Abstract
Objectives/Hypothesis:
Oxidative stress has been postulated to play an important role in chronic rhinosinusitis. Nrf2 is a transcription factor that is involved in the regulation of multiple antioxidant genes and its function has been previously shown to be important in sinonasal inflammation. Although the sinonasal implications of whole body Nrf2−/− has been reported, the function of sinonasal epithelial expression of Nrf2 has not been studied. The primary aim of this study is to generate a mouse model that is genetically deficient in epithelial specific Nrf2 and to understand its role in regulating sinonasal inflammation.
Study Type:
Basic Science
Methods:
An epithelial specific Nrf2 knock-out mouse was generated by crossing Krt5-cre(K5) with Nrf2 flox/flox. A papain induced model of rhinosinusitis was performed in the resulting K5 Nrf2−/− mouse. Immunohistochemistry was performed to quantify goblet cell hyperplasia. Mucosal cellular infiltrates were quantified using flow cytometry and tissue cytokines were measured using ELISA. Lastly, the cellular source of type 2 cytokines was determined using intracellular cytokine staining.
Results:
Papain sensitized mice lacking epithelial specific Nrf2 demonstrate increased goblet cell hyperplasia, significant tissue eosinophilia, and statistically significant increase in mucosal IL-13 when compared to Nrf2 wildtype mice. Lastly, mucosal T cells were identified as the cellular source of IL-13.
Conclusions:
We demonstrate enhanced severity of eosinophilic sinonasal inflammation from disruption of the epithelial specific Nrf2 pathway. The responsiveness of Nrf2-directed antioxidant pathways may act as a major determinant of susceptibility to eosinophilic inflammation and may have potential as a therapeutic target for CRS.
Keywords: Nrf2, mouse model, chronic rhinosinusitis, sinonasal, epithelial
Background
Chronic rhinosinusitis (CRS) is one of the most common medical problems in the United States, estimated to affect between 30 and 40 million Americans each year. It is the single most common self-reported chronic health condition and accounts for billions of dollars in health care costs and lost work days annually 1,2. Medical therapy and surgery are successful in treating the majority of patients with sinusitis; however, patients with eosinophilic polypoid CRS recalcitrant to these therapies are increasingly prevalent. Within this population, public health studies have found poor quality of life in terms of increased bodily pain and more interference with normal social activities compared to patients with other chronic diseases such as angina pectoris, congestive heart failure, chronic obstructive pulmonary disease, and chronic back pain 3. Although the pathogenesis of CRS was commonly believed to involve sinus ostial obstruction and persistent bacterial infection, over the years, the rhinologic literature has evolved suggesting a significant inflammatory component that has been largely attributed to cytokines and inflammatory cells mediated by the adaptive immune system. Recent literature suggests an increase in expression of cytoprotective enzymes in CRS patients, suggesting a role for oxidative stress in CRS pathophysiology 4,5.
The airway epithelium plays an essential role in oxygen exchange and is unique in that there is constant exposure to airborne environmental, allergen, and infectious exposures. These agents, as a result, can activate oxidative stress mechanisms either directly or indirectly through recruitment of inflammatory cells which release reactive oxygen species (ROS) 6,7. While oxidative stress has been associated with multiple health conditions of the lower airway including chronic inflammatory disorders such as asthma and COPD; its role in chronic rhinosinusitis is less clear 6–8.
The nuclear factor-E2 p45-related factor 2 (Nrf2) gene encodes a basic-leucine zipper transcription factor, which upon activation in response to inflammatory or oxidative stress detaches from its cytosolic inhibitor, KEAP1, translocates to the nucleus, and binds to the antioxidant response element (ARE) and transactivates a robust antioxidant and anti-inflammatory host defense transcriptional program. Our group has previously demonstrated along with others that enhancement of Nrf2 activity using sulforaphane, a compound naturally found in broccoli sprouts, improves epithelial tight junctional proteins JAM-A, ZO-1, and e-cadherin cell surface localization in human sinonasal epithelial cells stimulated with various environmental stimuli including particulate matter (PM), house dust mite, and cigarette smoke 9–13. Most recently, we demonstrated that mice deficient in Nrf2 are more susceptible to Ova-induced sinonasal inflammation, epithelial barrier dysfunction, and epithelial derived inflammatory cytokine production 14. In contrast, using a genetic mouse model of Nrf2 activation or via sulforaphane resulted in a reduction of Ova-induced sinonasal inflammation 15.
The primary objective of this study is to examine how Nrf2 regulates inflammation in the sinonasal epithelium. The hypothesis tested here is that intact epithelial Nrf2 expression is critical in regulating sinonasal inflammation and its loss of function is associated with a dysfunctional inflammatory state. To test this hypothesis, we generated an epithelial specific Nrf2 knockout mouse and utilizes a papain-induced model of rhinosinusitis. We used flow cytometry and immunohistochemistry to analyze the mucosal inflammatory cell infiltrate and function and ELISA to quantify tissue cytokines.
Methods
Animals
Animals (9 total in each group, 5 male, 4 female) were housed in a specific pathogen-free facility with single ventilated cages. All animal experiments were approved by the Johns Hopkins Institutional Animal Care and Use Committee (IACUC).
Generation of Epithelial Specific Nrf2 Knockout (K5 Nrf2−/−) Mice
Krt5-cre mice were gifted from Dr. Randall Reed’s lab and were generated as previously described 16. Krt5, while specifically found in keratinocytes has also been shown to be expressed in the airway epithelium by several groups including our lab 17–19. These mice were crossed to Nrf2 flox/flox mice, which were generated in the lab of S.B.. In brief, Nrf2 flox/flox mice were generated as previously described 20. The distribution of cre recombinase expression was verified via a LSL-tdTomato reporter. The resulting Krt5-cre Nrf2−/− (referred to from here on as K5 Nrf2−/−) mouse, which contains an epithelial specific Nrf2 deletion, was compared to wildtype Nrf2 flox/flox mice (intact Nrf2 system).
Papain Induced Model of Rhinosinusitis
The cysteine protease inducible murine model of type 2 sinonasal inflammation provides an excellent experimental platform to dissect molecular mechanisms involved in CRS pathophysiology as previously described 21. Mice were treated on days 0-2 and days 7-11, and sacrificed 24 hours after the final treatment.
Sinonasal Histopathology
Sinonasal mucosa was extracted from mouse heads as previously described by our group 22. In brief, mouse heads were fixed with paraformaldehyde and decalcified for 2 days before embedment in paraffin. Coronal paraffin sections were cut on a microtome and stained with hematoxylin and eosin (H&E) or alcian blue/periodic acid schiff. Images were acquired using the Zeiss Axio Imager.A2 microscope and measurements made using the Axiovision 4.8 software (Carl Zeiss Micro-imaging, Thornwood, NJ).
Flow Cytometric Analysis of Mucosal Inflammatory Cells
Mouse sinonasal tissue was isolated as described above and were minced and incubated for 45 minutes in 6 ml of dissociation buffer consisting of RPMI 1640, penicillin/streptomycin, β-mercaptoethanol, 0.2 mg/ml DNase I (Sigma Aldrich, St. Louis, MO), and 50 μg/ml Liberase TL (Roche Diagnostics, Indianapolis, IN). Single-cell suspensions were made and tissue was forced through a 70 μm cell strainer. Dissociation was stopped by the addition of 600 μl of fetal bovine serum. Cells were stained with Zombie Aqua fixable viability dye (Biolegend, San Diego, CA) prior to immunostaining.
For intracellular cytokine staining, single-cell suspensions were incubated 12 hours in 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 1 mM ionomycin. Cells were treated with 3 μg/ml brefeldin A for the final 3 hours of the stimulation and stained with monoclonal antibodies to identify eosinophils, basophils, innate lymphoid cells type 2 (ILC2) using the following gating schemes: Eosinophils (Lin−, CD45+, CD11b+, CD11c−, and Siglec-F+) T cells (Lin−, CD45+, CD11b−, CD3+), basophils (Lin−, CD45+, CD3−, FcεRIα+, c-Kit−), mast cells (Lin−, CD45+, CD3−, FcεRIα+, c-Kit+), and ILC2s (Lin−, CD45+, CD3−, CD11b−, FcεRIα−, c-Kit−, CD25+, ICOS+). For identification of ILC2s, the lineage cocktail included: B-220, NK1.1, Gr-1, CD11b, CD11c, TER119, and FcεRIα. For all other cell types, the lineage cocktail used: B-220, NK1.1, Gr-1, and TER119.
Measurement of Tissue cytokines
Sinonasal mucosal tissue was dissected and snap frozen and then suspended in PBS containing 0.1% Triton X-100, complete mini protease inhibitor (Roche Diagnostics, Indianapolis, IN), and 1 mM PMSF. Tissue was homogenized and centrifuged for 2 minutes at 15,000xg. Supernatants were collected and analyzed for IL-5 and IL-13 concentration by ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis
Results depict average ± SEM. Statistical significance was determined by two-tailed unpaired T test with equal variance. Statistical significance was considered to be P<0.05. Outliers were excluded on the basis of the Grubb’s test. Results were analyzed with the use of the statistical software Prism 6 (GraphPad La Jolla, CA).
Results
Successful Generation of K5 Nrf2−/− Mice and Papain Induced Rhinosinusitis
The distribution of cre recombinase expression was verified via a LSL-tdTomato reporter which illuminates the sinonasal epithelium red on confocal microscopy, indicating that cre was activated and the gene recombination occurred (Figure 1). Our intranasal papain induced rhinosinusitis protocol was able to generate robust sinonasal inflammation with eosinophilia (Figure 2)
Figure 1:
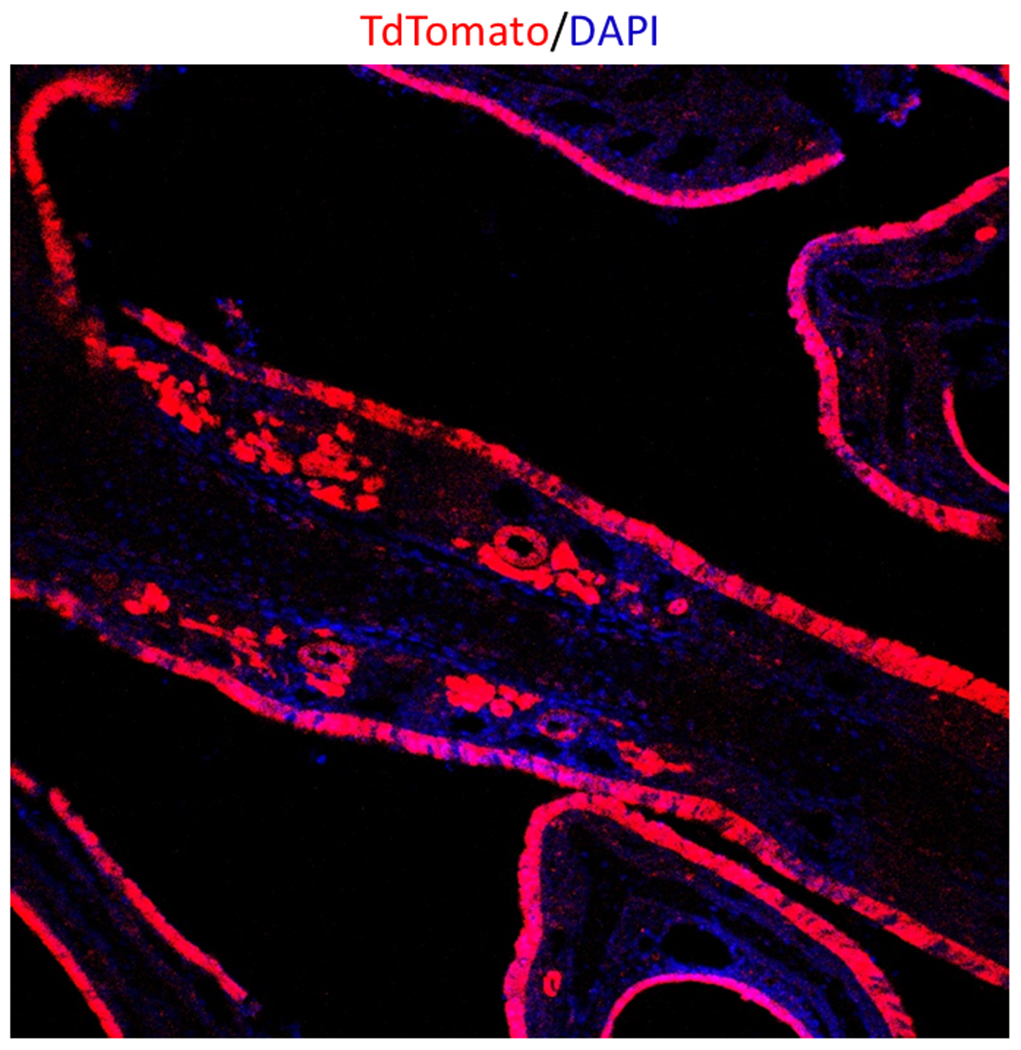
Confocal Microscopy (20X) Sinonasal epithelial expression of TdTomato indicating a successful cre recombinase reaction with Nrf2 flox/flox.
Figure 2:
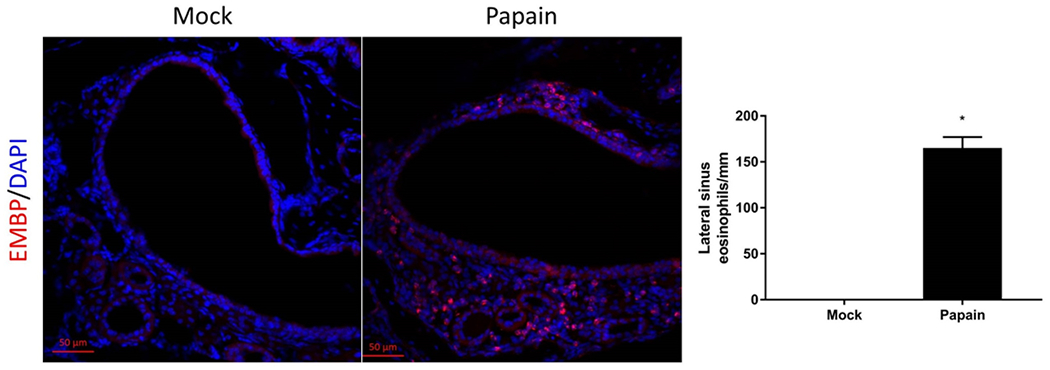
Cysteine protease papain induces eosinophilic inflammation in sinonasal mucosa. Confocal Microscopy (10X) of mouse sinonasal epithelium treated with papain to generate a murine model of rhinosinusitis. Note the large influx of eosinophilic major basic protein (EMBP, red) within lateral sinus, p<0.05. DAPI, blue
A Lack of Epithelial–Specific Nrf2 Signaling Dysregulates Sinonasal Inflammation Induced by Papain
Increased goblet cell hyperplasia
Type 2 immune responses are commonly associated with goblet cell hyperplasia and the “weep and sweep” response, which refers to the increased mucus production and mucociliary clearance needed to clear the airway of environmental irritants and pathogens 23. This IL-4/IL-13 dependent feature is also a common feature of CRS pathophysiology 24. To analyze goblet cell populations, sinonasal histological sections were analyzed from K5 Nrf2−/− and Nrf2flox/flox mice and were stained with alcian blue (Figure 3a). Alcian blue positive cells, were counted along the nasal septum, signifying goblet cells. Indeed, we found that K5 Nrf2−/− had a 2-fold statistically significant increase in goblet cells along the nasal septum (p<0.01) (Figure 3b).
Figure 3:
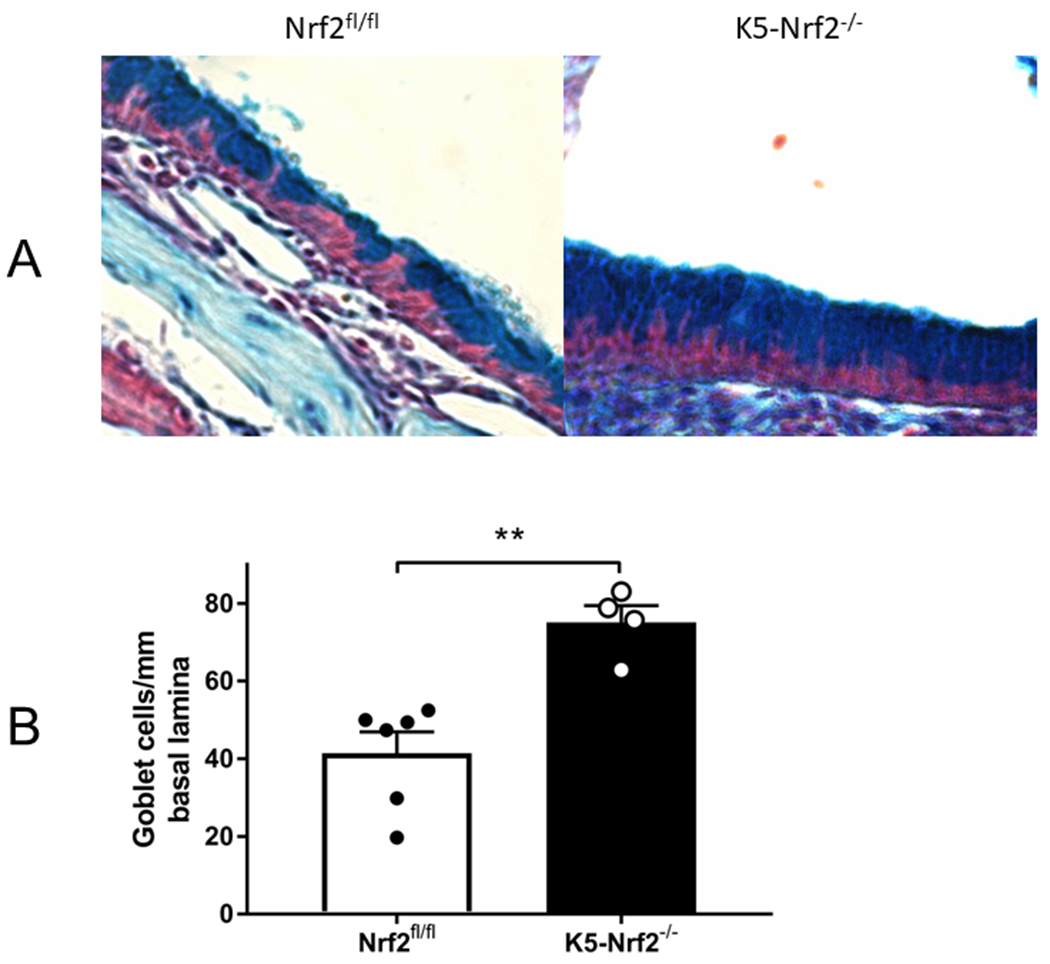
Goblet cell hyperplasia in K5 Nrf2−/− sinonasal mucosa (20X). (A) Representative photomicrograph of alcian blue stained histological sections from Nrf2 flox/flox (left) or K5 Nrf2−/− mice treated with papain (right). Note increased goblet cells in the right side (B) Goblet cells were counted along the nasal septum and normalized per mm of basal lamina. Data is presented as mean±SEM. **p<0.01.
Enhanced Tissue Eosinophilia
To evaluate the cellular infiltrates induced by enzymatically active papain, we analyzed mucosal inflammatory cells by flow cytometry and compared K5 Nrf2−/− and Nrf2flox/flox treated mice. The gating scheme that we used to identify specific cell populations is depicted in Figure 4. There were no statistically significant differences in basophils, mast cells, or ILC2s. Our results did demonstrate that K5 Nrf2−/− mice exhibit a 2 fold increase in eosinophilia (p<0.05) compared to Nrf2flox/flox mice, which have an intact Nrf2 signaling system (Figure 5).
Figure 4:
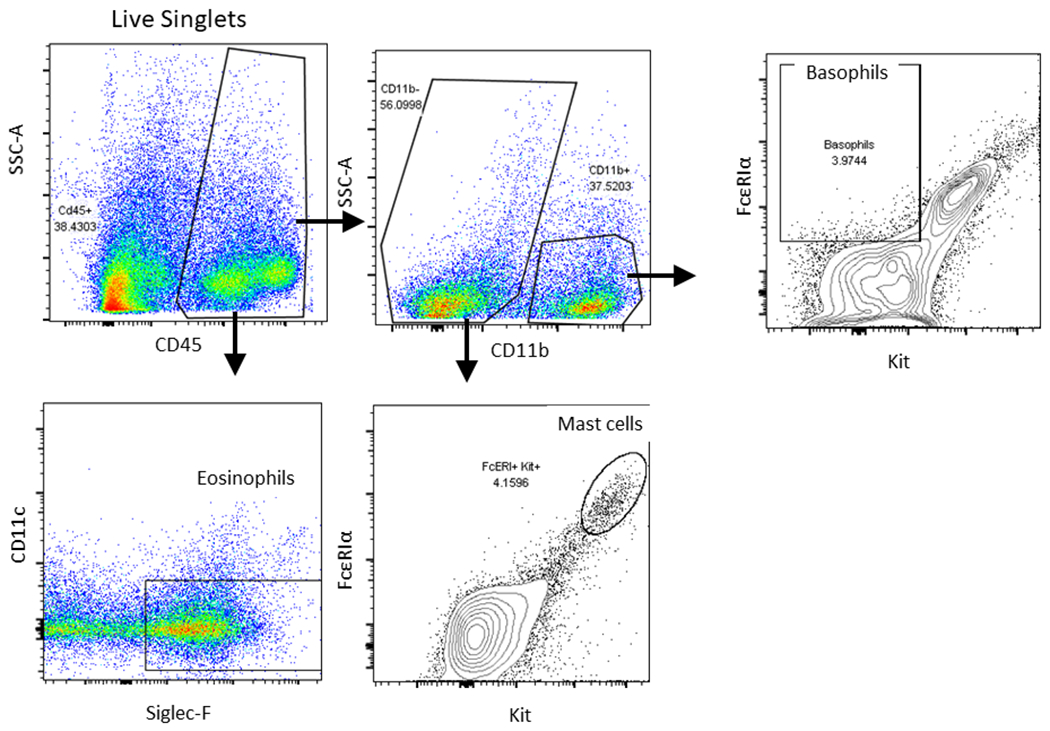
Flow cytometric gating scheme for identification of immune cell influx in sinonasal tissue. Eosinophils (Lin−, CD45+, CD11b+, CD11c−, and Siglec-F+), basophils (Lin−, CD45+, CD3−, FcεRIα+, c-Kit−), mast cells (Lin−, CD45+, CD3−, FcεRIα+, c-Kit+). Lineage cocktail: B220, NK1.1, Gr-1, and TER119.
Figure 5:
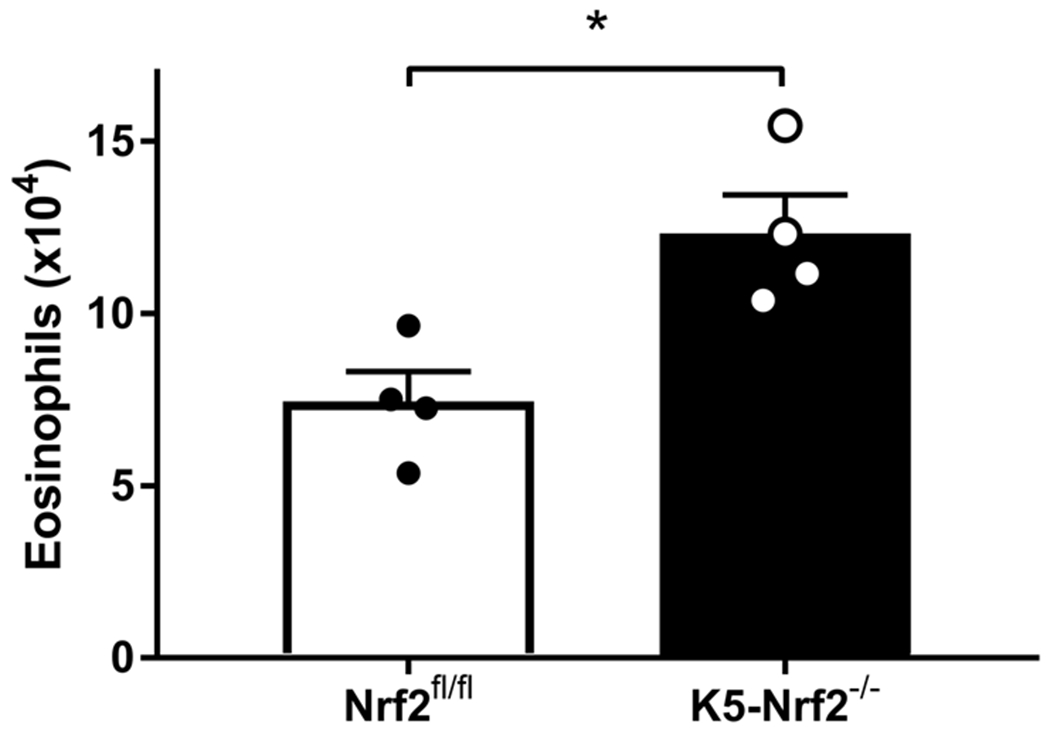
Flow cytometric analysis of sinonasal tissue for eosinophils. (Lin−, CD45+, CD11b+, CD11c−, and Siglec-F+) Data represented mean±SEM. *p<0.05
Increased IL-13 Production From Sinonasal Mucosal T cells
The type 2 cytokines IL-5 and IL-13 are involved in type 2 immune responses by promoting mucus production, IgE class switching, and stimulating eosinophil differentiation from myeloid progenitors in bone marrow respectively 25. Therefore, these cytokines were quantified from sinonasal tissue homogenates in both K5 Nrf2−/− and Nrf2flox/flox mice after papain treatment. K5 Nrf2−/− mice demonstrate a significant elevation in IL-13 (p<0.05) by almost 3 fold (Figure 6). IL-5 levels between the two groups did not achieve statistical significance.
Figure 6:
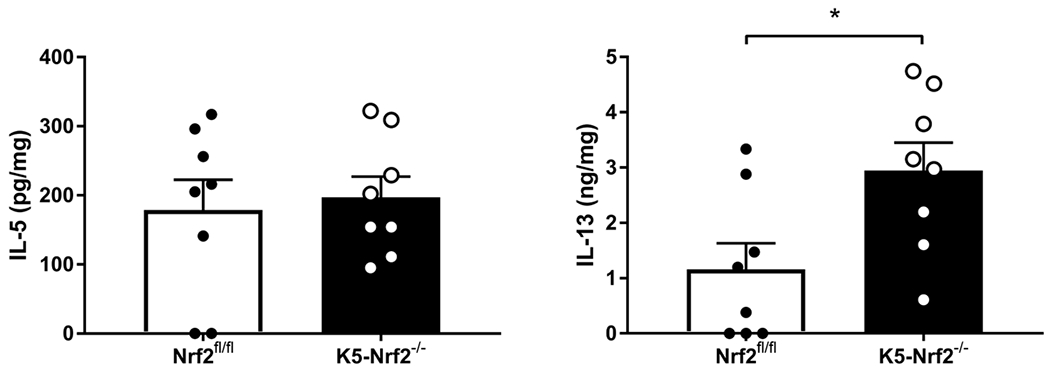
Local type 2 cytokine production in K5 Nrf2−/− and Nrf2 flox/flox mice induced by papain exposure. Concentrations of IL-5, and IL-13 in sinonasal tissue homogenates. Data represented mean±SEM. *p<0.05. In brief, there is a higher level of IL-13 overexpression in the K5 Nrf2−/− mouse. IL-5 levels did not change with epithelial specific knockout of Nrf2.
Next, intracellular cytokine staining and flow cytometry of IL-13 producing cells were used to determine the cellular source of IL-13 (Figure 7). We found a significant increase in IL-13 competent T cells (p<0.05) compared to basophil and ILC2 populations (Figure 8). These results indicate that epithelial specific Nrf2 signaling may regulates the activation and recruitment of IL-13 producing T cells in the sinonasal mucosa.
Figure 7:
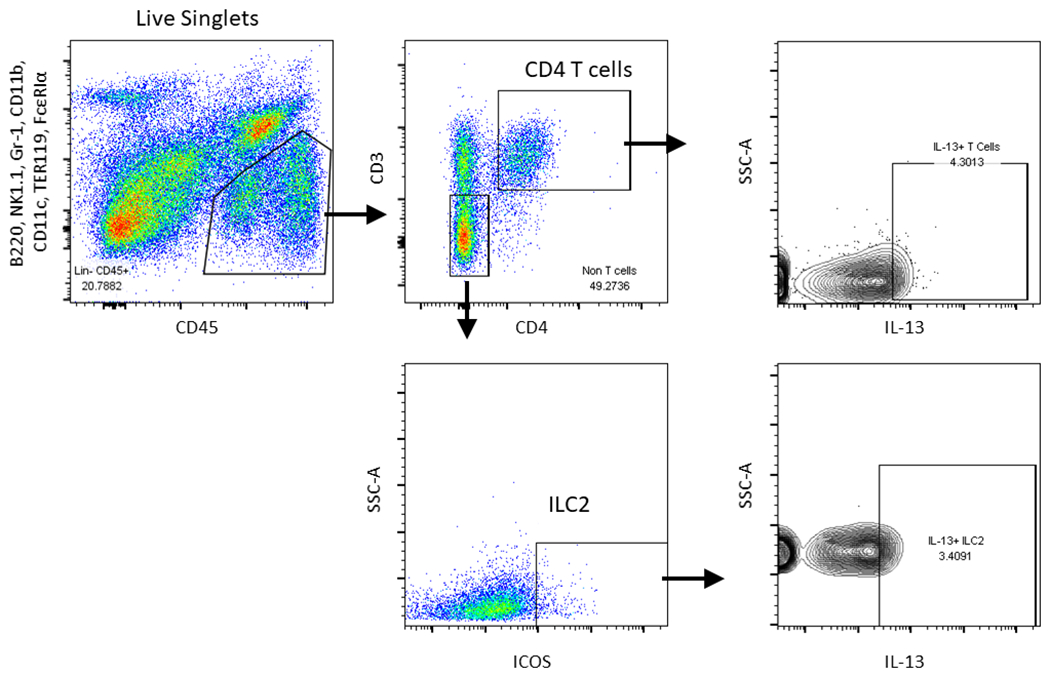
Flow cytometric gating scheme for identification of IL-13 producing immune cell influx in sinonasal tissue. T cells (Lin−, CD45+, CD11b−, CD3+) and ILC2s (Lin−, CD45+, CD3−, CD11b−, FcεRIα−, c-Kit−, CD25+, ICOS+) were identified as shown. Intracellular cytokine staining was performed in these populations for IL-13. Lineage cocktail: B220, NK1.1, Gr-1, and TER119. CD11b, CD11c, and FcεRIα.
Figure 8:
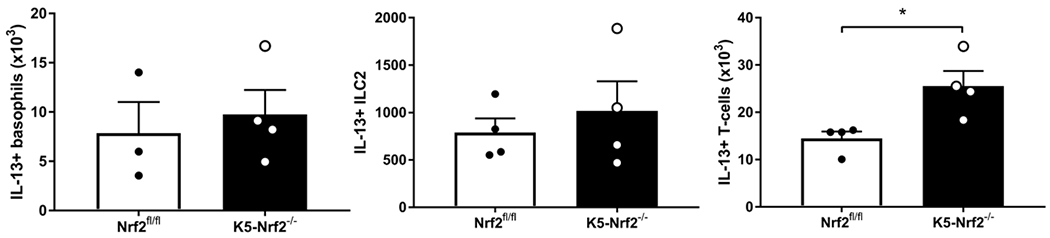
Papain induces expansion and recruitment of IL-13 producing T cell populations. IL-13 producing cells (basophils, ILC2, and T cells) were analyzed by flow cytometry in K5 Nrf2−/− and Nrf2 flox/flox mice. Data represented mean±SEM. *p<0.05.
Discussion
This study adds to our existing understanding of the role of Nrf2 in regulating the inflammation in chronic rhinosinusitis, specifically in the sinonasal epithelium. Previous work has demonstrated that Nrf2 is critical in regulating Ova induced sinonasal inflammation both from an epithelial barrier and inflammatory cytokine perspective 14,15. In addition, recent work has shown that Nrf2 activation can reverse sinonasal epithelial barrier dysfunction in CRS patients after stimulation with environmental triggers such as PM, tobacco smoke, and allergens 10,11,26. Our findings in this study, are additive to this body of work and further demonstrate that epithelial specific Nrf2 regulates the type 2 cellular and cytokine response in our mouse model of rhinosinusitis, which is very similar to what is seen in CRS. An epithelial specific deletion of Nrf2 alone is sufficient to create elevated sinonasal mucosal eosinophilia, goblet cell hyperplasia, and an increase in mucosal T cell production of IL-13. These findings further highlight how dysfunction in the sinonasal epithelium may help drive the Th2 inflammatory state seen in eosinophilic chronic rhinosinusitis.
The precise mechanisms by which the Nrf2 signaling pathway is protective against sinonasal inflammation remains poorly understood. Nrf2 is a redox transcription factor, which upon activation in response to inflammatory or oxidative stress detaches from its cytosolic inhibitor and transactivates a robust antioxidant and anti-inflammatory host defense transcriptional program. Therefore, these findings may suggest that excessive oxidative stress is generated during the inflammatory process and Nrf2’s ability to detoxify these reactive oxygen species helps control inflammation. CRS may represent a dysfunctional state of oxidative stress due to insufficient Nrf2 activity as seen in other inflammatory conditions such as COPD and asthma27. Further characterization of the Nrf2 signaling pathway in CRS patients will be crucial in future research studies.
These findings may have implications for the treatment of chronic rhinosinusitis, which remains primarily confined to oral corticosteroids and surgery. Nrf2 is a drug targetable pathway with known pharmacologic activators. Although several drugs are in the pipeline, there are two known agents, sulforaphane and dimethylfumarate. Sulforaphane is a naturally occurring compound found in broccoli sprouts and Dimethylfumarate is an FDA approved Nrf2 activating drug for use in relapsing multiple sclerosis. Several studies have explored the use of these compounds both in animal and human preclinical trials to enhance Nrf2 and reduce inflammation in diseases such as asthma and COPD 28,29.
In conclusion, our study in conjunction with previous data demonstrates that sinonasal epithelial specific Nrf2 levels are crucial in controlling inflammation and epithelial barrier stability. Future studies will be directed towards pharmacologic activation of the Nrf2 signaling pathway initially in mouse models of rhinosinusitis and eventually in human preclinical trials for the management of CRS.
Acknowledgments
Funding sources: NIH ES020859, NIH R01AI143731 (to M.R.)
Footnotes
Disclosures: N.R.L. is a patent co-inventor for methods treating vascular barrier dysfunction licensed to Navigen Pharmaceuticals which is unrelated to this work. N.R.L. holds a small amount of stock in Navigen Pharmaceuticals which is currently of no value.
Presented at the ARS meeting at the Triological Society COSM Meeting at National Harbor, MD 2018. This work was accepted as a Triological Thesis and was awarded the Edmund Prince Fowler Award.
Level of Evidence: N/A
References
- 1.Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol Suppl 2004; 193:3–5. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MP, Fishman P, Short SO, Sullivan SD, Yueh B, Weymuller EA Jr. Health care utilization and cost among adults with chronic rhinosinusitis enrolled in a health maintenance organization. Otolaryngol Head Neck Surg 2002; 127:367–376. [DOI] [PubMed] [Google Scholar]
- 3.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 1995; 113:104–109. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z, Wang Y, Zhang J et al. Expression of heme oxygenase-1 in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps: modulation by cytokines. Int Forum Allergy Rhinol 2015; 5:734–740. [DOI] [PubMed] [Google Scholar]
- 5.Mrowicka M, Zielinska-Blizniewska H, Milonski J, Olszewski J, Majsterek I. Evaluation of oxidative DNA damage and antioxidant defense in patients with nasal polyps. Redox Rep 2015; 20:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowler RP. Oxidative stress in the pathogenesis of asthma. Curr Allergy Asthma Rep 2004; 4:116–122. [DOI] [PubMed] [Google Scholar]
- 7.Jiang L, Diaz PT, Best TM, Stimpfl JN, He F, Zuo L. Molecular characterization of redox mechanisms in allergic asthma. Ann Allergy Asthma Immunol 2014; 113:137–142. [DOI] [PubMed] [Google Scholar]
- 8.Loukides S, Bakakos P, Kostikas K. Oxidative stress in patients with COPD. Curr Drug Targets 2011; 12:469–477. [DOI] [PubMed] [Google Scholar]
- 9.London NR Jr., Tharakan A, Lane AP, Biswal S, Ramanathan M Jr. Nuclear erythroid 2-related factor 2 activation inhibits house dust mite-induced sinonasal epithelial cell barrier dysfunction. Int Forum Allergy Rhinol 2017. [DOI] [PubMed] [Google Scholar]
- 10.Tharakan A, Halderman AA, Lane AP, Biswal S, Ramanathan M Jr. Reversal of cigarette smoke extract-induced sinonasal epithelial cell barrier dysfunction through Nrf2 Activation. Int Forum Allergy Rhinol 2016; 6:1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London NR Jr., Tharakan A, Rule AM, Lane AP, Biswal S, Ramanathan M Jr. Air pollutant-mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the Nrf2 pathway. J Allergy Clin Immunol 2016; 138:1736–1738 e1734. [DOI] [PubMed] [Google Scholar]
- 12.Hong Z, Guo Z, Zhang R et al. Airborne Fine Particulate Matter Induces Oxidative Stress and Inflammation in Human Nasal Epithelial Cells. Tohoku J Exp Med 2016; 239:117–125. [DOI] [PubMed] [Google Scholar]
- 13.Bui TT, Fan Y, Piao CH et al. Piper Nigrum extract improves OVA-induced nasal epithelial barrier dysfunction via activating Nrf2/HO-1 signaling. Cell Immunol 2019:104035. [DOI] [PubMed] [Google Scholar]
- 14.London NR Jr., Tharakan A, Mendiola M et al. Deletion of Nrf2 enhances susceptibility to eosinophilic sinonasal inflammation in a murine model of rhinosinusitis. Int Forum Allergy Rhinol 2019; 9:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.London NR Jr., Tharakan A, Mendiola M et al. Nrf2 activation via Keap1 deletion or sulforaphane treatment reduces Ova-induced sinonasal inflammation. Allergy 2019; 74:1780–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce AM, Gimenez-Conti IB, Schneider-Broussard R, Martinez LA, Conti CJ, Johnson DG. Increased E2F1 activity induces skin tumors in mice heterozygous and nullizygous for p53. Proc Natl Acad Sci U S A 1998; 95:8858–8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Reed RR, Lane AP. Acute inflammation regulates neuroregeneration through the NF-kappaB pathway in olfactory epithelium. Proc Natl Acad Sci U S A 2017; 114:8089–8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock JR, Onaitis MW, Rawlins EL et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 2009; 106:12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar PA, Hu Y, Yamamoto Y et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 2011; 147:525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am J Respir Cell Mol Biol 2011; 45:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tharakan A, Dobzanski A, London NR Jr. et al. Characterization of a novel, papain-inducible murine model of eosinophilic rhinosinusitis. Int Forum Allergy Rhinol 2018; 8:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho SH, Oh SY, Zhu Z, Lee J, Lane AP. Spontaneous eosinophilic nasal inflammation in a genetically-mutant mouse: comparative study with an allergic inflammation model. PLoS One 2012; 7:e35114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madden KB, Whitman L, Sullivan C et al. Role of STAT6 and mast cells in IL-4-and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol 2002; 169:4417–4422. [DOI] [PubMed] [Google Scholar]
- 24.Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol 2017; 12:331–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015; 75:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.London NR Jr., Tharakan A, Lane AP, Biswal S, Ramanathan M Jr. Nuclear erythroid 2-related factor 2 activation inhibits house dust mite-induced sinonasal epithelial cell barrier dysfunction. Int Forum Allergy Rhinol 2017; 7:536–541. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki M, Betsuyaku T, Ito Y et al. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2008; 39:673–682. [DOI] [PubMed] [Google Scholar]
- 28.Brown RH, Reynolds C, Brooker A, Talalay P, Fahey JW. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir Res 2015; 16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidel P, Roth M. Anti-inflammatory dimethylfumarate: a potential new therapy for asthma? Mediators Inflamm 2013; 2013:875403. [DOI] [PMC free article] [PubMed] [Google Scholar]


