Summary
Subset analysis of patients with sarcomatoid renal cell carcinoma (sRCC) included in the CheckMate 214 trial of ipilimumab-nivolumab vs sunitinib showed improved outcomes in sRCC with ipilimumab-nivolumab. The use of checkpoint inhibitor-based regimens in sRCC, for which therapeutic options were once limited, is further supported by additional clinical trials.
Keywords: sarcomatoid renal cell carcinoma, ipilimumab, nivolumab, checkpoint inhibitors, combination immunotherapy, renal cell carcinoma
In this issue of CLINICAL CANCER RESEARCH, Tannir and colleagues report on a subset analysis of CheckMate 214, specifically the subset of patients with advanced renal cell carcinoma and sarcomatoid features, and their clinical outcomes with ipilimumab-nivolumab versus sunitinib(1).
Sarcomatoid renal cell carcinoma (sRCC) refers to a form of RCC characterized histologically by the presence of spindle-shaped mesenchymal-looking cells typically in a portion of the tumor. sRCC may arise in any histologic subtype of RCC, likely via de-differentiation from a common cell of origin.. Clinically, sRCC is associated with aggressive disease and a poor prognosis. Compared with patients with non-sarcomatoid RCC, patients with sRCC more frequently have intermediate- or poor- International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk disease, shorter time to relapse, lower objective response rates (ORR), and less frequently receive 2nd or 3rd line treatments(2).
Therapeutic options for sRCC have historically been limited. In the phase II ECOG 8802 trial of doxorubicin and gemcitabine in sRCC, the combination achieved an ORR of just 16%, complete response rate (CR) of 2.7%, median overall survival (OS) of 8.8 months, and median progression free survival (PFS) of 3.5 months(2). The record of mTOR inhibitors in sRCC is also poor, with an estimated ORR of 13%(2). Sunitinib plus gemcitabine produced only somewhat more success, with ORR ranging 20–26%, CR around 3%, and median OS of approximately 10 months in phase II trials of the combination(2). Thus, there is a significant unmet need for effective therapies for sRCC.
In this issue, Tannir and colleagues report on a post-hoc analysis of the subgroup of patients with sRCC from CheckMate 214 with intermediate/poor-risk disease. The study previously demonstrated the long-term superiority of first-line ipilimumab-nivolumab versus sunitinib in advanced clear cell RCC(3). 145 patients with sRCC were identified post-hoc based on local pathology reports or central independent review. Central review determination of any percent sarcomatoid histology was considered sarcomatoid-positive based on consensus between three pathologists blinded on clinical outcomes, with discrepancies resolved on consensus review. 139 patients with intermediate/poor-risk disease were identified. Baseline characteristics of the sRCC subgroup were similar between the treatment cohorts based on gender, IMDC risk group, tumor PD-L1 levels, prior nephrectomy, and sites of metastases. Outcomes were assessed over a median follow up time of 47.7 months.
Confirmed ORR was 61% with ipilimumab-nivolumab in IMDC intermediate- or poor-risk sRCC (N=74) versus 23% with sunitinib (N=65, p<0.0001). Notably, CR with ipilimumab-nivolumab was 19% versus 3% with sunitinib. Median OS was not reached for ipilimumab-nivolumab versus 14.5 months for sunitinib (HR 0.45 (0.3–0.7), p=0.0004). Median PFS was 26.5 months with ipilimumab-nivolumab versus 5.1 months with sunitinib (HR 0.54 (0.3–0.9), p=0.0093). The OS, median PFS, and ORR benefits of ipilimumab-nivolumab vs sunitinib were seen regardless of tumor PD-L1 levels (≥1% versus < 1%), though the magnitude of the OS, median PFS, and ORR were higher in those with PD-L1 ≥1. The safety profile of ipilimumab-nivolumab in patients with sRCC was consistent with prior safety reports in the overall population (3).
These findings add to other subgroup analyses of sRCC in recent first-line phase 3 trials supporting the efficacy of immune checkpoint inhibitor (ICI)-based regimens compared to sunitinib. These include patients with sRCC treated with pembrolizumab-axitinib (N=51) in the subset analysis of the KEYNOTE-426 trial (12-month OS of 83.4%, ORR of 58.8%, CR of 11.8%)(4) and with atezolizumab-bevacizumab (N=68) in the subset analysis of IMmotion151 (12-month OS of 69%, ORR of 49%, CR of 10%)(5). Finally, the subset of patients with sRCC treated with JAVELIN Renal 101 trial also showed similarly improved outcomes with avelumab-axitinib (12-month OS 83%, ORR of 47%, CR of 4%)(6) [Table 1]. Indeed, given the ICI activity in sRCC in these combinations, ICI-based regimens have a crucial role in first-line treatment of sRCC.
Table 1.
Comparison of clinical outcomes of sarcomatoid renal cell carcinoma from major first line trials in metastatic renal cell carcinoma. NR: not reached; NA: not available
| Ipilimumab/Nivolumab CheckMate 214 (N=74) | Axitinib/Pembrolizumab KEYNOTE 426 (N=51) | Axitinib/Avelumab JAVELIN Renal 101 (N=47) | Atezolizumab/Bevacizumab IMmotion 151 (N=68) | |
|---|---|---|---|---|
| ORR | 61% | 59% | 47% | 49% |
| CR | 19% | 12% | 4% | 10% |
| Median PFS HR (95% CI) vs sunitinib | 26.5 months 0.54 (0.3–0.9) | NR 0.54 (0.29–1.00) | 7.0 months 0.57 (0.33–1.00) | 8.3 months 0.52 (0.34–0.79) |
| 12 month PFS | 57% (est.) | 57% | 35% (est.) | 39% |
| Median OS HR (95% CI) vs sunitinib | NR 0.45 (0.3–0.7) | NR 0.58 (0.21–1.59) | NA | 21.7 months 0.64 (0.41–1.01) |
| 12 month OS | 84% (est.) | 83% | 83% | 56% |
Notably, in the CheckMate 214 study, the ORR including CR in the sRCC subgroup were numerically greater than in the overall IMDC intermediate- or poor-risk population treated with ipilimumab-nivolumab (ORR 61% vs. 42% and CR 18% vs. 11%)(3). Inasmuch as sarcomatoid changes are thought to arise in the context of a pre-existing RCC, it is interesting that tumors with this form of de-differentiation appear to be particularly sensitive to ICI. Whether this reflects a heretofore unidentified subtype of RCC with a tendency towards sarcomatoid dedifferentiation and intrinsic features that render it more susceptible to an immune-mediated attack, or whether sarcomatoid dedifferentiation by itself is responsible for the heightened responsiveness to ICI is unclear. Although, if the latter, only the sarcomatoid component might be expected to respond.
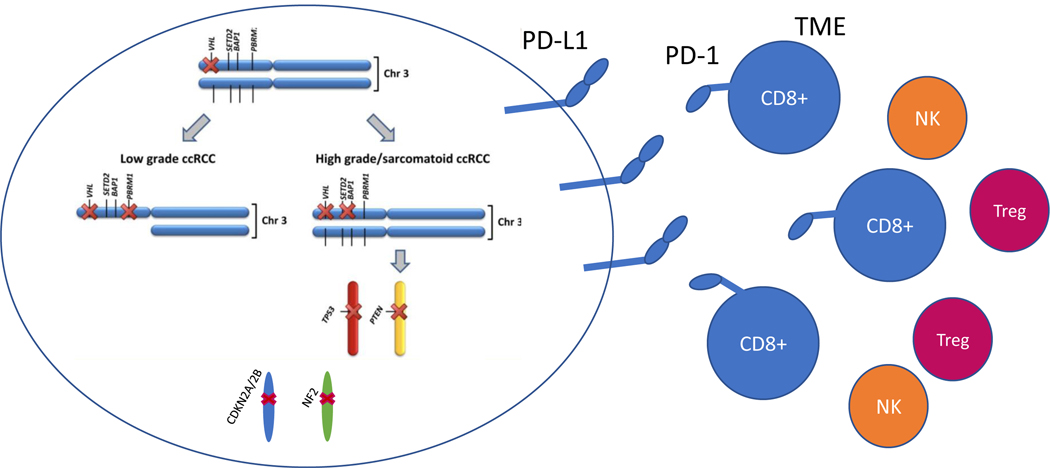
The susceptibility of sRCC to ICIs is consistent with our emerging understanding of the immune profile of sRCC. PD-L1 expression and intra-tumoral CD8-positive cell density are higher in sRCC versus non-sarcomatoid RCC(2), suggesting the presence of pre-existing intratumoral T cells exhausted by PD-L1 engagement [Figure 1]. Subset analysis of IMmotion151 also found higher PD-L1+ and T-effector gene expression in the sRCC group(5).
Figure 1.
Molecular mutations common in sarcomatoid renal cell carcinoma and interactions with tumor microenvironment driving sensitivity to immune checkpoint inhibitors. TME: tumor microenvironment; NK: natural killer cells; Treg: regulatory T-cells. Adapted from Wang et al., Clinical Cancer Research, 2017 (10).
An important factor relevant to ICI response is the tumor microenvironment (TME). An empiric characterization of the TME in RCC using matched trios of bulk tumor, patient-derived xenograft, and normal tissue, revealed a highly inflamed pan-RCC TME subtype characterized by extensive immune infiltrate(7). This inflamed subtype was enriched in aggressive RCCs with BAP1 mutations, which are particularly prevalent in sRCC (7). Interestingly, inflammation in the TME correlated with thrombocytosis and anemia (7), two validated IMDC criteria, suggesting that thrombocytosis and anemia may potentially represent systemic manifestations of an inflammatory response elicited by the tumor. This could potentially explain the higher frequency of IMDC risk factors in sRCC, which are characteristically inflamed.
Why did some patients with sRCC respond to ipilimumab-nivolumab while others did not? This heterogeneity in treatment response may relate to the genetic heterogeneity of sRCC. Mutations enriched in sRCC involve TP53; Hippo pathway members, in particular NF2; CDKN2A/2B; the PI3K regulator PTEN; and the deubiquitinase BAP1 (8–10) [Figure 1]. Transcriptional profiling of sRCC’s has also identified an enrichment of TGF-β signaling(10), potentially driving resistance to immunotherapies in a subset of these patients. Further work remains to understand the molecular drivers of ICI resistance.
In sum, these findings support the use of ipilimumab-nivolumab, and more broadly the incorporation of ICIs, in the treatment of sRCC. Given the multiplicity of pathways implicated, additional responses in sRCC may be gained from combination therapies. Elucidation of how the tumor and TME characteristics affect the traditional IMDC risk model remains key. The dramatic change in outcomes brought about with ICIs on sRCC, which are disproportionally associated with IMDC intermediate and poor risk features, represent a welcome complexity in the interpretation of the prognostic model in the ICI era. Mutations, gene expression, and TME features likely represent molecular determinants of response and may be helpful in the future to optimize treatment selection in metastatic renal cell carcinoma.
Acknowledgments
Funding Sources: J. Brugarolas is supported by an NCI SPORE grant (P50 CA196516).
Conflict of interest disclosures:
TZ: Research funding (to Duke) from Pfizer, Janssen, Acerta, Abbvie, Novartis, Merrimack, OmniSeq, PGDx, Merck, Mirati, and Astellas; consulting/speaking with Genentech Roche, Exelixis, Genomic Health, and Sanofi Aventis; and consulting/advisory board with AstraZeneca, Bayer, Pfizer, Foundation Medicine, Janssen, Amgen, BMS, Calithera, and MJH Associates. Stock ownership/employment (spouse) from Capio Biosciences, Archimmune Therapeutics, and Nanorobotics.
JKH: No disclosures.
NA: Consultancy to: Astellas, Astra Zeneca, Bayer, Bristol Myers Squibb, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Janssen, Merck, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics. Research funding to my institution: Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon.
JB: Research funding: Arrowhead Pharmaceuticals and Genentech/Roche; Consultancy: Arrowhead Pharmaceuticals, Exelixis, Nektar Therapeutics.
References:
- 1.Tannir NM, Signoretti S, Choueiri TK, McDermott DF, Motzer RJ, Abdallah F, et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debien V, Thouvenin J, Lindner V, Barthélémy P, Lang H, Flippot R, et al. Sarcomatoid dedifferentiation in renal cell carcinoma: From novel molecular insights to new clinical opportunities. Cancers (Basel). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, Arén Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020; 8(2): e000891; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J Clin Oncol. 2019; [Google Scholar]
- 5.Rini BI, Motzer RJ, Powles T, McDermott DF, Escudier B, Donskov F, et al. Atezolizumab plus Bevacizumab Versus Sunitinib for Patients with Untreated Metastatic Renal Cell Carcinoma and Sarcomatoid Features: A Prespecified Subgroup Analysis of the IMmotion151 Clinical Trial. Eur Urol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri TK, Larkin JMG, Pal SK, Motzer RJ, Venugopal B, Alekseev BY, et al. Efficacy and biomarker analysis of patients (pts) with advanced renal cell carcinoma (aRCC) with sarcomatoid histology (sRCC): Subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab plus axitinib (A + Ax) vs sunitinib (S). Ann Oncol. 2019; [Google Scholar]
- 7.Wang T, Lu R, Kapur P, Jaiswal BS, Hannan R, Zhang Z, et al. An empirical approach leveraging tumorgrafts to dissect the tumor microenvironment in renal cell carcinoma identifies missing link to prognostic inflammatory factors. Cancer Discov. 2018. September; 8(9): 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi M, Zhao S, Said JW, Merino MJ, Adeniran AJ, Xie Z, et al. Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma. Proc Natl Acad Sci U S A. 2016; February 23; 113(8): 2170–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malouf GG, Flippot R, Dong Y, Dinatale RG, Chen YB, Su X, et al. Molecular characterization of sarcomatoid clear cell renal cell carcinoma unveils new candidate oncogenic drivers. Sci Rep. 2020; 10: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Kim TB, Peng B, Karam J, Creighton C, Joon A, et al. Sarcomatoid renal cell carcinoma has a distinct molecular pathogenesis, driver mutation profile, and transcriptional landscape. Clin Cancer Res. 2017; November 1; 23(21): 6686–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]