in mice fed a LFD. As Mogat1 is typically expressed in low levels in non-steatotic livers and induced by HFD, it is possible that the lack of effect in mice on LFD is due to limited expression in normal liver at baseline. It is also possible that there is an interaction between the ASO and dietary fat content. However, since steatotic livers are more susceptible to IR injury (24), we sought to characterize the impact of Mogat1 deficiency in the response to IR injury specifically in steatotic livers.
One of the intriguing findings of this work is that the initial response to IR injury was not overtly altered by Mogat1 knockdown, but rather, the resolution of liver injury and regenerative response were compromised. We found that a variety of parameters, including plasma transaminase and bilirubin concentrations, liver necrosis, and oxidative damage were not significantly different at early reperfusion time points, but failed to resolve or were exacerbated in mice with Mogat1 knockdown at later time points. Additionally, Mogat1 knockdown was associated with increased systemic and hepatic inflammation as evidenced by increased hepatic expression and plasma concentrations of inflammatory cytokines and chemokines. Hepatic gene expression of Il-1β demonstrated an interesting pattern of change. While expected to increase in the early reperfusion time points (38, 39), it also displayed a second wave of elevation following a period of decline at the 24 h reperfusion time point. Few studies have evaluated inflammation beyond the 24 h reperfusion time point, thus, based on available literature, the natural course of Tnfα and Il-1β expression in the liver beyond 24 h following IR injury is unclear. Our RNA-seq data indicate that macrophage chemokines continue to be markedly upregulated at 24 h in ASO mice compared to CTRL mice. It is possible that this later influx of macrophages is responsible for the accumulation of Il-1β at 72 h. Additionally, neutrophil chemoattractants also continue to increase at 24 h. While macrophages are the main source of Il-1β, neutrophils also play a role in Il-1β release (40, 41). Thus, it is possible the neutrophil infiltration leads to further liver injury along with Il-1β accumulation at the later 72 h time point. While our current studies do not allow us to delineate the exact mechanism by which this occurs, it is in keeping with our overall finding that ASO mice have increased mortality and prolonged liver injury compared to CTRL mice. These findings suggest that while both groups experience significant liver injury following IR injury, CTRL mice are able to resolve these processes while mice with Mogat1 knockdown experience persistent liver injury. This prolonged period of inflammation and liver injury likely contributes to the inability to proceed with regeneration, resulting in increased mortality.
Our findings also suggest that the hepatic regenerative response to IR injury was negatively impacted by Mogat1 knockdown. Consistent with previous reports that showed the percentage of viable liver tissue increases after 24 h due to liver regeneration (42, 43), we found that the area of necrotic tissue decreased in CTRL mice between 24 and 72 h. While we observed no difference in necrosis between treatment groups at 24 h reperfusion, ASO treated mice had much larger necrotic areas compared to CTRL mice at 72 h, suggesting that the ability to replace necrotic tissue was impaired by Mogat1 knockdown. This idea is also supported by reduced expression of markers of regeneration, including Pcna, CyclinD1, and Ki67 with Mogat1 knockdown. Taken together, the temporal changes in the measured parameters of liver injury and failure to activate the regenerative response are consistent with the phenotype of increased late mortality that we observed in mice with Mogat1 knockdown. The mechanistic explanation for this failure to resolve inflammation and to initiate the regenerative response will require future investigation. However, our data suggest that lipid metabolism may play a significant role.
An interesting finding of our study was the observed increase in hepatic TAG content following IR injury in CTRL mice, which is remarkable since these livers were steatotic at baseline due to the high fat diet (Fig. 1B). Many studies have noted the development of transient steatosis following partial hepatectomy, and postulated that accumulation of liver fat is crucial to the regenerative process since suppression of hepatic fat accumulation may inhibit liver regeneration in that model (30). However, it is important to note that others have reported no effects of a general disruption of hepatic steatosis on liver regeneration (31, 44–47). Interestingly, the increase in TAG content we observed after IR surgery was blocked by Mogat1 knockdown. This is consistent with the role for MGAT1 in one of the pathways of TAG synthesis and could suggest that the inhibition of hepatic steatosis is involved mechanistically in the impaired regenerative capacity with Mogat1 knockdown. Interestingly, Mogat1 knockdown did not decrease liver TAG content in sham operated ASO mice compared to sham operated CTRL mice. At a gene expression level, decreases in TAG synthesis enzymes could be counteracted by reduction in lipolytic enzymes or mitochondrial oxidative enzymes. However, steady state lipid levels are a balance between synthesis and lipolysis. Thus, future studies evaluating TAG synthesis and turnover will require the use of tracer based approaches.
In addition to the role of hepatic TAG, Mogat1 deficiency could impair resolution and regeneration by impacting the synthesis or accumulation of other glycerophospholipids in related pathways. For example, we detected a significant decrease in PA hepatic content in ASO treated mice compared to CTRL mice at 72 h post reperfusion. PA is a component of the other pathway of TAG synthesis and there is evidence that PA has mitogenic effects that play a role in liver regeneration (28, 30, 48). PA stimulates cell proliferation through activation of the mTOR pathway (49, 50) and inhibition of PA synthesis impairs liver regeneration following acetaminophen toxicity (28). Though it is not yet clear why PA content was reduced with Mogat1 ASO, it is possible that impaired PA-mediated signaling and proliferation pathways may lead to a defective regenerative response. Additionally, we observed an increase in PC:PE ratio with increasing reperfusion time in CTRL mice but not ASO treated mice. The mechanism whereby this occurs is unclear, but it is known that changes in PC:PE ratio can impact liver regeneration, lipid droplet size, and energy homeostasis (29, 51). We also cannot exclude that other toxic lipids or free fatty acids (33, 52, 53) are accumulating with Mogat1 knockdown. While we found no difference in the hepatic content of free fatty acids palmitic or stearic acid, in the future, it may be illuminating to perform untargeted lipidomics and lipid flux studies in order to fully characterize this response.
Lastly, transcriptomic analyses also suggested that Mogat1 ASO led to a reduced expression of myriad enzymes involved in mitochondrial and intermediary metabolism including glycerophospholipid, fatty acid, branched chain amino acid, and arachidonic acid synthesis and degradation pathways. Although the expression of many of these genes was down-regulated by IR injury in CTRL mice as well, the magnitude of the effect was greater in the ASO-treated mice. Interestingly, we have previously shown that Mogat1 inhibition in fasted mice altered the transcriptional activity of peroxisome proliferator-activated receptor α (PPARα) (25), which is a critical regulator of lipid metabolic gene expression in liver. Indeed, pathway analyses identified PPAR signaling as being significantly suppressed by Mogat1 ASO after 4 h and 24 h of reperfusion in the present study as well. Impairments in these energy metabolic pathways could impede regeneration by causing an energy deficit that impacts cell proliferation and thus the regenerative response.
There are limitations to the present study that we must note. Female mice were not included in our study and very little is known about possible sex differences in the response to IR injury. Second, given the high mortality between 24 and 72 h, it is possible that a selection bias is occurring in the 72 h ASO group. However, given that the surviving ASO mice exhibit worse liver injury at that time point, it is probable that this bias is leading to an underestimation of the severity of the phenotype since the most severely injured mice likely succumbed earlier. We have also previously shown that the Mogat1 targeted ASO also decreased Mogat1 expression in adipose tissue (25), which could affect lipolysis (54) and the flux of fatty acids to the liver. There is also a possibility of off-target effects of ASO and it is known that ASOs can also accumulate in non-parenchymal liver cells (55). In the future, it will be important to validate our findings in genetic mouse models, specifically in mice with cell type specific deletion of Mogat1. Additionally, it will be important to determine if our findings are applicable to other models of liver injury including partial hepatectomy and cold IR injury, which could have significant implications for the field of liver transplantation.
In conclusion, we have found that Mogat1 knockdown is associated with poorer outcomes following hepatic IR injury in the setting of hepatic steatosis. Our data suggest that in the setting of Mogat1 knockdown, there is an inability to control oxidative stress and dampen down the pro-inflammatory state associated with IR injury. This inability to recover impedes liver regeneration and functional recovery resulting in late mortality. We also found significant changes related to hepatic lipid content and global changes in multiple metabolic pathways. Together, these finding suggest an important role for lipid and intermediary metabolism in the response to IR injury. These studies lay the groundwork for future studies aimed at understanding the relationship among hepatic steatosis, lipid metabolism, and regeneration following hepatic IR injury. A better understanding of the mechanisms underlying IR injury in steatotic livers may lead to identification of targets aimed at its prevention and treatment.
Supplementary Material
Acknowledgments
We thank the Genome Technology Access Center (GTAC) in the McDonnell Genome Institute at Washington University School of Medicine for help with genomic analysis. We thank the Metabolomics Facility at Washington University School of Medicine (NIH P30 DK056341) for performing mass spectrometry studies. We thank the Digestive Diseases Research Cores Center in the Division of Gastroenterology at Washing University School of Medicine for immunohistochemical studies. We thank Ionis Pharmaceuticals, Inc (Richard Lee, Mark J. Graham) for supplying the antisense oligonucleotides used in these studies.
Grants and financial support
Work in the authors’ lab was supported by grants from the NIH (R56 DK111735) and the American Diabetes Association (1-17-IBS-109) to BNF and core laboratories of Washington University School of Medicine (Digestive Diseases Research Cores Center, NIH P30DK052574 and the Nutrition Obesity Research Center P30 DK056341). KHHL is supported by NIH K12 HD076224-06. KSM is supported by NIH R00 DK056341. MRM is supported by a Pinnacle Research Award from the American Association for the Study of Liver Diseases Foundation. The Genome Technology Access Center in the McDonnell Genome Institute at Washington University School of Medicine is partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant# UL1TR002345 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The Metabolomics Facility at Washington University School of Medicine is supported by NIH P30 DK056341. The Digestive Diseases Research Cores Center in the Division of Gastroenterology at Washing University School of Medicine is supported by NIH P30DK052574
Abbreviations
- ALT
alanine transaminase
- ASO
antisense oligonucleotide
- AST
aspartate transaminase
- AU
arbitrary units
- CCL3
chemokine ligand 3
- DAG
diacylglycerol
- DGAT
diacylglycerol acyltransferase
- FFA
free fatty acid
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HFD
high fat diet
- HNE
hydroxynonenal
- IL
interleukin
- IP
intraperitoneal
- IR
ischemia reperfusion
- LFD
low fat diet
- MAG
monoacylglycerol
- MGAT
monoacylglycerol acyltransferase
- MIP
macrophage inflammatory protein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PCNA
proliferating cell nuclear antigen
- PE
phosphatidylethanolamine
- PPAR
peroxisome proliferator activated receptor
- TAG
triacylglycerol
- TNF
tumor necrosis factor
Footnotes
Potential conflicts of interest: Nothing to report.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl 2006;12:523–534. [DOI] [PubMed] [Google Scholar]
- 3.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249–1253. [DOI] [PubMed] [Google Scholar]
- 4.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 5.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, Stewart G, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl 2003;9:500–505. [DOI] [PubMed] [Google Scholar]
- 6.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, Gores GJ, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation 1996;62:1246–1251. [DOI] [PubMed] [Google Scholar]
- 7.Rinella ME, Alonso E, Rao S, Whitington P, Fryer J, Abecassis M, Superina R, et al. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl 2001;7:409–414. [DOI] [PubMed] [Google Scholar]
- 8.Escartin A, Castro E, Dopazo C, Bueno J, Bilbao I, Margarit C. Analysis of discarded livers for transplantation. Transplant Proc 2005;37:3859–3860. [DOI] [PubMed] [Google Scholar]
- 9.Feng S, Lai JC. Expanded criteria donors. Clin Liver Dis 2014;18:633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu MJ, Hickey AJ, Phillips AR, Bartlett AS. The impact of hepatic steatosis on hepatic ischemia-reperfusion injury in experimental studies: a systematic review. Biomed Res Int 2013;2013:192029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 2000;32:1280–1288. [DOI] [PubMed] [Google Scholar]
- 13.Selzner N, Selzner M, Jochum W, Amann-Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol 2006;44:694–701. [DOI] [PubMed] [Google Scholar]
- 14.Teramoto K, Bowers JL, Kruskal JB, Hara J, Iwai T, Endo M, Clouse ME. In vivo microscopic observation of fatty liver grafts after reperfusion. Transplant Proc 1994;26:2391. [PubMed] [Google Scholar]
- 15.Serafin A, Rosello-Catafau J, Prats N, Xaus C, Gelpi E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol 2002;161:587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson CD, Upadhya G, Conzen KD, Jia J, Brunt EM, Tiriveedhi V, Xie Y, et al. Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts. Liver Transpl 2011;17:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med 2011;17:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Z, Mulvihill MM, Mukhopadhyay P, Xu H, Erdelyi K, Hao E, Holovac E, et al. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology 2013;144:808–817 e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XJ, Cheng X, Yan ZZ, Fang J, Wang X, Wang W, Liu ZY, et al. An ALOX12–12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nat Med 2018;24:73–83. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 2004;43:134–176. [DOI] [PubMed] [Google Scholar]
- 21.Hall AM, Kou K, Chen Z, Pietka TA, Kumar M, Korenblat KM, Lee K, et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J Lipid Res 2012;53:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall AM, Soufi N, Chambers KT, Chen Z, Schweitzer GG, McCommis KS, Erion DM, et al. Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice. Diabetes 2014;63:2284–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soufi N, Hall AM, Chen Z, Yoshino J, Collier SL, Mathews JC, Brunt EM, et al. Inhibiting monoacylglycerol acyltransferase 1 ameliorates hepatic metabolic abnormalities but not inflammation and injury in mice. J Biol Chem 2014;289:30177–30188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liss KHH, McCommis KS, Chambers KT, Pietka TA, Schweitzer GG, Park SL, Nalbantoglu I, et al. The impact of diet-induced hepatic steatosis in a murine model of hepatic ischemia/reperfusion injury. Liver Transpl 2018;24:908–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutkewitte AJ, McCommis KS, Schweitzer GG, Chambers KT, Graham MJ, Wang L, Patti GJ, et al. Hepatic monoacylglycerol acyltransferase 1 is induced by prolonged food deprivation to modulate the hepatic fasting response. J Lipid Res 2019;60:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe Y, Hines IN, Zibari G, Pavlick K, Gray L, Kitagawa Y, Grisham MB. Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med 2009;46:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews WR, Guido DM, Fisher MA, Jaeschke H. Lipid peroxidation as molecular mechanism of liver cell injury during reperfusion after ischemia. Free Radic Biol Med 1994;16:763–770. [DOI] [PubMed] [Google Scholar]
- 28.Lutkewitte AJ, Schweitzer GG, Kennon-McGill S, Clemens MM, James LP, Jaeschke H, Finck BN, et al. Lipin deactivation after acetaminophen overdose causes phosphatidic acid accumulation in liver and plasma in mice and humans and enhances liver regeneration. Food Chem Toxicol 2018;115:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling J, Chaba T, Zhu LF, Jacobs RL, Vance DE. Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 2012;55:1094–1102. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Rudnick DA. Elucidating the metabolic regulation of liver regeneration. Am J Pathol 2014;184:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudnick DA, Davidson NO. Functional Relationships between Lipid Metabolism and Liver Regeneration. Int J Hepatol 2012;2012:549241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers 2015;1:15080. [DOI] [PubMed] [Google Scholar]
- 33.Neuschwander-Tetri BA. Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Curr Gastroenterol Rep 2010;12:49–56. [DOI] [PubMed] [Google Scholar]
- 34.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015;21:739–746. [DOI] [PubMed] [Google Scholar]
- 35.Li XL, Man K, Ng KT, Lee TK, Lo CM, Fan ST. Insulin in UW solution exacerbates hepatic ischemia / reperfusion injury by energy depletion through the IRS-2 / SREBP-1c pathway. Liver Transpl 2004;10:1173–1182. [DOI] [PubMed] [Google Scholar]
- 36.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation 1997;95:313–315. [DOI] [PubMed] [Google Scholar]
- 37.Masoud WG, Abo Al-Rob O, Yang Y, Lopaschuk GD, Clanachan AS. Tolerance to ischaemic injury in remodelled mouse hearts: less ischaemic glycogenolysis and preserved metabolic efficiency. Cardiovasc Res 2015;107:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol 1991;260:G355–362. [DOI] [PubMed] [Google Scholar]
- 39.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology 1998;27:1172–1177. [DOI] [PubMed] [Google Scholar]
- 40.Sadatomo A, Inoue Y, Ito H, Karasawa T, Kimura H, Watanabe S, Mizushina Y, et al. Interaction of Neutrophils with Macrophages Promotes IL-1beta Maturation and Contributes to Hepatic Ischemia-Reperfusion Injury. J Immunol 2017;199:3306–3315. [DOI] [PubMed] [Google Scholar]
- 41.Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, Rathkey JK, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat Commun 2020;11:2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konishi T, Schuster RM, Lentsch AB. Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2018;314:G471–G482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konishi T, Schuster RM, Lentsch AB. Liver repair and regeneration after ischemia-reperfusion injury is associated with prolonged fibrosis. Am J Physiol Gastrointest Liver Physiol 2019;316:G323–G331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, Crooke RM, et al. Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models. Hepatology 2008;48:1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sydor S, Gu Y, Schlattjan M, Bechmann LP, Rauen U, Best J, Paul A, et al. Steatosis does not impair liver regeneration after partial hepatectomy. Lab Invest 2013;93:20–30. [DOI] [PubMed] [Google Scholar]
- 46.Zou Y, Bao Q, Kumar S, Hu M, Wang GY, Dai G. Four waves of hepatocyte proliferation linked with three waves of hepatic fat accumulation during partial hepatectomy-induced liver regeneration. PLoS One 2012;7:e30675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vetelainen R, van Vliet AK, van Gulik TM. Severe steatosis increases hepatocellular injury and impairs liver regeneration in a rat model of partial hepatectomy. Ann Surg 2007;245:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T, Wang X, Zhou H, Jiang H, Mai K, He G. The Mitotic and Metabolic Effects of Phosphatidic Acid in the Primary Muscle Cells of Turbot (Scophthalmus maximus). Front Endocrinol (Lausanne) 2018;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joy JM, Gundermann DM, Lowery RP, Jager R, McCleary SA, Purpura M, Roberts MD, et al. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr Metab (Lond) 2014;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 2001;294:1942–1945. [DOI] [PubMed] [Google Scholar]
- 51.van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr 2017;1859:1558–1572. [DOI] [PubMed] [Google Scholar]
- 52.Liangpunsakul S, Chalasani N. Lipid mediators of liver injury in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 2019;316:G75–G81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007;45:1366–1374. [DOI] [PubMed] [Google Scholar]
- 54.Liss KHH, Lutkewitte AJ, Pietka T, Finck BN, Franczyk M, Yoshino J, Klein S, et al. Metabolic importance of adipose tissue monoacylglycerol acyltransferase 1 in mice and humans. J Lipid Res 2018;59:1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, Schmidt K, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res 2014;42:8796–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.