Abstract
Background:
Beta blockers have been associated with anti-tumorigenic effects, potentially by reducing adrenergic-mediated stress responses. Preclinical studies have additionally shown that beta blockade may enhance the efficacy of cancer immunotherapy. We investigated lung cancer patients who concomitantly used beta blockers and immune checkpoint inhibitors, with the hypothesis that beta blockade would positively impact clinical outcomes.
Methods:
We retrospectively reviewed the health records of 109 patients who were treated at Northwestern University between January 2014 through August 2018 with immune checkpoint inhibitors for non-small cell lung cancer (NSCLC). Comparisons of overall survival (OS) and progression-free survival (PFS) were performed using Kaplan-Meier analysis with log-rank test, and a univariate regression analysis was performed with a Cox proportional hazards model
Results:
Among 109 patients treated with immune checkpoint inhibitors for NSCLC, 28 of them were concomitantly prescribed beta blockers. Use of beta blockers was associated with increased PFS, with hazard ratio (HR) of 0.58 and 95% confidence interval (CI) of 0.36-0.93. There was not a significant increase in overall survival (OS) among patients who took beta blockers (HR 0.66, 95% CI 0.38-1.17). In a regression model, beta blockers were identified as predictive of PFS, as were non-squamous histology, tumor PD-L1 positivity, and lower line of treatment.
Conclusions:
Our data suggests beta blocker use may be associated with improved PFS among patients treated with immune checkpoint inhibitors for NSCLC. This was a small study and these findings should be further validated in prospective clinical studies.
Keywords: beta blocker, lung cancer, immunotherapy, stress response
MicroAbstract
This study retrospectively assessed the effect of beta blockers on clinical outcomes in lung cancer patients who were treated with immunotherapy. Beta blocker use was associated with improved progression-free survival in these patients. These findings align with prior work regarding the importance of stress signaling in anti-cancer immunity and should be further explored in prospective studies.
Introduction
The stress response has been increasingly recognized as a contributor to tumorigenesis and cancer progression1,2. Stress can markedly elevated catecholamine levels, which in turn activate downstream pathways via adrenergic receptors (ARs)3. Beta-adrenergic signaling has specifically been shown to influence diverse cancer-related processes, including angiogenesis, tumor invasion, and metastatic spread3. Clinical benefit from beta blocker therapy has accordingly been observed in both preclinical models of cancer and in clinical studies.
Large retrospective studies have demonstrated an association between beta blocker use and improved survival outcomes in malignant melanoma4, breast cancer5-7, epithelial ovarian cancer8, and colorectal cancer9. However, other groups have reported conflicting results, including in melanoma10 and in a population-based study of multiple solid tumor types11. Clinical data regarding beta blockade in lung cancer has been limited. One study found a positive survival effect of beta blockers among patients who received definitive radiotherapy for non-small cell lung cancer (NSCLC)12. Stress hormone-mediated activation of beta-ARs in NSCLC has also been shown to mediate resistance to EGFR inhibitors through induction of interleukin 613.
In addition to beta blockers, antidepressant medications are able to modulate the stress response, and preclinical data have suggested that both selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) possess anti-tumor effects14. SSRIs in particular have been associated with reduced risk of developing colorectal cancer15 and decreased time to progression in ovarian cancer16. This effect has been equivocal in other tumor types17, and a retrospective review of patients with NSCLC demonstrated no impact of antidepressant use on survival outcomes18.
Since these studies, the treatment of NSCLC has been transformed by the development of immune checkpoint inhibitors19, which hinder immune regulatory pathways and thus promote anti-tumor immune activity. Interestingly, beta-adrenergic signaling has been shown to be closely intertwined with immune function. It has specifically been linked to reduced proliferation of CD8+ T cells20, as well as to increased immune suppressive activity via regulatory T cells21 and myeloid-derived suppressor cells22. These findings are consistent with more general observations that the stress response can hinder the anti-tumor immune response23.
Based on this data, it has been speculated that inhibiting beta-adrenergic signals may enhance the efficacy of cancer immunotherapy. In mouse models of melanoma, inhibition of beta-adrenergic signaling led to increased tumor infiltration by cytotoxic T cells24. Importantly, these immunologic changes then were linked to improved efficacy of immune checkpoint inhibitors25. Another study found that beta blocker usage correlated with improved overall survival in patients with melanoma who received immunotherapy, and further found that markers of immune activity were elevated in mice after administration of beta blockers26.
Comparable studies have not yet been reported in lung cancer. We hypothesized that a similar clinical benefit would be seen in NSCLC. We therefore performed a retrospective analysis of patients with NSCLC to determine the impact of beta blocker use on clinical outcomes after treatment with immune checkpoint inhibitors.
Materials and methods
Patients and data collection
The Pathology Department at Northwestern University Feinberg School of Medicine maintains a database of patients who underwent pathologic analysis for lung cancer. This database was searched for patients with stage IV NSCLC (per American Joint Committee on Cancer, eighth edition), who were treated with either ICIs or combination chemotherapy with ICIs from January 2014 to August 2018. A total of 109 patients were retrospectively identified.
We used patient medical records to ascertain information on beta blocker usage, which was defined by documented use of any beta blocker at the time of initiating ICI treatment. Patients were excluded if beta blockers were discontinued within 3 months of starting ICIs. The type of beta blocker and indication were noted. The following information was also obtained from patient records: demographic data, smoking status, performance status, line of treatment, tumor positivity for PD-L1, and survival outcomes. PD-L1 tumor status was measured using the VENTANA PD-L1 SP-142 clone (Ventana Medical Systems Inc., Tucson, AZ) as assessed by Northwestern University pathologists. Positive PD-L1 staining was defined as greater than 50% of tumor cells.
Statistical analysis
Comparison of clinical characteristics between the beta blocker and non-beta blocker groups was carried out using chi-squared or Fisher’s exact test. Progression free survival (PFS) was measured from the date of ICI initiation to the date of first documented disease progression based on iRECIST criteria27. Overall survival was measured from the date of ICI initiation to the date of death or last follow-up. Kaplan-Meier analysis with the log-rank test was used to determine difference between survival outcomes. These calculations were performed in GraphPad Prism version 7 (La Jolla, CA). The Cox proportional hazards regression model was used to determine association between clinical variables and survival outcomes in a univariate analysis. This analysis was performed using R28. P-values are represented by * p < 0.05, ** p < 0.01, *** p < 0.001.
Results
Patient characteristics
A total of 109 patients were identified based on the eligibility criteria, and 28 of these patients were simultaneously on beta blockers. Three additional patients were discontinued from beta blockers within 3 months of ICI. Patients who were prescribed beta blockers were more likely to be older (p = 0.010, Table 1). There were no differences the two groups in regards to sex, race, BMI, smoking history, tumor pathology, and ECOG performance status. Tumor PD-L1 status was available for 66.4% of patients, and there were no differences between the two groups in regards to PD-L1 positivity. There was also no difference between the groups regarding choice of ICI and line of treatment (Table 1). Among patients who were using beta blockers, 4 out of 28 (14.3%) were on a non-selective beta blocker.
Table 1.
| No beta blocker (n = 81) | Beta blocker (n = 28) | p-value | |
|---|---|---|---|
| Age (years) | 65.7±12.5 | 73.7±10.1 | 0.01 |
| Sex (%) | 0.80 | ||
| Male | 42.0 | 39.3 | |
| Female | 58.0 | 60.7 | |
| Race (%) | 0.39 | ||
| White | 64.2 | 64.3 | |
| Black | 16.0 | 25.0 | |
| Other | 19.8 | 10.7 | |
| BMI (kg/m2) | 26.1±5.9 | 28.1±5.8 | 0.12 |
| Smoking history (%) | 0.65 | ||
| Never | 24.7 | 14.3 | |
| < 15 pack-years | 14.8 | 21.4 | |
| 15-29 pack-years | 21.0 | 21.4 | |
| > 30 pack-years | 39.5 | 42.9 | |
| Histology (%) | 0.71 | ||
| Adenocarcinoma | 79.0 | 85.7 | |
| Squamous cell carcinoma | 12.3 | 7.1 | |
| Other | 8.6 | 7.1 | |
| Treatment (%) | 0.45 | ||
| Nivolumab | 49.4 | 46.4 | |
| Pembrolizumab | 35.8 | 46.4 | |
| Atezolizumab | 14.8 | 7.1 | |
| Concurrent chemotherapy (%) | 0.95 | ||
| Yes | 11.1 | 10.7 | |
| No | 88.9 | 89.3 | |
| Line of treatment (%) | 0.33 | ||
| First | 29.6 | 39.3 | |
| Second | 58.0 | 57.1 | |
| Third or higher | 12.3 | 3.6 | |
| ECOG performance status (%) | 0.33 | ||
| 0 | 17.3 | 7.1 | |
| 1 | 59.3 | 64.3 | |
| 2 | 19.8 | 17.8 | |
| 3 | 3.7 | 10.7 | |
| CNS disease (%) | 0.49 | ||
| Yes | 35.8 | 28.6 | |
| No | 64.2 | 71.4 | |
| Tumor PD-L1 status (%) | 0.79 | ||
| Positive | 25.9 | 25.0 | |
| Negative | 39.5 | 46.4 | |
| Unknown | 34.6 | 28.6 | |
Survival outcomes with beta blocker use
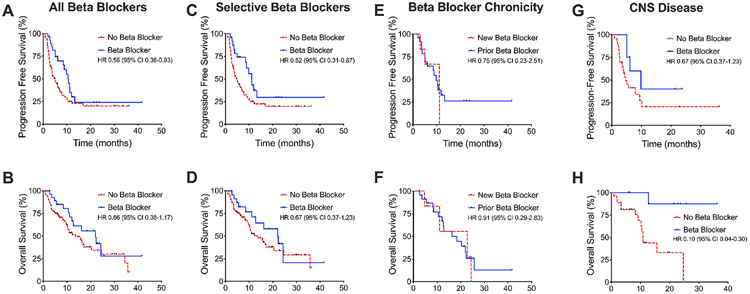
The median follow-up time for patients taking beta blockers was 12.3 months, while the median follow-up time for patients not taking beta blockers was 8.5 months. Patients who took beta blockers concurrently with receiving ICIs had prolonged progression-free survival (PFS) compared to those not using beta blockers, with a hazard ratio (HR) of 0.58 and 95% confidence interval (CI) of 0.36-0.93 (Figure 1A). There was no statistically significant difference in overall survival (OS) between the two groups (HR 0.66, 95% CI 0.38-1.17, Figure 1B). There remained a significant difference in PFS when comparing patients on selective beta blockers with patients not on any beta blockers (HR 0.52, 95% CI 0.31-0.87, Figure 1C-D).
Figure 1. Survival outcomes in association with beta blocker use.
(A) Use of beta blockers and immune checkpoint blockade was associated with longer PFS but (B) not OS in a Kaplan-Meier analysis. (C) Similar analysis performed showed difference in PFS but (D) not OS for the subset of patients with selective beta blockers. (E) There was no difference in PFS or (F) OS between patients who started beta blockers within 3 months of starting immunotherapy or prior to 3 months. (G) There was no statistically significant difference in PFS among patients with intracranial metastases, but (H) there was improved OS with beta blocker use.
Patients who were initiated on beta blockers within 3 months of starting ICIs were compared to those who were chronically on beta blocker therapy (i.e. greater than 3 months prior to starting ICIs). Only 6 patients were newly started beta blockers with ICIs, but no difference in PFS (HR 0.75, 95% CI 0.23-2.51) or OS (HR 0.91, 95% CI 0.29-2.83) was detected between these groups (Figure 1E-F).
Finally, 37 patients were noted to have intracranial metastases, a finding traditionally associated with poor patient survival 29. Of these patients, 8 were taking beta blockers concomitantly with their cancer treatment. Patients on beta blocker therapy did not have statistically significant improvement in PFS (HR 0.46, 95% CI 0.19-1.10) but did have improved OS (HR 0.10, 95% CI 0.04-0.30, Figure 1G-H).
Use of other stress-related medications
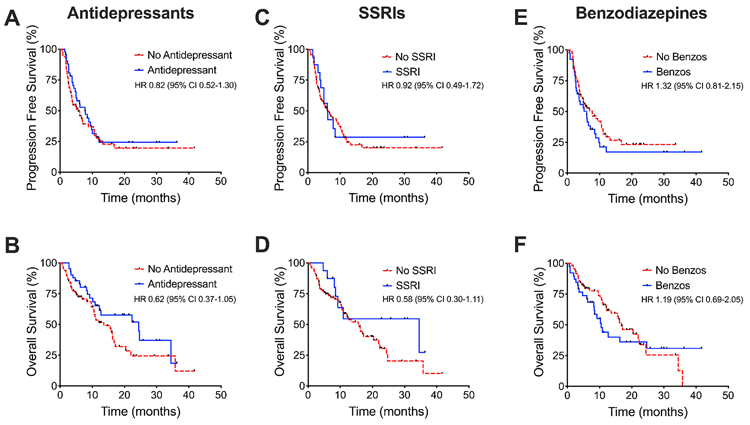
Patients were also assessed for concomitant use of other medications that may assess the stress response. These included antidepressant medications, which were defined as selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and the atypical antidepressants bupropion and mirtazapine. Antidepressant medications were utilized by 38.5% of patients and SSRIs were prescribed to 14.7% of patients. In addition, 35.8% of patients used benzodiazepines.
With all these drug classes, no statistically significant difference in PFS or OS was found between patients taking or not taking these medications (Figure 2). Patients on antidepressants did not exhibit difference in PFS (HR 0.82, 95% CI 0.52-1.30) or OS (HR 0.62, 95% CI 0.37-1.05). Similar results were seen with SSRIs in regards to PFS (HR 0.92, 95% CI 0.49-1.72) and OS (HR 0.58, 95% CI 0.30-1.11). Finally, benzodiazepine use was associated with non-significant signals towards worse PFS (HR 1.32, 95% CI 0.81-2.15) and OS (HR 1.19, 95% CI 0.69-2.05).
Figure 2. Survival outcomes in association with other stress-related medications.
Kaplan Meier plots showing no differences in (A) PFS or (B) OS between patients taking and not taking antidepressant medications. Similar results were obtained when categorizing by (C-D) selective serotonin reuptake inhibitors use and (E-F) benzodiazepine use.
Clinical covariates
Finally, a univariate Cox proportional hazards regression model was applied to relevant clinical variables to determine their association with progression-free survival. Beta blocker use was again shown to have a statistically significant association with prolonged PFS (p = 0.046, Table 2). In addition, non-squamous histology (p = 0.006), tumor PD-L1 positivity (p = 0.017), and lower line of treatment (p < 0.001) were all associated with increased PFS.
Table 2.
| Univariate Cox | Multivariate Cox | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Beta blocker use (yes vs. no) | 0.58 | 0.33-0.99 | 0.046 | 0.48 | 0.23-1.01 | 0.054 |
| Age (continuous) | 0.99 | 0.97-1.01 | 0.360 | 0.98 | 0.98-1.02 | 0.195 |
| Sex (male vs. female) | 1.09 | 0.68-1.74 | 0.715 | 0.96 | 0.49-1.88 | 0.895 |
| BMI (continuous) | 0.96 | 0.92-0.99 | 0.027 | 0.97 | 0.93-1.02 | 0.263 |
| Smoking (former or current vs. never) | 0.94 | 0.54-1.64 | 0.833 | 0.85 | 0.39-1.82 | 0.670 |
| ECOG performance status (0/1 vs. 2/3) | 0.95 | 0.57-1.61 | 0.861 | 0.85 | 0.38-1.87 | 0.683 |
| CNS metastasis (yes vs. no) | 0.99 | 0.60-1.61 | 0.958 | 1.03 | 0.51-2.10 | 0.926 |
| Tumor PD-L1 status (positive vs. negative) | 0.45 | 0.23-0.87 | 0.017 | 0.57 | 0.26-1.26 | 0.166 |
| Line of treatment (1st vs. 2nd or higher) | 0.33 | 0.19-0.58 | <0.001 | 0.33 | 0.13-0.87 | 0.025 |
| Concurrent chemotherapy (yes vs. no) | 0.42 | 0.15-1.15 | 0.092 | 0.40 | 0.08-2.04 | 0.270 |
Discussion
We sought to investigate the relationship between beta blocker use and clinical outcomes with immune checkpoint inhibitors in NSCLC. In a retrospective study, we found that treatment with beta blockers was associated with improved progression-free survival in these patients. To our knowledge, this is the first study to demonstrate a benefit in progression-free survival with combined beta blockers and ICIs in NSCLC.
These findings are consistent with a growing body of evidence suggesting that beta adrenergic signaling plays an important role in tumorigenesis, cancer progression, and tumor adaptations to immune surveillance. At the cellular level, beta-ARs can stimulate the mitogen-activated protein kinase (MAPK) pathway and downstream proliferative signals30, and also activate cyclic AMP and protein kinase A (PKA), which in turn leads to enhanced expression of vascular endothelial growth factor and immune checkpoints31. In addition, beta-ARs have been shown to regulate immune responses, including by suppressing activity of CD4+ and CD8+ T lymphocytes32,33. Stress in general has been shown exert a negative effect on antitumor activity23. However, other medications related to psychologic and physiologic stress, such as antidepressants and benzodiazepines, were not associated with improved survival. This result implies that the benefit seen with beta blockers is mediated directly through adrenergic receptors, rather than through a broader effect on patient stress.
The beta blocker and non-beta blocker groups were overall well-matched in terms of demographics. The only statistically significant difference was in age, with patients taking beta blockers being older on average. This age discrepancy would if anything be expected to negatively skew outcomes in this group34, making it possible that our results underestimate the impact of beta blockers. Conversely, we note that patients taking beta blockers had a higher rate of receiving ICIs as first line therapy (39% vs 30%), potentially biasing results in favor of the beta blocker group. Multiple studies have shown that PFS is markedly longer when ICIs are used in the first line as opposed to second line setting35,36. However, the difference in first-line use was not statistically significant in our analysis, and there was no difference in the use of concurrent chemotherapy.
Due to a low sample size, we were unable to assess for difference in responses between non-selective and selective beta blockers. Though conflicting results have been published in the literature, most prior studies have demonstrated a greater anti-tumor effect with non-selective beta blockers5,8. These agents, such as propranolol and carvedilol (which also has alpha-adrenergic antagonism), have the benefit of acting against both beta-1 and beta-2 ARs. Several preclinical studies have suggested that the beta-2 AR is primarily responsible for the deleterious effects of stress hormones. Given that both beta-1 and beta-2 AR are expressed in lung cancer tissue13, and that even selective beta blockers have some affinity for beta-2 ARs, it is possible that any beta blocker may be sufficient to exert the desired effect. We also speculate that the clinical benefit we observed may have been greater if a higher proportion of patients were taking non-selective beta blockers.
Both increased stress and adrenergic signaling have specifically been shown to facilitate cancer metastasis, especially to the brain37. Meanwhile, brain metastases have been shown to portend particularly poor prognosis in patients with NSCLC38. We thus examined the effect of incidental beta blocker use in this population. Our analysis was limited by low sample size, but it interestingly demonstrated that beta blockers conferred a strong overall survival benefit, a finding that merits further investigation.
Limitations of our study include the retrospective design and the relatively modest sample size. The multivariate analysis was further limited by unavailability of PD-L1 tumor expression data for a significant minority of patients. Both of these factors may explain the lack of factors, including beta blocker use, that were predictive in our multivariate model. Given the importance of PD-L1 as a biomarker for ICIs, we did not feel it would be useful to omit this variable from our analysis. This cohort also had a high percentage of patients who were administered immune checkpoint inhibitors as second- or later-line treatment. It is unclear if results would be different in a cohort reflecting current guidelines, in which more patients would receive ICIs with or without chemotherapy as first-line therapy.
Application of these findings could potentially be quickly implemented and cost-effective, given the extensive prior experience with beta blockers for other indications. Clinical trials are already underway evaluating the benefit of adjunctive beta blockers with ICIs in melanoma39, and should be pursued in non-small cell lung cancer. If validated in prospective studies, beta blockers could prove to be an important tool in abetting the effect of immunotherapy.
Clinical Practice Points.
Previous investigations have demonstrated that the stress response can adversely affect the anti-tumor immune response.
Beta blockers can blunt stress signaling and have been shown to affect multiple cancer-related processes in preclinical models.
Several retrospective studies have suggested a positive benefit of beta blockers in cancer patients, including those with melanoma treated with immunotherapy. However, the impact of beta blocker usage has not been significantly explored in lung cancer.
We performed a retrospective cohort study of 109 patients with non-small cell lung cancer who were treated with immune checkpoint inhibitors.
We found that concomitant use of beta blockers was associated with improved progression-free survival in this cohort, with a hazard ratio of 0.58.
This effect was seen with beta blockers but not with other medications associated with the stress response.
Given the widespread use of beta blockers and their general tolerability, the potential ability of beta blockers to abet the efficacy of immunotherapy could represent a cost-effective and easily implementable therapeutic tool.
The potential antitumor effects of beta blockers should be validated prospectively in clinical trials.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare they have no conflicts of interest.
References
- 1.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006;6:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol 2010;6:1863–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 2012;18:1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemeshow S, Sorensen HT, Phillips G, et al. beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2011;20:2273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol 2011;29:2635–44. [DOI] [PubMed] [Google Scholar]
- 6.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011;29:2645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powe DG, Voss MJ, Zanker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010;1:628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins JL, Thaker PH, Nick AM, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 2015;121:3444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen L, Hoffmeister M, Arndt V, Chang-Claude J, Brenner H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer 2014;120:1178–86. [DOI] [PubMed] [Google Scholar]
- 10.McCourt C, Coleman HG, Murray LJ, et al. Beta-blocker usage after malignant melanoma diagnosis and survival: a population-based nested case-control study. Br J Dermatol 2014;170:930–8. [DOI] [PubMed] [Google Scholar]
- 11.Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol 2011;72:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 2013;24:1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson MB, Sun H, Diao L, et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: Implications for combinations with beta-blockers. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielecka AM, Obuchowicz E. Antidepressant drugs as a complementary therapeutic strategy in cancer. Exp Biol Med (Maywood) 2013;238:849–58. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Tamim H, Shapiro S, Stang MR, Collet JP. Use of antidepressants and risk of colorectal cancer: a nested case-control study. Lancet Oncol 2006;7:301–8. [DOI] [PubMed] [Google Scholar]
- 16.Christensen DK, Armaiz-Pena GN, Ramirez E, et al. SSRI use and clinical outcomes in epithelial ovarian cancer. Oncotarget 2016;7:33179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coogan PF, Palmer JR, Strom BL, Rosenberg L. Use of selective serotonin reuptake inhibitors and the risk of breast cancer. Am J Epidemiol 2005;162:835–8. [DOI] [PubMed] [Google Scholar]
- 18.Abdel Karim NF, Hassan R, Siddiqi NI, et al. Impact of tricyclic antidepressants, selective serotonin reuptake inhibitors, and other antidepressants on overall survival of patients with advanced lung cancer from 2004 to 2014: University of Cincinnati experience. J Int Med Res 2019;47:6016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slota C, Shi A, Chen G, Bevans M, Weng NP. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun 2015;46:168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guereschi MG, Araujo LP, Maricato JT, et al. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur J Immunol 2013;43:1001–12. [DOI] [PubMed] [Google Scholar]
- 22.Jin J, Wang X, Wang Q, et al. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One 2013;8:e74497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004;5:617–25. [DOI] [PubMed] [Google Scholar]
- 24.Jean Wrobel L, Bod L, Lengagne R, Kato M, Prevost-Blondel A, Le Gal FA. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget 2016;7:77825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bucsek MJ, Qiao G, MacDonald CR, et al. beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res 2017;77:5639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kokolus KM, Zhang Y, Sivik JM, et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2018;7:e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. The Lancet Oncology 2017;18:e143–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2014. at http://www.R-project.org/.)
- 29.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D, Ma Q, Wang Z, et al. beta2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFkappaB pathway. Mol Cancer 2011;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006;12:939–44. [DOI] [PubMed] [Google Scholar]
- 32.Qiao G, Chen M, Bucsek MJ, Repasky EA, Hylander BL. Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response. Front Immunol 2018;9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahle M, Stachetzki U, Krause A, Pierer M, Hantzschel H, Baerwald CG. Regulation of beta2-adrenergic receptors on CD4 and CD8 positive lymphocytes by cytokines in vitro. Cytokine 2001;16:205–9. [DOI] [PubMed] [Google Scholar]
- 34.Tas F, Ciftci R, Kilic L, Karabulut S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett 2013;6:1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- 36.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 37.Choy C, Raytis JL, Smith DD, et al. Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative beta-blockade. Oncol Rep 2016;35:3135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Current Oncology 2013;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Propranolol Hydrochloride and Pembrolizumab in Treating Patients With Stage IIIC-IV Melanoma That Cannot Be Removed by Surgery. 2017. at https://clinicaltrials.gov/ct2/show/NCT03384836.)