Abstract
Purpose:
Increased β-adrenergic receptor (β-AR) signaling has been shown to promote the creation of an immunosuppressive tumor microenvironment. Preclinical studies have shown that abrogation of this signaling pathway, particularly β2-AR provides a more favorable anti-tumor microenvironment that enhances the activity of anti-PD-1 checkpoint inhibitors. We hypothesize that blocking stress-related immune suppressive pathways would improve tumor response to immune checkpoint inhibitors in patients. Here we report the results of dose escalation of a non-selective β-blocker (propranolol) with pembrolizumab in patients with metastatic melanoma.
Experimental Design:
A 3 + 3 dose-escalation study for propranolol BID with pembrolizumab (200 mg every 3 weeks) was completed. The primary objective was to determine the recommended phase 2 dose (RP2D). Additional objectives included safety, antitumor activity and biomarker analyses. Responders were defined as patients with complete or partial response per immune-modified RECIST at 6 months.
Results:
Nine metastatic melanoma patients received increasing doses of propranolol in cohorts of 10, 20 and 30 mg BID. No dose-limiting toxicities were observed. Most common treatment-related adverse events (TRAEs) were rash, fatigue and vitiligo, observed in 44% patients. One patient developed two ≥ grade 3 TRAEs. Objective response rate was 78%. While no significant changes in treatment-associated biomarkers were observed, an increase in IFN-γ and a decrease in IL-6 was noted in responders.
Conclusions:
Combination of propranolol with pembrolizumab in treatment-naïve metastatic melanoma is safe and shows very promising activity. Propranolol 30 mg BID was selected as RP2D in addition to pembrolizumab based on safety, tolerability and preliminary antitumor activity.
Introduction
Use of immune-checkpoint inhibitors has revolutionized the treatment of melanoma. A recent 5-year follow-up of patients with treatment-naïve metastatic melanoma treated with pembrolizumab has shown an overall survival rate of greater than 40% [1]. Similarly, the CheckMate 067 trial showed a 44% 5 year survival and 45% objective response with nivolumab monotherapy in untreated unresectable stage III or IV melanoma [2]. Although response rates and overall survival are numerically higher with combination of anti-PD-1 and anti-CTLA4 checkpoint inhibitors, 59% of patients developed grade 3 or 4 treatment-related adverse events (TRAEs) with combination nivolumab and ipilimumab, as opposed to 23% with nivolumab monotherapy [2]. On the other hand, with pembrolizumab monotherapy, ≥ grade 3 TRAEs were seen in only 17% of patients [1]. These data highlight an unmet need for better combination therapies using anti-PD1 checkpoint inhibitors that result in improved outcome and manageable toxicity profiles.
Increased β-adrenergic receptor (β-AR) signaling, driven by sympathetic nerves of the autonomic nervous system, has been shown to increase tumor growth in mouse melanoma models. Several studies have shown that β-adrenergic blockade with non-selective β-blocker propranolol in mouse melanoma models decreases tumor growth and metastasis [3, 4]. Propranolol exerts an anti-tumor effect by favorably modulating the tumor microenvironment (TME) by decreasing myeloid-derived suppressor cells (MDSC) and increasing CD8+ T-cell and natural killer cells in the TME [5]. Additionally, Bucsek et al. have shown a synergistic action of anti-PD1 antibody and propranolol using a B16-OVA melanoma model in mice experiencing chronic stress. Stress conditions were created by housing mice under the standard housing temperature at 22°C required for experimental mouse colonies [4]. Additionally, prior research in murine models and healthy volunteers has shown that stress-induced sympathetic nervous system leads to increase in levels of pro-inflammatory cytokines inflammation [6–8], which may be a key biological mechanism how stress affects health and promotes tumor progression. Pharmacologically blocking this sympathetic activation attenuates the inflammatory response to stress [6]. These preclinical observations form the foundation of our clinical investigation of this combination therapy in patients with metastatic melanoma.
Multiple retrospective studies have shown an improvement in overall survival (OS) in cancer patients treated with β-blockers [9–11]. A prospective study in patients with early stage melanoma treated with adjuvant propranolol reported an improvement in disease free survival [12]. Kokolus et al. in a retrospective study has shown a statistically significant improvement in OS in patients with metastatic melanoma treated concurrently with non-selective β-blocker and immunotherapy compared to patients treated with immunotherapy alone. Interestingly, no benefit was seen in patients who were treated with β1-selective blockers [13]. In our retrospective analysis, we did not see an improvement in OS in patients concurrently treated with β-blocker and checkpoint inhibitor, which could be due to a mix of different tumor histologies analyzed in the study, in addition to a small number of patients on non-selective β-blockers. No increase in ≥ grade 3 adverse events was observed in this cohort [14].
Based on preclinical data showing synergistic activity of anti-PD1 antibody and propranolol, and retrospective data showing efficacy and safety of this combination, we decided to prospectively evaluate the combination of propranolol and pembrolizumab in patients with metastatic melanoma. We hypothesized that the addition of the non-selective β-blocker propranolol to pembrolizumab may improve its anti-tumor efficacy by decreasing adrenergic stress, which is likely to be increased in patients with a new diagnosis of cancer.
Methods
Study Population
Eligible patients were recruited from the melanoma clinic at Roswell Park Comprehensive Cancer Center (Roswell Park), Buffalo, New York between January 2018 and August 2019. Eligibility criteria included adult patients (aged ≥ 18 years), Eastern Cooperative Oncology Group (ECOG) performance status of 0 (indicating no symptoms) or 1 (indicating mild symptoms) with treatment naïve, histologically confirmed unresectable stage III or IV melanoma (any histological subtype), with good organ function, and measurable disease on computed tomography (CT; preferred) or magnetic resonance imaging scans per immune-modified Response Evaluation Criteria In Solid Tumors (imRECIST) guidelines and absence of symptomatic brain metastases. Key exclusion criteria were prior therapy with PD-1/PD-L1 inhibitors, chronic autoimmune disease or other immunodeficiency syndromes, contraindication to use of β-blockers (uncontrolled depression, grade III or IV heart failure, severe asthma or COPD, uncontrolled type 1 or type 2 diabetes mellitus with HbA1C > 8.5 or fasting plasma glucose > 160 mg/dl, symptomatic peripheral arterial disease or Raynaud’s syndrome), and current or past use of β-blockers or calcium channel blockers in the last 2 years.
The study was approved by the Institutional Review Board at Roswell Park and registered on the clinicaltrials.gov website (NCT03384836). Informed consent was obtained from all participants prior to enrollment. The study was performed in accordance with the Declaration of Helsinki and the International Council for Harmonisation/Good Clinical Practice.
Study Design
This trial was an open label, single arm, non-randomized, single center, dose finding phase Ib study. Dose-escalation followed a “3 + 3” design, and no intra-patient dose-escalation was allowed. Eligible patients were treated with standard of care pembrolizumab 200 mg every 3 weeks i.v. and progressively increasing propranolol dosing from 10 mg (dose level 1), 20 mg (dose level 2) to 30 mg (dose level 3) twice a day, until 2 years on study or disease progression or dose-limiting toxicities (DLT). A total of 9 patients were accrued between January 2018 and August 2019. At the cutoff date of February 3, 2020, a total of 4 patients continue to be on the study treatment per protocol.
Study objectives and endpoints
The primary objective of the study was to assess the safety and efficacy [ORR (overall response rate within 6 months of starting therapy)] of combination of pembrolizumab with increasing doses of propranolol for unresectable stage III and metastatic melanoma and to select the recommended phase II dose (RP2D) based on safety, efficacy and biomarker analyses.
Safety outcomes were assessed by physical examination, laboratory findings, vital signs, and electrocardiogram. Adverse events (AE) were graded by Common Terminology Criteria for Adverse Events (CTCAE) v4.03. No AE due to propranolol doses of 10 mg-30 mg twice a day were anticipated. Nevertheless, a serious AE/DLT due to propranolol was defined as any life-threatening adverse event (e.g. symptomatic bradycardia or symptomatic hypotension) which would mandate recruitment per the 3 + 3 design. Otherwise a DLT was defined as grade 3 and higher immune-related adverse event (irAE) pneumonitis, colitis, hepatitis, nephritis, anemia, myositis, cardiomyositis, as defined by CTCAE v4.03, new onset diabetic ketoacidosis, Guillain-Barre syndrome or any other condition which the investigator believed to be an immune mediated adverse event and necessitated stopping therapy. Endocrinopathies were not included as DLTs, as the hormones will be replaced.
Objective response was defined as confirmed complete response (CR) or confirmed partial response (PR) among all treated patients with measurable disease at baseline.
The secondary objectives were to analyze efficacy as progression-free survival (PFS) and OS. PFS was measured from treatment initiation to time of disease progression or death, while OS was measured from the date of starting treatment until date of death or censoring. Exploratory objectives included analysis of biomarkers over time on study.
Assessments
Patients were assessed for tumor response according to imRECIST every 12 weeks (+/−14 days) for the first 6 months, and then per physician discretion until confirmed disease progression or toxicities. Safety assessments occurred at each clinic visit.
Exploratory analyses
Baseline tumor tissues, archival or fresh biopsy, were analyzed. Participants underwent serial blood collection into heparin and EDTA tubes for analysis of several biomarkers in peripheral blood. Patients completed the validated perceived stress scale (PSS) questionnaire [15] at baseline and additional time points to measure and quantify patient reported stress level perception. The results are reported as low stress (scores 0–13), moderate stress (scores 14–26) and high stress (scores 27–40) [15].
Tissue Collection and analyses:
Participants underwent tumor tissue collection at baseline for diagnosis, prior to study enrollment. 2/9 patients had a fresh biopsy and archival tissue was used for 7/9 patients. Formalin fixed paraffin embedded tissues were sectioned at 4 μm for multispectral immunofluorescence staining with antibodies against the following markers: CD8 (Dako, clone CD8/144B, dil 1:250), CD4 (Dako, clone 4B12, ready to use), Foxp3 (Abcam, clone 236A/E7, dil 1:125), CD14 (Cell Marque, clone EPR3563, dil 1:100), CD15 (Dako, clone Carb-3, dil 1:50), PDL1 (Abcam, clone SP142, dil 1:100), TIM3 (Cell Signaling Technology, clone D5D5R™, dil 1:100), OX40 (Cell Signaling Technology, clone E9U7O, dil 1:50), CTLA4 (BioCare, clone UMAB249, dil 1:50), PD1 (Cell Signaling Technology, clone EH33, dil 1:100 ), LAG3 (Cell Signaling Technology, clone D2G4O™, dil 1:250) and DAPI. Multispectral staining was performed after antibodies were optimized for standard immunohistochemistry and uniplex immunofluorescence staining. Slides were imaged using the Vectra Polaris spectral imaging system (PerkinElmer). Slides were initially scanned at × 4, visualized using Phenochart viewer (PerkinElmer) and five tumor areas per case were selected for scanning at high resolution ( × 20). Each fluorophore from PerkinElemer Opal™ kit was measured using a separate filter cube corresponding to its emission wavelength. The images were unmixed using a spectral library and individual fluorophores were separated with inForm™ software. The immune cell populations were quantified using cell segmentation and phenotype cell tool from inForm 1.1 (PerkinElmer). Threshold for positive staining and accuracy of phenotypes were confirmed by pathologist supervision (AKW). The individual markers from the panel were quantified and plotted as the average of the positive staining cells across the regions of interest.
Flow cytometry of peripheral blood:
Flow cytometry was used to quantify MDSC and regulatory T cell (Treg) populations in freshly isolated peripheral blood samples from heparinized tubes. An eight-color panel comprised of CD11b FITC, CD16 PE, CD45 PerCP, CD33 PECy7, HLADR APC, CD14 APCH7, CD15 V450; a lineage dump consisting of CD3, CD19, and CD56 (all conjugated to BV510) was used to measure eMDSC, mMDSC, and gMDSC subsets (Panel 1). Separately, a six-color panel comprised of CD8 FITC, CD25 PE, CD4 PerCP, CD3 PECy7, CD45 APCH7, and CD127 BV421 was employed to measure T cell subsets (Panel 2). WinList software (version 8.0; Verity Software House) was employed for the analysis of flow cytometric data. Analyzed data were reported as absolute cell count (cells per μL), or separately as the percentage of CD45+ events. MDSC populations were quantified according to the phenotypic definitions established by Bronte et al [16]. In brief, to quantify eMDSC, mononuclear cells (defined on the basis of their CD45 expression profile and light scatter characteristics) were sequentially gated to bivariate plots of HLADR vs. DUMP, CD14 vs. CD15, and CD33 vs. CD11b; where eMDSC were further defined as HLADRlow/-, DUMP-, CD14-, CD15-, CD33+, and CD11b+. To quantify mMDSC, mononuclear cells were sequentially gated to bivariate plots of CD11b vs. CD15 and HLADR vs. CD14; where mMDSC were further defined as CD11b+, CD15-, HLADRlow/-, and CD14+. To quantify gMDSC, mononuclear cells were sequentially gated to bivariate plots of CD14 vs. CD15 and CD11b vs. SSC-A; where gMDSC were further defined as CD14-, CD15+, and CD11b+. Separately, T cell subsets were quantified using Panel 2. Helper T cells were defined as CD3+, CD4+, CD8- and cytotoxic T cells were defined as CD3+, CD4-, CD8+. To quantify Tregs, CD3+, CD4+, CD8- T cells were gated to a bivariate plot of CD25 vs. CD127; where Tregs were further defined as CD25+, CD127(dim).
Chemokines/cytokines in peripheral blood:
Plasma was collected from EDTA blood and stored as aliquots at −80°C. 29-plex MILLIPLEX® MAP Human Cytokine/Chemokine Magnetic Bead Panel 96-Well Plate Assay was used to examine blood plasma levels of cytokines and chemokines. Wash buffer, sheath fluid, serum matrix, samples and standards were prepared in accordance with manufacturer’s protocol. The resultant data was analyzed using Upstate BeadView software for median fluorescence intensity (MFI) using a 5-parameter logistic curve-fitting method to calculate analyte concentrations in both samples and control wells.
Statistical Analysis
One of the primary objectives was to identify a RP2D based on safety, efficacy and the biomarker profile. As such, a standard 3 + 3 design was considered, with 3 dose levels, and requiring up to n = 18 subjects. Patients receiving at least one dose of pembrolizumab will be evaluable for response and safety.
In the primary analysis, adverse events and objective response are summarized by dose level using frequencies and relative frequencies.
For intra-dose analysis, peripheral blood biomarkers were summarized by dose-level and time-point using mean plots (+/− standard error). For intra-dose-level comparisons, the markers were modeled as a function of time-point and a random subject effect using a linear mixed model. An F-test about the main effect of time was used to evaluate whether marker expression changes over time. Additionally, the mean level at each time-point was compared to baseline using Dunnet adjusted tests. For inter-dose-level comparisons, percent change was calculated from baseline for each biomarker. The mean percent change was compared between dose levels using an ANOVA model, with pairwise comparisons made using a Tukey adjustment. All model assumptions were verified graphically, and transformations were applied as appropriate. All analyses were completed in SAS v9.4 (Cary, NC) at a significance level of 0.05.
Results
Patient characteristics
As of the data cut-off date of February 3, 2020, nine patients with metastatic cutaneous melanoma, that were treatment-naïve to PD-1/PD-L1 and CTLA-4 inhibitors completed enrollment for the phase I safety study. The median age of patients on the study was 65 years (35–96). Six patients were female (67%), and all patients were Caucasian. At baseline, 6 patients had an ECOG performance score of 0 (67%), 5 patients had M1c disease (56%) and 3 patients had elevated LDH (33%). The baseline PSS score ranged from 6–30, with a median score of 13. Four of 9 patients (44%) remain on study treatment. Primary reasons for study discontinuation were adverse events in 2 patients (22%) and disease progression in 3 patients (33%). Baseline patient and disease characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of the patient population
| Characteristics | Total Patients (N=9) |
|---|---|
| Median age at diagnosis (range), year | 65 (35–76) |
| Males, N (%) | 3 (33%) |
| ECOG, N (%) | |
| 0 | 6 (67%) |
| 1 | 3 (33%) |
| Elevated baseline LDH, N (%) | 3 (33%) |
| Metastatic stage, N (%) | M1b: 4 (44%) M1c: 5 (56%) |
| PD-L1 positive tumor (>1%), N (%) | 7/8 (88%)* |
| Median baseline PSS score | 13 |
ECOG, Eastern Cooperative Oncology Group; LDH, Lactate dehydrogenase; PD-L1, Programmed cell death-Ligand 1; PSS, Perceived Stress Scale
- PD-L1 could not be assessed in one patient.
Safety and tolerability
Treatment-related adverse events (TRAEs) occurred in all 9 patients. All but 1 patient had TRAEs that were grade 2 or lower. The most commonly reported TRAEs were fatigue, rash and vitiligo which occurred in 4/9 (44%) patients.
Serious TRAEs leading to discontinuation of therapy occurred in 2 patients, both in the 20 mg BID cohort: hemophagocytic lymphohistiocytosis (HLH) and labyrinthitis. Treatment-related AEs are shown in Table 2. There were no deaths on study treatment. Two Grade ≥ 3 AEs were reported in 1 patient. That patient developed a grade 3 increase in alanine aminotransferases (ALT), which was treated with oral prednisone. Subsequently he was found to have hepatitis C for which he was treated successfully. Later, he had a hospital admission complicated by necrotizing fasciitis, deep vein thrombosis, and HLH. Details of all AEs are shown Supplementary Table 1.
Table 2.
Treatment-related adverse events (TRAEs) while on the study
| 10 mg BID (N=3) | 20 mg BID (N=3) | 30 mg BID (N=3) | Total (n = 9) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Adverse event | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 |
| General disorders | Chills | 1(11.1%) | 1(11.1%) | 2(22.2%) | |||||
| Fatigue | 2(11.1%) | 1(11.1%) | 1(11.1%) | 4 (44.4%) | |||||
| Edema | 1(11.1%) | 1(11.1%) | 2(22.2%) | ||||||
| Alopecia | 1(11.1%) | 1(11.1%) | |||||||
| Hyperhidrosis | 1(11.1%) | 1(11.1%) | |||||||
| Night sweats | 1(11.1%) | 1(11.1%) | |||||||
| Gastrointestinal disorder | Abdominal distension | 1(11.1%) | 1(11.1%) | ||||||
| Diarrhea | 2(22.2%) | 2(22.2%) | |||||||
| Nausea | 2(22.2%) | 1(11.1%) | 3(33.3%) | ||||||
| Vomiting | 1(11.1%) | 1(11.1%) | |||||||
| Anorexia | 1(11.1%) | 1(11.1%) | |||||||
| Infections and infestations | Hemophagocytic Lymphohistiocytosis | 1(11.1%) | 1(11.1%) | 1(11.1%) | 1(11.1%) | ||||
| Investigations | Autoimmune thyroiditis | 1(11.1%) | 1(11.1%) | ||||||
| Hyperthyroidism | 1(11.1%) | 1(11.1%) | |||||||
| Hypothyroidism | 1(11.1%) | 1(11.1%) | |||||||
| Elevated Alanine aminotransferase | 1(11.1%) | 1(11.1%) | 1(11.1%) | 1(11.1%) | |||||
| Elevated Aspartate aminotransferase | 1(11.1%) | 1(11.1%) | |||||||
| Nervous system disorders | Labyrinthitis | 1(11.1%) | 1(11.1%) | ||||||
| Dizziness | 1(11.1%) | 1(11.1%) | |||||||
| Insomnia | 1(11.1%) | 1(11.1%) | |||||||
| Dysphonia | 1(11.1%) | 1(11.1%) | |||||||
| Skin and subcutaneous tissues | Vulvovaginal pruritus | 1(11.1%) | 1(11.1%) | ||||||
| Pruritus | 1(11.1%) | 1(11.1%) | 2(22.2%) | ||||||
| Rash | 2(22.2%) | 1(11.1%) | 1(11.1%) | 4 (44.4%) | |||||
| Vitiligo | 2(22.2%) | 1(11.1%) | 1(11.1%) | 4 (44.4%) | |||||
| Vascular disorders | Hypotension | 1(11.1%) | 1(11.1%) | ||||||
No DLTs were observed at any of the three dose levels. Hence propranolol at all three dose levels was considered for RP2D.
Preliminary antitumor activity
By the cutoff date of February 3, 2020, the median follow-up was 15.6 (range, 5.4–24.2) months. The median number of pembrolizumab cycles received were 7.5 (2–32). Objective responses were noted at all three dose levels (Table 3). Objective response rate was 7/9 (78%) on the study.
Table 3.
Best responses per immune-modified RECIST and current status of patients on study as of cutoff date
| Dose group | Patient number | Best response | Follow up (in months) | Time to best response (in months) | Time to progression (in months) | Current status |
|---|---|---|---|---|---|---|
| 10 mg | P1 | Stable disease | 24.2 | 2.8 | 8.2 | Received radiation to the enlarging lymph node. Currently on pembrolizumab and propranolol off study |
| P2 | Partial response | 23.9 | 3.0 | 10.2 | Progressive disease, and currently being treated with later lines of therapy | |
| P3 | Partial response | 23.1 | 2.8 | - | Continues on treatment per protocol | |
| 20 mg | P4 | Partial response | 18.9 | 3.0 | - | Off study due to toxicity. Continues to maintain response |
| P5 | Partial response | 15.5 | 5.8 | 11.6 | Off study due to toxicity. Patient had metastatectomy for Progressive disease | |
| P6 | Progressive disease | 7.9 | - | 1.1 | Patient died | |
| 30 mg | P7 | Partial response | 9.0 | 2.8 | - | Continues on treatment per protocol |
| P8 | Partial response | 6.7 | 2.8 | - | Continues on treatment per protocol | |
| P9 | Partial response | 5.3 | 2.8 | - | Continues on treatment per protocol |
Fig. 1 shows the spider plot for tumor response for all the nine patients on the study (P1-P9). In dose level 1, two patients experienced tumor response, and one patient had stable disease (SD) as best response. The patient with SD (P1) had a mixed response (increase in size of axillary lymph node and decrease in size of subcutaneous lesions), ultimately coming off study to receive radiation to the growing axillary lymph node and progressing per imRECIST. The patient continues to remain on combination of pembrolizumab and propranolol off study (total follow up of 24.2 months).
Figure 1.
Spider plot of the change in the sum of tumor diameters over time for the nine evaluable patients on study. 0 on the X-axis corresponds to the time of the baseline scan and percentage change in tumor size from baseline is shown on the Y-axis.
In dose level 2, two patients had a PR, and one patient came off study due to the development of rapid disease progression. Both patients with PR discontinued combination therapy due to toxicity. One patient has maintained PR (total follow up of 18.9 months), whereas the other patient had PD and underwent metastasectomy for the residual metastatic lesion.
In dose level 3, all three patients have experienced PR and continue on study treatment. As of the cut-off date, a total of 4 patients continue on treatment per protocol. One patient has died due to disease progression.
Perceived Stress Scale
We hypothesized that patients reporting increased stress would be more likely to respond due to blockade of the elevated β2-AR signaling. At baseline, 5/9 patients had low PSS scores, 3/9 had moderate scores and 1/9 had a high score. Responses were seen at all PSS score levels. (Supplementary Fig. 1A). We observed a trend towards decrease in PSS scores over time in dose levels 1 and 3. In dose level 2, although subsequent PSS scores were lower than baseline, a similar trend could not be conclusively established, due to lack of sufficient data points in that cohort (Supplementary Fig. 1B).
Chemokine and cytokine analysis
Intra-dose comparison:
In dose level 1, 3-week (p = 0.002), 6-week (p = 0.008), 9-week (p = 0.023) and 12-week (p = 0.018) levels of IP-10 were significantly higher than at baseline. Immunosuppressive chemokine, eotaxin showed significant time effect in dose level 2 (p = 0.04) with a large increase in expression around 12 weeks compared to baseline (2/3 patients). Additionally, there was a significant decrease at 12 weeks of immunostimulatory cytokine TNFβ compared to baseline in dose level 2 (p = 0.003) (1/3 patients). Supplementary Fig. 2A shows variation in levels of IP-10, eotaxin and TNFβ in the three dose levels.
Inter-dose comparison:
There was a decrease in expression of IL-12p70 in dose level 3 compared to dose levels 1 (p = 0.007) and 2 (p = 0.012) at week 6 (Supplementary Fig. 2C)
Responder vs. non-responder:
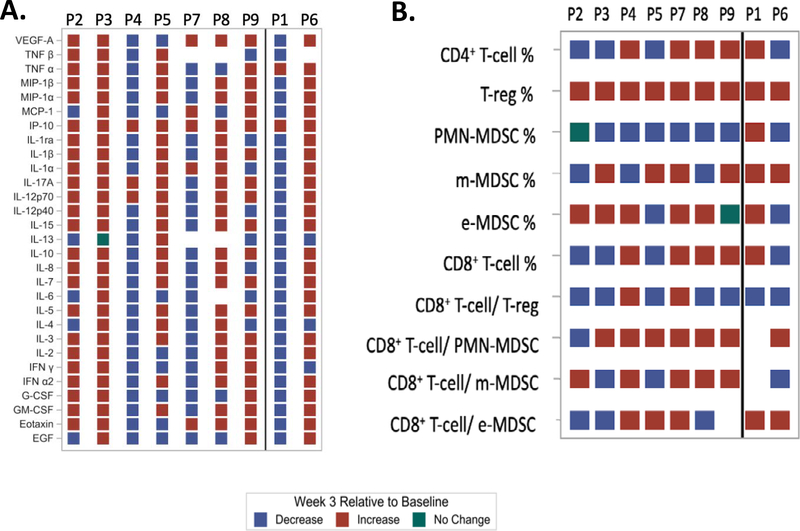
At week 3, compared to baseline, IFN-γ was increased in dose level 1 and 2/3 patients in dose level 3 (all these were responders) and decreased in both non-responders. Interestingly, IL-6 decreased in 5/6 responders (value was not available for one responder) and increased in 1 non-responder (although decreased in non-responder with mixed response; P1). An increase in IP-10 was observed among all patients. Fig. 2A shows the heat map of chemokine changes in responders and non-responders. Supplementary Table 2A shows the absolute quantitative change in chemokines from baseline to week 3 on study.
Figure 2.
Heat map showing changes (increase, decrease or no change) in (A) chemokines and cytokines and (B) cellular fractions at 3 weeks compared to baseline while on study treatment at different dose levels of propranolol separated for responders (P2,3,4,5,7,8,9) and non-responders (P1,6). Percentage of cellular fractions refers to the absolute number expressed as a percentage of total CD45+ cells.
Flow cytometry
Intra-dose comparison:
Supplementary Fig. 2B shows variation in CD8+T-cell %, m-MDSC %, Treg %, PMN-MDSC % (expressed as a percentage of total CD45+ cells), ratio of CD8+T-cell/ PMN-MDSC in the 3 dose levels. At all dose levels, there was a trend towards an initial increase in CD8+ T-cells/ total CD45+ cells (CD8+T-cell %) until week 3. There was also a trend of early increase in the m-MDSC % and T-reg % at all dose levels. Interestingly, in dose level 1, 30-week m-MDSC % and PMN-MDSC % were significantly higher (p < 0.01; p < 0.01 respectively), whereas 30-week ratio of CD8+/PMN-MDSC was lower (p = 0.01) when compared to baseline (Supplementary Fig. 2B). A trend in early decrease in PMN-MDSC % were seen in the initial 3 weeks in dose levels 2 and 3.
Inter-dose comparison:
Relative to baseline, significant decrease in PMN-MDSC % at 3 weeks was observed for dose level 2 compared to dose levels 1 (p = 0.004) and 3 (p = 0.007). Relative to baseline, highest increase in CD8+ T-cell % and ratio of CD8+T-cell/ Treg was seen in dose level 3, although not statistically significant (Supplementary Fig. 2C).
Responder vs. non-responder:
There was significant heterogeneity among responders at different dose levels. Interestingly, at week 3, the ratio of CD8+T-cell/ m-MDSC increased compared to baseline in 3/3 responders in dose level 3 and decreased in non-responders. An increase in CD8+ T-cell % compared to baseline was seen in 3/3 responders in dose level 3 vs. decrease in non-responder (although increased in non-responder with mixed response; P1). An increase in Treg % was seen in all patients. Fig. 2B shows the heat map of cellular changes in responders and non-responders. Supplementary Table 2B shows the quantitative change in peripheral blood cellular fractions from baseline to week 3 on study.
Tissue Biomarkers
As shown in Supplementary Fig. 3, multispectral staining revealed marked variability in the composition and the distribution of immune infiltrate at the baseline. In order to further characterize the CD8+T-cells and analyze impact of exhaustion markers on response to treatment [17], multispectral analysis of T-cell activation and exhaustion markers was performed using antibodies for CD8, OX40, CTLA4, PD1, LAG3 and TIM3. Representative multiplex and monoplex images are shown (Supplementary Fig. 4A). Phenotyping was performed using InForm software to delineate activated T-cells, cytotoxic T-cells, partially exhausted T-cells and exhausted T-cells with the underlying marker analysis illustrated for a representative tumor (Supplementary Figure 4B) at baseline. Supplementary Table 3 shows the percentage of different cellular fractions in the study patients in the regions of interest selected for the analysis. Non-responders (P1 and P6) had a lower number of CD8+ cytotoxic T cells, however three of the patients who responded to the therapy had comparable levels of CD8+ cytotoxic T cells, indicating that in a subset of patients, anti-tumor immune response can be activated independently of the baseline level. The number of m-MDSC was highly variable among responders and non-responders. The patient with rapid disease progression had the highest number of PMN-MDSC (P6). Non-responders exhibited high expression of PD-L1 on m-MDSC or PMN-MDSCs relative to patients who benefited from the therapy. PD-L1+ melanoma cells varied from 0.34%−29.34% and did correlate with the response. Other cellular fractions or ratios as shown in Supplementary Table 3 were not predictive of clinical response; however, our analyses is limited by the small size of a Phase I study.
Notably, one of the non-responders (P6), had a clear cell morphology with minimal immune infiltration (Supplementary Fig. 5). Interestingly, this patient also had < 1% PDL1+ melanoma cells, a higher infiltration of PMN-MDSC and a lower CD8+T-cell infiltration compared to other patients.
Recommended phase II dose
Propranolol 30 mg twice a day was chosen as the RP2D in this study in combination with pembrolizumab as there were no DLTs at this level, preliminary anti-tumor efficacy was observed in all three patients at this dose level, and these patients demonstrated a trend toward higher CD8+T-cell %, ratios of CD8+T-cell/ Treg and CD8+T-cell/ PMN-MDSC. This data is intriguing, however, due to the small size and significant heterogeneity in biomarkers, any statistically sound and meaningful interpretation of biomarkers to decide a RP2D is difficult.
DISCUSSION
Our phase I study shows that the combination of propranolol and pembrolizumab is safe in treatment-naïve metastatic melanoma. As the dose-effect relationship of propranolol on immune regulation is not well defined, another objective of the study was to select the RP2D of propranolol in combination with pembrolizumab based on toxicity, response and biomarker analyses. No DLTs were observed on the study. Observed frequency of adverse events were not higher than expected with pembrolizumab monotherapy alone. However, two patients developed rare toxicities: HLH and ototoxicity. One patient developed HLH after 3 cycles of pembrolizumab, during admission for necrotizing fasciitis from methicillin-resistant staphylococcus aureus (MRSA). Although rare, cases of HLH from pembrolizumab monotherapy have been reported in literature [18, 19]. Staphylococcus aureus is a known risk factor for secondary HLH and could have been a contributing cause [20]. HLH resolved with steroids, without requirement of additional immunosuppression. No further episodes of HLH relapse have been observed until last follow up. Another patient developed grade 2 ototoxicity (hearing loss/ labyrinthitis) after 2 cycles of pembrolizumab, which was treated with intratympanic and oral steroids. This patient had no evidence of leptomeningeal disease but still has residual hearing loss. The mechanism(s) underlying these rare irAE remains unclear. β-adrenergic receptors present in the inner ear epithelium play an important role in ion transportation [21]. Some β-blockers have been implicated in hearing loss [22]. Additionally, in rats, anti-PD1 have shown to be directly toxic to hair cell and Organ of Corti [23]. It is also interesting to note that the inner ear is rich in melanocytes. Vogt-Koyanagi-Harada disease, an autoimmune disease targeting melanocytes frequently involves the inner ear [24]. Ototoxicity in our patient could also be due to a cross-reactive autoimmune response of the patient’s T-cells to melanocytes in the inner ear. Autoimmune hearing loss, though rare, has been reported as a toxicity of pembrolizumab monotherapy [25]. With regard to HLH observed in one patient, we note that HLH was observed in the context of a severe Staphylococcus aureus wound infection. Staphylococcus aureus has also been associated with secondary HLH [26].
In treatment-naïve patients, the objective response rate has been reported as 46% with pembrolizumab monotherapy using immune-related response criteria, and median PFS as 11.6 months [1]. In our study, responses were observed at all 3 dose levels of propranolol with an objective response in 7/9 patients (78%). Five out of 7 patients continue to demonstrate maintenance of tumor response. After a median follow up of 15.6 months, 2/9 (22%) patients have gone on to receive second-line systemic therapy. Median PFS and OS has not been reached. Four patients continue on study treatment per protocol, and another patient continues on combination treatment off study.
Interestingly, compared to baseline, we saw an early increase in the levels of m-MDSC in peripheral blood of patients at several time points and at all doses. Low baseline blood m-MDSC levels has been shown to be predictive of response to anti-CTLA4 therapy [27]. However, the prognostic value and kinetics of m-MDSC during anti-PD1 therapy remains unclear. Similar to our findings, another study of patients with metastatic urothelial cancer treated with pembrolizumab reported an increase in m-MDSC during therapy [28]. At week 3, we noted an increase in IFN-γ (4/7 patients) and a decrease in IL-6 (5/6 patients) in responders. IL-6 also decreased in the patient with mixed response. These findings are consistent with our findings in 4T1 and AT3 mammary mouse models where reducing adrenergic stress or use of β2-AR−/− mice was associated with decreased plasma levels of pro-tumor cytokines and increased anti-tumor cytokines, decrease in IL-6, and increase in IFN-γ, yet no significant changes in IP-10 were observed [5]. The increase in IP-10 as seen in this phase I trial cohort could be an effect of pembrolizumab. This raises an interesting question if treatment-induced biomarkers could predict outcomes and help select a bioequivalent propranolol dose in humans; however, sample size in each cohort is too small for any meaningful biomarker interpretation. Though these peripheral blood findings are interesting, differences induced in peripheral blood do not always display parallel changes intra-tumorally [29]. The observation that the one patient with rapid disease progression had little T-cell infiltrate also suggest that serial tumor biopsies on treatment could provide important information relative to disease response and mechanisms of propranolol action in the TME.
Responses were observed irrespective of baseline PSS score levels. Interestingly, although a new cancer diagnosis is considered a stressful event [30–33], a majority of the patients had a low PSS score. Over a period of time, we observed a decrease in median PSS scores may have been associated with development of coping skills while undergoing treatment, or a positive experience of disease response, which occurred in most patients. Ultimately, an assessment of whether β2-AR blockade could influence outcomes in patient reporting moderate or high stress scores is limited by the small number of patients and a lack of control group without propranolol intervention.
The primary intent of this phase I dose escalation study was to investigate the safety of combining pembrolizumab with propranolol, that it did not increase the expected toxicities compared to pembrolizumab monotherapy alone, and to ensure that the combination does not compromise the historical efficacy of pembrolizumab monotherapy. Lower doses of propranolol were used due to lack of prospective evidence that the cardiovascular effects of pan-β blockade correlate with pharmacodynamic impact of propranolol on immune pathways. We note the provocative findings from Hiller et al. where breast cancer patients received intra-patient dose escalation of propranolol from 80 to 160 mg daily that suggested that patients with evidence of clinical β-blockade had higher tumor immune infiltration with CD68+macrophages and CD8+T-cells [34]. However, this was a post-hoc analysis and the clinical trial was not designed to answer the question about the appropriate dose of β-blocker to achieve this effect on immune cells. Since our clinical trial focused on studying the safety of this combination, we used lower doses of propranolol to ensure that there are no increased toxicities or compromise of efficacy compared to pembrolizumab alone. Imbedded in our ongoing phase II study is an evaluation of heart rate induced exercise as a biomarker of outcome.
Our study has several caveats that must be addressed in future, larger studies. Since this is a phase I study with the primary endpoint to determine safety of the combination, the biomarker analyses could only be exploratory in nature, and the study was not designed to evaluate the impact of treatment on TME. Due to lack of a control group for comparison, we could not attribute any immunological effects specifically to propranolol alone in the combination. Interpretation of some immunological biomarkers was limited due to lack of longitudinal samples for some patients as a result of disease progression or toxicities. The study design, sample size and objectives are consistent with Phase I oncology trials and was not designed as a formal assessment of efficacy. The response rate noted met our predetermined goal of not negatively impacting the single agent activity of pembrolizumab. We recognize that the remarkable response rate in this study is provocative and should be interpreted with caution until the larger multi-center phase II study, currently ongoing is completed.
To our knowledge, this effort is the first prospective clinical trial to show that the combination of propranolol with pembrolizumab is safe, and additionally suggests preliminary synergistic antitumor activity in treatment-naïve metastatic melanoma. Based on safety, response and biomarkers, propranolol at a 30 mg BID dose was chosen as the RP2D dose. A larger phase II, multi-center, single-arm study is ongoing to confirm and validate the findings from this study.
Supplementary Material
Statement of Translational Relevance.
Nerves of the sympathetic nervous system, and β-adrenergic receptor signaling are now known to play a role in cancer promotion and suppression of the immune system. The β2-receptor signaling pathway has been shown to create an immunosuppressive tumor microenvironment by decreasing CD8+T-cells and increasing the number and proliferation of myeloid-derived suppressor cells. Retrospective observational and prospective trials suggest a therapeutic role for β-blockers in patients with cancer. This is the first prospective phase I study combining pembrolizumab with a non-selective β-blocker, propranolol, to inhibit the stress pathway in patients with metastatic melanoma to determine safety and tolerability along with biological and correlative endpoints. The combination was tested in nine patients and found to be safe with objective response in 7/9 patients. These results strongly support our expanded phase II study investigating propranolol and pembrolizumab in patients with metastatic melanoma and additional studies of β2-adrenergic blockade in other advanced malignancies.
Acknowledgements
We thank the patients, their families and the study personnel for participating in this study. The research reported in this publication was supported by the Roswell Park Alliance Foundation Grant (to S. Gandhi) and Melanoma Fund (generous gifts from patients) (to M. Ernstoff), Roswell Park Comprehensive Cancer Network and National Cancer Institute (NCI) grants P30CA016056, NCI grant R01CA205246 (E. Repasky), Harry J Lloyd Charitable Trust (to E. Repasky), National Center for Advancing Translational Sciences of the National Institute of Health under award number 5KL2TR0013-05 and UL1TR0012-05 (to S. Gandhi). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement: The authors report no relevant financial or non-financial conflict of interest.
Disclaimer
The authors take full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the article for publication, and the writing of the article.
This article was prepared while Marc S. Ernstoff was employed at Roswell Park Comprehensive Cancer Center. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
References
- 1.Robert C, et al. , Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol, 2019. 20(9): p. 1239–1251. [DOI] [PubMed] [Google Scholar]
- 2.Larkin J, et al. , Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med, 2019. 381(16): p. 1535–1546. [DOI] [PubMed] [Google Scholar]
- 3.Jean Wrobel L, et al. , Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget, 2016. 7(47): p. 77825–77837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucsek MJ, et al. , beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res, 2017. 77(20): p. 5639–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadpour H, et al. , beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest, 2019. 129(12): p. 5537–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bierhaus A, et al. , A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A, 2003. 100(4): p. 1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steptoe A, et al. , Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond), 2001. 101(2): p. 185–92. [PubMed] [Google Scholar]
- 8.Goebel MU, et al. , Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med, 2000. 62(4): p. 591–8. [DOI] [PubMed] [Google Scholar]
- 9.Diaz ES, Karlan BY, and Li AJ, Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol, 2012. 127(2): p. 375–8. [DOI] [PubMed] [Google Scholar]
- 10.Powe DG, et al. , Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget, 2010. 1(7): p. 628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang HM, et al. , Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol, 2013. 24(5): p. 1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Giorgi V, et al. , Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med, 2011. 171(8): p. 779–81. [DOI] [PubMed] [Google Scholar]
- 13.Kokolus KM, et al. , Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology, 2018. 7(3): p. e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi S, et al. , Impact of concomitant medication use and immune-related adverse events on response to immune checkpoint inhibitors. Immunotherapy, 2020. 12(2): p. 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen S, Kamarck T, and Mermelstein R, A global measure of perceived stress. J Health Soc Behav, 1983. 24(4): p. 385–96. [PubMed] [Google Scholar]
- 16.Bronte V, et al. , Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun, 2016. 7: p. 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graves M, et al. , Monitoring Patient Response to Pembrolizumab With Peripheral Blood Exhaustion Marker Profiles. Front Med (Lausanne), 2019. 6: p. 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadaat M and Jang S, Hemophagocytic lymphohistiocytosis with immunotherapy: brief review and case report. J Immunother Cancer, 2018. 6(1): p. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah D, et al. , Pembrolizumab associated hemophagocytic lymphohistiocytosis. Ann Oncol, 2017. 28(6): p. 1403. [DOI] [PubMed] [Google Scholar]
- 20.Zhou M, et al. , Clinical features and outcomes in secondary adult hemophagocytic lymphohistiocytosis. QJM, 2018. 111(1): p. 23–31. [DOI] [PubMed] [Google Scholar]
- 21.Kim BG, et al. , beta1- and beta2-adrenergic stimulation-induced electrogenic transport by human endolymphatic sac epithelium and its clinical implications. Sci Rep, 2017. 7: p. 42217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Ghamdi BS, et al. , Use of beta blockers is associated with hearing loss. Int J Audiol, 2018. 57(3): p. 213–220. [DOI] [PubMed] [Google Scholar]
- 23.Kuzucu I, et al. , Investigation of the Ototoxic Effect of Pembrolizumab Using a Rat Model. Cureus, 2019. 11(11): p. e6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavezzo MM, et al. , Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis, 2016. 11: p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zibelman M, Pollak N, and Olszanski AJ, Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer, 2016. 4: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito S, et al. , Secondary hemophagocytic syndrome in a patient with methicillin-sensitive Staphylococcus Aureus bacteremia due to severe decubitus ulcer. Intern Med, 2006. 45(5): p. 303–7. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C, et al. , Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother, 2014. 63(3): p. 247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ornstein MC, et al. , Serial measurements of myeloid derived suppressor cells (MDSC) in metastatic urothelial carcinoma (mUC) patients (pts) treated with immune checkpoint inhibitors (CI). Journal of Clinical Oncology, 2017. 35(15_suppl): p. e16005–e16005. [Google Scholar]
- 29.Orecchioni S, et al. , Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br J Cancer, 2018. 118(10): p. 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen BL, et al. , Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer, 2008. 113(12): p. 3450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antoni MH and Dhabhar FS, The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer, 2019. 125(9): p. 1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutgendorf SK, et al. , Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun, 2011. 25(2): p. 250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abuatiq A, et al. , Perceptions of Stress: Patient and Caregiver Experiences With Stressors During Hospitalization. Clin J Oncol Nurs, 2020. 24(1): p. 51–57. [DOI] [PubMed] [Google Scholar]
- 34.Hiller JG, et al. , Preoperative beta-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin Cancer Res, 2020. 26(8): p. 1803–1811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.