Abstract
Background:
Marijuana has vasoconstrictive properties and its use has been associated with increased blood pressure in the general population. Yet, there are limited data on marijuana use and adverse outcomes among women with hypertension in pregnancy, even though these disorders are associated with severe maternal and fetal morbidity and mortality. Since marijuana is currently the most commonly used illicit drug in pregnancy, there is an urgent need to better understand the potential association between marijuana use and hypertension in pregnancy.
Objective:
To determine the adverse prenatal effects of marijuana use in women with hypertension in pregnancy.
Study design:
We conducted a retrospective cohort study among individuals with hypertension in pregnancy that delivered ≥23 weeks’ gestation at Oregon Health & Science University (October 2013-September 2018). The primary exposure assessed was marijuana use, identified by chart review of documented patient self-report or positive urine toxicology screen. Individuals were stratified into two groups by marijuana use: use during pregnancy versus never used. Primary outcomes included composite adverse maternal and neonatal outcomes. Secondary outcomes included individual maternal outcomes, rarer neonatal outcomes and severe features of preeclampsia. Differences were analyzed by Fisher’s exact, t-test, and logistic regression. Significance was determined by alpha = 0.05 for primary outcomes and alpha = 0.01 for secondary outcomes.
Results:
From 11,825 deliveries, 1,613 (13.6%) were classified with hypertension in pregnancy. A total of 117 individuals (7.3%) used marijuana during pregnancy, 1,110 (68.2%) had never used marijuana and 396 (24.6%) had unknown marijuana use and were excluded, leaving 1,217 individuals in this analysis. Women using marijuana in pregnancy were more likely to be younger, non-Hispanic White, publicly insured and using other substances compared to women who did not use marijuana. There were no differences in the overall distribution of hypertensive disorders, including preeclampsia with severe features, in women who used marijuana versus those who did not (p = .80). In multivariable analyses, after adjusting for maternal factors and other substance use, marijuana use was not associated with adverse maternal (aOR 1.23, 95% CI 0.43–3.50, p = .69) or neonatal (aOR 0.90, 95% CI 0.28–2.89, p = .86) outcomes.
Conclusions:
Marijuana use in pregnancy was not associated with maternal or neonatal outcomes or worsened hypertensive disease among women with hypertension in pregnancy after adjusting for maternal characteristics, including use of other substances. Our data highlight the need to consider use of other substances when evaluating the association between marijuana use in pregnancy and adverse pregnancy outcomes.
Keywords: Cannabis, marijuana, hypertension, pregnancy, outcomes
Introduction
Approximately 10% of pregnancies in the United States are complicated by hypertension in pregnancy, with an increasing rate of preeclampsia with severe features in recent decades [1-4]. This heterogeneous, systemic disease process predisposes women to increased maternal, fetal and neonatal mortality and morbidity, including renal and hepatic impairment, placental abruption, pulmonary edema, seizure, stroke, fetal growth restriction, and preterm birth [3,5].
Currently, marijuana is the most commonly used illicit drug during pregnancy under federal regulations [6] Overall, 2–5% of women use marijuana during pregnancy, but rates have been reported to be as high as 28% among young, urban and socioeconomically disadvantaged women [6]. Marijuana has vasoconstrictive properties similar to nicotine and its use has been associated with adverse cardiovascular and cerebrovascular events [7-9]. In the general population, acute marijuana use is associated with an increase in systolic blood pressure, but data in pregnancy are lacking [10-12]. The available research on marijuana use in pregnancy is limited in part by research methodology, polysubstance use, and reliance on patient self-report, making it challenging to determine the independent effect of marijuana use only on adverse pregnancy outcomes [13-16]. Additionally, women underreport their marijuana use up to 60–70%, and there is no biological validation for self-reported dosing [17-19].
Prior studies have found no association between the independent use of marijuana and the development of hypertensive disorders in pregnancy [16,18,20,21]. However, these studies looked only at the association between marijuana use and gestational hypertension or preeclampsia rather than the full range of hypertensive disease in pregnancy and adjusted only for concurrent tobacco and/or alcohol use. As women with hypertension in pregnancy are at increased risk for adverse pregnancy outcomes and marijuana use is increasing in pregnancy, it is important to assess the effect of marijuana use in pregnant women with hypertension. Therefore, the objective of our study was to determine the association between marijuana use in pregnancy and maternal and neonatal outcomes among women with hypertension in pregnancy.
Materials and methods
This was a retrospective cohort study among women with hypertension in pregnancy, who delivered ≥23 weeks’ gestation at Oregon Health & Science University (OHSU) from October, 2013 to September, 2018. The study cohort was initiated following publication of the 2013 American College of Obstetricians & Gynecologists (ACOG) Executive Summary on Hypertension in Pregnancy, which updated the diagnostic criteria for preeclampsia [22]. Institutional Review Board approval was received before study initiation (IRB #15070). Participants were screened for hypertension in pregnancy utilizing ICD-9-CM and ICD- 10-CM hospital discharge codes after delivery. Diagnoses were confirmed through individual chart review by two physicians, with disagreements adjudicated by a third physician (RMB). Women in the study cohort were categorized based on the primary exposure of marijuana use in pregnancy: marijuana use during pregnancy (current use at time of delivery or quit during pregnancy) versus no marijuana use (never used marijuana). Marijuana use in pregnancy was determined by review of the patient’s electronic medical record through patient self-report or positive urine toxicology screen. In this population it is routine to ask about drug use during pregnancy, but if the presence or absence of marijuana use during pregnancy was not specifically documented, the patient was excluded from the analysis. Notably, in the state of Oregon, urine toxicology screens are not routinely performed in women who report marijuana use in pregnancy.
Within the study cohort, individuals were categorized into five groups, utilizing ACOG criteria for hypertension in pregnancy: (1) chronic hypertension (CHTN); (2) gestational hypertension (GHTN); (3) preeclampsia without severe features (PE); (4) preeclampsia with severe features (PE-SF), which included those with a diagnosis of both PE-SF and hemolysis elevated liver enzymes and low platelet count (HELLP) syndrome; and (5) CHTN with superimposed preeclampsia [22]. Severe features included: systolic blood pressure (BP) ≥160mmHg or diastolic BP ≥110 mmHg, alanine transaminase (ALT) or aspartate transaminase (AST) ≥2x the upper limit of normal, creatinine (Cr) >1.1 mg/dL, platelet count <100 k/μL, pulmonary edema, severe headache or visual disturbances, and severe right upper quadrant or epigastric pain predelivery. Demographic, pregnancy, delivery and neonatal data were abstracted and maintained utilizing REDCap (Research Electronic Data Capture). Maternal data included age, race/ethnicity, body mass index (BMI) at first prenatal visit, parity, diabetes status, and insurance type. Additionally, alcohol, tobacco, amphetamine, marijuana and opioid use were included based on self-reported data recorded during routine prenatal care or admission for delivery or based on a positive urine drug screen during pregnancy. At least 96.5% of maternal characteristics or outcome data were available for all individuals in the final analytic sample.
The study authors reviewed individual charts to screen for severe features of preeclampsia, utilizing inpatient data during hospitalization for delivery. Blood pressure data were abstracted as the peak systolic and diastolic measurement before delivery. Severe features of preeclampsia were abstracted as dichotomous (yes/no) variables or as continuous data (peak [ALT, AST, BP, Cr] or nadir [platelets]) before delivery.
The primary outcomes comprised a composite of adverse maternal outcomes including acute kidney injury (serum Cr >1.1 mg/dL), blood transfusion, eclampsia, intensive care unit (ICU) admission, pulmonary edema, liver infarction/rupture, stroke, oliguria (<500mL urine in 24 h), vision loss, cardiomyopathy or placental abruption. Secondary maternal outcomes included the above as individual outcomes. Primary neonatal outcomes included common adverse endpoints (neonatal ICU [NICU] admission, preterm birth <37 weeks, preterm birth <34 weeks, neonatal intubation, respiratory distress syndrome, low birth weight less than 2,500 g and very low birth weight less than 1,500 g), in addition to a composite of less common adverse neonatal outcomes (5-min Apgar score <7, arterial cord pH <7, stillbirth, neonatal demise, neonatal seizure, bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, hypoxic ischemic encephalopathy, retinopathy of prematurity and neonatal sepsis). These less common neonatal outcomes were also evaluated individually as secondary outcomes. We also assessed the association between marijuana use and severe features of preeclampsia (severe systolic BP [SBP], severe diastolic BP [DBP], any severe feature [including BP] or any severe feature [excluding BP]) as secondary outcomes.
Differences in laboratory values or clinical variables between groups were assessed by t-test or Fisher’s exact test as appropriate. Multivariable logistic regression was utilized to adjust for the following confounders: race/ethnicity (non-Hispanic White vs. other), maternal age, pre-pregnancy BMI, insurance type (private vs. public), nulliparity, tobacco, alcohol, opioid (heroin, methadone or buprenorphine) or amphetamine use (determined a priori or p < .05 during univariable analyses). All of the above-listed substances were coded as a dichotomized variable: any use during pregnancy versus never used. Results were reported as a fully adjusted odds ratio, with a 95% confidence interval (CI). Significance was determined by alpha = 0.05 for primary outcomes and alpha = 0.01 for secondary outcomes. Our study was powered (alpha = 0.05, beta = 0.80) to detect a 12% difference in adverse pregnancy outcomes assuming a baseline rate of 20%. The data that support the findings of this study are available from the corresponding author (KG) upon reasonable request.
Results
Among 11,825 deliveries from October 2013 to September 2018, 1,613 (13.6%) were classified with hypertension in pregnancy ≥23 weeks’ gestation (Figure 1). Among this cohort, 117 individuals (7.3%) used marijuana in pregnancy (82 [5.1%] used at the time of delivery and 35 [2.2%] quit during pregnancy), 1,110 (68.2%) had never used marijuana, and 396 (24.6%) had missing data and were excluded. This resulted in a final cohort of 1,217 individuals. Of these women, 232 (19.1%) had a diagnosis of CHTN, 344 (28.3%) GHTN, 254 (20.9%) PE, 252 (20.7%) PE-SF, and 135 (11.1%) CHTN with superimposed PE.
Figure 1.
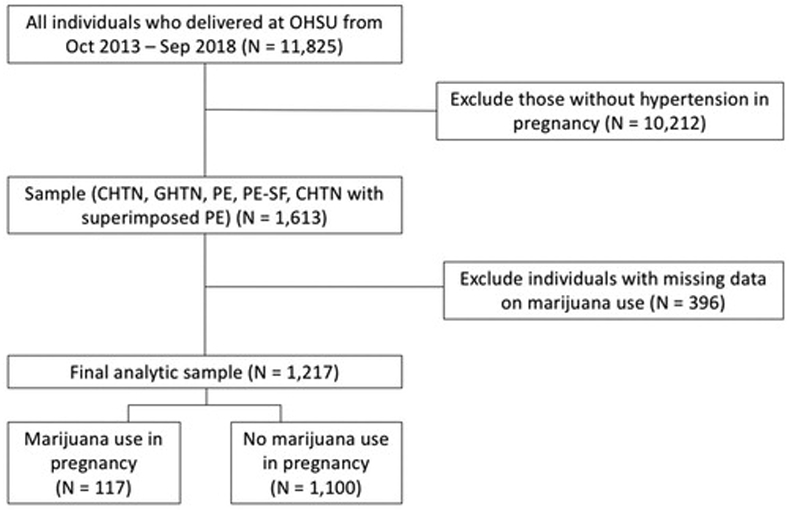
Flow diagram for study inclusion. CHTN: chronic hypertension; GHTN: gestational hypertension; PE: preeclampsia; PE-SF: preeclampsia with severe features.
Maternal characteristics of study participants, stratified by marijuana use, are shown in Table 1. Women who used marijuana in pregnancy were more likely to be younger, non-Hispanic White, non-Hispanic Black or multiracial/other, have public health insurance coverage and co-use of other substances including tobacco, alcohol, opioids and amphetamines. The distribution of hypertensive disorders was no different in women who used marijuana in pregnancy versus those who did not (p = .80). Selection bias was also assessed by comparing the maternal characteristics of individuals in the final analytic sample to those with missing data on marijuana use in pregnancy (Supplemental Table 1).
Table 1.
Maternal characteristics for women with hypertension in pregnancy, stratified by marijuana use in pregnancy.
| Marijuana use in pregnancy (n = 117) |
Never used marijuana (n = 1,100) |
p-value* | |
|---|---|---|---|
| Maternal characteristics | |||
| Age (mean years ± SD) | 27.2 ± 6.0 | 30.7 ± 6.2 | <.001 |
| Pre-pregnancy BMI (mean kg/m2 ± SD) | 31.0 ± 8.9 | 31.6 ± 8.6 | .49 |
| Nulliparous | 51.3% | 51.4% | 1.0 |
| Diabetes Mellitus (Type 1 or 2) | 4.3% | 7.0% | .33 |
| Race | 0.002 | ||
| Non-Hispanic White | 75.2% | 66.8% | |
| Hispanic | 6.0% | 15.4% | |
| Non-Hispanic Black | 4.3% | 3.8% | |
| Asian | 0% | 4.0% | |
| Multiracial and Othera | 14.5% | 10.0% | |
| Insurance type | |||
| Private (individual or group) | 13.7% | 50.1% | <.001 |
| Public (Medicaid or Medicare) | 86.3% | 49.9% | |
| Substance Useb | |||
| Tobacco | 43.6% | 6.6% | <.001 |
| Alcohol | 15.4% | 3.5% | <.001 |
| Opioids | 21.4% | 1.7% | <.001 |
| Amphetamines | 25.6% | 0.9% | <.001 |
| Hypertension in Pregnancy | |||
| Chronic hypertension (CHTN) | 18.0% | 19.1% | .80 |
| Gestational hypertension (GHTN) | 28.2% | 28.3% | |
| Preeclampsia (PE) | 24.8% | 20.5% | |
| Preeclampsia with severe features (PE-SF) | 20.5% | 20.7% | |
| CHTN with superimposed preeclampsia | 8.6% | 11.4% |
SD: standard deviation; BMI: body mass index.
t-test (comparison of means); Fisher’s exact test (categorical data).
American Indian/Alaska Native, Native Hawaiian or other Pacific Islander, more than one race or other.
Current use at time of delivery or quit during pregnancy.
We first assessed whether marijuana use during pregnancy was associated with adverse maternal and neonatal outcomes among women with hypertension in pregnancy (Table 2). The composite of adverse maternal outcomes was not statistically higher among the group of women who used marijuana versus those who did not (18.0% vs. 11.5%, p = .051). Cardiomyopathy was more common among women who used marijuana in pregnancy (1.7% vs. 0%, p = .009), but these individuals were also co-using amphetamines in pregnancy. For adverse neonatal outcomes, women who used marijuana were more likely to have a newborn requiring NICU admission (43.6% vs. 30.5%, p = .005). No other primary or secondary adverse neonatal outcomes or the composite of other rarer adverse neonatal outcomes were significantly different between those who used marijuana versus those who did not.
Table 2.
Adverse maternal and neonatal outcomes among women with hypertension in pregnancy, stratified by marijuana use in pregnancy.
| Marijuana use in pregnancy (n = 117) |
Never used marijuana, quit prior to or for pregnancy (n = 1,100) |
p-value* | |
|---|---|---|---|
| Adverse maternal outcomes | |||
| Acute kidney injury (creatinine >1.1 mg/dL) | 7.7% | 5.7% | .53 |
| Blood transfusion | 4.3% | 4.1% | .81 |
| Eclampsia | 2.6% | 0.3% | .014 |
| Intensive care unit (ICU) admission | 2.6% | 1.4% | .25 |
| Pulmonary edema | 3.4% | 0.8% | .029 |
| Liver infarction or rupture | 0.9% | 0.1% | .18 |
| Stroke | 0% | 0.1% | 1.0 |
| Oliguria | 1.7% | 1.2% | .65 |
| Vision loss | 0% | 0.2% | 1.0 |
| Cardiomyopathy | 1.7% | 0% | .009 |
| Placental abruption | 4.3% | 2.1% | .18 |
| Composite adverse maternal outcome (any of above) | 18.0% | 11.5% | .051 |
| Individual adverse neonatal outcomes | |||
| Neonatal intensive care unit (ICU) admission | 43.6% | 30.5% | .005 |
| Preterm birth <37 weeks | 27.4% | 22.6% | .25 |
| Preterm birth <34 weeks | 8.6% | 7.7% | .72 |
| Neonatal intubation | 6.8% | 4.3% | .24 |
| Respiratory Distress Syndrome | 13.7% | 11.8% | .55 |
| Low birth weight <2,500g | 27.4% | 19.9% | .071 |
| Very low birth weight <1,500g | 5.1% | 4.2% | .63 |
| Other rare adverse neonatal outcomes | |||
| 5-minute Apgar score <7 | 12.8% | 6.0% | .010 |
| Arterial cord pH <7 | 0% | 1.0% | .61 |
| Stillbirth | 0% | 0.6% | 1.0 |
| Neonatal demise | 3.4% | 0.8% | .029 |
| Neonatal seizure | 0% | 0.8% | 1.0 |
| Bronchopulmonary dysplasia | 0% | 0.3% | 1.0 |
| Necrotizing enterocolitis | 0.9% | 0.3% | .33 |
| Intraventricular hemorrhage | 1.7% | 0.6% | .21 |
| Hypoxic ischemic encephalopathy | 0% | 0.4% | 1.0 |
| Retinopathy of prematurity | 2.6% | 2.0% | .73 |
| Neonatal sepsis | 0% | 0.6% | 1.0 |
| Composite rare adverse neonatal outcomesa | 15.4% | 10.9% | .17 |
Fisher’s exact test (categorical data).
Includes 5-min Apgar <7, arterial cord pH <7, stillbirth, neonatal demise, neonatal seizure, bronchopulmonary dysplasia, necrotizing enterocolitis, intra-ventricular hemorrhage, hypoxic ischemic encephalopathy, retinopathy of prematurity, and neonatal sepsis.
After multivariable adjustment for maternal characteristics (race, maternal age, pre-pregnancy BMI, nulliparity and insurance type) in addition to other substance use (tobacco, alcohol, opioid or amphetamine use during pregnancy), the association between marijuana use and NICU admission was no longer statistically significant (Table 3; OR 1.30, 95% CI 0.78 – 2.15, p = .31). Other multivariable analyses assessing marijuana use in pregnancy and the odds of the composite adverse maternal outcome, other primary individual neonatal outcomes, or the composite of rare adverse neonatal outcomes, found no statistically significant associations (Table 3).
Table 3.
Multivariable logistic regression for adverse pregnancy outcomes with marijuana use in pregnancy.
| Fully adjusted OR* | 95% CI | p-value | |
|---|---|---|---|
| Composite adverse maternal outcomea | 1.23 | 0.43–3.50 | .69 |
| Neonatal intensive care unit (ICU) admission | 1.30 | 0.78–2.15 | .31 |
| Preterm birth <37 weeks | 0.93 | 0.38–2.26 | .87 |
| Preterm birth <34 weeks | 1.10 | 0.26–4.69 | .89 |
| Neonatal intubation | 1.65 | 0.32–8.55 | .55 |
| Respiratory Distress Syndrome | 0.83 | 0.27–2.56 | .75 |
| Low birth weight <2,500g | 1.36 | 0.56–3.30 | .50 |
| Very low birth weight <1,500g | 1.58 | 0.29–8.55 | .60 |
| Composite rare adverse neonatal outcomeb | 0.90 | 0.28–2.89 | .86 |
| Systolic BP ≥160 mmHg | 0.72 | 0.31–1.70 | .46 |
| Diastolic BP ≥110 mmHg | 0.75 | 0.29–1.97 | .56 |
| Any severe feature (including BP)c | 1.0 | 0.46–2.18 | .99 |
| Any severe feature (excluding BP) | 1.15 | 0.49–2.71 | .74 |
Adjusted for race, maternal age, pre-pregnancy BMI, nulliparity, and insurance type, tobacco, alcohol, opioid, and amphetamine use during pregnancy.
Includes acute kidney injury (serum creatinine >1.1 mg/dL), blood transfusion, eclampsia, maternal ICU admission, pulmonary edema, liver infarction or rupture, stroke, oliguria (<500 mL urine in 24 h), vision loss, cardiomyopathy or placental abruption.
Includes 5-min Apgar <7, arterial cord pH <7, stillbirth, neonatal demise, neonatal seizure, bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, hypoxic ischemic encephalopathy, retinopathy of prematurity, and neonatal sepsis.
Includes systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg, alanine transaminase (ALT) or aspartate transaminase (AST) ≥2x upper limit of normal, severe persistent right upper quadrant or epigastric pain not accounted for by alternative diagnoses, creatinine ≥1.1 mg/dL or ≥2x baseline in the absence of other renal disease, platelet count <100,000 × 109/L, pulmonary edema, new-onset severe headache or visual disturbances pre-delivery.
Women who used marijuana were not more likely to develop preeclampsia with severe features compared to women who did not use marijuana. However, during univariable analyses those who used marijuana were more likely to have a severe DBP (≥110 mmHg) compared to those who did not use marijuana (29.9% vs. 20.5%, p = .024). After multivariable adjustment for maternal characteristics and other substance use, the odds of severe DBP was no longer significantly associated with marijuana use (OR 0.75, 95% CI 0.29 – 1.97). There was no significant difference between women who used marijuana versus those who did not for severe SBP (≥160 mmHg) (37.6% vs. 38.0%, p = 1.0) or any severe features of preeclampsia including or excluding BP (56.4% vs. 52.3%, p= .44 and 26.5% vs. 23.3%, p= .36, respectively) when performing univariable analyses.
Discussion
These results demonstrate that marijuana use in pregnancy is not independently associated with adverse maternal and neonatal outcomes among women with hypertension in pregnancy. Although univariable analyses demonstrated an association with higher rates of NICU admission and severe DBP, with multivariable modeling, marijuana use was not associated with adverse maternal or neonatal outcomes or higher rates of severe range blood pressures compared to women who did not use marijuana. Additionally, preeclampsia with severe features was not increased among women who used marijuana compared to those who did not.
The univariable analysis found NICU admission was more common among women who used marijuana compared to those who did not, but the association was no longer significant after multivariable analysis, which included adjustment for other substance use. Furthermore, our analyses found no association between marijuana use in pregnancy and other adverse neonatal outcomes, including preterm birth, low birth weight or stillbirth.
Additionally, the association between marijuana use and an increase in DBP was unexpected, given prior studies in non-pregnant adults have described an association between marijuana use and an increase in systolic BP only [10-12]. However, the association between marijuana use and severe range DBP was not statistically significant after adjusting for use of other substances.
The association between marijuana use and adverse pregnancy outcomes among women with hypertension in pregnancy has not been previously studied, while existing literature on the association between maternal marijuana use and adverse maternal and neonatal outcomes is limited and conflicting. Similar to our study findings, the National Academy of Sciences, Engineering and Medicine (NASEM) concluded there was insufficient evidence demonstrating an association between prenatal marijuana use and preterm birth or stillbirth [18]. A secondary analysis of the Stillbirth Collaborate Research Network dataset also found no significant association between marijuana use and the composite adverse pregnancy outcome, which included small for gestational age, spontaneous preterm birth and hypertensive disorders in pregnancy after adjusting for tobacco use, clinical and socioeconomic factors (adjusted OR 1.29, 95% CI 0.56 – 2.96) [15]. Conversely, unlike our study findings, the 2017 NASEM report felt there was sufficient evidence to support an association between maternal marijuana use and low birth weight infants [15]. These findings suggest marijuana use later in pregnancy may restrict fetal growth, highlighting the importance of understanding the timing and frequency of maternal marijuana use during pregnancy.
Although these prior studies assessed a cohort of pregnant women with and without hypertensive disorders in pregnancy from a diverse range of medical centers, which was distinct from the population in our analysis, a similar null association between marijuana use and adverse pregnancy outcomes was found when adjusting for other substance use. It is important to recognize the high prevalence of concomitant substance use among women who use marijuana in pregnancy [15,16,21,23]. Prior studies have adjusted for concurrent tobacco use only or excluded individuals with polysubstance use, making it challenging to determine the independent effect of marijuana use only on adverse pregnancy outcomes [15,16]. Additional studies are needed to assess independent marijuana use, including dosage, timing, and method of administration, on both adverse maternal and neonatal outcomes in pregnancy, especially in pregnancies complicated by hypertension.
This study has a number of strengths. To our knowledge it is the first study to assess the association between prenatal marijuana use and pregnancy outcomes among women diagnosed with hypertension in pregnancy. In addition, the most current diagnostic criteria for hypertension in pregnancy were utilized, as the study cohort was initiated following publication of the 2013 ACOG Executive Summary on Hypertension in Pregnancy [22]. Finally, results were interpreted in the context of other maternal substance use including tobacco, alcohol, opioid and amphetamine use.
Our study also has several limitations. First, a majority of the marijuana use during pregnancy was self-reported, which can skew the associations toward the null, given pregnant women are known to underreport marijuana use [19,24]. Second, data on the quantity, timing, duration and mode of administration was unavailable making it difficult to determine a causal association with adverse pregnancy outcomes. Our study also had limited power to detect modest differences in adverse maternal and neonatal outcomes between women who used marijuana and those who did not. Thus, a larger study is recommended to evaluate the association between marijuana use in pregnancy and less frequent adverse outcomes. Lastly, the higher percentage of non-Hispanic White individuals in this study limits the applicability of our findings to more heterogeneous populations in the United States. Therefore, future studies are needed to assess the association between independent marijuana use and adverse pregnancy outcomes among women of diverse racial and ethnic backgrounds.
In conclusion, this study found that marijuana use was not associated with adverse maternal or neonatal outcomes or more severe features of preeclampsia among women with hypertension in pregnancy. Furthermore, concomitant substance use was common and confounded the association between marijuana use and adverse maternal and neonatal outcomes, highlighting the need to reduce the use of marijuana and other substances during pregnancy. Given the recent rise in marijuana use among pregnant women and its potential association with hypertension and adverse pregnancy outcomes, this is an important topic for future research and investigation.
Supplementary Material
Acknowledgement
A version of the study findings were presented at the Society for Maternal-Fetal Medicine’s 39th Annual Pregnancy Meeting, February 11th-16th, 2019, Las Vegas, NV.
Funding
This research was supported by the Oregon Clinical & Translational Research Institute grant [CTSA Award No.: UL1TR002369]. The grant supported the use of REDCap (Research Electronic Data Capture) for data abstraction.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data for this article is available online at https://doi.org/10.1080/14767058.2020.1785422
References
- [1].Berg CJ, Mackay AP, Qin C, et al. Overview of maternal morbidity during hospitalization for labor and delivery in the United States:1993–1997 and 2001–2005. Obstet Gynecol. 2009;113(5):1075–1081. [DOI] [PubMed] [Google Scholar]
- [2].Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wagner SJ, Barac S, Garovic VD. Hypertensive pregnancy disorders: current concepts. J Clin Hypertens (Greenwich). 2007;9(7):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yoder SR, Thornburg LL, Bisognano JD. Hypertension in pregnancy and women of childbearing age. Am J Med. 2009;122(10):890–895. [DOI] [PubMed] [Google Scholar]
- [5].Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance-United States, 1991–1999. MMWR Surveill Summ. 2003;52(2):1–8. [PubMed] [Google Scholar]
- [6].Committee on Obstetric Practice. Committee Opinion No. 722: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2017;130(4):e205–e209. [DOI] [PubMed] [Google Scholar]
- [7].Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know. Am J Cardiol. 2014;113(1):187–190. [DOI] [PubMed] [Google Scholar]
- [8].Wolff V, Armspach JP, Lauer V, et al. Ischaemic strokes with reversible vasoconstriction and without thunderclap headache: a variant of the reversible cerebral vasoconstriction syndrome? Cerebrovasc Dis. 2015; 39(1):31–38. [DOI] [PubMed] [Google Scholar]
- [9].Wolff V, Lauer V, Rouyer O, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke. 2011;42(6):1778–1780. [DOI] [PubMed] [Google Scholar]
- [10].Alshaarawy O, Elbaz HA. Cannabis use and blood pressure levels: United States National Health and Nutrition Examination Survey, 2005–2012. J Hypertens. 2016;34(8):1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goyal H, Awad HH, Ghali JK. Role of cannabis in cardiovascular disorders. J Thorac Dis. 2017;9(7): 2079–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(S1):58S–63s. [DOI] [PubMed] [Google Scholar]
- [13].Conner SN, Bedell V, Lipsey K, et al. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. 2016; 128(4):713–723. [DOI] [PubMed] [Google Scholar]
- [14].Gunn JK, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Metz TD, Allshouse AA, Hogue CJ, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am J Obstet Gynecol. 2017; 217(4):478.e1–478-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Warshak CR, Regan J, Moore B, et al. Association between marijuana use and adverse obstetrical and neonatal outcomes. J Perinatol. 2015;35(12):991–995. [DOI] [PubMed] [Google Scholar]
- [17].Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213(6):761–778. [DOI] [PubMed] [Google Scholar]
- [18].National Academies of Sciences Engineering and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- [19].Shiono PH, Klebanoff MA, Nugent RP, et al. The impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter study. Am J Obstet Gynecol. 1995;172(1):19–27. [DOI] [PubMed] [Google Scholar]
- [20].Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215(4):506.e1–506.e7. [DOI] [PubMed] [Google Scholar]
- [21].Leemaqz SY, Dekker GA, McCowan LM, et al. Maternal marijuana use has independent effects on risk for spontaneous preterm birth but not other common late pregnancy complications. Reprod Toxicol. 2016; 62:77–86. [DOI] [PubMed] [Google Scholar]
- [22].American College of Obstetricians and Gynecologists. Task force on hypertension in pregnancy. Hypertens Pregnancy. 2013. Available from: http://www.spog.org.pe/web/phocadownloadpap/HypertensioninPregnancy.pdf [DOI] [PubMed] [Google Scholar]
- [23].Passey ME, Sanson-Fisher RW, D’Este CA, et al. Tobacco, alcohol and cannabis use during pregnancy: clustering of risks. Drug Alcohol Depend. 2014;134: 44–50. [DOI] [PubMed] [Google Scholar]
- [24].Young-Wolff KC, Tucker LY, Alexeeff S, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California From 2009–2016. JAMA. 2017;318(24):2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


