Abstract
Background:
Anhedonic symptoms of posttraumatic stress disorder (PTSD) reflect deficits in reward processing that have significant functional consequences. Although recent evidence suggests that disrupted integrity of fronto-limbic circuitry is related to PTSD development, including anhedonic PTSD symptoms (post-trauma anhedonia; PTA), little is known about potential structural biomarkers of long-term PTA as well as structural changes in fronto-limbic pathways associated with recovery from PTA over time.
Methods:
We investigated associations between white matter microstructure, gray matter volume, and PTA in 75 recently traumatized individuals, with a subset of participants (n = 35) completing follow-up assessment 12 months after trauma exposure. Deterministic tractography and voxel-based morphometry were used to assess changes in white and gray matter structure associated with changes in PTA.
Results:
Reduced fractional anisotropy (FA) of the uncinate fasciculus at around the time of trauma predicted greater PTA at 12 months post-trauma. Further, increased FA of the fornix over time was associated with lower PTA between 1- and 12-months post-trauma. Increased gray matter volume of the ventromedial PFC and precuneus over time was also associated with reduced PTA.
Conclusions:
Microstructure of the uncinate fasciculus, an amygdala-prefrontal white matter connection, may represent a biomarker of vulnerability for later PTA. Conversely, development and recovery from PTA appears to be facilitated by white and gray matter structural changes in a major hippocampal pathway, the fornix. The present findings shed new light on neuroanatomical substrates of recovery from PTA and characterize white matter biomarkers of risk for posttraumatic dysfunction.
Keywords: Anhedonia, Trauma, Tractography, Prospective, MRI
Introduction
Anhedonia, or an inability to experience pleasure, emerges in a substantial number of trauma victims. In the immediate aftermath of trauma exposure, post-trauma anhedonia (PTA) symptoms are potent predictors of posttraumatic stress disorder (PTSD) development (Feeny, Zoellner, Fitzgibbons, & Foa, 2000; Malta, Wyka, Giosan, Jayasinghe, & Difede, 2009). Emerging evidence suggests anhedonia is a unique PTSD symptom dimension, as identified in factor analytic studies of PTSD symptom expression (Liu et al., 2014; Pietrzak et al., 2015; Yang et al., 2017). These symptoms are orthogonal to the fear and hyperarousal aspects of PTSD (Armour, 2015; Breslau, Reboussin, Anthony, & Storr, 2005; Byrne, Harpaz-Rotem, Tsai, Southwick, & Pietrzak, 2019; Pietrzak, Goldstein, Southwick, & Grant, 2011; Yang et al., 2017) and are reflective of deficits in reward-related processes. PTA and more general reward-processing deficits have significant functional consequences, such as increased risk of substance use (Debell et al., 2014; Fani et al., 2020; Nickerson et al., 2014) and suicidality (Spitzer, Zuromski, Davis, Witte, & Weathers, 2018). Importantly, a majority of individuals show a general recovery from PTA over time, suggesting potential inherent mechanisms of resilience to chronic reward-processing deficits in response to psychological stress. Although a number of prospective neuroimaging studies of trauma have investigated potential neuromarkers of future PTSD diagnoses, few studies have investigated how changes in brain structure are associated with trauma recovery. Characterization of the neural substrates of recovery from trauma, specifically changes in PTA, could inform identification of neurobiological targets for early intervention strategies. Therefore, the present study investigated changes in brain structure associated with changes in PTA symptoms to shed light on the neural mechanisms that underlie risk and recovery from posttraumatic symptoms, and provide a fuller understanding of the neural etiology of trauma and stress-related disorders.
Prior work has demonstrated that PTSD is associated with alterations in white matter pathways that interconnects neural circuitry critical for fear- and reward-related processes that are dysfunctional in PTSD (Fenster, Lebois, Ressler, & Suh, 2018; Harnett, Goodman, & Knight, 2020). Specifically, fractional anisotropy (FA) of the uncinate fasciculus (amygdala to ventromedial prefrontal cortex; PFC) and cingulum bundle (PFC to posterior cingulate and hippocampus) is reduced in individuals with PTSD compared to non-PTSD controls (Costanzo et al., 2016; Fani et al., 2016; Koch et al., 2017; Olson et al., 2017; Sanjuan, Thoma, Claus, Mays, & Caprihan, 2013). In addition, the fornix (which connects the hippocampus to deep-brain nuclei), may also be involved in PTSD; prior research has demonstrated increased fornix and stria terminalis FA over time within treatment-resistant PTSD patients (Kennis et al., 2015) and fornix FA has been found to be positively related to trait anxiety (Modi et al., 2013). Recent research on white matter predictors of PTSD demonstrated the reduced microstructural integrity of the uncinate fasciculus and dorsal cingulum bundle, but greater integrity of the fornix/stria terminalis, are associated with greater acute and long-term PTSD symptom severity (Harnett, Ference, Knight, & Knight, 2018). Thus white matter microstructure of these pathways may play a role in the development and expression of posttraumatic symptoms.
Despite growing interest in characterizing white matter decrements that are associated with PTSD, surprisingly little prior research has linked microstructure of these tracts to PTA. In a previous report, we demonstrated that the presence of PTA, up to six months after a traumatic event, was predicted by reduced FA of the uncinate fasciculus (Fani et al., 2019). To our knowledge, this is the only study to directly investigate the relationship of white matter microstructure, including fear- and reward-related circuitry, with PTA. Research in other populations and with other modalities, however, suggest anhedonia symptoms may also be related to white matter pathways such as the cingulum and fornix. A recent study found that FA of the dorsal cingulum was negatively related to anhedonic symptoms in individuals with depression (Coloigner et al., 2019). Similarly, prior studies have suggested a role for the fornix in reward processing (Brown & Winocur, 1973; LeGates et al., 2018). The fornix provides a connection between the nucleus accumbens and hippocampus, regions that are involved with reward-related behaviors. Acute or prolonged stress/trauma can affect the strength of these connections. Thus, the fornix may play a key role in PTA within humans; however, limited work to date has assessed the microstructure of these tracts in humans in relation to PTSD. Taken together, the integrity of these white matter pathways may be critical to the development of PTA following a traumatic event.
A key gap in our understanding of the neurostructural correlates of PTA is the lack of longitudinal data assessing both changes in PTA and how these changes correspond with change in white matter pathways over time. In the aftermath of trauma, posttraumatic symptoms show changes in severity over time; these changes can be related to psychological recovery or deterioration (Bonanno & Mancini, 2012). However, no prior study to our knowledge has investigated how changes in white matter microstructure correspond with long-term changes in posttraumatic symptoms such as PTA. Identifying white matter correlates of recovery from PTA and PTSD symptoms would provide quantifiable neural targets for current treatment approaches. Given the prior findings implicating the uncinate fasciculus, cingulum bundle, and fornix in PTSD, it may be that changes in the microstructure of these tracts over time is associated with recovery from PTA or other posttraumatic symptoms.
In the present study, we sought to investigate white matter correlates of PTA risk and recovery. To assess white matter markers of PTA risk, we examined the relationship between microstructure of select white matter tracts of interest (quantified via FA) in the acute aftermath of trauma and 12-months post-trauma and its relationship with both acute (~1-month) and future (12-month) PTA. Given our prior work (Fani et al., 2019; Harnett et al., 2018), we hypothesized that FA of uncinate fasciculus and dorsal cingulum would be negatively associated with 12-month PTA. We investigated white matter markers of PTA recovery by assessing relationships between the change in white matter FA and PTA over time (from 1- to 12- months post-trauma). We conducted secondary analyses of gray matter morphometry data to further characterize brain structure changes associated with PTA. Finally, we completed exploratory follow-up analyses to assess whether the observed relationships with PTA were distinct from other posttraumatic symptom clusters or otherwise associated with trait-like anhedonia.
Methods and Materials
An initial sample of 83 participants were recruited from the emergency department (ED) at Grady Memorial Hospital as part of a larger study investigating biological markers of PTSD development following trauma. Once patients signed informed consent, trained assessors collected demographic information and assessments of prior trauma, substance abuse, depression and PTSD symptoms, as well as details concerning the presenting trauma. Participants were queried about past and current medical conditions and medications. Participants who had experienced a DSM-IV criterion A trauma in the past 24 hours were eligible for the study but were not included if they had current suicidal ideation or attempt in the last 3 months, MRI contraindications (e.g., metal or implanted devices, current pregnancy), prior hospitalization for mental health conditions, loss of consciousness as a result of the trauma and/or current intoxication, or otherwise altered mental status. Participants were also assessed for traumatic brain injury by ED physicians using the Glasgow Coma Scale, and patients with scores of less than 15 were excluded from the study.
Given the present univariate analyses were focused on white matter microstructural changes, we excluded participants based on outliers for diffusion weighted imaging (DWI) property outliers. Eight participants were excluded from initial analyses due to outlier values in the DWI data. Therefore, 75 participants were included in initial analyses (Age: M = 35.24, SD = 12.53; 45 Males). A subset (n = 28) of participants in the present study were also included in a previous report of white matter predictors of PTA (Fani et al., 2019). The prior report found that acutely assessed FA of the uncinate fasciculus was a prospective marker of 6-month PTA. In the current study, we utilized multi-scanner data collection (see Magnetic Resonance Imaging below) with a significantly increased sample size and assessment of PTA at a later timepoint (i.e., 12-months post-trauma). We also utilized a different analytic method to derive and analyze the DTI data, leveraging recent developments in image processing and deterministic tractography. Further, we assessed longitudinal changes in both white and gray matter structure, unlike our previous study.
Traumas experienced by participants at the time of assessment were primarily motor vehicle and motorcycle accidents (n = 48); other traumas included pedestrians versus automobile incidents (n = 10) and industrial or home accidents, bicycle accidents, non-sexual assault, stabbing, gunshot wound, animal attack, and sexual assault (n =17). In total, 30 participants met diagnostic criteria for PTSD at the 1-month assessment (40%). In addition, a subset of 35 participants’ data were available from a follow-up MRI obtained twelve months after trauma who had complete corresponding psychometric data (see Table 1 for clinical and demographic information). Nine participants included in the follow-up met diagnostic criteria for PTSD at the 12-month follow-up (26%). All participants provided informed consent as approved by the Emory University Institutional Review Boards and Grady Memorial Hospital Research Oversight Committee.
Table 1.
Demographic and clinical information
| Mean (SD) or Count (%) | |
|---|---|
| Age | 35.24 (12.53) |
| Gender | 45 (60%) M |
| 30 (40%) F | |
| Race | |
| Black | 55 (73.3%) |
| White | 13 (17.3%) |
| Mixed-race | 4 (5.3%) |
| Other | 3 (4%) |
| Clinical Characteristics 1 month post-trauma (n=75) |
|
| mPSS total | 15.35 (11.22) |
| ⌊Anhedonia | 1.97 (2.38) |
| ⌊Re-experiencing | 4.19 (3.74) |
| ⌊Avoidance | 3.51 (3.19) |
|
⌊Arousal 12 months post-trauma (n = 62) |
5.68 (3.94) |
| mPSS total | 8.60 (10.24) |
| ⌊Anhedonia | 1.10 (2.06) |
| ⌊Re-experiencing scores | 1.81 (3.01) |
| ⌊Avoidance scores | 2.11 (2.76) |
| ⌊Arousal scores | 3.58 (3.64) |
Note: mPSS = Modified PTSD Symptom Scale, M = Male, F = Female.
Psychometric assessment
Participants completed clinical assessments at both 1- and 12-months post-trauma as described in previous reports (Fani et al., 2019; Stevens et al., 2017; van Rooij et al., 2018). A modified version of the PTSD Symptom Scale (mPSS) was administered at these timepoints to assess the severity of posttraumatic symptom clusters, as defined in the DSM-IV (i.e., re-experiencing, avoidance/emotional numbing, and hyperarousal) (Falsetti, Resnick, Resick, & Kilpatrick, 1993; Foa & Tolin, 2000). For the present study, a PTA subscale was derived from the mPSS as previously described (Fani et al., 2019). Briefly, three items reflecting loss of interest, emotional detachment and restricted range of positive emotion were summed to derive a PTA symptom severity score. Prior factor analytic studies have repeatedly demonstrated that these three items cluster together and comprise an anhedonia dimension of PTSD (Kashdan, Elhai, & Frueh, 2006; Liu et al., 2014; Yang et al., 2017). Of note, in the DSM-IV, these three items are often combined with avoidance items to create a summed avoidance/emotional numbing subscale. We separated PTA items from avoidance to investigate these subscales separately. The Beck Depression Inventory-II (BDI-II), a self-report measure of depression symptoms in the past two weeks, was also administered at the 1 and 12 month timepoints (Beck, Steer, & Brown, 1996). Three items from the BDI-II scale were combined that reflected anhedonic symptoms, as indicated by findings of studies that demonstrate a two factor model fit (non-anhedonic vs anhedonic symptoms) with this measure (Joiner, Brown, & Metalsky, 2003; Leventhal, Chasson, Tapia, Miller, & Pettit, 2006). The derived anhedonia subscale of the BDI was used in secondary analyses. Finally, participants were also asked about whether they had sought psychiatric or psychological help as a result of their traumatic incident at the 1 and 12-month assessments. These data were used in supplementary analyses to determine the potential impact of treatment seeking behaviors on longitudinal changes in PTA in the present sample (see supplemental material).
Magnetic resonance imaging
The full details of the MRI data acquisition and processing are available in the supplemental materials. Briefly, diffusion weighted and T1-weighted images were collected from three 3T Siemens MRI systems (Table S1). Motion, eddy current, and susceptibility effects in the diffusion weighted data were reduced using a combination of FSL, ROBEX, and ANTs (Andersson & Sotiropoulos, 2016; Avants, Tustison, & Song, 2009; Iglesias, Liu, Thompson, & Tu, 2011; Smith et al., 2004; Wang et al., 2017; Zhang, Brady, & Smith, 2001). Q-space diffeomorphic reconstruction was completed using DSI Studio (August 2018 build) (Yeh & Tseng, 2011). Deterministic tractography in DSI studio was completed to reconstruct a priori tracts of interest from a group connectome of the 75 participants’ data from the 1-month assessment (Yeh, Verstynen, Wang, Fernández-Miranda, & Tseng, 2013). Specifically, the uncinate fasciculus, dorsal cingulum bundle, posterior (hippocampal) cingulum bundle, and fornix were reconstructed within the left and right hemisphere resulting in eight tracts bilaterally (Figure 1a). FA from these ROIs was extracted and averaged across hemispheres for statistical analyses.
Figure 1. White matter tract reconstruction and associations of white matter integrity with recovery from posttraumatic anhedonia.
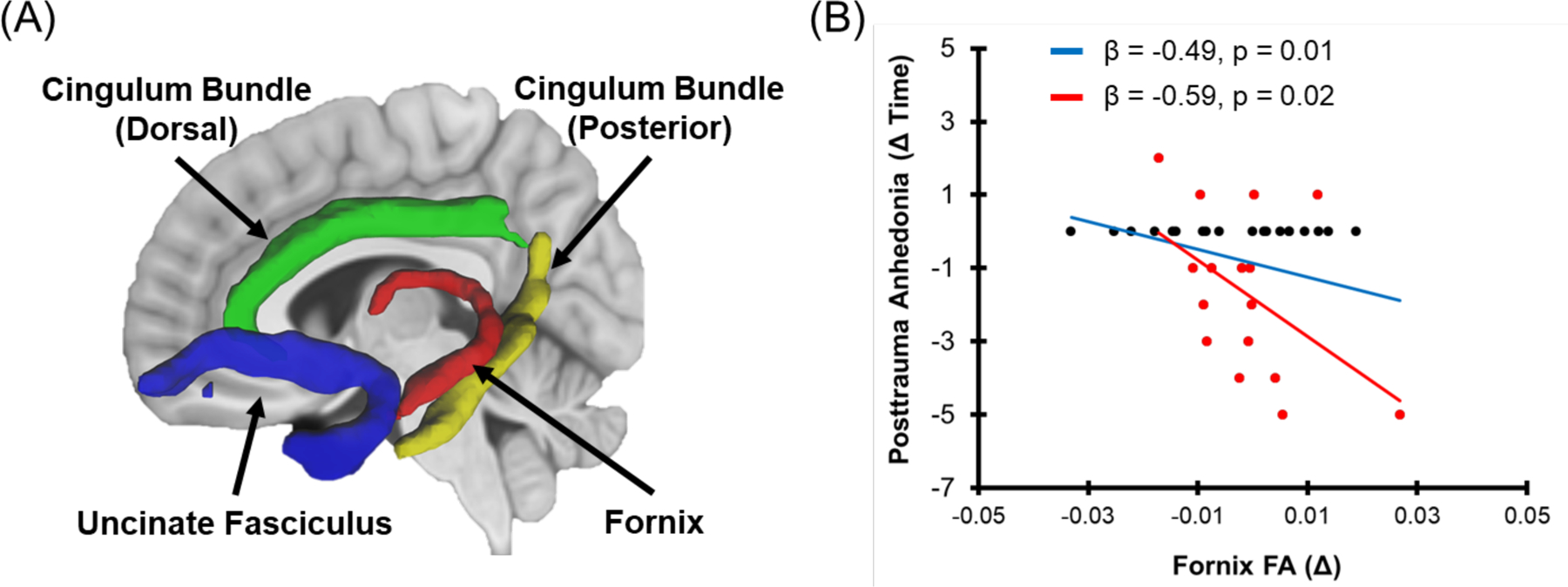
(A) Deterministic tractography was completed to reconstruct a priori tracts of interest (uncinate fasciculus – blue, dorsal cingulum bundle – green, posterior cingulum bundle – yellow, and fornix – red). (B) Changes in fractional anisotropy (FA) within the fornix were associated with recovery in posttraumatic anhedonia (data are 12-months minus 1-month). Individual dots represent participant scores for FA for the respective tracts and posttraumatic anhedonia and greater values represent relative increases from 1- to 12- month assessments. Red dots represent participants who showed a non-zero change in posttraumatic anhedonia from 1- to 12- months while black dots represent individuals who showed no change in posttraumatic anhedonia. The solid red line represents the linear line of best fit for participants who showed a non-zero change, while the solid blue line represents the linear line of best for all participants in the analysis.
As a follow-up to the white matter analyses, we completed a supplemental voxel based morphometry (VBM) analysis of the T1-weighted data using standard FSL routines (i.e., FSLVBM) (Andersson, Jenkinson, & Smith, 2007; Douaud et al., 2007; Good et al., 2001). 3dttest++ in the Analysis for Functional NeuroImages (AFNI) was completed using the 1- and 12- month VBM data with a continuous predictor variable for the change in posttraumatic anhedonia and covariates for scanner effects. To correct for voxel-wise multiple comparisons, 3dClustSim for permutation testing within 3dttest++ was used to determine the corresponding cluster extent (k = 400 voxels or 3200mm3) at a cluster forming threshold of p = 0.005 to maintain α = 0.05.
Statistical analyses
Statistical analyses were completed using IBM SPSS version 24 and AFNI (Cox, 1996). The present analyses were predominately focused on identifying neural markers of risk and recovery from PTA. Thus we completed three separate multiple regression models asses the relationship between FA of our a priori white matter tracts of interest and PTA. We first evaluated the relationships between FA of white matter tracts (assessed during the acute phase) and both 1- and 12-month PTA symptoms. Multiple linear regression analyses were completed that included dummy-coded covariates for scanner and FA of the white matter tracts (assessed ~1-month post-trauma) to assess the association between FA and both 1- and 12-month PTA (two separate models). We then assessed the relationship between changes in FA of white matter tracts and changes in PTA over time. A multiple linear regression was completed to test the relationship between changes in FA and PTA (between 1 and 12 months). Multiple linear regression analyses were completed that included dummy-coded covariates for scanner and the change in FA for each white matter tract associated with the change in PTA scores. A nominal p-value threshold of 0.05 was used to determine statistical significance for our a priori models. Based on the multiple linear regressions of change in FA and PTA over time, we conducted an exploratory VBM analysis (described above) to assess the relationship between changes in gray matter volume and PTA over time. We further completed separate exploratory regression analyses using BDI-II anhedonia scores to determine if observed relationships were specific to PTA or may be more related to trait anhedonia. We also completed similar exploratory regression analyses for comparison with other posttraumatic symptom clusters in the mPSS (i.e., re-experiencing, avoidance, arousal).
Results
White matter microstructural associations with 1- and 12-month PTA
Multiple regression analyses did not reveal a statistically significant relationship between FA of the tracts of interest and acute (i.e., 1-month) PTA (all ps > 0.05). Uncinate fasciculus FA was significantly negatively associated with 1-month re-experiencing symptoms (Table S2). Additionally, uncinate fasciculus FA varied significantly and negatively with 12-month PTA severity, consistent with our a priori hypothesis (Table 2). Follow-up regression analysis indicated no significant relationships between uncinate fasciculus FA and 12-month BDI-II anhedonia scores (Table S5).
Table 2.
White matter microstructural associations with PTA symptoms.
| 1-Month PTA | 12-Month PTA | Δ (12 – 1-Month) PTA | ||||
|---|---|---|---|---|---|---|
| F-statistic (p-value) | 0.92 (0.49) | 1.29 (0.28) | 2.61 (0.04) | |||
| R2 | 0.075 | 0.12 | 0.36 | |||
| Structurea | β | T-statistic (p-value) | β | T-statistic (p-value) | β | T-statistic (p-value) |
| Uncinate fasciculus | −0.23 | −1.31 (0.20) | −0.37 | −1.95 (0.03+) | 0.08 | 0.44 (0.66) |
| Dorsal cingulum bundle | −0.12 | −0.77 (0.44) | −0.08 | −0.46 (0.65) | −0.26 | −1.61 (0.12) |
| Posterior cingulum bundle | −0.15 | −0.85 (0.40) | −0.16 | −0.80 (0.43) | 0.33 | 1.88 (0.07) |
| Fornix/stria terminalis | 0.20 | 1.33 (0.19) | 0.26 | 1.58 (0.12) | −0.49 | −2.72 (0.01) |
Note: PTA = Posttrauma anhedonia. Bold values indicate significant at p < 0.05.
1-tailed significance test was used.
For analyses of 1- and 12- month PTA, FA for each tract assessed at 1-month after trauma was the predictor variable. For analyses of the change in PTA, the change in FA for each tract was the predictor.
White and grey matter changes associated with recovery from posttrauma anhedonia and other PTSD symptoms.
Regression analyses indicated that changes in fornix FA varied negatively with changes in PTA between 1- and 12-months (β = −0.49, p = 0.01) (Figure 1b; Table 2) but no relationship was observed with the change in BDI-II anhedonia scores (Table S5) or with PTSD avoidance symptoms (Table S3). Changes in dorsal cingulum bundle FA showed a significant negative relationship with changes in hyperarousal symptoms (Table S4).
As a follow-up to the white matter analyses, we also assessed gray matter volume changes associated with changes in PTA using VBM. Increases in gray matter volume within the vmPFC and the precuneus were significantly associated with reductions in PTA (β = −0.72 and −0.57 respectively) (Tables S6–S7; Figure 2). The change in gray matter volume within the precuneus was also related to changes in re-experiencing symptoms such that increased precuneus gray matter volume over time was associated with reduced PTA over time (Table S7). Gray matter volume changes within the vmPFC [t(31) = −0.91, β = −0.16, p = 0.37] or precuneus [t(31) = −0.99, β = −0.17, p = 0.33] were not associated with the change in BDI-II anhedonia scores.
Figure 2. Gray matter volume (GMV) changes associated with recovery in posttraumatic anhedonia.
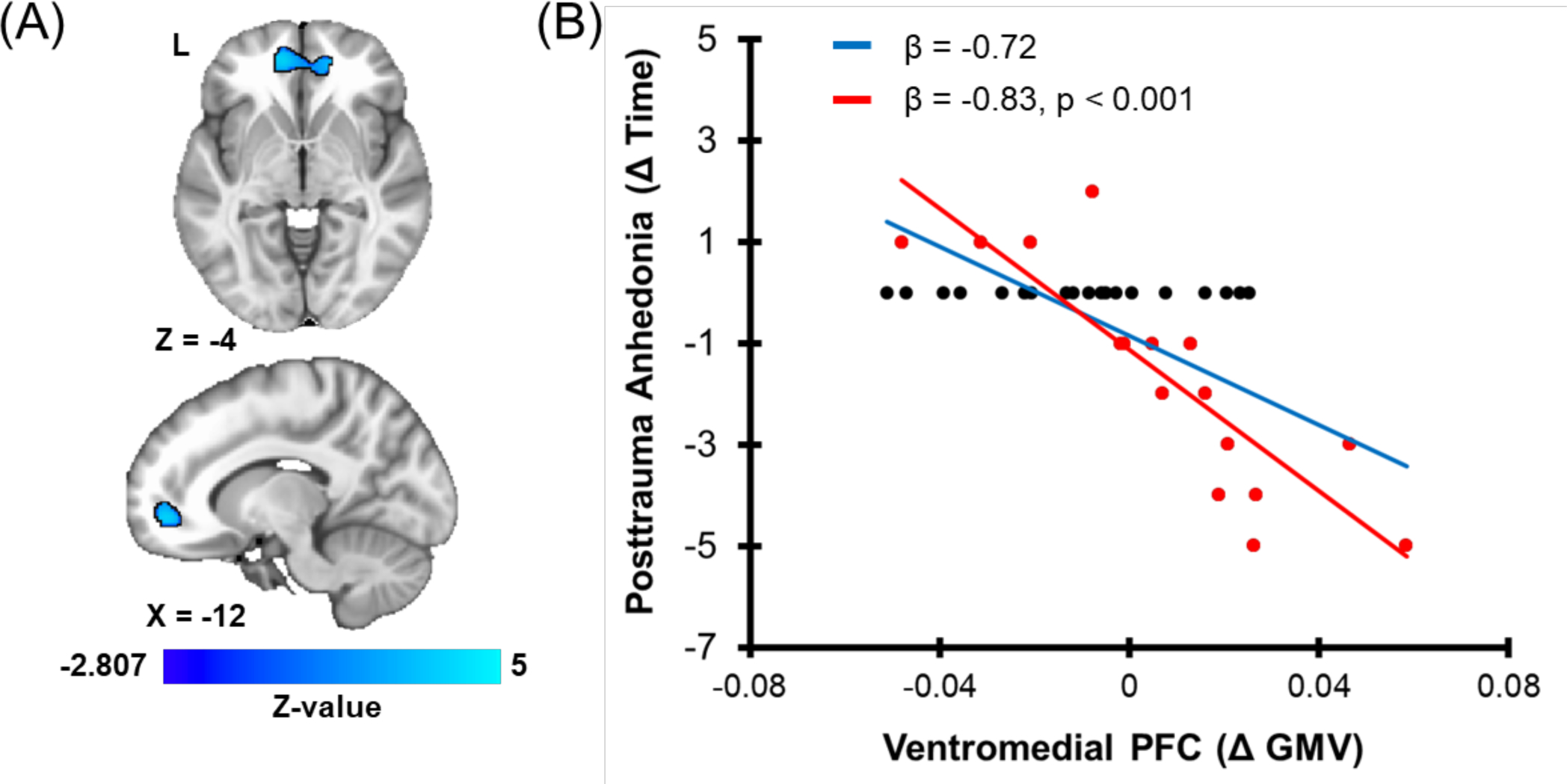
Individual dots represent participant scores for GMV for the cluster in the ventromedial prefrontal cortex (PFC) and posttraumatic anhedonia (data are 12-months minus 1-month). Greater values represent relative increases from 1- to 12- month assessments. Red dots represent participants who showed a non-zero change in posttraumatic anhedonia from 1 to 12 months while black dots represent individuals who showed no change in posttraumatic anhedonia. The solid red line represents the linear line of best fit for participants who showed a non-zero change, while the solid blue line represents the linear line of best for all participants in the analysis.
Subgroup analyses
Of the 35 participants included in the prior analyses, 19 participants (54%) showed no change in PTA. We therefore completed supplementary regression analyses for the fornix, vmPFC, and precuneus to determine if the same effects were observed only in individuals that showed a change in PTA. Regression analyses included the brain region and a dummy-coded covariate for scanner (three separate models). PTA varied significantly with changes in FA of the fornix [t(13) = −2.70, β = −0.59, p = 0.02] and gray matter volume of both the vmPFC [t(13) = −5.73, β = −0.83, p < 0.001] and precuneus [t(13) = −2.81, β = −0.59, p = 0.02] clusters (Figures 1 and 2).
We also tested for potential gender differences in the FA or gray matter volume of the assessed brain regions with a series of independent-samples t-tests. We did not observe any gender differences in FA of the white matter tracts (all ps > 0.05). A trend-level difference in the change in gray matter volume of the vmPFC over time was observed [t(33) = 1.88, p = 0.07] such that men showed greater decreases (M = −0.01, SD = 0.03), while women showed greater increases (M = 0.01, SD = 0.02), in vmPFC volume.
Discussion
The present prospective study investigated neural correlates of risk and recovery from PTA in a sample of recently traumatized individuals recruited from the ED. We investigated the relationship between white matter microstructure within weeks of trauma exposure and long-term (12-months post-trauma) PTA symptoms. We further investigated the relationship between changes in PTA symptoms and in white matter microstructure over time. We conducted secondary analyses of gray matter volume to further characterize the structural correlates of recovery from PTA with a multi-modal approach. Acutely assessed (i.e., ~1-month post-trauma) FA of the uncinate fasciculus was negatively associated with future (i.e., 12-month) PTA symptoms. Further, increased FA of the fornix between the ~1-month and 12-month post-trauma MRI sessions was associated with reductions in PTA between the 1 and 12-month assessments. Secondary analyses of gray matter structural MRI data revealed that increases in gray matter volume of the vmPFC were also associated with reductions in PTA between the 1 and 12-month assessments. Notably, none of the present findings were also associated with hedonic capacity/trait-like anhedonia, as assessed with the BDI-II. The current results demonstrate that PTA, which reflects emotional numbing and social reward deficits that emerge following trauma, has important structural correlates, and that changes in brain structure may partially underlie recovery from PTA.
Consistent with our prior reports (Fani et al., 2019; Harnett et al., 2018), lower FA of the uncinate fasciculus was associated with greater acute (~1-month post-trauma) re-experiencing symptoms and future (i.e., 12-months post-trauma) anhedonia symptoms. Importantly, changes in FA of the fornix were uniquely associated with changes in PTA symptoms over time. Changes in gray matter volume of the vmPFC and precuneus were also associated with changes in PTA. The present findings suggest that microstructure of uncinate fasciculus is predictive of future development of PTA symptoms. Further, recovery from PTA appears to be partially due to changes in the fornix, a major hippocampal structural pathway. These data are also largely in line with prior prospective investigations of PTSD which suggest that pre- and early peri-traumatic amygdala – ventromedial PFC circuitry, and changes in hippocampal circuitry over time, contribute to the development of posttraumatic dysfunction (Admon et al., 2013, 2009; Stevens et al., 2017). The findings provide novel insight into structural brain changes related to changes in PTSD symptoms.
In the present sample, decreases in PTA between 1- and 12- months corresponded with increases in microstructure (i.e., FA) of the fornix. The fornix is a white matter pathway that connects the hippocampus to other subcortical structures, such as the hypothalamus and nucleus accumbens (Kelley & Domesick, 1982). The hippocampus is critical for the encoding of contextual and affective information particularly for fear learning processes (Corcoran, 2005; Harnett et al., 2016; Phillips & LeDoux, 1992). Further, the nucleus accumbens is known to play a critical role in the motivation, anticipation, and pleasurable experience of reward (Ikemoto & Panksepp, 1999; Knutson, Adams, Fong, & Hommer, 2001; Sabatinelli, Bradley, Lang, Costa, & Versace, 2007). Given the fornix connects these brain regions, the fornix may be a critical pathway for hippocampal-accumbens dependent reward learning and processing. In line with this view, prior research in animal models demonstrates that lesions of the fornix disrupt reward learning, but these behaviors may be rescued with intracranial self-stimulation (Yoganarasimha & Meti, 1999). Further, interactions between the hippocampus and accumbens appear to underlie the formation of appetitive memories (Trouche et al., 2019). Taken together, the previous literature suggests that reduced structural connectivity (e.g., reduced fornix integrity) between the hippocampus and nucleus accumbens may contribute to a decreased ability to properly encode or retrieve the potential pleasure of stimuli and future events. The present findings suggest that strengthening of subcortical pathways between the hippocampus and deep-brain nuclei may reflect changes in reward-related processes, that in turn contribute to reductions in PTA over time.
PTA symptom recovery (i.e., decreased symptoms between 1- and 12-months) was also associated with increases in gray matter volume of both the vmPFC and precuneus. The vmPFC has previously been implicated in PTSD given its role in regulation of the amygdala-mediated expression of emotional responses and the observed dysfunction in vmPFC activity in PTSD patients (Hayes, Hayes, & Mikedis, 2012; Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015). Importantly, the vmPFC also plays a role in the assessment of reward value and decision-making in addition to its role in emotion regulation (Hiser & Koenigs, 2018). However, limited research has investigated how changes in vmPFC structure over time are related to changes in PTSD symptoms or reward processing. A prior study found that changes in the orbitofrontal cortex, proximal to the vmPFC cluster in the present report, was related to changes in PTSD severity (Sekiguchi et al., 2013), such that reductions in volume were associated with greater PTSD severity. It may be that increased synaptogenesis and dendritic branching, which have been associated with increased VBM (Keifer et al., 2015), underlies our finding of increased volume in the vmPFC over time. We propose that these structural findings represent increased neural plasticity in the vmPFC over time, which may facilitate greater assessment of reward values, and may contribute to a reversal of reward-processing deficits and a decrease in PTA.
Our data also demonstrate the relevance of uncinate fasciculus microstructure in predicting future posttraumatic symptoms. The present analyses build upon previous findings examining acutely assessed white matter microstructure associations with PTA and other PTSD symptoms. First, we replicated a prior finding and observed that uncinate fasciculus FA was negatively correlated with acute (i.e., 1-month) re-experiencing symptoms (Harnett et al., 2018). The convergence of these findings in two independent samples suggest that re-experiencing of a recent traumatic experience may be partially dependent on structural connectivity of the vmPFC and amygdala. Second, we extended previous findings indicating that uncinate fasciculus FA was predictive of 6 month PTA symptoms (Fani et al., 2019), showing that reduced FA of this tract was predictive of more chronic (12 month) PTA symptoms. While the initial report utilized diffusion tensor imaging (DTI) and probabilistic tractography, the present report utilized an updated processing pipeline combined with a model-free reconstruction and deterministic tractography approach to the diffusion weighted data that may outperform these other methods in some respects (Maier-Hein et al., 2017; Yeh et al., 2013). By pooling data across scanners, we further demonstrated the robustness of the predictive utility of uncinate fasciculus FA for long-term PTA with respect to both acquisition and processing method from the present sample. Several reports have found uncinate fasciculus microstructure to vary with traditional PTSD symptom clusters (Costanzo et al., 2016; Harnett et al., 2018; Koch et al., 2017; Olson et al., 2017; Santhanam et al., 2019), although to date only one prior study has investigated its role in PTA symptoms (Fani et al., 2019). Further, few studies have utilized a longitudinal design to identify whether white matter microstructure assessed acutely post-trauma is predictive of future PTSD symptoms. Prior research found reduced uncinate fasciculus FA in individuals that developed PTSD compared to those that did not following trauma exposure (Sun et al., 2013). Consistent with the prior research, we found that acutely assessed FA of the uncinate fasciculus was related to anhedonic symptoms at 12 months post-trauma. Considered in the context of prior literature, our findings support the potential for acutely assessed white matter microstructure as an informative biological marker of later posttraumatic dysfunction.
The present findings should be interpreted in light of several limitations. A portion of the present sample did not exhibit PTA and did not change in PTA over time. Although we performed follow-up regression analyses on only individuals that showed any change in PTA, these analyses reduced the sample size. Further, the range of variability in PTA may be impacted by our measure of PTA in the present study, given that it was derived from the avoidance/numbing subscale of the mPSS. More detailed measures of PTA may provide for finer resolution of the associated variability between PTA and brain structure. Another limitation to consider is the present sample comprised individuals presenting to an emergency department, most of whom were motor vehicle crash survivors. A wider range of trauma types (e.g., military combat or exposure to war-zones) should be included in future studies to assess the generalizability of these findings. Finally, follow-up data was only available for a small subset of participants one year following trauma exposure, and it would also be useful to assess participants beyond 12-months post-trauma. Specifically, although we observed changes in brain structure over time, it remains unclear if the observed changes in brain structure are persistent (e.g., last beyond 12 months).
In conclusion, we investigated white matter predictors of PTA and white and gray matter correlates of change in PTA symptoms over time. We found that reduced uncinate fasciculus FA was associated with greater acute re-experiencing and future anhedonia symptoms. Increases in FA of the fornix uniquely mirrored recovery in PTA over time, and increases in gray matter volume of the vmPFC and precuneus were further associated with recovery in PTA severity. Our data reveal a promising neural marker of future posttraumatic dysfunction and also indicate that hippocampal-accumbens structural pathways may partially underlie general recovery from anhedonia after trauma. Taken together, our findings suggest unique neural markers of risk and recovery for post-trauma anhedonia and provide new insights into the structural pathophysiology of PTSD.
Supplementary Material
Funding:
This research was supported by the National Institute of Mental Health K00 MH119603, K23 MH101380, R01 MH094757, R21 MH106902, F32 MH101976, K01 MH102415, and U01 MH110925.
Footnotes
Disclosures: The authors have no financial conflicts of interest to disclose.
Data Sharing: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, & Hendler T (2013). Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Human Brain Mapping, 34(11), 2808–2816. 10.1002/hbm.22100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, & Hendler T (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 14120–14125. 10.1073/pnas.0903183106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, & Smith S (2007). Non-linear registration aka spatial normalisation. FMRIB Technical Report TRO7JA2. Retrieved from http://fmrib.medsci.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf [Google Scholar]
- Andersson Jesper L.R., & Sotiropoulos SN (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour C (2015). The underlying dimensionality of PTSD in the diagnostic and statistical manual of mental disorders: Where are we going? European Journal of Psychotraumatology, 6 10.3402/ejpt.v6.28074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Tustison N, & Song G (2009). Advanced Normalization Tools (ANTS). Insight Journal, 1–35. Retrieved from ftp://ftp3.ie.freebsd.org/pub/sourceforge/a/project/ad/advants/Documentation/ants.pdf [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bonanno GA, & Mancini AD (2012). Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychological Trauma: Theory, Research, Practice, and Policy, 4(1), 74–83. 10.1037/a0017829 [DOI] [Google Scholar]
- Breslau N, Reboussin BA, Anthony JC, & Storr CL (2005). The structure of posttraumatic stress disorder: Latent class analysis in 2 community samples. Archives of General Psychiatry, 62(12), 1343–1351. 10.1001/archpsyc.62.12.1343 [DOI] [PubMed] [Google Scholar]
- Brown RJ, & Winocur G (1973). The Fornix as a reward pathway. Physiology and Behavior, 11(1), 47–52. 10.1016/0031-9384(73)90121-2 [DOI] [PubMed] [Google Scholar]
- Byrne SP, Harpaz-Rotem I, Tsai J, Southwick SM, & Pietrzak RH (2019). Latent typologies of DSM-5 PTSD symptoms in U.S. military veterans. Psychiatry Research, 273, 266–273. 10.1016/j.psychres.2018.12.094 [DOI] [PubMed] [Google Scholar]
- Coloigner J, Batail JM, Commowick O, Corouge I, Robert G, Barillot C, & Drapier D (2019). White matter abnormalities in depression: A categorical and phenotypic diffusion MRI study. NeuroImage: Clinical, 22 10.1016/j.nicl.2019.101710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA (2005). Hippocampal Inactivation Disrupts the Acquisition and Contextual Encoding of Fear Extinction. Journal of Neuroscience, 25(39), 8978–8987. 10.1523/JNEUROSCI.2246-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ME, Jovanovic T, Pham D, Leaman S, Highland KB, Norrholm SD, & Roy MJ (2016). White matter microstructure of the uncinate fasciculus is associated with subthreshold posttraumatic stress disorder symptoms and fear potentiated startle during early extinction in recently deployed Service Members. Neuroscience Letters, 618, 66–71. 10.1016/j.neulet.2016.02.041 [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, & Goodwin L (2014). A systematic review of the comorbidity between PTSD and alcohol misuse. Social Psychiatry and Psychiatric Epidemiology. 10.1007/s00127-014-0855-7 [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, … James A (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain, 130(9), 2375–2386. 10.1093/brain/awm184 [DOI] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, & Kilpatrick DG (1993). The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. The Behavior Therapist, 16, 161–162. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2011-20330-001&site=ehost-live [Google Scholar]
- Fani N, Jain J, Hudak LA, Rothbaum BO, Ressler KJ, & Michopoulos V (2020). Post-trauma anhedonia is associated with increased substance use in a recently-traumatized population. Psychiatry Research, 285, 112777 10.1016/j.psychres.2020.112777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, … Ressler KJ (2016). Structural and functional connectivity in posttraumatic stress disorder: Associations with FKBP5. Depression and Anxiety, 33(4), 300–307. 10.1002/da.22483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Michopoulos V, van Rooij SJH, Clendinen C, Hardy RA, Jovanovic T, … Stevens JS (2019). Structural connectivity and risk for anhedonia after trauma: A prospective study and replication. Journal of Psychiatric Research, 116, 34–41. 10.1016/j.jpsychires.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny NC, Zoellner LA, Fitzgibbons LA, & Foa EB (2000). Exploring the roles of emotional numbing, depression, and dissociation in PTSD. Journal of Traumatic Stress, 13(3), 489–498. 10.1023/A:1007789409330 [DOI] [PubMed] [Google Scholar]
- Fenster RJ, Lebois LAM, Ressler KJ, & Suh J (2018). Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nature Reviews Neuroscience. 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, & Tolin DF (2000). Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 13(2), 181–191. 10.1023/A:1007781909213 [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, & Frackowiak RSJ (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14(1 I), 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Harnett NG, Ference EW, Knight AJ, & Knight DC (2018). White matter microstructure varies with post-traumatic stress severity following medical trauma. Brain Imaging and Behavior, 1–13. 10.1007/s11682-018-9995-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett NG, Goodman AM, & Knight DC (2020). PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Experimental Neurology. 10.1016/j.expneurol.2020.113331 [DOI] [PubMed] [Google Scholar]
- Harnett NG, Shumen JR, Wagle PA, Wood KH, Wheelock MD, Baños JH, & Knight DC (2016). Neural mechanisms of human temporal fear conditioning. Neurobiology of Learning and Memory, 136, 97–104. 10.1016/j.nlm.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(1), 9 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, & Koenigs M (2018). The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biological Psychiatry. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Liu CY, Thompson PM, & Tu Z (2011). Robust brain extraction across datasets and comparison with publicly available methods. IEEE Transactions on Medical Imaging, 30(9), 1617–1634. 10.1109/TMI.2011.2138152 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, & Panksepp J (1999). The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 10.1016/S0165-0173(99)00023-5 [DOI] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, & Metalsky GI (2003). A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Research, 119(3), 243–250. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, & Frueh BC (2006). Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour Research and Therapy, 44(3), 457–467. 10.1016/j.brat.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Keifer OP, Hurt RC, Gutman DA, Keilholz SD, Gourley SL, & Ressler KJ (2015). Voxel-based morphometry predicts shifts in dendritic spine density and morphology with auditory fear conditioning. Nature Communications, 6 10.1038/ncomms8582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, & Domesick VB (1982). The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anterograde and retrograde-horseradish peroxidase study. Neuroscience, 7(10), 2321–2335. 10.1016/0306-4522(82)90198-1 [DOI] [PubMed] [Google Scholar]
- Kennis M, Van Rooij SJH, Tromp DPM, Fox AS, Rademaker AR, Kahn RS, … Geuze E (2015). Treatment Outcome-Related White Matter Differences in Veterans with Posttraumatic Stress Disorder. Neuropsychopharmacology, 40(10), 2434–2442. 10.1038/npp.2015.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 21(16). 10.1523/jneurosci.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SBJ, Van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2017). Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: A diffusion tensor imaging study. Journal of Psychiatry and Neuroscience, 42(5), 331–342. 10.1503/jpn.160129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, & Thompson SM (2018). Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature. 10.1038/s41586-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, & Pettit JW (2006). Measuring hedonic capacity in depression: A psychometric analysis of three Anhedonia Scales. Journal of Clinical Psychology, 62(12), 1545–1558. 10.1002/jclp.20327 [DOI] [PubMed] [Google Scholar]
- Liu P, Wang L, Cao C, Wang R, Zhang J, Zhang B, … Elhai JD (2014). The underlying dimensions of DSM-5 posttraumatic stress disorder symptoms in an epidemiological sample of Chinese earthquake survivors. Journal of Anxiety Disorders, 28(4), 345–351. 10.1016/j.janxdis.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Maier-Hein KH, Neher PF, Houde JC, Côté MA, Garyfallidis E, Zhong J, … Descoteaux M (2017). The challenge of mapping the human connectome based on diffusion tractography. Nature Communications, 8(1). 10.1038/s41467-017-01285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malta LS, Wyka KE, Giosan C, Jayasinghe N, & Difede JA (2009). Numbing symptoms as predictors of unremitting posttraumatic stress disorder. Journal of Anxiety Disorders, 23(2), 223–229. 10.1016/j.janxdis.2008.07.004 [DOI] [PubMed] [Google Scholar]
- Modi S, Trivedi R, Singh K, Kumar P, Rathore RKS, Tripathi RP, & Khushu S (2013). Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: Preliminary evidence from a DTI based tractography study. Behavioural Brain Research, 238(1), 188–192. 10.1016/j.bbr.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry, 77(3), 276–284. 10.1016/j.biopsych.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson A, Barnes J. Ben, Creamer M, Forbes D, McFarlane AC, O’Donnell M, … Bryant RA (2014). The temporal relationship between posttraumatic stress disorder and: Problem alcohol use following traumatic injury. Journal of Abnormal Psychology, 123(4), 821–834. 10.1037/a0037920 [DOI] [PubMed] [Google Scholar]
- Olson EA, Cui J, Fukunaga R, Nickerson LD, Rauch SL, & Rosso IM (2017). Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A TBSS and tractography study. Depression and Anxiety, 34(5), 437–445. 10.1002/da.22615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential Contribution of Amygdala and Hippocampus to Cued and Contextual Fear Conditioning. Behavioral Neuroscience, 106(2), 274–285. 10.1037/0735-7044.106.2.274 [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, & Grant BF (2011). Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders, 25(3), 456–465. 10.1016/j.janxdis.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Tsai J, Armour C, Mota N, Harpaz-Rotem I, & Southwick SM (2015). Functional significance of a novel 7-factor model of DSM-5 PTSD symptoms: Results from the National Health and Resilience in Veterans Study. Journal of Affective Disorders, 174, 522–526. 10.1016/j.jad.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, & Versace F (2007). Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. Journal of Neurophysiology, 98(3), 1374–1379. 10.1152/jn.00230.2007 [DOI] [PubMed] [Google Scholar]
- Sanjuan PM, Thoma R, Claus ED, Mays N, & Caprihan A (2013). Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: A diffusion tensor imaging study. Psychiatry Research - Neuroimaging, 214(3), 260–268. 10.1016/j.pscychresns.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam P, Teslovich T, Wilson SH, Yeh PH, Oakes TR, & Weaver LK (2019). Decreases in white matter integrity of ventro-limbic pathway linked to post-traumatic stress disorder in mild traumatic brain injury. Journal of Neurotrauma, 36(7), 1093–1098. 10.1089/neu.2017.5541 [DOI] [PubMed] [Google Scholar]
- Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, … Kawashima R (2013). Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Molecular Psychiatry, 18(5), 618–623. 10.1038/mp.2012.51 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. In NeuroImage (Vol. 23). 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Spitzer EG, Zuromski KL, Davis MT, Witte TK, & Weathers F (2018). Posttraumatic Stress Disorder Symptom Clusters and Acquired Capability for Suicide: A Reexamination Using DSM-5 Criteria. Suicide and Life-Threatening Behavior, 48(1), 105–115. 10.1111/sltb.12341 [DOI] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, … Ressler KJ (2017). Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma. Biological Psychiatry, 81(12), 1023–1029. 10.1016/j.biopsych.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang Z, Ding W, Wan J, Zhuang Z, Zhang Y, … Xu J (2013). Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PLoS ONE, 8(12). 10.1371/journal.pone.0083473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-dos-Santos V, … Dupret D (2019). A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space. Cell, 176(6), 1393–1406.e16. 10.1016/j.cell.2018.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, … Jovanovic T (2018). The Role of the Hippocampus in Predicting Future Posttraumatic Stress Disorder Symptoms in Recently Traumatized Civilians. Biological Psychiatry, 84(2), 106–115. 10.1016/j.biopsych.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Peterson DJ, Gatenby JC, Li W, Grabowski TJ, & Madhyastha TM (2017). Evaluation of field map and nonlinear registration methods for correction of susceptibility artifacts in diffusion MRI. Frontiers in Neuroinformatics, 11 10.3389/fninf.2017.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang L, Cao C, Cao X, Fang R, Zhang J, & Elhai JD (2017). The underlying dimensions of DSM-5 PTSD symptoms and their relations with anxiety and depression in a sample of adolescents exposed to an explosion accident. European Journal of Psychotraumatology, 8(1). 10.1080/20008198.2016.1272789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, & Tseng WYI (2011). NTU-90: A high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. NeuroImage, 58(1), 91–99. 10.1016/j.neuroimage.2011.06.021 [DOI] [PubMed] [Google Scholar]
- Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, & Tseng WYI (2013). Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE, 8(11), e80713 10.1371/journal.pone.0080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganarasimha D, & Meti BL (1999). Amelioration of fornix lesion induced learning deficits by self-stimulation rewarding experience. Brain Research, 845(2), 246–251. 10.1016/S0006-8993(99)01957-5 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


