Abstract
Introduction:
Breakthroughs in cancer immunotherapy have spurred interest in the development of vaccines to mediate prophylactic protection and therapeutic efficacy against primary tumors or to prevent relapse. However, immunosuppressive mechanisms employed by cancer cells to generate effective resistance has hampered clinical translation of therapeutic cancer vaccines. To enhance vaccine efficacy, the immunomodulatory properties of cytoreductive therapies such as chemotherapy and radiation could amplify a cancer-specific immune response.
Areas covered:
Herein, the authors discuss therapeutic cancer vaccines that harness whole cells and antigen targeted vaccines. First, recent advancements in both autologous and allogenic whole cell vaccines and combinations with checkpoint blockade and chemotherapy are reviewed. Next, tumor antigen targeted vaccines using peptide-based vaccines and DNA-vaccines are discussed. Finally, combination therapies using antigen targeted vaccines are reviewed.
Expert opinion:
A deeper understanding of the immunostimulatory properties of cytoreductive therapies has supported their utility in combination therapies involving cancer vaccines as a potential strategy to induce a durable anti-tumor immune response for multiple types of cancers. Based on current evidence, combination therapies may have synergies which depend on the identity of the cytotoxic agent, vaccine target, dosing schedule, and cancer type. Together, these observations suggest that combining cancer vaccines with immunomodulatory cytoreductive therapy is a promising strategy for cancer therapy.
1|. Introduction
Harnessing the immune system for cancer therapy has long been the goal in oncology and is now being realized in clinical practice with cancer immunotherapy [1]. Recent clinical successes have transformed the treatment of aggressive and difficult-to-treat cancers, such as melanoma and lymphoma [2]. Most notable has been the ability of the checkpoint inhibitors to achieve a significant increase in survival for patients with metastatic cancer, for which conventional therapies have failed [3]. In the context of advances towards understanding how tolerance, immunity and immunosuppression regulate antitumor immune responses, alongside the advent of targeted therapies, these successes suggest that active immunotherapy represents a path to obtain a robust and long-lasting response in cancer patients [4].
The discovery of CD8+ and CD4+ T cells specific for cancer-testis or differentiation antigens expressed in patient tumor samples was the harbinger of systematic efforts to characterize tumor-specific or tumor-associated antigens to broaden the effectiveness of immunotherapies using cancer vaccines [5–7]. These efforts focused on actively helping educate the immune system to identify and eliminate cancer cells. Histopathological evaluations of tumor sections have indicated a strong positive association between patient survival, the presence of intratumoral T cells and production of the cytotoxic molecule interferon-γ (IFN-γ) [8–10]. Vaccination might reasonably be expected to amplify the frequency and strength of these pre-existing responses or perhaps induce de novo reactions. Similar to conventional vaccines for preventing infectious disease, some cancer vaccines have been developed as effective prophylactic (or preventative) agents. This strategy has been deployed with considerable success in the clinic for the prevention of cancers of viral origin, such as hepatitis B virus and human papillomavirus (HPV), where the etiological agent is known, and is now part of the recommended vaccination schedule in children and young adults [11–13].
In contrast, the development of therapeutic vaccines as a monoagent to treat existing disease has proved elusive. Unlike prophylactic cancer vaccines, which confer protection against a known agent, therapeutic vaccines seek to activate an immune response against tumor antigens expressed selectively or exclusively by cancer cells [14]. In designing therapeutic cancer vaccines, the greatest difficulty has been in identifying the combinations of tumor antigens that might be incorporated in a vaccine formulation as expression on cancer cells alone may be inadequate for predicting the ability of a vaccine to generate a protective T cell response [15]. Even when an antigen is identified, it may not be sufficiently immunogenic or uniformly expressed on the tumor to generate a potent and durable immune response [16]. Beyond expression of checkpoint molecules and the mutational burden in cells comprising the tumor, the determinants of an immune response are not well understood [17]. Additionally, the optimal adjuvant that can be used safely and effectively in a cancer vaccine formulation in humans is not yet clear [18]. The desired adjuvant (or adjuvant combination) will be one capable of triggering the maturation of dendritic cells to a state where they can facilitate the generation of tumor-reactive, cytotoxic T cells [19]. Finally, although it is likely that formulations for human immunization will eventually be optimized, the effectiveness of a tumor-specific T cell may still be limited by the immunosuppression mechanisms deployed by tumors to escape immune cells [20].
Despite the current limitations, conferring immunity against cancer relapse in patients that are in remission can be lifesaving. Therefore, oncologists are employing the strategy of using two or more therapeutic agents to target multiple cancer cell survival pathways, which has been successfully employed as a standard treatment regimen for multiple types of cancers for decades [21]. For example, in advanced non-small cell lung cancer (NSCLC) patients, 42% of patients with programmed death ligand 1 (PD-L1) expressing tumors fail to respond to anti PD-L1, Pembrolizumab [22,23]. However, checkpoint inhibitor in combination with chemotherapy is associated with significantly longer overall survival and progression-free survival as compared to chemotherapy or PD-1 inhibition alone in NSCLC patients [24]. Similarly, in a recent phase III clinical trial (NCT00861614), patients with castration-resistant prostate cancer that had metastasized to the bone marrow after unsuccessful docetaxel treatment, received a combination administration of a single dose of bone directed radiotherapy with anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), Ipilimumab, or placebo every 3 weeks for up to 4 doses [25]. Patients that were co-administered Ipilimumab exhibited a survival benefit as compared to the placebo arm [26]. Furthermore, the effect of cytotoxic chemotherapy and radiation on the cells of the immune system or in modifying the tumor microenvironment to enhance the immune clearance of tumor cells is only now being characterized [27]. Although conventional wisdom might suggest that non-specific cytotoxic chemotherapy might have deleterious effect on immune mechanisms, such effects may be more nuanced than previously believed and strongly drug-, dose- and/or schedule-dependent [28,29]. The use of combinations of these therapies, in a scientifically guided manner has proved to broaden the anti-tumor response.
To stimulate a durable immune response, the combination of therapeutic cancer vaccines with traditional cancer treatments including radiation and chemotherapy, or targeted immunotherapies is a promising strategy. Towards the goal of enhancing immune responses as potentially curative therapies, a number of cancer vaccine platforms including irradiated whole cancer-cell vaccines, peptide-based vaccines, and virus-vector vaccines, in combination with immunotherapies, are in various phases of clinical and pre-clinical development. Combination strategies with radiation and chemotherapy have been demonstrated to induce tumor cell death and enhance breadth of tumor-specific T cell response and those with immune checkpoint blockade act to accentuate the vaccine mediated cytotoxic T cell response. As the same agent may prove inhibitory, benign or even stimulatory depending on the stage of immune response being targeted and the dose/schedule being used, great care must be exercised when designing strategies and suboptimal dosing schedules. In this review, we examine existing cancer vaccine approaches and highlight the enhanced therapeutic potential of combination cancer vaccine strategies. We first discuss existing cancer vaccine approaches including those that failed to show efficacy in clinical trials. We then discuss various combination approaches involving both pre-clinical cancer vaccine platforms and existing vaccines platforms. Lastly, we conclude with some thoughts on the future directions for both cancer vaccines as monotherapies and cancer vaccine combinations.
2|. Engineered cell-based combination cancer vaccines
Dendritic cells (DC) are specialized immune cells that play a vital role in promoting an antigen-specific immune response [30–33]. Cancer vaccines that harness DCs have been developed to initiate and shape the tumor-specific immune response and/or boost existing spontaneous antitumor T cell responses. The common strategy for cancer vaccination is to harness the DC function of priming naïve T cells and boost a memory T cell response against tumor associated antigens (TAAs) that are expressed by cancer cells. While there are a variety of different DC vaccine formulations, common elements include – (i) a source of TAAs such as tumor cell lysate or irradiated tumor cells and (ii) a pro-inflammatory chemoattractant, such as granulocyte-macrophage colony stimulating factor (GM-CSF), to recruit and activate DCs.
2.1 |. Autologous DC vaccines
Autologous DC vaccines, derived from patient-specific immune cells, are currently the only type of FDA approved cell-based cancer vaccine. An example of this approach is Provenge, which is approved for the treatment of castration-resistant metastatic prostate cancer (mCRPC) [34]. It is a patient-specific cell therapy in which the patient’s peripheral blood mononuclear cells (PBMCs) are cultured with a recombinant fusion protein comprised of prostatic acid phosphatase (PAP) and GM-CSF, which induces dendritic cell (DC) differentiation and presentation of PAP-derived epitopes. These PAP-epitope derived DCs are administered to patients and induce a PAP-specific cytotoxic T cell response [34–36]. Using autologous DCs mitigates concerns of off-target immune responses and reduces the risk of the development of autoimmune-like disorders, which is observed with PD-1/PD-L1 or CTLA-4 checkpoint blockade therapy [37,38]. While this approach has been shown to improve overall survival as compared to chemotherapy alone, the therapy has limited scalability, since PBMCs must first be isolated from the patient and differentiated into DCs in vitro.
For hematological malignancies, such as acute myeloid leukemia (AML), AML-DCs have the advantage of expressing the full spectrum of antigens [39]. In this approach, hybridomas of AML cells and autologous patient derived DCs have been developed as a potential cell-based cancer vaccine. Notably, among 17 patients vaccinated in complete remission after chemotherapy, 12 (71%) remain in remission at a median follow-up of 57 months. Vaccination was associated with expansion of TAA-specific T cells that lasted more than 6 months [40]. The multicenter study testing this approach is ongoing (NCT01096602) and is estimated to be completed in 2024.
Tumor biopsies are often used to assess the histopathology of the tumor. In addition to characterizing the cytological composition of the tumor, such biopsies may also be used as a source for collecting tumor cells for a cell-based vaccine. The subsequent formulation may be deployed via an injection or implantation [41–43]. One of the major benefits of this approach is that it eschews the need for defined TAAs and could promote a cytotoxic T cell response against multiple TAAs. To manufacture autologous DC vaccines at scale, Ali et al. developed a macroporous poly(lactic-co-glycolic acid) (PLG) scaffold, designed to mediate sustained release of GM-CSF to recruit DCs [44]. Cytosine-guanosine oligonucleotide (CpG-ODN) was incorporated onto the PLG matrix to enhance the fraction of plasmacytoid DCs (pDCs) and promoted local production of tumor necrosis factor α (TNF- α) nd IFN-γ. Tumor cell lysates from freeze-thawing murine B16 melanoma cells were loaded into these scaffolds and resulted in systemic cytotoxic T cell response against melanoma antigens. This scaffold was able to mediate prophylactic protection against B16 melanoma if administered before B16 melanoma inoculation and provide therapeutic protection against B16 melanoma if administered after inoculation [45]. This platform, termed WDVAX, in now in phase I clinical trials (NCT01753089) for metastatic melanoma.
2.2 |. Allogeneic DC vaccines
Allogenic DC vaccines are an alternative approach to autologous vaccines. The main advantage of this approach is that it precludes the need for isolation and transformation of PBMCs into DCs prior to each administration, allowing for less patient-to-patient variability and enhanced scalability. However, they differ from autologous vaccines in that they do not necessarily contain patient specific TAAs. Thus, even if they can induce an immune response, they may not be able to mediate effective tumor lysis. Commonly used allogenic cells are established cancer cell lines known to express specific TAAs. These allogenic cells are generally transfected to express chemoattractant molecules, such as GM-CSF, prior to administration. The first GM-CSF transfected allogenic pancreatic cancer vaccine, termed GVAX was studied in a 2001 phase I clinical trial. In this dose escalation study, patients with pancreatic cancer were administered irradiated GM-CSF expressing pancreatic cancer cell doses ranging from 1 × 107 to 5 × 108 cells. The highest dose was determined to increase eosinophil and macrophage infiltration into tumors and did not cause any significant adverse effects [46]. As a result of this successful phase I clinical trial, a phase II clinical trial was initiated in which patients with pancreatic cancer received 5 × 108 irradiated GM-CSF secreting allogenic pancreatic cancer cells administered to 3 different lymph node regions. Patients were administered 5 doses over the course of a year. The overall survival (OS) of patients administered the allogeneic cancer vaccine was 15 to 20 months which is comparable to standard tumor resection. Further, it was found that the vaccine lead to increased induction and maintenance of mesothelin specific T cells which was correlated with longer disease-free survival [47]. However, despite promising phase II clinical data, two phase III clinical trials (Identifiers: NCT00089856, NCT00133224) did not demonstrate therapeutic efficacy and were halted early.
Cancer vaccines against hematological cancers are an attractive therapeutic option, especially for older patients who have fewer treatment options available and are at a higher risk of treatment-related mortality from allogenic hematopoietic stem cell transplantation. For the treatment of acute myeloid leukemia (AML), an allogeneic DC cancer vaccine, DCP-001, was developed by differentiating CD34+ acute myeloid leukemia (AML) cells into DCs. These AML-derived DCs are morphologically and phenotypically identical to myeloid derived-DCs and induced a strong antigen-specific immune response against AML-associated antigens [48]. A phase I clinical trial (NCT01373515) initiated to determine the safety profile of this vaccine when administered intradermally to AML patients within 2 months after having achieved complete remission or patients who have stable disease for at least two months. Patients received 4 bi-weekly vaccinations of DCP-001 cells and T cell reactivity to AML-related antigens WT-1, PRAME, NY-ESO-1, and MAGE-A3 were accessed by IFNγ ELISpot analysis. WT-1 and PRAME are both expressed by DCP-001, and NY-ESO-1 and MAGE-A3 were included to access epitope spreading. Four of eight patients showed DCP-001 induced or enhanced T cell response to at least one of the AML-associated antigens. Further, antibody responses against blast antigens were evaluated in ten patients, five of whom showed vaccination-induced humoral responses. Overall, patients whose immune-monitoring data demonstrated positive response to the vaccine showed statistically significant improvement in survival over patients who did not respond favorably [49]. As a result of these promising phase I clinical trial results, a phase II clinical trial (NCT03697707) was initiated in 2018.
2.3|. Combination DC cancer vaccines and approved chemo/immune therapies
Promoting tumor-specific immunity with a vaccine and subsequent amplification of tumor-specific T cells with checkpoint blockade therapy is an attractive hypothesis. The effectiveness of such an approach has been tested using clinically approved PD-1/PD-L1 and CTLA4 blockade using Pembrolizumab or Ipilimumab respectively with cell-based vaccines in preclinical mouse studies and early stage clinical trials [50–53]. In pre-clinical studies, anti-CTLA-4 checkpoint therapy in combination with cancer vaccines (e.g. GVAX) demonstrated synergistic reduction in tumor size and increase in the antitumor immune response in a mouse model of melanoma and prostate cancer. The timing of the combination of anti-CTLA-4/GVAX vaccine has been identified as an important parameter in cancer vaccines. For example, in the prostate cancer model Pro-TRAMP, it has been demonstrated that anti-CTLA-4 mAbs should be administered after vaccination for an additive effect. The effect of timing on treatment efficacy is likely due to higher anti CTLA-4 mAb concentrations in sites of high CTLA-4 expression, such as the gut, since the expression of CTLA-4 on TAA-specific T cells is not upregulated until after vaccination. Therefore, if anti CTLA-4 is administered before or concurrent to vaccination, the anti CTLA-4 mAbs may be unavailable to bind to TAA-specific T cells. Furthermore, the addition of low-dose cyclophosphamide depletes immune cells, including regulatory T cells (Tregs), and thereby augments the anti-tumor efficacy of GVAX + anti-CTLA-4 combination therapy [51]. In the CT26 murine model of colorectal cancer, dual PD-1 and CTLA-4 checkpoint blockade in combination with GVAX lead to tumor rejection of 100% of mice. This same combination leads to rejection of ID8-VEGF ovarian carcinoma in 75% of mice [52]. Results from mouse studies suggests that anti-CTLA-4 and anti-PD-1 mAbs increase the frequency of activated T cells and the effector T cell/Treg ratio in vaccinated tumors. While studies have focused on the scheduling of anti-CTLA-4 mAbs with vaccination, this variable has been less studied for the combination of anti-PD-1 mAbs and vaccine [54]. The pre-clinical studies with combination checkpoint blockade and GVAX supported clinical trials for patients with metastatic pancreatic cancer (NCT01417000, NCT02243371, NCT02004262) with several that are still ongoing (NCT03161379, NCT03190265, NCT03153410, NCT03006302, NCT02648282, NCT02451982) in which GVAX was combined with cyclophosphamide and/or nivolumab seem promising.
There are also a number of clinical trials in which Provenge is used in combination with cytoreductive therapies including chemotherapy, radiation, immune checkpoint blockade and secondary hormone therapy to better treat mCRPC (NCT00779402, NCT02793765, NCT01807065). Cytoreductive strategies provide a transient decrease in tumor size whereas the Provenge cancer vaccine decreases tumor growth rate. The combination of these approaches could allow for better therapeutic outcomes than a monotherapy. However, many cytoreductive therapies impair immune cell number and function. Therefore the timing and dose of combination therapies will be an important factor to consider when designing clinical studies [55].
3|. Cancer-antigen specific subunit cancer vaccines
While whole-cancer cell vaccines are promising, their manufacturing process is labor-intensive and has limited scalability. For example, Provenge is administered intravenously in a three-dose schedule at two-week intervals. Each dose requires leukapheresis three days prior to administration to allow time to differentiate peripheral blood monocytes into DCs. This manufacturing process is not only time consuming and expensive, but also operationally prohibitive in many clinical settings, especially in low-resource settings. As an alternative, peptide and DNA-based cancer vaccines are a potentially cheaper, and more accessible treatment option than whole cell-based vaccines. These vaccines operate in a manner similar to cell-based cancer vaccines, that is, by recruiting DCs and facilitating the expansion of cytotoxic T cells against TAAs. In contrast to whole cell-based vaccines, antigen targeted cancer vaccines are defined and might be manufactured at scale for off-the-shelf use, without the need for cell collection from the patient. However, antigen targeted cancer vaccines require that patient tumors express specific TAAs, which in most types of cancers limits their utility to a subset of patients. Further, the initial cytotoxic T cell response from an antigen targeted vaccine is more restricted than that from whole cell-lysate. Most TAA targets for antigen-targeted cancer vaccines can be grouped into the categories: oncofetal mutations, germline/cancer testis mutations, and lineage differentiation antigens [56].
3.1 |. Oncofetal mutations
Oncofetal antigens are primarily expressed during fetal development but may be mutated and/or over-expressed by cancer cells and is associated with head-and-neck [57], hepatocellular [58], colorectal [59], squamous esophageal [60], and breast carcinoma [61]. Oncofetal TAA targets for vaccine development include carcinoembryonic antigen (CEA), insulin-like growth factor II mRNA-binding protein 3 (IMP-3) [62] and alpha-fetoprotein (AFP) [63]. In a recent pre-clinical study, Hirayama et al. generated IMP3 derived long peptides (IMP-3-LPs) capable of eliciting both TAA specific effector and helper T cells. IMP-3-LPs-specific Th-cells responded to autologous DCs loaded with the recombinant IMP-3 proteins in vitro, suggesting that these IMP-3-LPs can be successfully processed by DCs. Co-culturing IMP-3 specific Th-cells with IMP-3 effector T cells on autologous DCs pulsed with both short peptides derived from IMP-3 and IMP-3-LPs augmented the expansion of IMP-3 effector T cells. Further, IMP-3-LPs were able to induce IMP-3 specific Th-cells from PBMCs isolated from head-and-neck cancer patients [62]. Together, these results highlight the potential for IMP-3 derived peptide-based cancer vaccines in the treatment of head-and-neck cancer. In a separate study, Hensel et al. developed a recombinant adeno-associated virus vector encoding CEA (rAAV-CEA) to mediate prophylactic protection against CEA syngeneic MC38-CEA colon adenocarcinoma model. The rAAV-CEA was administered intramuscularly, followed by multiple administrations of GM-CSF encoding plasmid to the same intramuscular site. Subsequent MC38-CEA tumor challenge in mice resulted in tumor free survival. Notably, tumor challenge in mice with MC38 cells which did not express CEA did not result in enhancement in survival, indicating CEA specific immunity [64].
3.2 |. Germline/cancer testis mutations
Similar to oncofetal mutations, germline/cancer testis mutations may be upregulated by cancer cells and is generally restricted to immune privileged germline cells. Common therapeutic vaccine target mutations include Wilms tumor 1 (WT1) protein, melanoma-associated antigen (MAGE) superfamily, and cancer/testis antigen 1 (NY-ESO-1). A National Cancer Institute consensus study on prioritization of cancer antigens ranked the WT1 protein as the top immunotherapy target in cancer, which is overexpressed on multiple tumor types, including acute myeloid leukemia [65,66]. A multivalent WT1 peptide vaccine (galinpepimut-S) has been developed and tested in acute myeloid leukemia (AML) patients in phase 1 and 2 clinical trials [67]. In the most recent phase 2 trial (NCT01266083), patients in complete remission received 6 vaccinations administered over 10 weeks with the potential to receive 6 additional monthly doses if they remained in remission. Immune responses (IRs) were evaluated after the 6th and 12th vaccinations by CD4+ T cell proliferation, CD8+ T cell IFN-γ secretion, or the CD8-relevant WT1 peptide major histocompatibility complex tetramer assay. In terms of compliance, 14 patients (64%) completed ≥6 vaccinations, and only 9 (41%) received all 12 vaccine doses. Fifteen patients (68%) relapsed, and 10 (46%) died. The vaccine was well tolerated, with the most common toxicities being grade 1/2 injection site reactions (46%), fatigue (32%), and skin induration (32%). Median disease-free survival from first complete remission was 16.9 months, whereas the overall survival from diagnosis was estimated to be ≥ 5 years. Nine of 14 tested patients (64%) had an IR in ≥1 assay (CD4 or CD8) [67].
There has been extensive pre-clinical research showing efficacy of MAGE-based therapeutic peptide cancer vaccines in the treatment of cancers including melanoma [68–70], NSCLC [71], and breast cancer [72,73]. Deuperret et al. developed a MAGE-A immunogen with cross-reactivity for multiple MAGE-A isoforms. The general domain structure of MAGE-A family is conserved between mice and humans; however, the sequence homology is poor. As a result, separate consensus vaccines were developed for proof-of-concept murine cancer models and for human pre-clinical studies. Mice were vaccinated with the murine MAGE-A consensus vaccine, and the vaccine induced robust CD8+ IFNγ responses to all 6 isoforms predicted to cross-react with this vaccine. To test the antitumoral response, melanoma was induced in Tyr::CreER;BrafCA/þ;Ptenlox/lox mice by administration of topical 4-OHT (tamoxifen). In this melanoma model, the murine MAGE-A consensus vaccine was able to extend survival by 50 days as compared to control cohort [74]. Similar to targeting MAGE epitopes, pre-clinical studies with NY-ESO-1 derived peptides have been conducted in combination with an adjuvant [75–78]. In one study, Albershardt et al. engineered a lentiviral vector, LV305, to deliver NY-ESO-1 to dendritic cells in vivo. Mice immunized with LV305 developed NY-ESO-1 specific cytotoxic T cells within 2 weeks post-immunization, which conferred protection against NY-ESO-1 expressing CT26 lung metastasis. Further, adoptive cell transfer of NY-ESO-1 cytotoxic T cells conferred protection in tumor-bearing recipient mice, confirming transferable immunity [78]. Notably, in 2014 a phase I clinical trial (NCT02122861) to determine the safety profile of LV305 therapeutic cancer vaccine was initiated. There were no significant side effects noted in any of the 30 patients as a result of the cancer vaccine. Anti-NY-ESO-1 specific Th-cells and cytotoxic T cells were induced in 57% of evaluable sarcoma patients and one patient with synovial sarcoma achieved a partial response lasting more than 3 years. Further, the induction of anti-NY-ESO-1 immune response was found to improve 1-year survival over patients in which vaccination failed to elicit a T cell response [79].
3.3 |. Cell lineage differentiation antigens
Cell lineage differentiation antigens are among the first identified cancer antigens. These include MART-1, gp100, prostate-specific antigen (PSA), and tyrosinase (Tyr). Expression of MART-1 and gp100 is most associated with melanoma. It was first recognized in 1994 that HLA-A*02 restricted cytotoxic T cells derived from tumor infiltrating lymphocytes (TILs) of melanoma patients recognized nonmutated proteins expressed by most melanoma cells. The most frequently recognized proteins were MART-1 and gp100, which was recognized by TILs from 90% and 40% patients, respectively [80,81]. As a result of these early investigations, the MART-127–35 peptide, AAGIGLTV, was among the first to be employed in humans in 1999. In a phase I clinical trial, 23 HLA-A*0201 patients with metastatic melanoma received subcutaneous administration of the MART-127–35 peptide, doses ranging from 0.1 to 10mg, emulsified in incomplete Freund’s adjuvant (IFA). Dosing schedule consisted of 4 doses separated by 3-week intervals. While the phase I clinical trial did not induce any clinically significant toxicities, it also failed to demonstrate any therapeutic efficacy at all doses. Furthermore, an analysis of PBMCs from the peripheral blood indicated that there was no correlation between MART-1 specific T cell activation and vaccine dose [82]. Given current understanding of tolerogenic immune cells, in particular the role of myeloid derived suppressor cells (MDSCs) and the role of regulatory T cells in the maintenance of the immunosuppressive tumor microenvironment, the lack of clinical efficacy might not be surprising.
Despite these suboptimal initial results, the development of cell lineage differentiation targeted vaccines has persisted due to their expression on a variety of cancers. In melanoma, recent studies have focused on developing vaccine strategies which both expand cancer antigen-specific T cells while also suppressing MDSCs. In a pre-clinical study, Yan et al. developed a novel synthetic consensus DNA vaccine against Tyr and tested its efficacy in the highly metastatic and poorly immunogenic B16-F10 murine melanoma model. The DNA vaccine was administered three times at 2-week intervals either in a prophylactic setting 7 days prior B16-F10 inoculation or in a therapeutic setting 7 days after B16-F10 inoculation. The induction of IFN-γ producing T cells by vaccination was assessed by ELISpot assay with T cells isolated from spleen of transfected mice. These studies confirmed the vaccine was able to illicit expansion of Tyr-specific T cells, with Tyr epitopes ‘DWRDAEKCDICTDEY’ and ‘AKHTISSDYVIPIGT’ being dominant. In a prophylactic setting, the vaccine slowed the recruitment of MDSCs to the tumor and was associated with a reduction in the concentration of immunosuppressive IL-10, and MDSC chemoattractant molecules MCP-1 and CXCL5, and decreased tumor growth rate. In a therapeutic setting, similar effects contributed to improving overall survival [83].
3.4|. Co-administration of cancer antigen vaccines with immunomodulators
TAA targeted cancer vaccines have been shown to induce antigen specific T cell and antibody responses in pre-clinical studies and in clinical trials. Recent efforts have focused on characterizing the effects of combining TAA targeted cancer vaccines with existing cancer therapeutics which can modulate the immunosuppressive tumor microenvironment and further improve outcomes.
The presence of TILs is correlated with favorable outcomes with checkpoint blockade therapy, which is a logical prelude for combination with TAA targeted cancer vaccines [84]. Conniot et al. combined a nanoparticle-based cancer vaccine with PD-1 blockade and OX40 co-stimulation. The nanoparticle delivered the Melan-A/MART-126–35 MHC I-restricted peptide and Melan-A/MART-151–73 MHC II-restricted peptide to DCs in the draining lymph nodes, which were shown to potentiate cytotoxic and helper T cell responses, respectively. OX40 is a co-stimulatory receptor member of the TNF family, expressed on activated T cells. Once activated, OX40 induces expansion, trafficking, and pro-inflammatory cytokine production by effector T cells. In mice inoculated with Ret-melanoma cells, this combination therapy restricted melanoma growth and prolonged survival when administered as a prophylactic. When administered as a therapeutic, it was found that the infiltration of MDSCs compromises the effect of the combination therapy. To overcome this limitation, ibrutinib, a small molecule inhibitor which has been shown to limit the generation and migration of MDSCs, was utilized to enhance the effect of the combination therapy. The combination of nanoparticle vaccine, anti-PD-1, OX-40 stimulation, and ibrutinib was able to greatly extend survival in both the ret-melanoma and B16-F10 models of murine melanoma as compared to the combination therapy without ibrutinib [85]. In another study, Sahin et al. developed a personalized melanoma vaccine by developing a computational model to predict cancer neo-epitopes in melanoma patients. First, non-synonymous mutations were identified by comparative exome and RNA sequencing of tumor biopsies and healthy blood cells. The mutations were ranked according to expression level of mutation-encoding RNA and predicted high binding affinity to autologous HLA class I / class II. 10 mutations were selected per patient and engineered into synthetic RNAs and used as the basis of an RNA vaccine to be administered to DCs in draining lymph nodes, which in mouse studies, showed efficient uptake by DCs and antigenicity [86]. In clinical trials, the vaccine was well tolerated in all patients, with each patient developing T cells against at least 3 mutations and pre-existing weak responses against 1/3 of immunogenic neo-epitopes were augmented upon vaccination. One patient experienced multiple relapses and progressing metastases at the start of vaccination despite a strong T cell response against six neo-antigens. For this patient, a compassionate pembrolizumab treatment program was initiated which, strikingly, lead to an 80% decrease in size of multiple melanoma lesions and, eventually, complete response. Notably, neo-epitope specific T cell subsets were PD-1+ and post-vaccine lesions were shown to upregulate PD-L1 [87].
Combination therapies involving chemotherapeutics have also shown promise in treating various types of cancers. For example, numerous clinical studies have shown that checkpoint inhibitors in combination with chemotherapy are associated with significantly longer overall survival and progression-free survival as compared to chemotherapy alone in patients with advanced non-small cell lung carcinoma [24]. This is not entirely unexpected as many chemotherapeutics increase the number of tumor infiltrating cytotoxic T cells or reduce the number of Tregs [88–90]. Therefore, combining chemotherapeutics and TAA-targeted cancer vaccines may have a synergistic effect which is corroborated by the results of recent pre-clinical studies [91–93]. In one such study, Shah et al. developed an AML-vaccine comprised of a macroporous PEG-alginate-based scaffold and incorporation of GM-CSF, CpG-ODN, and AML-associated antigens in the form of either freeze-thaw lysates derived from bone marrow of AML-infected mice or WT1 peptide [93]. Both vaccine formulations were associated with expansion of WT1 tetramer specific cytotoxic T cells in the peripheral blood within a week of vaccination. Notably, both vaccine formulations provided prophylactic protection against MLL-AF9 AML in 100% of mice when administered 10 days prior to AML inoculation and mice survived an AML re-challenge approximately 3 months later. Next, the WT1 AML-vaccine was tested in a therapeutic model of established AML in combination with standard induction chemotherapy (iCt) regimen of cytarabine (Ara-C) and doxorubicin [94]. Leukemia burden was reduced in mice after iCt treatment, but AML relapsed between day 25 and 35. The WT1 AML-vaccine alone provided prolonged survival, but ultimately did not improve overall survival. However, the iCt and WT1 AML-vaccine combination therapy was effective in mediating therapeutic protection in 100% of mice. Interestingly, combining iCt with an antigen-free vaccine durably depleted leukemia in all mice, dependent on both encapsulation and release of GM-CSF and CpG-ODN, and in which immunogenic cell death of AML cells recruited to the cryogel scaffold may have contributed to the efficacy of this strategy.
In a study to stimulate the endogenous immune response to overcome established advanced tumors, Moynihan et. al. used combination cancer vaccine and immunotherapy to potentiate an immune response [95]. The study was motivated by the development of a strategy to efficiently target peptide vaccines to lymph nodes, and the clinical success with checkpoint blockade therapy [96]. The combinations consisted of A (tumor-antigen-specific antibody), I (MSA–IL-2), P (anti-PD-1) and V (amphiphile–vaccine). Component V was a potent lymph-node (LN)-targeted vaccine composed of peptide antigens and CpG DNA conjugated to albumin-binding lipids that reversibly bind to interstitial albumin and efficiently traffic to LNs, leading to robust T cell responses. In multiple syngeneic tumor models, this quaternary combination immunotherapy cured a majority of mice with established tumors and elicited long-lived protective T cell memory responses. Notably, all four components incorporated in AIPV were required for treatment of several difficult-to-treat tumor models. The results from the multiple tumor models revealed distinct hierarchies of importance for the four components. For example, the monoclonal antibody component was a critical contributor to efficacy in the B16F10 model, but it was the least important component in the DD-Her2/neu model. A key aspect of AIPV treatment was that this set of four agents collectively mounted an integrated response that overcomes tumor resistance mechanisms in all of the models evaluated here, suggesting that the appropriate combination of immune effectors can overcome a range of obstacles present in tumor microenvironments.
4|. Conclusions
The studies reviewed here highlight advances in the development of cancer vaccines and synergies afforded by combining cancer vaccines with chemotherapy and immune checkpoint blockade. Therapeutic cancer vaccinations strategies can be broadly categorized by their delivery vehicle and antigen target, each with their own distinct properties.
DC vaccines induce expansion of T cells with a broad T cell receptor repertoire, allowing for a more sustained and robust immune response. Thus far, the only FDA approved DC vaccine formulation is Provenge [34]. While this is an attractive personalized cancer vaccine cell therapy, the labor-intensive manufacturing process and a modest improvement in overall survival has hindered its applicability. Vaccines such as WDVAX and allogenic DC vaccines such as GVAX and DCP-001 are attractive alternatives as they avoid the need for personalized cell-manufacturing, and are scalable therapies [44–46]. Further, new DC vaccine formulations preclude identification of specific cancer antigens, which may broaden their applicability to a larger number of cancer patients.
DNA- or peptide-based cancer antigen targeted vaccines act on DCs in the lymph nodes. The vaccines expand antigen specific T cells which mediate cancer cell lysis, which in turn allows for DC priming of tumor-associated antigens in a manner analogous to DC vaccines. While these therapies are generally scalable, their scope is limited by variability in expression levels of antigen targets and are therefore available to a subset of cancer patients. In addition, since distribution of expressed cancer antigens is dependent on the cancer type, different formulations must be designed and tested to treat different cancers. To allow antigen targeted vaccines to reach more patients, the development of personalized cancer vaccine strategies is an important research area [87,97]. Beyond reaching more patients, personalized vaccines incorporate multiple antigen targets, thus enhancing T cell receptor repertoire of initial immune response. However, since the antigen targets of these vaccines would likely differ between patients, the regulatory approval of such an approach may require a new mechanism.
In many of the clinical studies, cancer vaccines elicited a robust T cell response but were unable to mediate sustained tumor regression, in part, due to the immunosuppressive tumor microenvironment. Thus, combining cancer vaccines with immunomodulators such as chemotherapy or checkpoint blockade was thought to enhance their efficacy. Indeed, pre-clinical and early clinical data suggests that combining therapeutic cancer vaccines with either chemotherapy or checkpoint blockade allows for enhanced synergy when compared to a monotherapy. Based on existing research, the combination of cancer vaccines with chemotherapy or immune checkpoint blockade represent the most probable path towards clinical translation of therapeutic cancer vaccines.
5|. Expert Opinion
Vaccination as a means to prevent cancer or cancer relapse has been a long-sought goal of cancer therapy, supported by decades of research in preclinical models and in patients that T cells can be educated to target tumor cells. For cancers with a microbial etiology, vaccination has been highly effective in reducing the incidence of disease [98]. However, vaccination against established malignancy has been largely disappointing [99]. Until recently, it was generally believed that when used in combination with a cancer vaccine, cytoreductive therapy would invariably have a negative effect on vaccine-mediated immune responses and antitumor activity. However, a greater depth of understanding has suggested that the immunomodulatory properties of cytoreductive regimens might be exploited to enhance vaccine-mediated antitumor effects [100]. This synergy can be mediated by multiple mechanisms, depending on the type of cytotoxic agent and the specific vaccine employed, as well as the dosing schedule of each modality. Therefore, an increasing amount of clinical and pre-clinical data supports the use of a combination approach involving immunotherapy and front-line chemotherapy drugs as the standard method to effectively treat multiple types of cancers [101]. Therapeutic cancer vaccines have been shown to be immunogenic in clinical trials, and many of them have demonstrated efficacy in at least small numbers of patients [102]. Dendritic cell-based therapeutic cancer vaccines have been approved for clinical use, and their combination with checkpoint inhibitors is highly developed for clinical applications. Cancer-specific subunit cancer vaccines offer the possibility of an off-the-shelf approach for therapeutic cancer vaccines. Cancer vaccines are an effective means to address ‘immune ignorant’ tumors, which have a poor prognosis regardless of any current intervention. The efficient delivery of vaccine components will support the successful development of methods to activate tumor specific immune responses [103].
Given the complex interactions between cancer cells and the many components of their environment, it is reasonable to postulate that the future of immunotherapy lies in the combination of complementary immunotherapeutic strategies with chemotherapeutics or other oncogenic pathway inhibitors. The optimal approach will likely vary substantially between tumor types and may even be patient specific. This is a particularly important consideration as the rate of progress in the understanding of tumor immunology and clinical application of immunomodulatory agents has varied substantially between different types of cancers [104]. Tumors may be heterogeneous and develop clinical resistance to monotherapies and efficient antitumor strategies must focus on hitting different targets concurrently. In general, greater host and disease heterogeneity are associated with fewer options and poorer outcomes. Combination strategies will need to account for the unique genetic, epigenetic, and complexity of the cancer. By modulating inhibitory molecules, regulatory immune cells, and the metabolic resources and demands of T cells, vaccine-stimulated T cells might be induced to be fully functional within the immunosuppressive tumor microenvironment. In making therapy decisions identifying reliable biomarkers to improve patient selection, standardizing metrics for monitoring toxicities and comprehensive knowledge about the timing and dose of combination therapies will be important factors for successful development. The process will likely be iterative, as new cancer vaccine technologies are developed and more targeted immunotherapies are available which may lower toxicities while providing durable protection against cancer relapse.
Fig. 1|. Targets of Cancer Immunotherapies.
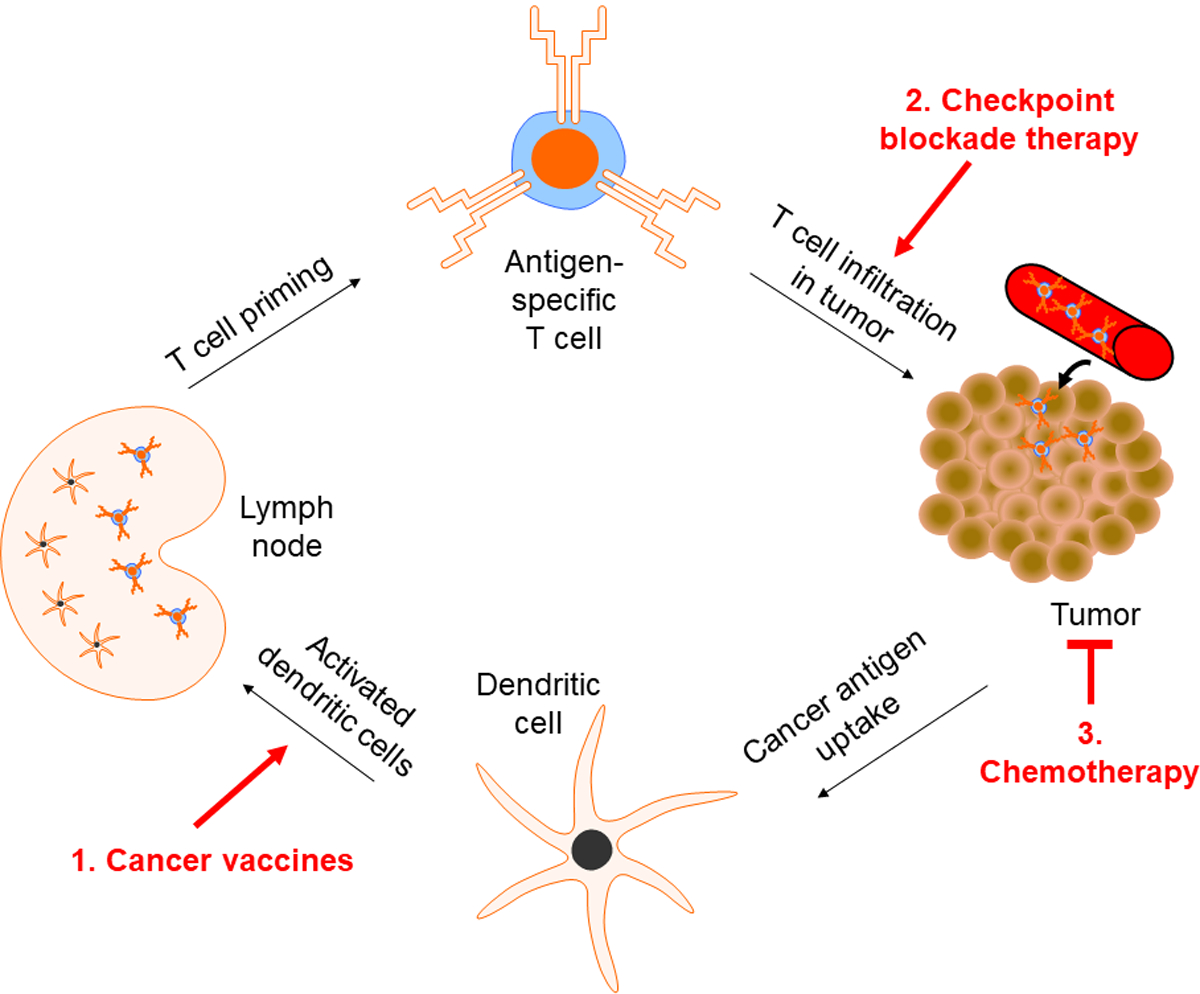
To generate a sustained T cell response against tumors, dendritic cells (DCs) uptake and present cancer antigens to T cells. Subsequently, T cells migrate to the tumor site and seek to induce cancer cell death. (1) Cancer vaccines aim to facilitate the process of antigen presentation of DCs by providing an initial source of cancer antigens. T cells that recognize these cancer antigens are activated and migrate to the tumor site to initiate cancer-cell lysis. (2) Many cancer cells upregulate immunosuppressive checkpoint blockade ligands to inactivate T cells. Thus, combining therapeutic cancer vaccines with checkpoint blockade therapy may enhance anti-tumoral efficacy and allow for better clinical outcomes. (3) Chemotherapies are generally cytoreductive and may enhance the effect of cancer vaccines by reducing concentration of immunosuppressive regulatory T cells within the tumor microenvironment thereby accentuating the vaccine mediated cytotoxic T cell response.
Table 1|.
Selected Pre-Clinical Cancer Vaccine Results
| Components | Cancer Type | Key Takeaways | Reference Number |
|---|---|---|---|
| PLG scaffold loaded with tumor lysis, GM-CSF, and CpG-ODN | Melanoma (B16-F10) | Vaccine formulation provided both prophylactic and therapeutic protection in mice. | 45 |
| GVAX cancer vaccine and anti-CTLA-4 combination | Prostate cancer (Pro-TRAMP) | Timing of checkpoint blockade and cancer vaccine administration are important. Administration of anti-CTLA-4 before or concurrent to GVAX showed no enhancement in anti-tumoral response whereas administration after GVAX showed improved survival. | 51 |
| Viral vector encoding CEA and GM-CSF plasmid | Colon adenocarcinoma (MC38-CEA) | Viral vector encoding CEA administered in combination with GM-CSF plasmid provided prophylactic protection against MC38-CEA colon adenocarcinoma model. | 64 |
| Lentiviral vector encoding NY-ESO-1 | Lung Cancer (CT26 expressing NY-ESO-1) | Viral vaccine generated NY-ESO-1 specific T-cells and provided prophylactic protection against NY-ESO-1 expressing lung cancer model. | 78 |
| DNA vaccine against tyrosinase | Melanoma (B16-F10) | DNA vaccine was effective in both prophylactic and therapeutic setting against B16-F10 melanoma. | 83 |
| PEG-alginate-based scaffold loaded with GM-CSF, CpG-ODN, and cancer antigen in combination with cytarabine and doxorubicin | Acute myeloid leukemia | Vaccine provides prophylactic protection against leukemia. In a therapeutic setting, when administered alongside standard induction chemotherapy regimen of cytarabine and doxorubicin, the therapy provided long-lasting protection against leukemia. | 94 |
PLG, poly(lactic-co-glycolic acid); GM-CSF, granulocyte-macrophage colony stimulating factor; CpG-ODN, Cytosine-guanosine oligonucleotide; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CEA, carcinoembryonic antigen; NY-ESO-1, cancer/testis antigen 1; WT1, Wilms tumor 1
Funding:
The authors are funded by the American Cancer Society (via grant number IRG-15-172-45-IRG) and the National Institutes of Health (via a National Cancer Institute grant T32 CA153915 and a National Institute of Arthritis and Musculoskeletal and Skin Diseases grant T32 AR064194).
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
6| References
- 1.Mellman I, Coukos G, Dranoff G: Cancer immunotherapy comes of age. Nature 2011, 480:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farkona S, Diamandis EP, Blasutig IM: Cancer immunotherapy: the beginning of the end of cancer? BMC medicine 2016, 14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer 2012, 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng D, Schultz L, Pardoll D, Mackall C: Cancer Immunology In Abeloff’s Clinical Oncology. Edited by: Elsevier; 2020:84–96. e85. [Google Scholar]
- 5.Wu R, Forget M-A, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi L: Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer journal (Sudbury, Mass.) 2012, 18:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD: Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nature medicine 1999, 5:677–685. [DOI] [PubMed] [Google Scholar]
- 7.Yee C, Greenberg P: Modulating T-cell immunity to tumours: new strategies for monitoring T-cell responses. Nature Reviews Cancer 2002, 2:409–419. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN: Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New England journal of medicine 2003, 348:203–213. [DOI] [PubMed] [Google Scholar]
- 9.John SY, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ: Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer research 2004, 64:4973–4979. [DOI] [PubMed] [Google Scholar]
- 10.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman W-H, Pagès F: Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer research 2011, 71:1263–1271. [DOI] [PubMed] [Google Scholar]
- 11.Franco EL, Harper DM: Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine 2005, 23:2388–2394. [DOI] [PubMed] [Google Scholar]
- 12.Martin D, Gutkind J: Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. Oncogene 2008, 27:S31–S42. [DOI] [PubMed] [Google Scholar]
- 13.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C: US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. JNCI: Journal of the National Cancer Institute 2015, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH: Therapeutic cancer vaccines. The Journal of clinical investigation 2015, 125:3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T: Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014, 515:572–576. [DOI] [PubMed] [Google Scholar]; **Describes novel method of identifying novel neo-epitopes in murine tumor models.
- 16.Gilboa E: How tumors escape immune destruction and what we can do about it. Cancer Immunology, Immunotherapy 1999, 48:382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havel JJ, Chowell D, Chan TA: The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature Reviews Cancer 2019, 19:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moyer TJ, Zmolek AC, Irvine DJ: Beyond antigens and adjuvants: formulating future vaccines. The Journal of clinical investigation 2016, 126:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overwijk WW: Cancer vaccines in the era of checkpoint blockade: the magic is in the adjuvant. Current opinion in immunology 2017, 47:103–109. [DOI] [PubMed] [Google Scholar]
- 20.Majzner RG, Mackall CL: Clinical lessons learned from the first leg of the CAR T cell journey. Nature medicine 2019, 25:1341–1355. [DOI] [PubMed] [Google Scholar]
- 21.Pusuluri A, Wu D, Mitragotri S: Immunological consequences of chemotherapy: Single drugs, combination therapies and nanoparticle-based treatments. Journal of Controlled Release 2019, 305:130–154. [DOI] [PubMed] [Google Scholar]
- 22.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. : Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. New England Journal of Medicine 2016, 375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 23.Shen X, Zhang L, Li J, Li Y, Wang Y, Xu Z-X: Recent Findings in the Regulation of Programmed Death Ligand 1 Expression. Frontiers in Immunology 2019, 10:1337–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addeo A, Banna GL, Metro G, Di Maio M: Chemotherapy in Combination With Immune Checkpoint Inhibitors for the First-Line Treatment of Patients With Advanced Non-small Cell Lung Cancer: A Systematic Review and Literature-Based Meta-Analysis. Frontiers in Oncology 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, Sonpavde G, Sternberg CN, Yegnasubramanian S, Antonarakis ES: Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. European urology 2019, 75:88–99. [DOI] [PubMed] [Google Scholar]
- 26.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H, et al. : Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet. Oncology 2014, 15:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozpiskin OM, Zhang L, Li JJ: Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 2019, 9:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss T, Weller M, Roth P: Immunological effects of chemotherapy and radiotherapy against brain tumors. Expert review of anticancer therapy 2016, 16:1087–1094. [DOI] [PubMed] [Google Scholar]
- 29.Hader M, Frey B, Fietkau R, Hecht M, Gaipl US: Immune biological rationales for the design of combined radio-and immunotherapies. Cancer Immunology, Immunotherapy 2020, 69:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung S, Unutmaz D, Wong P, Sano G-I, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. : In Vivo Depletion of CD11c+ Dendritic Cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity 2002, 17:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mempel TR, Henrickson SE, von Andrian UH: T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004, 427:154–159. [DOI] [PubMed] [Google Scholar]
- 32.Torralba D, Baixauli F, Villarroya-Beltri C, Fernández-Delgado I, Latorre-Pellicer A, Acín-Pérez R, Martín-Cófreces NB, Jaso-Tamame ÁL, Iborra S, Jorge I, et al. : Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nature Communications 2018, 9:2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palucka K, Banchereau J: Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheever MA, Higano CS: PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clinical Cancer Research 2011, 17:3520. [DOI] [PubMed] [Google Scholar]; **Overviews of clinical data leading to approval of Provenge vaccine.
- 35.Arlen Philip M, Skarupa L, Pazdur M, Seetharam M, Tsang Kwong Y, Grosenbach Douglas W, Feldman J, Poole Diane J, Litzinger M, Steinberg Seth M, et al. : Clinical Safety of a Viral Vector Based Prostate Cancer Vaccine Strategy. Journal of Urology 2007, 178:1515–1520. [DOI] [PubMed] [Google Scholar]
- 36.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. : Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010, 28:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Y, Walsh AM, Canavan M, Wechalekar MD, Cole S, Yin X, Scott B, Loza M, Orr C, McGarry T, et al. : Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PloS one 2018, 13:e0192704–e0192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinmann SC, Pisetsky DS: Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford, England) 2019, 58:vii59–vii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avigan D, Rosenblatt J: Vaccine therapy in hematologic malignancies. Blood 2018, 131:2640–2650. [DOI] [PubMed] [Google Scholar]
- 40.van de Loosdrecht AA, van Wetering S, Santegoets SJ, Singh SK, Eeltink CM, den Hartog Y, Koppes M, Kaspers J, Ossenkoppele GJ, Kruisbeek AM: A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunology, Immunotherapy 2018, 67:1505–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Describes phase I study of novel allogenic DC vaccine, DCP-001.
- 41.Simons JW, Jaffee EM, Weber CE, Levitsky HI, Nelson WG, Carducci MA, Lazenby AJ, Cohen LK, Finn CC, Clift SM, et al. : Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer research 1997, 57:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 42.Shirota H, Klinman DM: CpG-conjugated apoptotic tumor cells elicit potent tumor-specific immunity. Cancer immunology, immunotherapy : CII 2011, 60:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curry WT, Jr., Gorrepati R, Piesche M, Sasada T, Agarwalla P, Jones PS, Gerstner ER, Golby AJ, Batchelor TT, Wen PY, et al. : Vaccination with Irradiated Autologous Tumor Cells Mixed with Irradiated GM-K562 Cells Stimulates Antitumor Immunity and T Lymphocyte Activation in Patients with Recurrent Malignant Glioma. Clinical cancer research : an official journal of the American Association for Cancer Research 2016, 22:2885–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ: Infection-mimicking materials to program dendritic cells in situ. Nature materials 2009, 8:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Describes development of PLG scaffold designed to release GM-CSF to recruit DCs.
- 45.Ali OA, Emerich D, Dranoff G, Mooney DJ: In situ regulation of DC subsets and T cells mediates tumor regression in mice. Science translational medicine 2009, 1:8ra19–18ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]; **PLG based cancer vccine mediates prophylactic and therapeutic protection against B16 melanoma in mice.
- 46.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. : Novel Allogeneic Granulocyte-Macrophage Colony-Stimulating Factor–Secreting Tumor Vaccine for Pancreatic Cancer: A Phase I Trial of Safety and Immune Activation. Journal of Clinical Oncology 2001, 19:145–156. [DOI] [PubMed] [Google Scholar]; **Phase I study of GVAX, a GM-CSF transfected allogenic pancreatic cancer cell based therapeutic cancer vaccine.
- 47.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. : A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Annals of surgery 2011, 253:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santegoets SJAM Schreurs MWJ, Masterson AJ Liu YP, Goletz S, Baumeister H, Kueter EWM, Lougheed SM, van den Eertwegh AJM, Scheper RJ, et al. : In vitro priming of tumor-specific cytotoxic T lymphocytes using allogeneic dendritic cells derived from the human MUTZ-3 cell line. Cancer Immunology, Immunotherapy 2006, 55:1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Loosdrecht AA, van Wetering S, Santegoets SJAM, Singh SK, Eeltink CM, den Hartog Y, Koppes M, Kaspers J, Ossenkoppele GJ, Kruisbeek AM, et al. : A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunology, Immunotherapy 2018, 67:1505–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li B, VanRoey M, Wang C, Chen T-hT, Korman A, Jooss K : Anti–Programmed Death-1 Synergizes with Granulocyte Macrophage Colony-Stimulating Factor–Secreting Tumor Cell Immunotherapy Providing Therapeutic Benefit to Mice with Established Tumors. Clinical Cancer Research 2009, 15:1623. [DOI] [PubMed] [Google Scholar]
- 51.Wada S, Jackson CM, Yoshimura K, Yen H-R, Getnet D, Harris TJ, Goldberg MV, Bruno TC, Grosso JF, Durham N, et al. : Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. Journal of Translational Medicine 2013, 11:89–89. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Relative timing of administration of GVAX cancer vaccine and checkpoint blockade, anti-CTLA-4, is an important parameter in enhancing cancer vaccine efficacy.
- 52.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G: Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Research 2013, 73:3591–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Enhanced efficacy of GVAX vaccine when combined with dual PD-1 and CTLA-4 checkpoint blockade.
- 53.Santos PM, Adamik J, Howes TR, Du S, Vujanovic L, Warren S, Gambotto A, Kirkwood JM, Butterfield LH: Impact of checkpoint blockade on cancer vaccine–activated CD8+ T cell responses. Journal of Experimental Medicine 2020, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Versteven M, Van den Bergh JM, Marcq E, Smits EL, Van Tendeloo VF, Hobo W, Lion E: Dendritic cells and programmed death-1 blockade: a joint venture to combat cancer. Frontiers in immunology 2018, 9:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mouraviev V, Mariados N, Albala D, Concepcion RS, Shore ND, Sims RB, Emberton M, Pieczonka CM: The Rationale for Optimal Combination Therapy With Sipuleucel-T for Patients With Castration-resistant Prostate Cancer. Reviews in urology 2014, 16:122–130. [PMC free article] [PubMed] [Google Scholar]
- 56.Hollingsworth RE, Jansen K: Turning the corner on therapeutic cancer vaccines. NPJ vaccines 2019, 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerk SA, Finkel KA, Pearson AT, Warner KA, Zhang Z, Nör F, Wagner VP, Vargas PA, Wicha MS, Hurt EM: 5T4-targeted therapy ablates cancer stem cells and prevents recurrence of head and neck squamous cell carcinoma. Clinical Cancer Research 2017, 23:2516–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Pan L, Yao M, Cai Y, Dong Z, Yao D: Expression of oncofetal antigen glypican-3 associates significantly with poor prognosis in HBV-related hepatocellular carcinoma. Oncotarget 2016, 7:42150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalyan A, Kircher S, Shah H, Mulcahy M, Benson A: Updates on immunotherapy for colorectal cancer. Journal of gastrointestinal oncology 2018, 9:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warnecke-Eberz U, Metzger R, Hölscher AH, Drebber U, Bollschweiler E: Diagnostic marker signature for esophageal cancer from transcriptome analysis. Tumor Biology 2016, 37:6349–6358. [DOI] [PubMed] [Google Scholar]
- 61.Cua S, Tan HL, Fong WJ, Chin A, Lau A, Ding V, Song Z, Yang Y, Choo A: Targeting of embryonic annexin A2 expressed on ovarian and breast cancer by the novel monoclonal antibody 2448. Oncotarget 2018, 9:13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirayama M, Tomita Y, Yuno A, Tsukamoto H, Senju S, Imamura Y, Sayem MA, Irie A, Yoshitake Y, Fukuma D, et al. : An oncofetal antigen, IMP-3-derived long peptides induce immune responses of both helper T cells and CTLs. Oncoimmunology 2016, 5:e1123368–e1123368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamai T, Mizukoshi E, Kumagai M, Terashima T, Iida N, Kitahara M, Shimakami T, Kitamura K, Arai K, Yamashita T, et al. : A novel α-fetoprotein-derived helper T-lymphocyte epitope with strong immunogenicity in patients with hepatocellular carcinoma. Scientific Reports 2020, 10:4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hensel JA, Khattar V, Ashton R, Ponnazhagan S: Recombinant AAV-CEA Tumor Vaccine in Combination with an Immune Adjuvant Breaks Tolerance and Provides Protective Immunity. Molecular therapy oncolytics 2018, 12:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Describes development of rAAV-CEA cancer vaccine and its ability to mediate prophylactic protection against murine MC38-CEA colon adenocarcinoma.
- 65.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. : The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clinical cancer research : an official journal of the American Association for Cancer Research 2009, 15:5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG, Voillet V, Gottardo R, Ragnarsson GB, Bleakley M: T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nature Medicine 2019, 25:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maslak PG, Dao T, Bernal Y, Chanel SM, Zhang R, Frattini M, Rosenblat T, Jurcic JG, Brentjens RJ, Arcila ME, et al. : Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv 2018, 2:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Batchu RB, Gruzdyn O, Potti RB, Weaver DW, Gruber SA: MAGE-A3 With Cell-Penetrating Domain as an Efficient Therapeutic Cancer Vaccine. JAMA Surgery 2014, 149:451–457. [DOI] [PubMed] [Google Scholar]
- 69.Gérard C, Baudson N, Ory T, Louahed J: Tumor mouse model confirms MAGE-A3 cancer immunotherapeutic as an efficient inducer of long-lasting anti-tumoral responses. PloS one 2014, 9:e94883–e94883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Junwei W, Xiumin Z, Jing Y, Shoujing Y, Zengshan L: In vivo enhancement of the MAGE-specific cellular immune response by a recombinant MAGE1-MAGE3-TBHSP70 tumor vaccine. Cancer Cell International 2016, 16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gérard C, Baudson N, Ory T, Louahed J: Tumor Mouse Model Confirms MAGE-A3 Cancer Immunotherapeutic As an Efficient Inducer of Long-Lasting Anti-Tumoral Responses. PloS one 2014, 9:e94883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sypniewska RK, Hoflack L, Tarango M, Gauntt S, Leal BZ, Reddick RL, Gravekamp C: Prevention of metastases with a Mage-b DNA vaccine in a mouse breast tumor model: potential for breast cancer therapy. Breast Cancer Research and Treatment 2005, 91:19–28. [DOI] [PubMed] [Google Scholar]
- 73.Castro F, Leal B, Denny A, Bahar R, Lampkin S, Reddick R, Lu S, Gravekamp C: Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. British journal of cancer 2009, 101:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duperret EK, Liu S, Paik M, Trautz A, Stoltz R, Liu X, Ze K, Perales-Puchalt A, Reed C, Yan J, et al. : A Designer Cross-reactive DNA Immunotherapeutic Vaccine that Targets Multiple MAGE-A Family Members Simultaneously for Cancer Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2018, 24:6015–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Describes development of MAGE-A cancer vaccine with cross-reactivity for multiple MAGE-A isoforms.
- 75.Parvanova I, Rettig L, Knuth A, Pascolo S: The form of NY-ESO-1 antigen has an impact on the clinical efficacy of anti-tumor vaccination. Vaccine 2011, 29:3832–3836. [DOI] [PubMed] [Google Scholar]
- 76.Li M, Shi H, Mu Y, Luo Z, Zhang H, Wan Y, Zhang D, Lu L, Men K, Tian Y, et al. : Effective inhibition of melanoma tumorigenesis and growth via a new complex vaccine based on NY-ESO-1-alum-polysaccharide-HH2. Molecular cancer 2014, 13:179–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue W, Metheringham RL, Brentville VA, Gunn B, Symonds P, Yagita H, Ramage JM, Durrant LG: SCIB2, an antibody DNA vaccine encoding NY-ESO-1 epitopes, induces potent antitumor immunity which is further enhanced by checkpoint blockade. OncoImmunology 2016, 5:e1169353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albershardt TC, Campbell DJ, Parsons AJ, Slough MM, ter Meulen J, Berglund P: LV305, a dendritic cell-targeting integration-deficient ZVexTM-based lentiviral vector encoding NY-ESO-1, induces potent anti-tumor immune response. Molecular Therapy - Oncolytics 2016, 3:16010. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Describes development of LV305 cancer vaccine to provide protection against NY-ESO-1 expressing CT26 lung cancer model.
- 79.Somaiah N, Block MS, Kim JW, Shapiro GI, Do KT, Hwu P, Eder JP, Jones RL, Lu H, ter Meulen J, et al. : First-in-Class, First-in-Human Study Evaluating LV305, a Dendritic-Cell Tropic Lentiviral Vector, in Sarcoma and Other Solid Tumors Expressing NY-ESO-1. Clinical Cancer Research 2019:clincanres.1025.2019. [DOI] [PubMed] [Google Scholar]
- 80.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA: Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proceedings of the National Academy of Sciences of the United States of America 1994, 91:3515–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA: Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. The Journal of experimental medicine 1994, 180:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cormier JN, Salgaller ML, Prevette T, Barracchini KC, Rivoltini L, Restifo NP, Rosenberg SA, Marincola FM: Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. The cancer journal from Scientific American 1997, 3:37–44. [PMC free article] [PubMed] [Google Scholar]
- 83.Yan J, Tingey C, Lyde R, Gorham TC, Choo DK, Muthumani A, Myles D, Weiner LP, Kraynyak KA, Reuschel EL, et al. : Novel and enhanced anti-melanoma DNA vaccine targeting the tyrosinase protein inhibits myeloid-derived suppressor cells and tumor growth in a syngeneic prophylactic and therapeutic murine model. Cancer Gene Therapy 2014, 21:507–517. [DOI] [PubMed] [Google Scholar]; **Describes development of DNA vaccine against Tyr and its effectiveness in both prophylactic and therapeutic protection against
- 84.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. : PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Presence of TILs is assoicated with favorable outcomes with checkpoint blockade therapy.
- 85.Conniot J, Scomparin A, Peres C, Yeini E, Pozzi S, Matos AI, Kleiner R, Moura LIF, Zupančič E, Viana AS, et al. : Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nature Nanotechnology 2019, 14:891–901. [DOI] [PubMed] [Google Scholar]
- 86.Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C, Türeci Ö, Sahin U: Intranodal Vaccination with Naked Antigen-Encoding RNA Elicits Potent Prophylactic and Therapeutic Antitumoral Immunity. Cancer Research 2010, 70:9031. [DOI] [PubMed] [Google Scholar]
- 87.Sahin U, Derhovanessian E, Miller M, Kloke B-P, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, et al. : Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547:222–226. [DOI] [PubMed] [Google Scholar]
- 88.Liang Y, Lü W, Zhang X, Lü B: Tumor-infiltrating CD8+ and FOXP3+ lymphocytes before and after neoadjuvant chemotherapy in cervical cancer. Diagnostic pathology 2018, 13:93–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao Q, Wang S, Chen X, Cheng S, Zhang Z, Li F, Huang L, Yang Y, Zhou B, Yue D, et al. : Cancer-cell-secreted CXCL11 promoted CD8+ T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. Journal for ImmunoTherapy of Cancer 2019, 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN: Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer. Frontiers in immunology 2019, 10:1654–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bauer C, Sterzik A, Bauernfeind F, Duewell P, Conrad C, Kiefl R, Endres S, Eigler A, Schnurr M, Dauer M: Concomitant gemcitabine therapy negatively affects DC vaccine-induced CD8+ T-cell and B-cell responses but improves clinical efficacy in a murine pancreatic carcinoma model. Cancer Immunology, Immunotherapy 2014, 63:321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petrizzo A, Mauriello A, Luciano A, Rea D, Barbieri A, Arra C, Maiolino P, Tornesello M, Gigantino V, Botti G, et al. : Inhibition of tumor growth by cancer vaccine combined with metronomic chemotherapy and anti-PD-1 in a pre-clinical setting. Oncotarget; Vol 9, No 3 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shah NJ, Najibi AJ, Shih T-Y, Mao AS, Sharda A, Scadden DT, Mooney DJ: A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nature Biomedical Engineering 2020, 4:40–51. [DOI] [PubMed] [Google Scholar]; **Describes developent of alginate-based cancer vaccine against murine AML.
- 94.Zuber J, Radtke I, Pardee T, Zhao Z, Rappaport A, Luo W, McCurrach M, Yang M-M, Dolan M, Kogan S, et al. : Mouse models of human AML accurately predict chemotherapy response. Genes & development 2009, 23:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM: Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nature medicine 2016, 22:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ: Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507:519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. : An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grenier JM, Yeung ST, Khanna KM: Combination Immunotherapy: Taking Cancer Vaccines to the Next Level. Front Immunol 2018, 9:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Romero P, Banchereau J, Bhardwaj N, Cockett M, Disis ML, Dranoff G, Gilboa E, Hammond SA, Hershberg R, Korman AJ, et al. : The Human Vaccines Project: A roadmap for cancer vaccine development. Sci Transl Med 2016, 8:334ps339. [DOI] [PubMed] [Google Scholar]
- 100.Chen G, Emens LA: Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother 2013, 62:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong KK, Li WA, Mooney DJ, Dranoff G: Advances in therapeutic cancer vaccines In Advances in immunology. Edited by: Elsevier; 2016:191–249. vol 130.] [DOI] [PubMed] [Google Scholar]
- 102.Schreiber TH, Raez L, Rosenblatt JD, Podack ER: Tumor immunogenicity and responsiveness to cancer vaccine therapy: the state of the art. Semin Immunol 2010, 22:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM: Improving vaccine and immunotherapy design using biomaterials. Trends in immunology 2018, 39:135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galon J, Bruni D: Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nature Reviews Drug Discovery 2019, 18:197–218. [DOI] [PubMed] [Google Scholar]


