Abstract
Infusion of viral-specific T cells (VSTs) is an effective treatment for viral infection after stem cell transplant. Current manufacturing approaches are rapid, but growth conditions can still be further improved. To optimize VST cell products, we designed a high-throughput flow cytometry-based assay using 40 cytokine combinations in a 96 well plate to fully characterize T cell viability, function, growth, and differentiation. Peripheral blood mononuclear cells (PBMC) from six consenting donors were seeded at 100,000 cells/well with pools of CMV peptides from IE-1 and pp65, and combinations of IL15, IL6, IL21, IFNα, IL12, IL18, IL4, and IL7. Ten-day cultures were tested by 13 color flow cytometry to evaluate viable cell count, lymphocyte phenotype, memory markers, and IFNγ and TNFα expression. Combinations of IL15/IL6 and IL4/IL7 were optimal for the expansion of viral-specific CD3+ T cells, (18-fold and 14-fold respectively compared with unstimulated controls). CD8+ T cells expanded 24-fold in IL15/IL6, and 9-fold in IL4/IL7 cultures (p< 0.0001). CD4+ T cells expanded 27-fold in IL4/IL7 and 15-fold in IL15/IL6 (p< 0.0001). CD45RO+ CCR7- effector memory T cells were the preponderant cells (76.8% and 72.3% in IL15/IL6 and IL15/IL7 cultures, respectively). Cells cultured in both cytokine conditions were potent, with 19.4% of CD3+ cells cultured in IL15/IL6 producing IFNγ (7.6% producing both TNFα and IFNγ), and 18.5% of CD3+ cells grown in IL4/IL7 (9% producing both TNFα and IFNγ). This study shows the utility of this single plate assay to rapidly identify optimal growth conditions for VST manufacture using only 107 PBMC.
Keywords: Cytokine, T cell, Process Development, Antiviral, Cellular Therapy
Introduction
Adoptive T cell immunotherapies are increasingly used to treat infection and malignant disease. A common technique involves culturing T cells with antigen-presenting cells (APC) exposed to peptide antigens in the presence of a cytokine cocktail. Several immunomodulatory cytokines are currently used to promote T cell division and differentiation, but optimal conditions for the growth and function of peptide stimulated T cell products to have yet to be fully defined. Cell products used in clinical trials have typically supplemented T cell cultures with the growth-promoting cytokines IL2, IL15, IL4, and IL7 [1-3]. However, the search to optimize culture conditions is limited by the time and labor needed to screen multiple cytokine combinations. Therefore, we established a flow cytometry-based approach to rapidly evaluate many cytokine combinations in a single 96 well plate to measure T cell phenotype and potency on a limited number of cells.
Manufacture of viral-specific T cell products expands a heterogeneous pool of pre-existing memory T cells from peripheral donor blood, with the final product containing a polyclonal mixture of CD4+ helper and CD8+ cytotoxic T cells[4]. This diversity increases the complexity of manufacture, as CD4+ T cells and CD8+ T cells respond differently to cytokine stimulation. For example, IL4 enhances the survival of resting T cells and induces CD4+ Th2 helper differentiation[5-7], while IL15 promotes survival and diversity of CD8+ memory T cells[8, 9]. IL2 is a canonical T cell growth cytokine which continues to be used in clinical trials due to its effectiveness in expanding T cells derived from tumor-infiltrating lymphocytes[10]. However, other cytokines also have essential functions:
IL6 may enhance Th17 development [11].
Our manufacturing methods have transitioned from culturing T cells in 24 well plates with IL2, and APC transduced with viral antigens[18-20] to a simplified culture containing a combination of IL4 and IL7 in G-Rex gas permeable devices with soluble mixes of peptides[3, 21, 22]. This system rapidly expands functionally competent T cells specific for multiple viruses. Ten-fold expansions of 1.5x107 donor PBMCs cultured in a G-Rex can provide sufficient VST CD3+ T cells to treat multiple patients in the third-party “off the shelf” setting[3, 23-26]. Manufactured T cells are currently characterized for safety, phenotype, and potency in three separate assays: ELISPOT for IFN-γ release, 51Cr release to measure cytotoxicity, and flow cytometry to identify cellular phenotype. However, routinely available 13 color flow cytometry panels make it possible to measure intracellular cytokines, surface marker phenotype, and correlates of cytotoxicity and alloreactivity, in a single assay to define product quality. Flow cytometric assays minimize culture volume reducing the number of cells needed for validation while increasing the number of testable conditions that can be applied to donor PBMC. Using this approach, we identified new cytokine combinations controlling phenotypic diversity, growth, and function of viral-specific T cell products. We found that high throughput screening by multicolor flow cytometry is affordable and practical for product development.
Materials and Methods
Blood collection
Peripheral blood was collected from de-identified platelet transfusion filters from donors according to IRB-approved protocol.
Cell culture
PBMCs were separated by Ficoll and spun at 800 xg for 25 minutes to purify lymphocytes. Cells were washed twice with complete RPMI, and 2x107 cells were re-suspended in 5 mL with 10 μL of 200 μg/mL of peptide libraries encompassing IE1 and PP65. Cells were incubated at 37 °C for 1 h. Five mL of complete media was added, and 100,000 cells/well were plated in 96-well round-bottom plates. Cytokines were added at the indicated concentration in a final volume of 200 μL. Cells were cultured for seven days. Plates were then spun down at 400xg, and the cells re-suspended in 200 μL of complete media. Samples were split into two plates of 100 μL each, and fresh media and cytokines were added at the indicated concentration to a final volume of 200 μL. Cells were cultured for three additional days before antigen re-stimulation and antibody staining. For culture within Grex-10 gas permeable chambers (Wilson Wolf; St. Paul, MN, 1-1.5x107 cells were isolated from PBMCs and re-suspended in 1 mL of complete media with 2 μL of 200 μg/mL of peptide libraries encompassing IE1 and PP65 and cultured for 1h at 37 °C. 29 mL of complete media was added, along with either 400 U/mL IL4 + 10 ng/mL IL7, or 10 ng/mL of IL15 + 100 ng/mL IL6. Cells were cultured for seven days, after which 15 mL of media was removed and replaced with fresh complete media and cytokines. Cells were cultured for three additional days before antigen re-stimulation in ELISPOT assays.
Intracellular Cytokine Staining
Plates were spun down at 400xg for 5 min and re-suspended in 100 μL of complete media containing the mix of IE1 and PP65 peptide libraries at 1.0 μg/mL final concentration with no peptide controls. Cells were incubated with peptides at 37 °C for 1h. Then 100 μL of complete media containing Brefeldin A or Monensin plus Brefeldin A was added. Cells were cultured for an additional 5h, after which cells were stained with antibodies for phenotyping.
Cr51 cytotoxicity assay
VSTs were collected and re-suspended in complete media to 2 x 106 cells per mL. Autologous target cells (Phytohemmaglutinin (PHA) Blasts) were collected, washed once with PBS, and re-suspended in once PBS to a concentration of 2.5 x 106 cells per mL. 10 μL of Cr51 was added along with 1 μL of either actin or IE1 + pp65 peptide pools per 100 μL of target suspension. Targets were incubated with chromium and peptides for 1h at 37 °C, after which targets were washed three times with complete media. Effectors were added in serial dilution in triplicate with 2 x 105 effectors as the top condition. After 1h, targets were re-suspended in complete media at a concentration of 5 x 104 cells per mL, and 100 μL of targets (5000 cells) were added per well into a 96-well U-bottom plate. Conditions included targets alone (spontaneous release), targets plus 1% Triton-X100 (max release), and 40:1, 20:1, 10:1 effector:target combinations using no peptide, actin peptide, or IE1+pp65 peptide pools. Cells were cultured for 4h, after which supernatant was collected for detection of Cr51 release. Replicates were averaged together, and cytotoxicity was calculated according to the formula {[(specific release)-(spontaneous release)]/[(max release)-(spontaneous release)]}.
ELISPOT assay
ELISPOT plates were coated with 100 μL of 1 μg/mL final concentration anti-IFNγ mAb (Clone 1-D1K; Mabtech, Cincinnati, OH) in sterile ELISPOT carbonate coating buffer (1.59g Na2CO3, 2.93g NaHCO3 per liter of sterile water) overnight at 4 °C. Plates were washed twice with 150 μL of coating buffer, and 100 μL of complete media was added to wells and incubated for 1h at 37 °C. Cells from G-rex10 culture vessels were plated at the indicated concentrations in 200 μL total volume with actin (1 μg/mL), IE1 and pp65 peptide pools (1 μg/mL), SEB (0.5 μg/mL), or media alone. Plates were incubated for 16h at 37 °C, after which cells were decanted, and plates were washed six times with 1x PBS/0.05% Tween 20. 100 μL of biotinylated anti-IFNγ mAb at 1 μg/mL (Clone 7-B6-1; Mabtech) in biotin buffer (2.5g biotin in 500 mL 1x PBS) was added to each well and incubated at 37 °C for 1 h. Plates were washed six times with 1x PBS/0.05% Tween 20. 100 μL of an avidin-peroxidase solution in 1x PBS/Tween 20 (APC; Millipore Sigma, Darmstadt, Germany) was added per well and incubated for 1h at room temperature. Plates were washed three times with 1x PBS/0.05% Tween 20 and three times with 1x PBS. Spots were developed using 100 μL of AEC substrate (3-amino-9-ethyl carbazole; Vector Labs, Burlingame, CA) for 4 minutes at room temperature and rinsing with tap water. Plates were dried overnight; individual wells were punched out onto adherent film for enumeration by an independent third party. Corrected spot counts were derived according to the formula {Raw spot value + 2 × [(Raw spot value × %confluence) / (100% - %confluence)]}.
Antibody staining
Cells were spun down at 400xg for 5 min and washed once in 100 μL 1x PBS. Cells were spun down again and re-suspended in 50 μL of 1x PBS containing Live Dead Aqua at a dilution of 1:500 and then stained for 20 min at 4 °C and washed with 100 μL of 1x PBS containing 2% FCS. Cells were fixed in 50 μL of 1x Cytofix/Cytoperm (BD Biosciences, San Jose, CA) for 30 minutes at 4 °C and washed twice in 1x Permwash (BD Biosciences, San Jose, CA), then re-suspended in 25 μL of staining solution containing 12.5 μL of 1x Permwash and 12.5 μL of Brilliant Violet staining solution (Biolegend, San Diego, CA). Markers for staining included CD62L V450 (Biolegend; clone DREG-56), CD4 BV570 (Biolegend; clone RPA-T4), CD45RO BV605 (Biolegend; clone UCHL1), CD8 BV711 (Biolegend; clone SK1), CD56 BV785 (Biolegend; clone 5.1H11), CCR7 FITC (Biolegend; G043H7), CD28 PE (Miltenyi Biotec, San Diego, CA; clone REA612) , CD95 PE Dazzle CF594 (Biolegend; clone DX2), CD3 PerCP Cy5.5 (Biolegend; clone OKT3), TNFα PE Vio770 (Miltenyi Biotec; clone cA2), IFNγ APC (Biolegend; clone RS.B3), CD107a APC H7 (Miltenyi Biotec; clone REA 792), CD45RA APC H7 (Biolegend; clone HI100). Cells were stained for at least 30 minutes at 4 °C, washed twice with 100 μL of 1x Permwash, and re-suspended in 55 μL of 1x Permwash. Cells were loaded onto a Sartorius IQue Screener Plus (Sartorius, Göttingen, Germany) to collect all samples. Data was analyzed in ForeCyt and Flowjo software (FlowJo, Ashland, OR), and statistics were analyzed in Graphpad Prism software (GraphPad, San Diego, CA).
High throughput screening of alternative cytokine combinations
The method allowed for up to 40 cytokine culture conditions to be tested on 1x107 PBMCs in a 96 well plate using the IQue Screener Plus, high throughput screener for all flow cytometry. This method combined initial culturing and antigen-specific expansion with staining and analysis in a single culture plate (Supplemental Figure 1). We selected flow cytometry as our method for analysis to combine measurements of cellular phenotype, viability, expansion, and effector function in a single 13 color panel. To test for antigen specificity, cells were split on day seven into two identical plates with fresh media and cytokines, and plates were subsequently challenged with or without peptide pools on day 10. We initially tested combinations of IL15, IL6, IL21, and IFNα as compared against IL4 and IL7 as a reference standard for four samples (plate layouts 1, 2; Supplemental Figure 2). We also tested modified layouts, which added IL12 and IL18, and combinations intermixing IL15, IL6, IL4, and IL7. Furthermore, we tested layouts with additional replicates of IL15 + IL6 and IL4 + IL7 and replicates challenged with irrelevant peptide pools as an additional measure of antigen specificity (plate layouts 3, 4; Supplemental Figure 2). Overall, the use of 96 well plates as both culture and staining vessels for flow cytometric analysis allowed us to test 92 different combinations of cytokines using four different plate layouts. Our workflow for the analysis of samples utilized a hierarchical gating strategy that categorized positive and negative gates based on initial FMO staining controls analyzed within Flowjo (Supplemental Figure 3). For phenotyping, we measured the frequency of living CD3+, CD3+ CD4+, and CD3+ CD8+ T cells present within the culture, along with CD3- CD56+ NK cells. Viability was measured by vital dye staining using Live Dead Aqua, while the cytotoxic function was compared by measuring IFNγ and TNFα intracellular cytokine production in viral wells pulsed with peptide pools over antigen non-specific background wells. Finally, T cell memory markers ’surface expression was used to judge the differentiation status of cells, including CD45RA, CD45RO, CCR7, CD28, CD95, and CD62L.
Results
High throughput screening of cytokine combinations
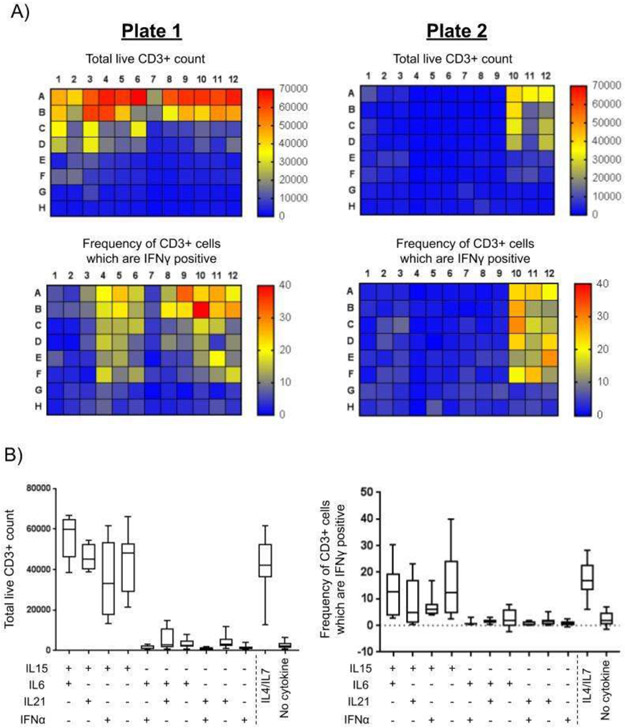
We first screened the growth and function of cells in all cytokine conditions. Representative heat map data are shown in Figure 1a and summarized in Supplementary Figure 4. The control culture of PBMCs without added cytokine did not support T cell growth. CD3+ T cell expansion in the standard cytokine mix of IL4 and IL7 (optimum results with 400 U/mL and 10 ng/mL respectively) achieved a 12.3-fold increase over control. IL15 alone or, in combination with other cytokines, achieved the best expansions at the highest dose of 10 ng/mL representing a 22.5-fold increase over control. Mixtures containing IL6 and IL21 produced only a modest expansion of 2.9-fold.
Figure 1. High throughput flow cytometry analysis identifies superior cytokine combinations.
A) Quantifications of phenotype and function of two plates from Sample 4 were analyzed by flow cytometry on Day 10, with entire contents of wells collected and visualized by heat map. The total count of viable CD3+ T cells was quantified from wells re-stimulated with media alone. The frequency of IFNγ+ CD3+ cells was derived from the frequency of IFNγ+ CD3+ cells after re-stimulation with IE1 and pp65 peptide pools and subtracted from media alone control wells. B) Wells containing the highest concentration of cytokines were compared between Samples 1-4 when cultured using plate layouts 1 and 2. The total recovered viable CD3+ count, and the frequency of CMV specific CD3+ IFNγ+ cells (n ≥ 8) were compared across each sample.
We then selected the combination of IL15 (10 ng/mL) and IL6 (100 ng/mL) for further investigation based on the favorable expansion of CD3+ T cells and their cytokine production in four experiments (Figure 1b). We confirmed that the addition of IL15 or IL7 was sufficient for the expansion of CD3+ T cells and that our original selection of IL15/IL6 was superior to all other combinations. Overall, culture is a combination of IL15/IL6 consistently promoted CD3+ T cell expansion and IFNγ production to levels similar to culture in IL4/IL7.
Selective cytokine culture imparts bias on the ratio of CD4 vs. CD8 cells in viral-specific T cell products
Six CMV reactive patient samples in IL15 alone, IL15 and IL6 and IL4 and IL7, were compared in replicate testing (Figure 2). Culture in IL15/IL6 expanded a median of 17.1-fold more CD3+ cells compared with no cytokine controls (p<0.0001). Culture in IL4/IL7 expanded a median of 13.8-fold more compared with no cytokine control (p<0.0001; Figure 3a). The viability of CD3+ cells on average was not significantly different between wells containing IL15/IL6 or IL4/IL7, with a median of 90% and 89%, respectively (p=0.966). CD3- CD56+ NK cells were present in cultures expanded with IL15/IL6, with a median of 6.6% of total cells recovered (4106 cells; Figure 2a). Less than 300 NK cells were recovered on average from wells containing IL4/IL7, representing 0.6% of total cells recovered.
Figure 2. Culture in IL15 and IL6 stimulates expansion of CMV specific CD3+ T cells equal or better than IL4 and IL7.
Cells were re-stimulated with CMV peptide pools for 6h and evaluated for phenotype and function by flow cytometry, with the entire well contents analyzed for the top dilutions of IL15 and IL6, IL4 and IL7, IL15 alone, and no cytokine controls. The median of individual replicates (n≥8) was analyzed across experiments, and 2way ANOVA analyzed individual patient samples (n=6), and statistics with Tukey’s correction with **** p < 0.0001. From wells re-stimulated with media alone, the total count of viable CD3+ T cells, the percentage of viability of all CD3+ cells, and viable CD56+ CD3- NK cell count were calculated from wells in (a). The median total count of viable CD3+ CD4+ cells, CD3+ CD8+ cells, and the ratio of CD4+/CD8+ cells were calculated from wells in (b). From wells re-stimulated with IE1 and pp65 peptide pools, the median frequency of CD3+ IFNγ+ cells and CD3+ IFNγ+ TNFα+ cells were calculated from wells in (c), along with the total number of CMV specific CD3+ IFNγ+ cells (d) and the frequency of IFNγ+ cells within CD4+ CD3+ and CD8+ CD3+ subtypes were analyzed (e).
Figure 3. VSTs cultured in IL15/IL6 show CMV-specific cytotoxicity.
VSTs cultured in the presence of IL15/IL6 were investigated for cytolytic activity as measured by Cr51 release assay (a) and by expression of CD107a (b). In (a), VSTs were measured for cytotoxicity against autologous PHA blasts pulsed with no peptide, actin, or IE1 and pp65 peptide pools. In (b), VSTs were measured for cytolytic activity by flow cytometry of CD107a expression after re-stimulation for 6h with no peptide, actin, or IE1 + pp65 peptide pools.
Interestingly, we identified a strong bias in the ratio of CD4+ to CD8+ cells in the final product, depending on the initial culture’s cytokines. Culture in IL15 /IL6 conditions favored outgrowth of CD8+ T cells compared with culture in IL4/IL7, which favored outgrowth of CD4+ T cells. Culturing cells in IL4/IL7 expanded 2.1-fold more CD4+ T cells than cells cultured in IL15/IL6 (Figure 2b; p<0.0001), while culturing cells in IL15/IL6 more than doubled the expansion of CD8+ cells compared with IL4/IL7, with 2.8-fold more CD8+ cells on average in IL15/IL6 vs. IL4/IL7 (Figure 2b; p<0.0001). The viability of CD4+ and CD8+ cells were not significantly different comparing culture in IL15/IL6 and IL4/IL7 (data not shown; CD4 viability p = 0.4381; CD8 viability p = 0.1033), suggesting the different cytokine combinations were stimulating outgrowth of either CD4+ or CD8+ cells, rather than preserving the selective survival of individual subsets.
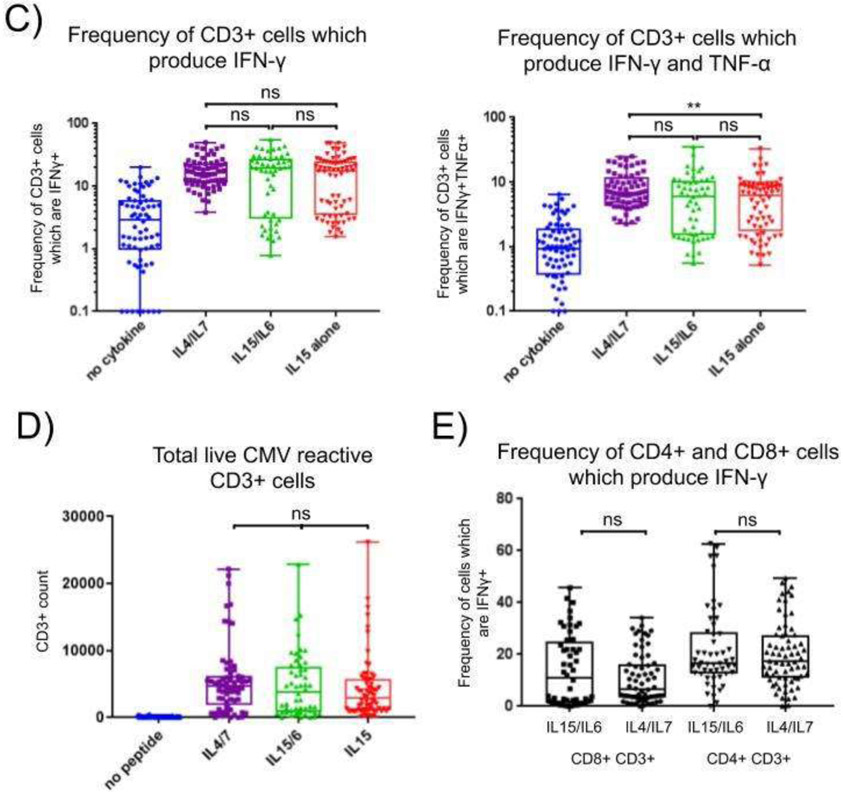
Both culture conditions expanded CD3+ T cells producing IFNγ in response to CMV peptide pool re-stimulation. The highest concentration of IL15+IL6 induced a median of 19.3% IFNγ producing CD3+ T cells (range 0.8%-55.3%), while IL4+IL7 induced a median of 16.3% of IFNγ producing CD3+ T cells in response to CMV peptides (range 3.8%-49.6%; Figure 2c; p=0.99). We also investigated the proportion of multi-cytokine producing cells, as evidence suggests these cells offer superior protection against viral infection when compared with cells producing a single cytokine. A median of 6.1% (range 0.6%-35.4%) of cells cultured in IL15/IL6 and 6.9% (range 2.3%-25.0%) of cells cultured in IL4/IL7 produced both IFNγ and TNFα in response to CMV peptides (Figure 2c; p=0.22). We observed no significant difference in the total number of CMV reactive CD3+ cells comparing IL15/IL6 with IL4/IL7 cytokine conditions (Figure 2d; p=0.45).
Limited production of cytokines by IL-15 expanded CD4+ T cells has been previously reported. Therefore, we compared the production of IFNγ by CD4+ and CD8+ subsets within the CD3+ T cell population and found culture in IL15/IL6 was sufficient to expand CMV specific CD8+ and CD4+ cells equivalent to culture in IL4/IL7 (Figure 2e). 6.6% (range 0%-34.2%) of CD8+ cells cultured in IL4/IL7 and 11.1% (range 0%-45.7%) of CD8+ cells cultured in IL15/IL6 produced IFNγ in response to CMV (p=0.46). Similarly, a median of 17.3% (range 0%-49.4%) and 16.6% (range 0%-62.7%) of CD4+ cells produced IFNγ in response to CMV peptides when cultured in IL4/IL7 and IL15/IL6, respectively (p=0.46). In summary, compared with IL4/IL7, cultures in IL15/IL6 produced a comparable number of CMV reactive CD3+ VSTs that were more skewed towards a CD8+ phenotype.
VSTs grown in IL15/IL6 show CMV-specific cytotoxicity
We compared the CMV specific cytotoxicity of cells grown in IL15/IL6 by traditional Cr51 release assays and CD107a expression in our flow-based assay in Figure 3. VSTs were expanded in IL15/IL6 as effectors and split between assays after ten days of expansion for Donors 3 and 4. Neither donor was reactive against autologous targets up to 10:1 E:T ratio. VST effectors were also challenged with autologous targets loaded with either actin peptide (negative control) or IE1+pp65 peptide pools. Both donors demonstrated CMV specific reactivity, with VSTs from Donor 4 reaching 26% CMV specific killing and Donor 3 reaching 18% CMV specific killing at 40:1 E:T ratio with 1% actin specific killing (Figure 3a). VSTs from these cultures were also re-stimulated with actin or IE1+pp65 peptide pools and analyzed by flow cytometry for CD107a expression. CD107a (LAMP1) can potentially be used as a surrogate marker for cytotoxicity as it is transiently expressed on the cell surface during degranulation[27]. A median of 8.3% of CD3+ VSTs derived from Donor 3 expressed CD107a in response to IE1+pp65 peptide re-stimulation, while a median of 17.6% of CD3+ VSTs from Donor 4 expressed CD107a after re-stimulation (Figure 3b). This demonstrated that CD107a is a potential alternative marker for cytotoxicity as part of the assay.
VSTs are effector memory in phenotype
T cell differentiation’s extent has been suggested to influence the persistence of adoptively transferred T cells[28, 29]. We characterized the surface phenotype of VSTs expanded by in vitro culture in IL15/IL6 and IL4/IL7 to identify the proportion of cells expressing different combinations of T cell memory markers in Figure 4. Pre-culture CD3+ T cells comprised on average 32% naive/stem cell memory cells, 30.4% effector memory cells , 22% central memory cells and 15.4% terminal effectors. Ten-day VST products had a preponderance of effector-memory CD3+ cells representing 72.3% and 76.9% of cells grown in IL4/IL7 and IL15/IL6. Terminal effector cells lacking both CCR7 and CD45RO, represented 11.3% in IL4/IL7, and 14.3% in IL15/IL6. Central memory cells represented a minority of cells after culture, 9.3%, and 6.6% in IL4/IL7 and IL15/IL6 cultures. There was a greater frequency of naive cells in IL4/IL7 cultures (7.0%) than in IL15/IL6 (2.3%). Both culture conditions substantially reduced the frequency of naive cells compared to pre-culture frequencies (32%). We also compared the memory phenotype of antigen-specific cells with antigen non-responsive cells. CD3+ IFNγ+ cells had a predominantly effector memory phenotype, while a small number of naive cells (4.2% in IL4/IL7 and 1.7% in IL15/IL6) remained within the IFN-γ negative (antigen non-reactive) fraction, suggesting that culture in IL4/IL7 was preserving a subset of naive cells within the final product.
Figure 4. T cell therapy products are effector memory in phenotype (CCR7-CD45RO+) Memory analysis.
Cells were analyzed for the expression of memory markers CCR7 and CD45RO and divided into four populations, both pre and post-culture with cytokines. The pre-culture memory phenotype of viable cells was quantified for all samples (a) according to the layout presented for representative Sample 1 (b). After ten day culture, samples were analyzed again for memory markers CCR7 and CD45RO and averaged samples cultured in IL15/IL6 and IL4/IL7 growth conditions (c) and analyzed using 2-way ANOVA with Tukey’s correction (* corresponds to p < 0.05). One representative sample was examined for the memory phenotype of CD3+ cells which were positive or negative for IFNγ after re-stimulation with CMV specific peptides.
Process development time substantially reduced by using the IQue
Process development of antigen-specific T cells such as VSTs has mostly been limited to testing specific conditions in 24-well plates or Grex-10 devices, limiting the systematic testing of an array of cytokines and growth conditions. Expanding and testing VSTs using such methods require approximately 20 hours of work and 34 hours of incubation per cytokine condition. In contrast, process development in 96 well plates and a 13 color flow cytometry panel required only 12 hours of work and 8 hours of incubation. Therefore, to evaluate 40 cytokine conditions, take 2160 hours per sample by existing methods, but only 20 hours using our improved approach (Table 1).
Table 1.
| Traditional PD in culture vessels | Traditional PD in plates | Microassay using flow cytometry | |
|---|---|---|---|
| Culture vessel | G-Rex 10 | 24-well plate | 96-well plate |
| Sample usage | 15 × 108 | 50 × 108 | 10 × 108 |
| Conditions per culture vessel | 1 | 24 | 48 duplicates |
| Time to Ficoll, culture and feed | 8 h culture | 10 h culture | 8 h culture |
| Time for analysis, ELISpot | 4 h setup, 8 h incubation | 4 h setup, 18 h incubation | Included in intracellular flow |
| Phenotype | 2 h setup, 1 h analysis | 2 h setup, 1 h analysis | Included in intracellular flow |
| Cytotoxicity | 4 h setup, 8 h incubation | 4 h setup, 8 h incubation | Included in intracellular flow |
| Viable count | 1 h setup | 1 h setup | Included in intracellular flow |
| Intracellular flow | NA | NA | 4 h setup, 6 h incubation |
| Total time investment for all conditions | 8 h culture, 38 h analysis (4416 h for 96 conditions) | 10 h culture, 38 h analysis (192 h for 96 conditions) | 8 h culture, 10 h analysis (36 h for 96 conditions) |
NA, not applicable; PD, process development.
Optimized cytokine conditions identified in the 96-well plate translates to clinical scale manufacturing
Miniaturized cell cultures may not reliably scale-up in a linear fashion. To test whether our system could predict the phenotype and function of clinical-sized products, we investigated whether IL15/IL6 cultures in Grex-10 culture vessels would recapitulate the data from our experiments in 96-well plates. At least 1x107 cells were seeded with IE1 and pp65 peptide pools in Grex-10 culture vessels with medium and either IL4/IL7 at 400 U/mL IL4/100 ng/mL IL7 or 10 ng/mL IL15/ 100 ng/mL IL6 (Figure 5). Cells grown in IL15/IL6 produced 638 ±297 spots per 100,000 cells in response to CMV peptides, while cells cultured in IL4 and IL7 produced a mean of 555 ±230 spots per 100,000 added cells in response to CMV peptide pool re-stimulation. The CMV response was antigen-specific, as cells produced less than ten spots on average in response to either actin or no peptide controls per 100,000 added cells. This demonstrated that cells grown in IL15/IL6 were functionally equivalent to cells grown in IL4/IL7 when cultured to clinical scale and that the high throughput screening method can reliably optimize product development.
Figure 5. Cells cultured in Grex-10 vessels with IL15/IL6 produce equivalent levels of IFNγ compared with culture in IL4/IL7.
Cells were grown in Grex-10 culture vessels with IL15 and IL6 or IL4 and IL7 for ten days and tested in ELISPOT assays for IFNγ production when re-stimulated with media alone, actin (1 μg/mL), IE1 and pp65 peptide pools (1 μg/mL), or SEB (0.5 μg/mL). A representative sample is given in (a), and the mean of four different samples (b) was compared using 2-way ANOVA with Tukey’s correction. *** p < 0.001; **** p < 0.0001.
Discussion
Here we describe a high throughput flow cytometric assay to rapidly and efficiently evaluate the growth of viral-specific T cells from donor PBMCs in multiple cytokine combinations. Among the combinations tested, we identified superior and comparable expansion and T cell effector function for cells cultured in IL4/IL7 and IL15/IL6. Culture with IL4/IL7 favored an expansion of CD4+ T cells, at the expense of CD8+ T cells, while culture with IL15/IL6 expanded both CD8+ and CD4+ T cells. We subsequently confirmed that the IL15/IL6 cytokine growth condition was equivalent to IL4/IL7 by IFNγ at clinical scales.
This flow cytometry approach allowed us to evaluate 40 cytokine combinations per plate using only 1x107 cells. Promising cytokine combinations were re-investigated with additional replicates in subsequent experiments, ultimately using only 3x107 total PBMCs to measure 90 total cytokine combinations. Importantly, both the culture conditions and the functional assay were modular by design, allowing for simple exchange of new cytokine combinations into the culture layout as inferior conditions were removed and introducing new flow cytometric markers into the functional assay. This flexibility helps process development through speed, replicating reproducibility, and efficiently using a limited starting product. The approach generates a large amount of data necessitating some restrictions on the parameters selected to study. Therefore, we selected specific cytokine combinations that were optimal for T cell expansion, the secretion of IFNγ and TNFα, maintenance of cytotoxicity, and central/effector memory status. Cytokine combinations of IL6, IFNα, and IL21, not including IL15, were excluded because they induced little CD3+ proliferation when compared with combinations of IL4 and IL7. When comparing IL7 and IL4 combined with other cytokines, we found that IL7 but not IL4 promoted VST growth. This is consistent with observations that IL4 promotes cell survival but only supports the growth of naïve T cells[5, 6, 30].
We also found that IL6 improved cell expansion in combination with IL15 without modifying effector function. Our results are consistent with knockout mouse experiments showing that IL6 reduces the threshold for TCR signaling in CD8+ T cells[31], promoting memory T cell expansion in response to antigen-specific peptide re-stimulation. The requirement for including IL15 or IL7 for memory T cell expansion is expected as both receptors share homology with IL2 and use the common γ-chain and its associated Jak/STAT signaling proteins[32-34]. Recombinant IL7 has been used clinically to expand T cell subsets in cases of lymphopenia[35, 36], and was included with IL4 for its pro-survival benefits for T cells[37].
We showed that culture in IL15 and IL6 supports robust antigen-specific CD4+ T cell expansion. This is in contrast to earlier studies suggesting that culture in IL15 was inferior to culture in IL4/IL7 because of a lack of antigen-specific CD4+ T cell expansion – despite superior total cell expansion – and excessive CD56+ NK cell growth [37]). We identified only a small (median of 6.6%) growth of NK cells in culture with either IL15/IL6 or IL15 alone. Production of an efficacious mix of viral-specific CD4+ and CD8+ is needed for T cell therapy products to enhance the cytotoxic CD8+ T cell response with “help” provided by anti-viral CD4+ T cells in the form of immune activation, recruitment, and inhibition of viral replication[38]. For CMV infections, CD8+ T cells responses correlate with the resolution of disease after HSCT[39], while the addition of CMV specific CD4+ T cells has also been demonstrated to help CD8+ T cell responses for some HSCT patients[40], and has been suggested to support CD8+ cell persistence[41]. We also discovered that some donors appeared to have a preference for expansion of CD3+ cells in IL4/IL7, while other donors favored expansion in IL15/IL6 (Supplemental Figure 5). Individual donors may present with a pre-existing bias favoring CD4+ CMV specific T cells, while other donors may have a bias favoring CD8+ CMV specific T cells. Importantly, our experiments demonstrated CMV specific VSTs expanded in both culture conditions, and multiple clinical trials have used VST cells cultured in IL4/IL7 to treat ongoing viral infections, including EBV related post-transplant lymphoproliferative disorder (PTLD) [42-45]. These successes may represent the relative abundance of antigen-specific memory T cells expanded by the memory VST protocol. Nevertheless, the combination of IL15/IL6 may provide a more balanced ratio of antigen-specific CD4+ to CD8+ T cells during the polyclonal expansion of T cell products against not only viral-specific antigens but also other targets, including tumor-associated antigens. Specifically, T cells expanded against tumor-associated antigens and antigen specific T cells derived from naïve cord blood require APCs as stimulators to promote differentiation and expansion of naïve (e.g. cord blood) T cells, and/or to improve stimulation of potentially exhausted T cells (e.g. from cancer patients). Our published clinical trials targeting lymphomas[46] and leukemias[47, 48] demonstrate that we can expand tumor-specific T cells from peripheral blood of healthy donors and cancer patients using peptide-pulsed antigen-presenting cells co-cultured with IL15, IL6, IL7, and IL12 as growth factors. Subsequently, TAA-specific T cells are further expanded using antigen-presenting cells cultured with IL7 (10 ng/mL) and IL2 (100 U/mL). Hence, all of these steps (DC generation, first, second, third stimulations) present unit operations that could be tested using a high throughput approach, such as the one presented here.
In conclusion, we have shown that this high throughput plate-based flow cytometric assay can effectively and reliably measure T cell growth, function, and phenotype to optimize VST product development. We show that IL15/IL6 is equivalent to IL4/IL7 in GMP culture conditions. The assay’s modular nature facilitates future investigations to optimize culture conditions with three or four cytokine combinations using IL15/IL6 and IL4/IL7 as a baseline.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (K23-HL136783-01) award to MK, a Children’s Cancer Foundation award to PJH, and a Board of Visitors grant to CB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
Sartorius loaned the Ique Screener Plus. C. Bollard serves as an advisor board for Cellectis, is a co-founder of Mana Therapeutics, owns stock in Torque Therapeutics and Neximmune, and serves on the board of directors for Cabaletta Bio. P. Hanley is a co-founder and serves on the board of directors for Mana Therapeutics and on the scientific advisory board of Cellevolve. M. Keller serves on an advisor board for Gilead Sciences. C. Lazarski has no additional disclosures.
References
- [1].Rosenberg SA, Restifo NP, Adoptive cell transfer as personalized immunotherapy for human cancer, Science 348(6230) (2015) 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, Martinez CA, Kennedy-Nasser A, Leung KS, Gottschalk SM, Krance RA, Brenner MK, Rooney CM, Heslop HE, Leen AM, Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant, Mol Ther 21(11) (2013) 2113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gerdemann U, Vera JF, Rooney CM, Leen AM, Generation of multivirus-specific T cells to prevent/treat viral infections after allogeneic hematopoietic stem cell transplant, J Vis Exp (51) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hanley PJ, Shaffer DR, Cruz CR, Ku S, Tzou B, Liu H, Demmler-Harrison G, Heslop HE, Rooney CM, Gottschalk S, Bollard CM, Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein-Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation, Cytotherapy 13(8) (2011) 976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vella A, Teague TK, Ihle J, Kappler J, Marrack P, Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4, The Journal of experimental medicine 186(2) (1997) 325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vella AT, Dow S, Potter TA, Kappler J, Marrack P, Cytokine-induced survival of activated T cells in vitro and in vivo, Proceedings of the National Academy of Sciences of the United States of America 95(7) (1998) 3810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu-Li J, Shevach EM, Mizuguchi J, Ohara J, Mosmann T, Paul WE, B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes, The Journal of experimental medicine 165(1) (1987) 157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Boyman O, Purton JF, Surh CD, Sprent J, Cytokines and T-cell homeostasis, Current opinion in immunology 19(3) (2007) 320–6. [DOI] [PubMed] [Google Scholar]
- [9].Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL, Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool, The Journal of clinical investigation 115(5) (2005) 1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jin J, Sabatino M, Somerville R, Wilson JR, Dudley ME, Stroncek DF, Rosenberg SA, Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment, J Immunother 35(3) (2012) 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kimura A, Kishimoto T, IL-6: regulator of Treg/Th17 balance, European journal of immunology 40(7) (2010) 1830–5. [DOI] [PubMed] [Google Scholar]
- [12].Fry TJ, Mackall CL, Interleukin-7: master regulator of peripheral T-cell homeostasis?, Trends in immunology 22(10) (2001) 564–71. [DOI] [PubMed] [Google Scholar]
- [13].Geiselhart LA, Humphries CA, Gregorio TA, Mou S, Subleski J, Komschlies KL, IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation, J Immunol 166(5) (2001) 3019–27. [DOI] [PubMed] [Google Scholar]
- [14].Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD, IL-7 is critical for homeostatic proliferation and survival of naive T cells, Proceedings of the National Academy of Sciences of the United States of America 98(15) (2001) 8732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ, Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function, The Journal of experimental medicine 201(1) (2005) 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Casey KA, Mescher MF, IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype, J Immunol 178(12) (2007) 7640–8. [DOI] [PubMed] [Google Scholar]
- [17].Li Y, Bleakley M, Yee C, IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response, J Immunol 175(4) (2005) 2261–9. [DOI] [PubMed] [Google Scholar]
- [18].Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE, Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients, Blood 92(5) (1998) 1549–55. [PubMed] [Google Scholar]
- [19].Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, Sixbey J, Gresik MV, Carrum G, Hudson M, Dilloo D, Gee A, Brenner MK, Rooney CM, Heslop HE, Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease, The Journal of experimental medicine 200(12) (2004) 1623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hanley PJ, Melenhorst JJ, Nikiforow S, Scheinberg P, Blaney JW, Demmler-Harrison G, Cruz CR, Lam S, Krance RA, Leung KS, Martinez CA, Liu H, Douek DC, Heslop HE, Rooney CM, Shpall EJ, Barrett AJ, Rodgers JR, Bollard CM, CMV-specific T cells generated from naive T cells recognize atypical epitopes and may be protective in vivo, Sci Transl Med 7(285) (2015) 285ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, Leung K, Carrum G, Gee AP, Vera JF, Krance RA, Brenner MK, Rooney CM, Heslop HE, Leen AM, Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT, Sci Transl Med 6(242) (2014) 242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Geyeregger R, Freimuller C, Stevanovic S, Stemberger J, Mester G, Dmytrus J, Lion T, Rammensee HG, Fischer G, Eiz-Vesper B, Lawitschka A, Matthes S, Fritsch G, Short-term in-vitro expansion improves monitoring and allows affordable generation of virus-specific T-cells against several viruses for a broad clinical application, PloS one 8(4) (2013) e59592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Keller MD, Darko S, Lang H, Ransier A, Lazarski CA, Wang Y, Hanley PJ, Davila BJ, Heimall JR, Ambinder RF, Barrett AJ, Rooney CM, Heslop HE, Douek DC, Bollard CM, T-cell receptor sequencing demonstrates persistence of virus-specific T cells after antiviral immunotherapy, British journal of haematology 187(2) (2019) 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keller MD, Bollard CM, Virus specific T-cell Therapies for Patients with Primary Immune Deficiency, Blood (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P, Dey BR, Grilley BJ, Gee AP, Brenner MK, Rooney CM, Heslop HE, Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation, Blood 121(26) (2013) 5113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM, Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals, Nature medicine 12(10) (2006) 1160–6. [DOI] [PubMed] [Google Scholar]
- [27].Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA, Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation, Journal of immunological methods 281 (1-2) (2003) 65–78. [DOI] [PubMed] [Google Scholar]
- [28].Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E, The who’s who of T-cell differentiation: human memory T-cell subsets, European journal of immunology 43(11) (2013) 2797–809. [DOI] [PubMed] [Google Scholar]
- [29].Busch DH, Frassle SP, Sommermeyer D, Buchholz VR, Riddell SR, Role of memory T cell subsets for adoptive immunotherapy, Seminars in immunology 28(1) (2016) 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Geginat J, Sallusto F, Lanzavecchia A, Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells, The Journal of experimental medicine 194(12) (2001) 1711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S, IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes, J Immunol 180(12) (2008) 7958–68. [DOI] [PubMed] [Google Scholar]
- [32].Carrette F, Surh CD, IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis, Seminars in immunology 24(3) (2012) 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fry TJ, Mackall CL, The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance, J Immunol 174(11) (2005) 6571–6. [DOI] [PubMed] [Google Scholar]
- [34].Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA, IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels, Immunity 4(4) (1996) 329–36. [DOI] [PubMed] [Google Scholar]
- [35].Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE, IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells, J Immunother 29(3) (2006) 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL, Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets, The Journal of experimental medicine 205(7) (2008) 1701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, Perna SK, Ennamuri S, Gottschalk S, Brenner MK, Heslop HE, Rooney CM, Leen AM, Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections, Mol Ther 20(8) (2012) 1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sant AJ, McMichael A, Revealing the role of CD4(+) T cells in viral immunity, The Journal of experimental medicine 209(8) (2012) 1391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reusser P, Riddell SR, Meyers JD, Greenberg PD, Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease, Blood 78(5) (1991) 1373–80. [PubMed] [Google Scholar]
- [40].Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H, Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy, Blood 99(11) (2002) 3916–22. [DOI] [PubMed] [Google Scholar]
- [41].Riddell SR, Reusser P, Greenberg PD, Cytotoxic T cells specific for cytomegalovirus: a potential therapy for immunocompromised patients, Rev Infect Dis 13 Suppl 11 (1991) S966–73. [DOI] [PubMed] [Google Scholar]
- [42].Houghtelin A, Bollard CM, Virus-Specific T Cells for the Immunocompromised Patient, Frontiers in immunology 8 (2017) 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harris KM, Davila BJ, Bollard CM, Keller MD, Virus-Specific T Cells: Current and Future Use in Primary Immunodeficiency Disorders, J Allergy Clin Immunol Pract 7(3) (2019) 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Naik S, Nicholas SK, Martinez CA, Leen AM, Hanley PJ, Gottschalk SM, Rooney CM, Hanson IC, Krance RA, Shpall EJ, Cruz CR, Amrolia P, Lucchini G, Bunin N, Heimall J, Klein OR, Gennery AR, Slatter MA, Vickers MA, Orange JS, Heslop HE, Bollard CM, Keller MD, Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes, The Journal of allergy and clinical immunology 137(5) (2016) 1498–1505 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McLaughlin LP, Bollard CM, Keller MD, Adoptive T Cell Therapy for Epstein-Barr Virus Complications in Patients With Primary Immunodeficiency Disorders, Frontiers in immunology 9 (2018) 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hont AB, Cruz CR, Ulrey R, O'Brien B, Stanojevic M, Datar A, Albihani S, Saunders D, Hanajiri R, Panchapakesan K, Darko S, Banerjee P, Fortiz MF, Hoq F, Lang H, Wang Y, Hanley PJ, Dome JS, Bollard CM, Meany HJ, Immunotherapy of Relapsed and Refractory Solid Tumors With Ex Vivo Expanded Multi-Tumor Associated Antigen Specific Cytotoxic T Lymphocytes: A Phase I Study, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37(26) (2019) 2349–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Weber G, Caruana I, Rouce RH, Barrett AJ, Gerdemann U, Leen AM, Rabin KR, Bollard CM, Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia--implications for immunotherapy, Clinical cancer research : an official journal of the American Association for Cancer Research 19(18) (2013) 5079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gerdemann U, Katari U, Christin AS, Cruz CR, Tripic T, Rousseau A, Gottschalk SM, Savoldo B, Vera JF, Heslop HE, Brenner MK, Bollard CM, Rooney CM, Leen AM, Cytotoxic T lymphocytes simultaneously targeting multiple tumor-associated antigens to treat EBV negative lymphoma, Mol Ther 19(12) (2011) 2258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.