Abstract
Iron-sulfur cluster proteins play key roles in a multitude of cellular processes. Iron-sulfur cofactors are assembled primarily in mitochondria and are then exported to the cytosol by use of an ABCB7 transporter. It has been shown that the yeast mitochondrial transporter Atm1 can export glutathione-coordinated iron-sulfur clusters, [2Fe–2S] (SG)4, providing a source of cluster units for cytosolic iron-sulfur cluster assembly systems. This pathway is consistent with the endosymbiotic model of mitochondrial evolution where homologous bacterial heavy metal transporters, utilizing metal glutathione adducts, were adapted for use in eukaryotic mitochondria. Herein, the basis for endosymbiotic evolution of the human cluster export protein (ABCB7) is developed through a BLAST analysis of transporters from ancient proteobacteria. In addition, a functional comparison of native human protein, versus a disease-causing mutant, demonstrates a key role for residue E433 in promoting cluster transport. Dysfunction in mitochondrial export of Fe–S clusters is a likely cause of the disease condition X-linked sideroblastic anemia.
Keywords: ABCB7, Atm1, [2Fe–2S](GS)4 cluster complex, Mitochondrial exporter, Endosymbiosis
1. Introduction
ATP-binding cassette (ABC) transporters use the energy from ATP hydrolysis to facilitate transmembrane movement of molecular species [1]. The human genome encodes 49 ABC transporters that are responsible for mediating translocation of various molecular substrates that include lipids, cations and anions, peptides, sugars, amino acids, drugs and hydrophobic compounds [2]. Mutations in 11 of the 49 transporters are associated with genetic diseases while many others have been extensively studied because they are drug targets [2]. The transporters are collected into 7 substrate-related families (A – G) depending on the class of substrate molecule, where the term “substrate” will usually refer to the species that is transported. A-type transporters (ABCA), for example, promote the movement of lipids, while the ABCB family has been implicated in multidrug resistance and the transport of ionic species.
The basic structure of a transporter consists of a dimer of two domains, each of which includes a nucleotide binding domain (NBD) and transmembrane domain (TMD) that are connected by coupling helices [3,4] (Fig. 1). The transporter proteins can exist as a homodimer, heterodimer, or one long chain. When multiple subunits exist, the individual chains contain one NBD and one TMD. More specifically, ABC transporters contain several conserved structural motifs, namely the Walker A and B sequences, the ABC signature motif, and the H and Q loops [1]. Five different mutations in ABCB7 have so far been found to result in disease conditions: namely, E208D, I400 M, V411L, E433K, and G682S [5–9]. The first four mutations result in sideroblastic anemia with cerebellar ataxia [7–10], but interestingly the fifth mutation results in ataxia with no anemia [5]. ABCB7 is important for proper hematopoiesis as it was observed in mice that the E433K mutation results in decreased heme biosynthesis [11]. In fact, a buildup of iron in mitochondria and a deficiency of cluster-bound forms of cytosolic Fe–S proteins have been observed in both yeast and human cells when the transport protein is knocked out or is dysfunctional as a result of disease-related substitutions [1,12–14].
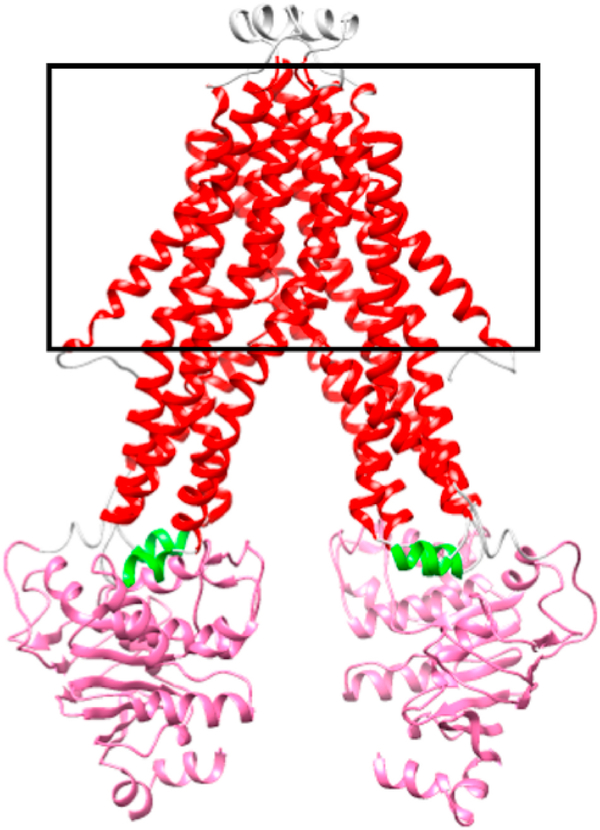
Fig. 1.
Model of human ABCB7. Human ABCB7 was modeled after S. cerevisiae Atm1p (PDB: 4MYC) using the Phyre 2 server. The black outline represents the inner mitochondrial membrane. The color coding is as follows: red – transmembrane helices (residues 135–169, 189–235, 245–289, 291–338, 350–401, 407–449); green – coupling helices (238–243, 340–345); magenta – nucleotide binding domain (459–607).
The Fe–S cluster assembly machinery is highly conserved from bacteria to man [15], and it has been shown that ABCB7 plays a role in the maturation of cytosolic iron-sulfur (Fe–S) cluster proteins, linking the mitochondrial and cytosolic Fe–S cluster assembly machineries [16]. Mitochondria evolved in eukaryotes by a cell taking up a bacterial cell, thought to be from the Rickettsiaceae family [17]. The crystal structure of an ABCB7 bacterial homologue, Novosphingobium aromaticivorans (Na) Atm1, was recently reported in complex with oxidized and reduced glutathione, and a metal-glutathione complex [18]. Interestingly, Novosphingobium aromaticivorans is from the class Alphaproteobacteria, the same class as Rickettsiaceae, suggesting the protein may be related to a bacterial precursor of human ABCB7. Atm1, a yeast ABCB7 homologue, has been shown to transport glutathione-coordinated Fe–S clusters [2Fe–2S](GS)4 [19–21]. The role of transporting glutathione-complexed iron-sulfur clusters most likely evolved from the ability of ancient organisms to export heavy metals as glutathione complexes [22], consistent with the endosymbiotic model and the evolution of cluster biosynthesis in mitochondria.
Phylogenetic analysis has previously demonstrated that genes that code for proteins involved in eukaryotic iron-sulfur cluster biosynthesis are most closely related to genes found in Proteobacteria, in comparison to all prokaryotes [23]. The physiological role of Atm1 in prokaryotes is heavy metal export via metal-glutathione complexes, as the protein is found in the cell membrane [18]. When the bacteria that became the mitochondria were taken up, the bacterial cell membrane became the inner mitochondrial membrane, where Atm1 is found in eukaryotes. Eukaryotes would no longer need Atm1 for the purpose of heavy metal export, as heavy metals would not be found in the mitochondria, and so the protein evolved to serve the distinct, but related purpose of transporting cluster complexes. A logical substrate in eukaryotes would likely be similar to the substrate in prokaryotes, a metal-glutathione complex like the [2Fe–2S](GS)4 complex, as proposed in this report. As described before, the crystal structure of Na Atm1 in complex with oxidized glutathione shows 2 binding sites located 5 Å apart that have been proposed to be a possible binding site for a [2Fe–2S](GS)4 cluster [18]. In eukaryotic homologs, these results further suggest that ABCB7 evolved from a metal-glutathione complex transporter to provide the cell with a mechanism to export glutathione-coordinated Fe–S clusters from mitochondria to the cytosol [20].
In addition to the crystal structure of the bacterial Na Atm1 transporter, a related structure of the Saccharomyces cerevisiae (Sc) Atm1 transporter has also been determined and a glutathione binding site modeled into the putative substrate binding domain [18,24]. The residue corresponding to E433 in the human protein is one of the residues that is proposed to interact with GSH in the yeast crystal structure [24] and lies in the substrate binding pocket of Na Atm1 that is partially formed by the TM helix suggested to be involved in communicating structural changes to the rest of the protein following substrate binding [18].
Recently we reported on the functional mechanism of the yeast Atm1 homologue [22]. In this paper we further elaborate on the export mechanism, the regulation of activity based on cellular availability of Fe–S cluster and MgATP, and the molecular basis of a human disease condition based on a natural E433K substitution. Sideroblastic anemia results from the impact of this derivative on cluster binding, transport, and ATPase activity, and is explored through a comparative study of native human ABCB7 and E433 point derivatives. In addition, the experiments and analysis described herein further test the hypothesis that such exporters accept glutathione-complexes metal cofactors as a natural substrate, derived from ancient heavy metal transporters, and that disease states arising from natural substitutions that impair transport can result in the cellular phenotypic changes of increased mitochondrial iron and a decrease in cytosolic holo forms of Fe–S dependent proteins.
2. Results
2.1. Influence of substrate on ATPase activity
ATP hydrolysis activity of native ABCB7 and substituted derivatives was analyzed by use of the ENZchek Phosphate Activity Assay Kit. This method provides a spectrophotometric method for quantification of inorganic phosphate released during ATPase activity by use of a coupled enzyme assay that uses inorganic phosphate to form a product with a new absorbance maximum at 360 nm that is linearly related to phosphate concentration in the range of 2–150 μM. All protein derivatives studied showed basal ATPase activity, and the effect of both glutathione and [2Fe–4Fe](GS)4 on the ATPase activity of native and derivative proteins was analyzed.
ATPase activity for native ABCB7 was stimulated in the presence of glutathione, with an overall change in ATPase activity (ΔVmax) of 0.68 ± 0.02 μM/min, relative to basal activity in the absence of substrate (Fig. 2) and a binding constant (KD) of 9.5 ± 0.5 mM was determined. However, it is noteworthy that the level of glutathione required to achieve the maximal stimulated activity exceeds the upper limit of the concentration of glutathione in the cell, 10 mM [25], and we do not consider glutathione to be a physiologically relevant substrate. Glutathione stimulation was not observed for any of the substituted derivatives.
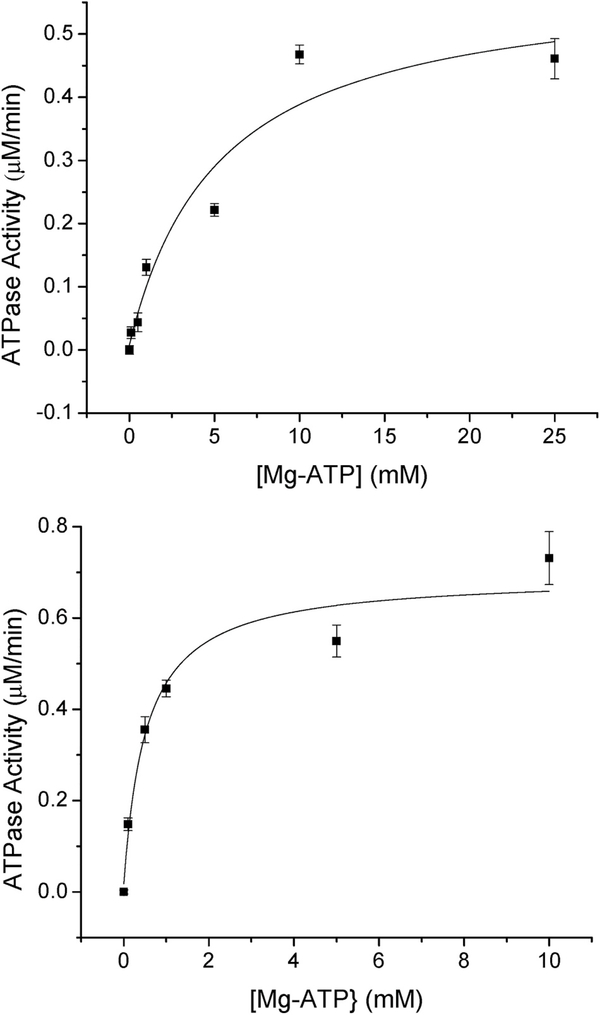
Fig. 2.
(Top) [2Fe–2S](GS)4 effect on ATP hydrolysis of native ABCB7. The dependence of [2Fe–2S](GS)4 cluster on ATPase activity for native ABCB7 was assayed using the EnzChek Phosphate Assay. The KD for cluster was determined to be 44 ± 13 nM and the increase in ATPase activity was determined to be 0.18 ± 0.01 μM/min (bottom). The dependence of [2Fe–2S](GS)4 cluster on ATPase activity for E433D ABCB7. The KD for cluster was determined to be 238 ± 88 nM and increase in ATPase activity was determined to be 0.22 ± 0.02 μM/min. Basal ATPase activity was subtracted from each point.
The [2Fe–2S](GS)4 cluster stimulated the activity of both native ABCB7 (Fig. 2) and the E433D derivative. Moreover, a lower cluster concentration was required to stimulate the activity of native ABCB7 than the E433D derivative, with KD’s of 44 ± 13 nM and 238 ± 88 nM (Table 1), respectively, supporting the view that the glutathione cluster complex is the endogenous substrate for the export protein. In the presence of 10 μM cluster the ATPase activity for native ABCB7 increased by 0.18 ± 0.01 μM/min, and by 0.22 ± 0.02 μM/min for E433D ABCB7. No stimulation was observed for any of the other derivatives in the presence of the glutathione-complexed cluster.
Table 1. ATPase stimulation of ABCB7 in the presence of [2Fe–2S](GS)4.
The effect of [2Fe–2S](GS)4 cluster on ATPase activity of native and D433 derivative proteins was assayed using the EnzChek Phosphate Assay Kit. The change in Vmax, representing the stimulation of ATPase activity, was observed to be cluster dependent for native and E433D ABCB7. Cluster did not stimulate the E433K and E433Q derivatives, consistent with cluster transport studies (Fig. 4).
| Protein | KD (nM) | Δ Vmax (μM/min) |
|---|---|---|
| native | 44 ± 13 | 0.18 ± .01 |
| E433D | 238 ± 88 | 0.22 ± .02 |
In summary, the stimulation of ATPase activity, a hallmark of substrate binding and coupling of the substrate and nucleotide binding domains, an essential prelude to substrate transport, is observed only for the native protein (or conservatively-substituted E433D derivative) with glutathione or glutathione-complexed species. Stimulation is lost in the other E433 derivatives, including the disease-causing E433K derivative.
2.2. Influence of substrate on Michaelis-Menten parameters for ATPase activity
The effect of cluster on the Michaelis-Menten profiles for Mg-ATP turnover was also assayed using the ENZchek phosphatase assay kit. In the presence of cluster, the KM for Mg-ATP decreased from 5.3 ± 1.0 to 0.54 ± 0.23 mM, although Vmax increased only marginally to 0.68 ± 0.03 μM/min (Fig. 3, Table 2). The E433D derivative behaved similar to native with regard to Vmax (0.35 ± 0.03 μM/min with no cluster present, versus 0.79 ± 0.03 μM/min in the presence of cluster) although the KM for Mg-ATP did not significantly change (2.1 ± 0.2 mM with cluster present versus 0.85 ± 0.28 mM with no cluster present) (Table 2). For the E433K and E433Q derivatives both the Km and Vmax remained similar to basal level (with a factor of ~2) following addition of cluster (Table 2).
Fig. 3.
(Top) Mg-ATP dependence on ATP hydrolysis for native ABCB7. The dependence of Mg-ATP on ATPase activity for native ABCB7 was assayed using the EnzChek Phosphate Assay Kit. The KM for Mg-ATP was determined to be 5.3 ± 1.0 mM and the Vmax for ATPase activity was determined to be 0.58 ± 0.04 μM/min (bottom) Mg-ATP dependence on ATP hydrolysis for native ABCB7 in the presence of 10 μM [2Fe–2S](GS)4. The dependence of Mg-ATP on ATPase activity for native ABCB7 was assayed using the EnzChek Phosphate Assay Kit in the presence of 10 μM [2Fe–2S](GS)4. The KM for Mg-ATP was determined to be 0.54 ± 0.23 mM and the Vmax for ATPase activity was determined to be 0.68 ± 0.03 μM/min.
Table 2. Michaelis-Menten parameters for ABCB7 variants in the presence of [2Fe–2S](GS)4.
The KM for Mg-ATP and Vmax of ATPase activity was determined using the EnzChek Phosphate Assay Kit in the presence and absence of 10 μM [2Fe–2S](GS)4 cluster. In the presence of cluster, the Km for Mg-ATP increased, while this was not observed in any of the substituted derivatives. The Vmax was observed to increase in both the native and E433D derivative in the presence of cluster. This observation is consistent with the cluster dependence on ATPase activity (Table 1) and the ability to transfer cluster (Fig. 4).
| Mg-ATP KM (mM) | Mg-ATP Vmax (μM/min) | Mg-ATP KM (mM) w/10 μM cluster | Mg-ATP Vmax (μM/min) w/10 μM cluster | fold increase in kcat/KM in presence of cluster | |
|---|---|---|---|---|---|
| native | 5.3 ± 1.0 | 0.58 ± 0.04 | 0.54 ± 0.23 | 0.68 ± 0.03 | 11.5 |
| E433D | 0.85 ± 0.28 | 0.35 ± 0.03 | 2.1 ± 0.2 | 0.79 ± 0.03 | 0.92 |
| E433K | 0.49 ± 0.11 | 0.37 ± 0.02 | 1.1 ± 0.3 | 0.32 ± 0.03 | 0.40 |
| E433Q | 0.71 ± 0.30 | 0.28 ± 0.04 | 1.1 ± 0.2 | 0.31 ± 0.01 | 0.72 |
These data demonstrate that with the exception of the conservative E433D derivative, the other E433 derivatives have lost the ability to couple substrate binding with stimulation of ATPase activity at the nucleotide binding domain. That is, these derivatives cannot lower the KM to promote MgATP binding and ATPase activity at physiological concentrations.
2.3. Cluster transport assay
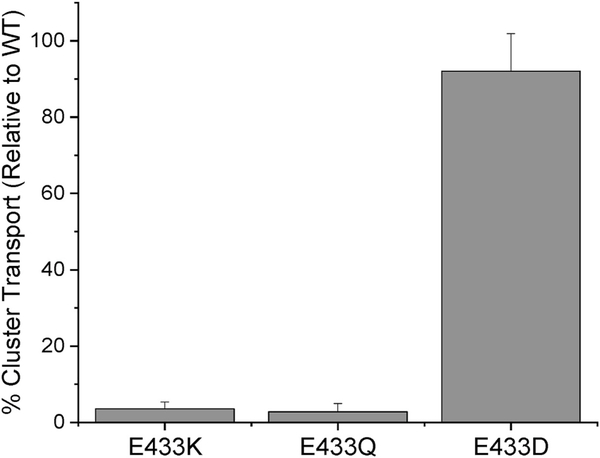
The ability of each substituted derivative to transport cluster was assayed via proteoliposome transport assays (Fig. 4) and each was unable to transport cluster as efficiently as native ABCB7. The E433D derivative was able to transport the most cluster relative to native (92 ± 10% of native), while the E433Q and E433K derivatives showed substantially diminished transport (2.8 ± 2.1% and 3.5 ± 1.8% of native, respectively). These data show that the carboxylate bearing amino acid residue at position 433 is critical for the transport function of ABCB7.
Fig. 4.
[2Fe-2](GS)4 Cluster transport in ABCB7 derivatives. The ability of each E433 derivative to transport [2Fe–2S](GS)4 cluster was assayed using proteoliposome transport assays in conjugation with a tiron assay to quantitate the amount of cluster transferred to the inside of the proteoliposome. Similar to the stimulation assays, the E433D derivative behaved most similarly to native protein, while cluster transport in the E433K and E433Q derivatives was greatly reduced.
2.4. BLAST analysis
To investigate which eukaryotic proteins were most similar to Novosphingobium aromaticivorans Atm1, a BLAST analysis was conducted with Na Atm1 while limiting the results to the Eukaryotic domain. The Basic Local Alignment Search Tool (BLAST) compares sequences (amino acid or nucleic acid), identifies finds regions of similarity, and calculates the statistical significance of matches. BLAST is commonly used to identify functional and evolutionary relationships when comparing sequences from genomic data bases. The goal of this study was to evaluate the similarity of the ancient heavy metal transporter from Na, which exported heavy-metal glutathione complexes, with other eukaryotic transporters that have been implicated in iron-sulfur cluster translocation. Such a functional connection would strengthen both the hypothesis that glutathione-complexes iron-sulfur clusters are the natural substrates for the ABCB7-type mitochondrial transporters and that such clusters are a natural component of the labile iron pool.
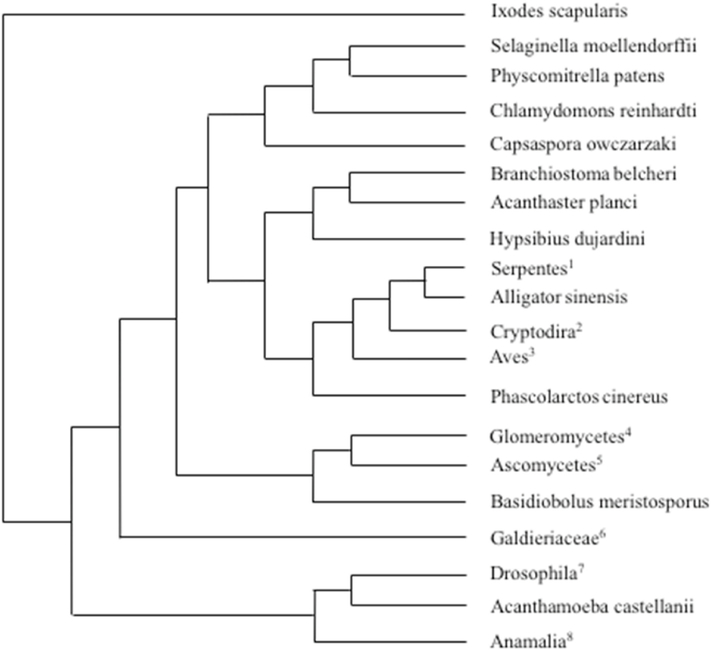
In the BLAST analysis conducted against Na Atm1, animals, plants, yeast, and an amoeba all appeared in the results. The organisms with protein sequences most closely matching the Na Atm1 sequence in the eukaryotic domain are Pantholops hodgsonli (Tibetan antelope), Nephila clavipes (Golden silk orb-weaver), Ixodes scapularis (Deer tick), Drosophila eugracilis (Fruit fly), and Selaginella moellendorffii (moss). The presence of such a wide array of organisms from the eukaryote domain suggests that the protein is conserved from prokaryotes to eukaryotes, similar to how the proteins involved in Fe–S cluster biosynthesis are conserved from bacteria to man (Fig. 5).
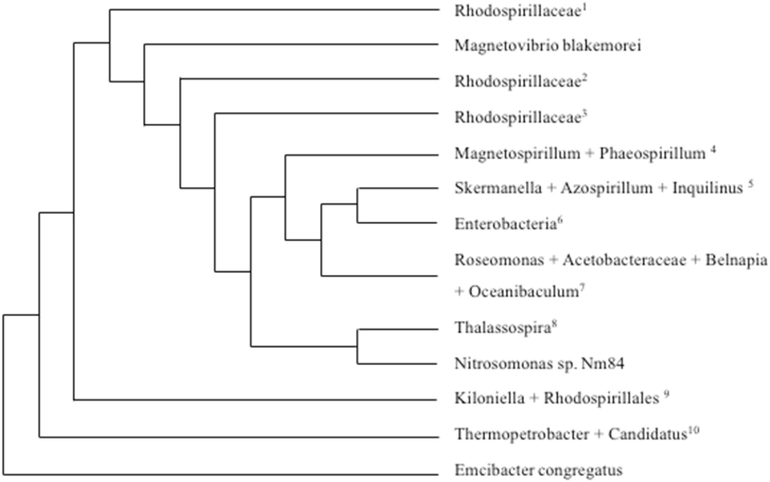
Fig. 5.
Phylogenetic tree of HsABCB7 blast matches in prokaryotes. BLAST was used to determine the proteins in the prokaryote domain that were most similar to human ABCB7. The distance of tree results is shown above, with the lowest common scientific name listed in the figure. For branches that containing different species, the species are listed below, with the numbers corresponding to the superscript number in the figure. Interestingly, all species found are of the phylum Proteobacteria, and a large majority are of the class Alphaproteobacteria, which contains the family Rickettsiaceae, believed to be the bacteria that evolved into mitochondria in the endosymbiotic theory [17]. 1: Ferrovibrio sp., Rhodospirillaceae bacterium SYSU D60009.2: Candidatus Endolissoclinum sp. TMED26, Rhodospirillaceae bacterium TMED23.3: Rhodospirillales bacterium CG15_BIG_FIL_POS-T_REV_8_21_14_020_66_15], Alphaproteobacteria bacterium MarineAlpha3_Bin2], Alphaproteobacteria bacterium MarineAlpha3_Bin1, Alphaproteobacteria bacterium MarineAlpha3_Bin4. 4: Magnetospirillum sp. 64–120, Magnetospirillum gryphiswaldense, Magnetospirillum moscoviense, Phaeospirillum molischianum, Magnetospirillum sp. LBB-42, Magnetospirillum marisnigri, Magnetospirillum sp. ME-1, Magnetospirillum sp. XM-1.5: Skermanella stibiiresistens, Skermanella aerolata, Azospirillum sp. TSO35–2, Azospirillum sp. TSO22–1, Inquilinus limosus. 6: Serratia marcescens, Serratia, Serratia sp. Nf2, Enterobacterales, Klebsiella oxytoca, Serratia sp. TKO39, Serratia ureilytica. 7: Roseomonas gilardii, Roseomonas, Roseomonas mucosa, Roseomonas rosea, Roseomonas sp. CPCC 101021, Roseomonas aestuarii, Roseomonas rhizosphaerae, Roseomonas deserti, Acetobacteraceae bacterium AT-5844, Roseomonas, Belnapia moabensis, Belnapia rosea, Oceanibaculum nanhaiense, Oceanibaculum indicum. 8: Thalassospira sp., Thalassospira tepidiphila, Thalassospira profundimaris, Thalassospira mesophila, Thalassospira alkalitolerans. 9: Kiloniella laminariae, Kiloniella spongiae, Kiloniella majae, Kiloniella sp., Kiloniella litopenaei, Rhodospirillales bacterium 47_12_T64.10: Thermopetrobacter sp. TC1, Candidatus Phaeomarinobacter ectocarpi.
Similarly, a BLAST analysis was conducted with Hs ABCB7 to identify those prokaryotic proteins that were most similar to human ABCB7, while limiting the results to the Prokaryotic domain. The organisms with protein sequences most closely matching to Hs ABCB7 in the prokaryotic domain are as follows; Skermanella stibiiresistens, Roseomonas gilardii, Roseomonas deserti, and Magnetospirillum sp. Interestingly, all organisms that showed up in the blast results are from the Proteobacteria phylum, and almost all, except for the enterobacteria, are from the Alphaproteobacteria class. This class contains the family Rickettsiaceae, which is hypothesized to contain the bacteria that led to the development of mitochondria according to the endosymbiotic theory (Fig. 6) [17].
Fig. 6.
Phylogenetic tree of Na Atm1 blast matches in eukaryotes. BLAST was used to determine the proteins in the eukaryote domain that were most similar to Novosphingobium aromaticivorans Atm1. The distance of tree results is shown above, with the lowest common scientific name listed in the figure. For branches that containing different species, the species are listed below, with the numbers corresponding to the superscript number in the figure. Animals, plants, and yeast all had proteins that were similar to Na Atm1 based on BLAST results. Animals and yeast were most highly represented, while only 5 species of plants, mosses, and algae, were in the BLAST results. Evolutionarily yeast is more closely related to animals than plants, which is most likely why more yeast and animals show up in the results than plants. 1: Pseudonaja textilis, Python bivittatus, Thamnophis sirtalis. 2: Terrapene mexicana triunguis, Chrysemys picta bellii, Chelonia mydas, Pelodiscus sinensis. 3: Manacus vitellinus, Cyanistes caeruleus, Charadrius vociferus, Fulmarus glacialis, Dromaius novaehollandiae, Tauraco erythrolophus, Apaloderma vittatum, Calidris pugnax, Haliaeetus albicilla, Phaethon lepturus, Aptenodytes forsteri, Chlamydotis macqueenii, Mesitornis unicolor, Leptosomus discolor, Chaetura pelagica, Cuculus canorus, Nestor notabilis, Amazona aestiva, Calypte anna. 4: Diversispora versiformis, Rhizophagus clarus, Rhizophagus irregularis, Rhizophagus diaphanous, Gigaspora rosea. 5: Byssochlamys spectabilis, Aspergillus nomius, Aspergillus flavus, Aspergillus oryzae, Aspergillus bombycis, Aspergillus arachidicola, Aspergillus ellipticus, Aspergillus heteromorphus, Aspergillus sclerotiicarbonarius, Aspergillus carbonarius, Aspergillus sclerotioniger, Aspergillus glaucus, Aspergillus ruber, Aspergillus cristatus, Aspergillus steynii, Penicillium vulpinum, Penicillium digitatum, Aspergillus candidus, Emergomyces pasteurianus, Hortaea werneckii, Cercospora beticola, Cercospora berteroae, Pseudocercospora musae, Sphaceloma murrayae. 6: Galdieria sulphuraria, Chondrus crispus. 7: Drosophila eugracilis, Drosophila obscura, Drosophila elegans. 8: Hyalella azteca, Nilaparvata lugens, Nephila clavipes, Pantholops hodgsonii.
3. Discussion
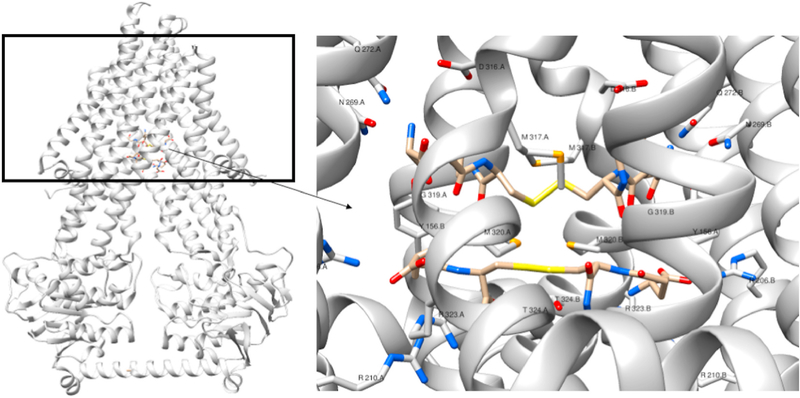
Recent interest in the function of the Atm1/ABCB7 type transporters [18,20–22,24] reflects their important role in cellular function, and acknowledges the uncertainty over the endogenous function of these transporters. Nevertheless, experimental evidence that glutathione-complexed clusters bind to such transporters, stimulate ATPase activity, and are translocated in liposomal assays, all point toward the proteins transporting a [2Fe–2S](GS)4 cluster [20–22,26]. Its bacterial homologue in Novosphingobium aromaticivorans is thought to be involved in heavy metal detoxification by exporting metals coordinated by glutathione [18]. Indeed, the two crystallographically-characterized binding sites for oxidized glutathione dimers are structurally positioned in a manner that could support docking of a [2Fe–2S](GS)4 complex (Fig. 7). In both the study of Na Atm1 and this report, a much greater variance was found in the experimentally determined KM for Mg-ATP than for Vmax, which is essentially constant. For ABCB7-promoted turnover of Mg-ATP, conducted in the presence of 10 μM cluster, the KM for Mg-ATP was found to decrease by 10-fold to 0.54 ± 0.23 mM. For Na Atm1, the KM for Mg-ATP was found to be similar to the value determined for glutathione conjugates of silver and mercury, 0.012 ± 0.001 mM and 0.12 ± 0.04 mM, respectively [18].
Fig. 7.
GSSG orientation in the binding sites of NaAtm1. Left: NaAtm1 (PBD:4mrs) complexed with oxidized glutathione to show how the molecules lie in the binding pocket of the TMD. The black outline represents the cell membrane. Right: Zoomed in view of the oxidized glutathione molecules in the binding pocked of NaAtm1. Potential residues that interact with the glutathione molecules are shown. These oxidized glutathione molecules are located 5 Å apart, which suggests that a [2Fe–2S] (GS)4 could occupy the same pocket in eukaryotic homologs.
Human ABCB7 follows a similar trend to Saccharomyces cerevisiae Atm1 concerning the kinetics of ATP hydrolysis. Both native proteins show increased ATPase activity in the presence of GSH (KD = 32 ± 9 μM) or [2Fe–2S](GS)4 (KD 100 ± 30 nM), and the KM for Mg-ATP is decreased in the presence of cluster (93-fold in the case of Atm1) [22]. Substitutional analysis of the analogous residue was also conducted. Of key interest for human ABCB7 is the E433K substitution, as it linked to sideroblastic anemia. A similar D398K substitution in the yeast protein Atm1 also resulted in loss of substrate stimulation in the presence of GSH or cluster, reflecting the loss of coupling between substrate binding site and the ATPase domains [22].
This decrease in Mg-ATP KM is the key factor driving transport of the cluster from mitochondria. Cellular ATP levels fluctuate between 0.5 and 3 mM in yeast [27] and range from 1 to 10 mM in animals [28], while [Mg2+] ranges from 17 to 20 mM in mammals [29] and lies around 5 mM in the cytosol of yeast cells, with 1.35 mM being unbound [30]. However, less is known concerning the concentration of Mg-ATP in different cellular compartments [31]. The only study that reported a mitochondrial concentration of Mg-ATP was conducted on sycamore cells, where it was determined that the concentration of Mg-ATP was 520 ± 60 μM [31]. The mitochondria of the sycamore cells only had a concentration of 2.4 ± 0.4 mM Mg2+, lower than what is expected in mammalian mitochondria, however the ATP levels of studied plants suggests a similar ATP level [31,32]. While it cannot be expected that the concentration of Mg-ATP in sycamore plants and humans are the same, nevertheless, it provides a good estimate. In the presence of cluster and GSH the KM for Mg-ATP dropped from 5.3 ± 1.1 mM to 0.54 ± 0.23 mM and 0.04 ± 0.01 mM, respectively, which is lower than the Mg-ATP concentration in the mitochondria. This decrease in KM for Mg-ATP was also observed in S. cerevisiae Atm1 [22].
Efficient cellular utilization of Mg-ATP requires ABC transporters to hydrolyze the nucleotide only when a substrate (glutathione-complexed cluster) is bound to the TMD. Otherwise the ATPase domain would be constantly active and turning over the available Mg-ATP pool. A previous report on Ppg, one of the most heavily studied ABC transporters, provides additional support for this idea through an analysis of structural changes in the NBD following binding of the substrate to be transported. Similar to most ABC transporters, Ppg can transport a wide variety of molecules, some of which stimulate ATPase activity while others inhibit. In the presence of substrates that stimulate ATPase activity, enhanced crosslinking is observed between the Walker A motif and the ABC signature motif, while in the presence of substrates that inhibit activity a decrease was observed in the rate of crosslinking [33]. Similar to all ABC transporters, the molecular substrate binds to the TMD. Subsequently, structural changes are communicated from the TMD to the NBD by residues in the coupling helices that connect the two domains [22]. Crystallographic studies of the bacterial Atm1 revealed two sets of TM helices (TM 2/3 and TM 4/5) in each helical chain that bordered the NBD. These domains are shifted in the presence of Hg (GSH)2, the proposed substrate, consistent with the proposed role for these coupling helices in mediating communication between the NBD and TMD [3,4]. When the metal-glutathione adduct binds in the pocket defined by the TMD, these shifts in the coupling helices are most likely responsible for communicating the structural changes to the NBD that result in a lowering of KM for Mg-ATP [22].
Of the known ABCB7 substitutions that result in sideroblastic anemia with ataxia, the E433K derivative causes the most severe form of the disease [11], and is observed herein to knockout cluster transport. Neither the glutathione-complexed [2Fe–2S] cluster nor glutathione alone were found to stimulate ATPase activity. The substituted protein did not exhibit a decrease in KM for Mg-ATP in the presence of cluster. Residues in TM5 and 6 that lie in the substrate-binding pocket of the bacterial Na Atm1 are observed to shift in the presence of the mercury-glutathione complex, as well as the residue homologous to E433 resides in TM6 of the human protein, which was suggested to communicate conformational changes to the rest of the protein in Na Atm1 [18]. Accordingly, this residue most likely plays a role in communicating substrate binding to the other domains of the protein. Both the charge and size of the side chain appear to be important for proper function, although in this case the charge may have a greater effect, since the aspartate derivative behaved similar to native, exhibiting stimulation of ATPase activity in the presence of cluster and the ability to transport cluster (92 ± 10% of native), while the E433Q and E433K mutations essentially knocked out cluster transport, and neither showed ATPase stimulation in the presence of cluster.
ABCB7 mutations that result in sideroblastic anemia with ataxia are rare, and new mutations that result in the disease have been discovered in the past few years [5]. Inasmuch as these mutations are located in different regions of the protein, there is unlikely to be a unique mechanism underlying the impact of point substitutions that result in disease. The most severe of these mutations, E433K, resides in the substrate-binding pocket and knocks out cluster transport and ATPase stimulation in the presence of both GSH and the cluster complex. The decrease in KM for Mg-ATP observed in the presence of cluster for native protein is also eliminated, suggesting the residue plays a key role in communication between the TMD and NBD. In the bacterial homologue, Rees and coworkers state that the binding site for GSSG is located near a helical irregularity from residues 314–317 which could potentially be involved in effecting conformational change [18]. These residues are located near residue T324, which is equivalent to E433 in ABCB7. Therefore, binding of the iron-sulfur glutathione complex could cause changes in the irregular helical region through contacts with the E433 residue, the most likely explanation underlying how the E433K mutation results in disease states associated with the human protein. Although debated, if the physiological role of ABCB7 is to export glutathione-coordinated clusters from the mitochondria, the E433K derivative prevents the protein from functioning in a transport role and supports a disease model where this natural disease-causing mutation is unable to transport cluster from the mitochondria for use in the cytosol.
BLAST analysis revealed an interesting link between ABCB7 and bacterial homologs. While conducting BLAST analysis of organisms with proteins most similar to ABCB7 in prokaryotes, all results were found in the same phylum, with a large majority coming from the class Alphaproteobacteria. Conversely, when BLAST analysis of Na Atm1 was conducted in the eukaryote domain, the proteins that matched were from a wide variety of organisms, including plants, animals, yeast, and amoeba. Since the Alphaproteobacteria class contains the bacteria that evolved into mitochondria, it is expected that the bacterial proteins most similar to human ABCB7 would be found in this class, as observed in the BLAST analysis [6]. However, after the first eukaryotic cells evolved, where bacteria formed the mitochondria, the cells would have evolved into the wide variety of eukaryotes containing proteins that mutated from the bacterial Atm1. This is consistent with why the BLAST analysis of eukaryotes contains proteins from plants, animals, yeast, and amoeba. Interestingly, the plants that showed up in the BLAST analysis were algae and mosses. Mosses are some of the ancient most plants that are still in existence today, and explains why they have proteins with a similar sequence to bacterial Atm1 [34]. Additionally, when a blast analysis of Na Atm1 was conducted with a limitation that omitted the phylum Proteobacteria, only 31 of 414 organisms that matched were found to be prokaryotes, while 383 organisms were eukaryotes (Table S1). This further demonstrates how the protein in Novosphingobium aromaticivorans is more closely related to proteins found in eukaryotes than bacterial proteins found outside the phylum Proteobacteria. Presumably, these prokaryotes evolved into mitochondria containing a protein similar to Na Atm1, as it is found in other Alphaproteobacteria.
In summary, the results presented herein support an endosymbiotic evolutionary model where the human mitochondrial exporter ABCB7 evolved from a subclass of bacterial heavy metal efflux proteins, with the metal in the form of glutathione adducts. Consistent with this hypothesis is the buildup of iron in the mitochondria, and deficiency of cluster-bound forms of cytosolic Fe–S proteins observed in both yeast and human cells when the transport protein is knocked out or is dysfunctional as a result of disease-related substitutions [1,12–14].
4. MATERIALS and METHODS
4.1. Site-directed mutagenesis
A pET28b (+) expression vector carrying the ABCB7 gene was transformed into Rosetta (DE3) cells. The QuikChange technique (Stratagene) was employed for the following amino acid substitutions: E433D, E433K, and E433Q. PCR reactions contained 50 ng of pASK-IBA2 template DNA, 2 units of Phusion DNA polymerase (New England Biolabs), 10x Phusion buffer (New England Biolabs), 125 ng of each primer, 0.2 mM dNTPs, and 3% DMSO. Primers for the mutations were purchased from Integrated DNA Technologies and are shown in Table S2. The thermocycle was identical to that described in the QuikChange manual (Stratagene). The post-thermocycle samples were incubated with 7.5 units of DpnI at 37 °C for 4 h. Subsequently, CaCl2-competent BL21 Rosetta cells were transformed via heat shock with the mutant constructs. Mutagenesis results were confirmed by nucleotide sequencing by GENEWIZ.
4.2. Protein growth and purification
Rosetta (DE3) cells containing the pET28B (+) vector with the ABC7 gene were grown in 10 mL LB overnight starter cultures with 100 mg/L kanamycin at 37 °C and used to inoculate 1 L of LB, which was subsequently grown to an OD550 of approximately 0.6. Cells were then induced with 100 μg/L isopropyl β-d-1-thiogalactopyranoside. Cells were pelleted after a 12 h induction period at room temperature. Cell pellets were stored at 80 °C until used.
Cell pellets were incubated for 30 min in 100 mM Tris, 150 mM NaCl, pH 8.0 with 1 mg/mL lysozyme and the solutions then sonicated and centrifuged at 5000 rpm (3000 g) for 30 min at 4 °C. The supernatant was removed and added to a HisPur Cobalt column equilibrated with 100 mM Tris, 150 mM NaCl, pH 8.0. The column was then washed with 100 mL of 100 mM Tris, 150 mM NaCl, 0.25% n-dodecyl β-d-maltoside (ddm), 100 mM imidazole, pH 8.0, and eluted with the same buffer except the use of 150 mM imidazole instead of 100 mM. Protein aliquots evaluated to be >95% pure by SDS-PAGE were concentrated using a 10 kDa membrane, aliquoted, flash frozen, and stored at −20 °C until used.
4.3. Liposome synthesis
Following previously published procedures [22], DOPG (Dioleoylphosphatidyl-Glycerol), DOPC (Dioleoylphosphatidyl-Choline), and DOPE (Dioleoylphosphatidyl-Ethanolamine) were purchased from Avanti Polar Lipids, Inc. An equimolar mixture of DOPG, DOPC and DOPE (133 μL, 131 μL and 124 μL, respectively) were vortexed and dried over Argon gas. The lipid mixture was stored under vacuum for one day to dry them completely. The lipid residue was dissolved in 1 mL of 50 mM HEPES, 100 mM NaCl, pH 7.5 and extruded 21 times through a 400 nm membrane.
4.4. ABCB7 incorporation into proteoliposomes
Incorporation of ABCB7 into proteoliposomes was based on prior protocols [21,35,36]. The previously described liposomes were diluted two-fold and a solution of 10% Triton-X titrated into the liposome mixture in 4 μL increments until a maximum OD550 was attained. Additional 2 μL increments of 10% Triton-X were added until the “loose” state was reached, at which the OD550 reached approximately −0.5. Purified ABCB7 (80 μL of 2.5 μM) was added per 1 mL of liposome and incubated for 15 min at 4 °C. For a negative control, 50 mM HEPES, 100 mM NaCl, pH 7.5 was added at the same volume as the protein. Aliquots of 75 mg of BioBeads were added to the proteoliposome solution at 4 °C at 0.5, 1, and 2 h and a final aliquot was added the next morning. Two hours after the final addition of BioBeads, they were separated from the proteoliposome solution. The reconstituted proteoliposome was removed from the buffer by ultracentrifugation at 80,000 rpm for 20 min, and the pellet was resuspended in 1 mL of 50 mM HEPES, 100 mM NaCl, pH 7.5. The resulting proteoliposome was maintained on ice and used immediately in a tiron assay.
4.5. ATPase assay
Experiments to quantify the stimulation of ABCB7 ATPase activity by glutathione and cluster were performed with native protein and substituted derivatives. ATPase activity was monitored by use of the ENZChek Phosphate Assay Kit in 60 μL reactions containing 1.7 μM protein, 5 μM MESG, 2.5 mM Mg-ATP, 5 μM purine nucleoside phosphorylase, and varying amounts of GSH/cluster in 50 mM Tris, 1 mM MgCl2, pH 7.5. The concentrations of GSH used were 0, 2, 3, 4, 5, 6, 7, and 8 mM GSH. The concentrations of cluster used were 0, 0.1, 0.5, 1, 5, and 10 μM cluster. To inhibit cluster breakdown in the absence of additional glutathione, the cluster was dissolved in degassed water and a fresh batch of cluster was used for each set of experiments. The reactions were monitored over 10 min at 360 nm, and the ATPase activity was calculated from the slope from 5 to 10 min. Data from the 0 mM trial for each set of experiments was subtracted from each trial in that series, resulting in a measure of ΔATPase activity. The change in activity (y) was then plotted vs concentration of GSH or cluster, and the data was fit to a standard one-site binding equation (1) (Fig. 2),
| (1) |
where KD is the apparent dissociation constant for the cluster (or glutathione) to the binding pocket and ΔVmax represents the maximum (or limiting) change in reaction velocity when the transporter is saturated with cluster (or glutathione).
Michaelis-Menten plots were generated by varying the concentration of Mg-ATP in both the presence and the absence of cluster (or glutathione) (Fig. 3). The final concentrations of MgATP used were 0, 0.1, 0.5, 1.0, 5.0, and 10.0 mM (and an additional 25.0 mM concentration in the presence of cluster), with 1.7 μM ABCB7 and either 10 μM cluster, 5 mM GSH, or the absence of both. Reactions were performed as described above, and the standard Michaelis-Menten equation (2) was then fit to the data, where V is the measured reaction velocity, Vmax is the maximum reaction velocity at saturating MgATP, KM is the Michaelis constant, and S is the [MgATP].
| (2) |
4.6. Cluster transport assay
To a solution of freshly made proteoliposome (0.5 mL in 50 mM HEPES and 100 mM NaCl at pH 7.5), Mg-ATP and [2Fe–2S](GS)4 cluster in GSH solution, pH 8.6, were added to obtain a final concentration of 10 mM Mg-ATP and 10 mM GSH-Fe-S cluster in 1 mM GSH. The solution was mixed and incubated for 1 h at 25 °C, and the reaction mixture was centrifuged for 5 min at 6000 rpm at 25 °C to remove precipitate formed from cluster hydrolysis. The decantate was centrifuged further at 80,000 rpm for 20 min at 4 °C to isolate the proteoliposome. The resulting pellet was resuspended in 275 μL of 50 mM HEPES, 100 mM NaCl, pH 7.5, and the proteoliposome was denatured by the addition of 82.5 μL of 2 M HCl and boiling at 95 °C for 15 min to release iron. To isolate the iron in solution, the denatured proteoliposome solution was centrifuged at 13,000 rpm for 10 min, and the solution was neutralized by the addition of 800 μL of 10 mM, pH 6.5 ME S buffer and 9.2 μL of 5 M NaOH to 100 μL of the decantate. A stock tiron solution (100 mM, 100 μL in MES buffer) was added to chelate the released ferric ions, and the solution was incubated at room temperature in the dark for 10 min before measuring the absorbance at 550 nm by use of a Cary Win UV Spectrophotometer. The negative control was subtracted from each trial and normalized to native.
4.7. Blast analysis
A protein Blast search was conducted on the human ABCB7 isoform 1 sequence from UniProtKB (O75027–1) using the Non-redundant protein sequences (nr) database and selecting for the prokaryote domain. The blastp (protein-protein BLAST) program was used, with 100 max target sequences, an expect threshold of 10, word size of 6. The scoring parameters used included a BLOSUM62 matrix, gap costs of existence: 11, extension: 1, and compositional adjustments set to conditional compositional score matrix adjustment. This was repeated with the protein sequence of Atm1 from Novosphingobium aromaticivorans obtained from UniProtKB (Q2G506–1). All parameters were held constant except the eukaryote domain was selected instead of the prokaryote domain. Phylogenetic trees were exported from the “Distance tree of results” report in the Blast analysis. Phylogenetic trees were labeled with the lowest common taxonomic classification.
Further protein blast analysis of ABCB7 was conducted using the same parameters as above but expanding the number of max target sequences to 500 and omitting only proteobacteria. The taxonomy report was analyzed to investigate the relationship of human ABCB7 to protein sequences in eukaryotes and prokaryotes not found in the proteobacteria phylum.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health [AI072443].
Abbreviations:
- NBD
Nucleotide binding domain
- TMD
Transmembrane domain
- ABC
ATP-binding cassette
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.abb.2020.108661.
References
- [1].Burke MA, Ardehali H, Mitochondrial ATP-binding cassette proteins, Transl. Res 150 (2007) 73–80. [DOI] [PubMed] [Google Scholar]
- [2].Vasiliou V, Vasiliou K, Nerbert DW, Human ATP-binding cassette (ABC) transporter family, Hum. Genom 3 (2009) 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wen PC, Tajkhorshid E, Conformational coupling of the nucleotide-binding and the transmembrane domains in ABC transporters, Biophys. J 101 (2011) 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hollenstein K, Dawson RJP, Locher KP, Structure and mechanism of ABC transporter proteins, Curr. Opin. Struct. Biol 17 (2007) 412–418. [DOI] [PubMed] [Google Scholar]
- [5].Protasova MS, Grigorenko AP, Tyazhelova TV, Andreeva TV, Reshetov DA, Gusev FE, Laptenko AE, Kuznetsova IL, Goltsov AY, Klyushnikov SA, Illarioshkin SN, Rogaev EI, Whole-genome sequencing identifies a novel ABCB7 gene mutation for X-linked congenital cerebellar ataxia in a large family of Mongolian ancestry, Eur. J. Hum. Genet 24 (2016) 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boratyn GM, Schaffer AA, Agarwala R, Altschul SF, Lipman DJ, Madden TL, Domain enhanced lookup time accelerated blast, Biol. Direct 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maguire A, Hellier K, Hammans S, May A, X-linked cerebellar ataxia and sideroblastic anaemia associated with a missense mutation in the ABC7 gene predicting V411L, Br. J. Haematol 115 (2001) 910–917. [DOI] [PubMed] [Google Scholar]
- [8].Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, Lill R, Bishop DF, Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation, Blood 96 (2000) 3256–3264. [PubMed] [Google Scholar]
- [9].Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM, Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A), Hum. Mol. Genet 8 (1999) 743–749. [DOI] [PubMed] [Google Scholar]
- [10].D’Hooghe M, Selleslag D, Mortier G, Van Coster R, Vermeersch P, Billiet J, Bekri S, X-linked sideroblastic anemia and ataxia: a new family with identification of a fourth ABCB7 gene mutation, Eur. J. Paediatr. Neurol 16 (2012) 730–735. [DOI] [PubMed] [Google Scholar]
- [11].Pondarre C, Campagna DR, Antiochos B, Sikorski L, Mulhern H, Fleming MD, ABCB7, the gene responsible for X-linked sideroblastic anemia with ataxia, is essential for hematopoiesis, Blood 109 (2007) 3567–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Khan AA, Quigley JG, Control of intracellular heme levels: heme transporters and heme oxygenases, Biochim. Biophys. Acta Mol. Cell Res 1813 (2011) 668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kiss K, Brozik A, Kucsma N, Toth A, Gera M, Berry L, Vallentin A, Vial H, Vidal M, Szakacs G, Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes, PloS One 7 (2012), e37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miyake A, Higashijima S.-i.,Kobayashi D, Narita T, Jindo T, Setiamarga DHE, Ohisa S, Orihara N, Hibiya K, Konno S, Sakaguchi S, Horie K, Imai Y, Naruse K, Kudo A, Takeda H, Mutation in the abcb7 gene causes abnormal iron and fatty acid metabolism in developing medaka fish, Dev. Growth Differ 50 (2008) 703–716. [DOI] [PubMed] [Google Scholar]
- [15].Frazzon J, Fick JR, Dean JR, Biosynthesis of iron-sulphur clusters is a complex and highly conserved process, Biochem. Soc. Trans 30 (2002) 680–685. [DOI] [PubMed] [Google Scholar]
- [16].Pondarre C, Antiochos BB, Campagna DR, Greer EL, Deck KM, McDonald A, Han AP, Medlock A, Kutok JL, Anderson SA, Eisenstein RS, Fleming MD, The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron-sulfur cluster biogenesis, Hum. Mol. Genet 15 (2006) 953–964. [DOI] [PubMed] [Google Scholar]
- [17].Emelyanov VV, Rickettsiaceae, rickettsia-like endosymbionts, and the origin of mitochondria, Biosci. Rep 21 (2001) 1–17. [DOI] [PubMed] [Google Scholar]
- [18].Lee JY, Yang JG, Zhitnitsky D, Lewinson O, Rees DC, Structural basis for heavy metal detoxification by an Atm1-type ABC exporter, Science 343 (2014) 1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qi W, Li J, Chain CY, Pasquevich GA, Pasquevich AF, Cowan JA, Glutathione complexed Fe-S centers, J. Am. Chem. Soc 134 (2012) 10745–10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qi W, Li J, Cowan JA, A structural model for glutathione-complexed iron-sulfur cluster as a substrate for ABCB7-type transporters, Chem. Commun 50 (2014) 3795–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li J, Cowan JA, Glutathione-coordinated [2Fe-2S] cluster: a viable physiological substrate for mitochondrial ABCB7 transport, Chem. Commun 51 (2015) 2253–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pearson SA, Wachnowski C, Cowan JA, Defining the mechanism of the mitochondrial Atm1p [2Fe-2S] cluster exporter, Metallomics 12, 2020, pp. 902–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F, Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers, PLoS Genet 5 (2009) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Srinivasan V, Pierik AJ, Lill R, Crystal structures of nucleotide-free and glutathione-bound mitochondrial ABC transporter Atm1, Science 343 (2014) 1137–1140. [DOI] [PubMed] [Google Scholar]
- [25].Penninckx MJ, An overview on glutathione in Saccharomyces versus nonconventional yeasts, FEMS Yeast Res 2 (2002) 295–305. [DOI] [PubMed] [Google Scholar]
- [26].Schaedler TA, Thornton JD, Kruse I, Schwarzlander M, Meyer AJ, van Veen HW, Balk J, A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly, J. Biol. Chem 289 (2014) 23264–23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ozalp VC, Pedersen TR, Nielsen LJ, Olsen LF, Time-resolved measurements of intracellular ATP in the Yeast Saccharomyces cerevisiae using a new type of nanobiosensor, J. Biol. Chem 285 (2010) 37579–37588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Beis I, Newsholme EA, The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates, Biochem. J 152 (1975) 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Romani AMP, Cellular magnesium homeostasis, Arch. Biochem. Biophys 512 (2011) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Okorokov LA, Lichko LP, Kulaev IS, Vacuoles: main compartments of potassium, magnesium, and phosphate ions in Saccharomyces carlsbergensis cells, J. Bacteriol 144 (1980) 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gout E, Rebeille F, Douce R, Bligny R, Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: unravelling the role of Mg2+ in cell respiration, Proc. Natl. Acad. Sci. U.S.A 111 (2014) E4560–E4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Usuda H, Adenine-nucleotide levels, the redox state of the NADP system, and assimilatory force in nonaqueously purified mesophyll chloroplasts from maize leaves under different light intensities, Plant Physiol 88 (1988) 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Loo TW, Bartlett MC, Clarke DM, Drug binding in human P-glycoprotein causes conformational changes in both nucleotide-binding domains, J. Biol. Chem 278 (2003) 1575–1578. [DOI] [PubMed] [Google Scholar]
- [34].Pruchner D, Nassal B, Schindler M, Knoop V, Mosses share mitochondrial group II introns with flowering plants, not with liverworts, Mol. Genet. Genom 266 (2001) 608–613. [DOI] [PubMed] [Google Scholar]
- [35].Poolman B, Doeven MK, Geertsma ER, Biemans-Oldehinkel E, Konings WN, Rees DC, Functional analysis of detergent-solubilized and membrane-reconstituted ATP-binding cassette transporters, Methods Enzymol 400 (2005) 429–459. [DOI] [PubMed] [Google Scholar]
- [36].Geertsma ER, Nik Mahmood NA, Schuurman-Wolters GK, Poolman B, Membrane reconstitution of ABC transporters and assays of translocator function, Nat. Protoc 3 (2008) 256–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.