Abstract
Mitochondria are recognized as the main source of ATP to cover the energy demands of the cell. ATP production occurs by oxidative phosphorylation when electrons are transported through the electron transport chain (ETC) complexes and develop the proton motive force across the inner mitochondrial membrane that is used for ATP synthesis. Studies since the 1960s have been concentrated on the two models of structural organization of ETC complexes known as “solid-state” and “fluid-state” models. However, advanced new techniques such as blue-native gel electrophoresis, mass spectroscopy, and cryogenic electron microscopy for analysis of macromolecular protein complexes provided new data in favor of the solid-state model. According to this model, individual ETC complexes are assembled into macromolecular structures known as respiratory supercomplexes (SCs). A large number of studies over the last 20 years proposed the potential role of SCs to facilitate substrate channeling, maintain the integrity of individual ETC complexes, reduce electron leakage and production of reactive oxygen species, and prevent excessive and random aggregation of proteins in the inner mitochondrial membrane. However, many other studies have challenged the proposed functional role of SCs. Recently, a third model known as the “plasticity” model was proposed that partly reconciles both “solid-state” and “fluid-state” models. According to the “plasticity” model, respiratory SCs can co-exist with the individual ETC complexes. To date, the physiological role of SCs remains unknown, although several studies using tissue samples of patients or animal/cell models of human diseases revealed an associative link between functional changes and the disintegration of SCs assembly. This review summarizes and discusses previous studies on the mechanisms and regulation of SC assembly under physiological and pathological conditions.
Keywords: mitochondria, inner mitochondrial membrane, electron transport chain complexes, respiratory supercomplexes, human diseases
Introduction
Mitochondria, known as the “powerhouse” of the cell, provide over 90% of ATP required in eukaryotic cells. Under physiological conditions, carbohydrates and lipids are metabolized to acetyl-CoA in mitochondria. Acetyl-CoA enters the tricarboxylic acid cycle producing the “reducing equivalents,” NADH and FADH2. Oxidative phosphorylation (OXPHOS) is described as oxidation of NADH and FADH2 through the electron transport chain (ETC) that is coupled with ATP synthesis. The ETC is composed of four complexes (CI, CII, CIII, and CIV) located in the inner mitochondria membrane (IMM). The ETC complexes transfer electrons from NADH and FADH2 to molecular oxygen accompanied by simultaneous pumping of protons from the matrix to the intermembrane space that generates an electrochemical proton gradient (proton motive force) across the IMM. The electrochemical energy created by ETC complexes stimulates FOF1-ATP synthase (CV) to produce ATP.
For a long time, the structural organization of ETC complexes has been an interesting subject to contemplate. Since the discovery of ETC complexes, two main models, known as the “solid-state” and “fluid-state” models, were proposed to explain the structural organization of ETC complexes in the IMM (Fig. 1). Pioneer studies in the early 1960s [1] suggested the capability of ETC and OXPHOS proteins to form a functional unit termed “oxysome,” which could facilitate electron transfer and ATP synthesis This model, later known as the “solid-state” model, could logically explain the structural proximity of ETC complexes to support their functional integrity. At the same time, elucidation of the physical and chemical properties of isolated ETC complexes revealed that individual complexes could function separately and undergo oxidation-reduction [2]. Based on the enzymatic activity and diffusion characteristics of individual ETC complexes, a “fluid-state” (or “random-collision”) model was proposed, in which all proteins and redox components of ETC and OXPHOS diffuse freely and randomly in the IMM [3]. Since 2000, analysis of digitonin-solubilized mitochondrial proteins by blue native polyacrylamide gel electrophoresis (BN-PAGE) [4] enabled to separate high molecular mass multiprotein complexes with relatively preserved protein-protein interactions. By using this technique, ETC complexes were found to assembly into the multiprotein complexes or respiratory chain supercomplexes (SCs) in yeast and mammalian mitochondria. These findings were further supported by other studies that demonstrated the existence of SCs by electron cryotomography in mitochondrial cristae isolated without treatment with digitonin [5]. Notably, only certain fractions of individual complexes are assembled into the SCs, and recently, a “plasticity” model was proposed to explain the structural organization of ETC complexes [6]. This model reconciles both “solid-state” and “fluid-state” models suggesting that SCs co-exist with individual ETC complexes. High-resolution structural analysis using cross-linking mass spectroscopy [7, 8] and cryogenic electron microscopy (cryo-EM) [9–12] presented a more detailed structural organization of SCs in mammalian mitochondria. In addition to the architecture of SCs, several proteins have been discovered that participate in the biogenesis, assembly, and stability of respiratory SCs in yeast [13, 14] and mammalian [15, 16] mitochondria.
Figure 1.
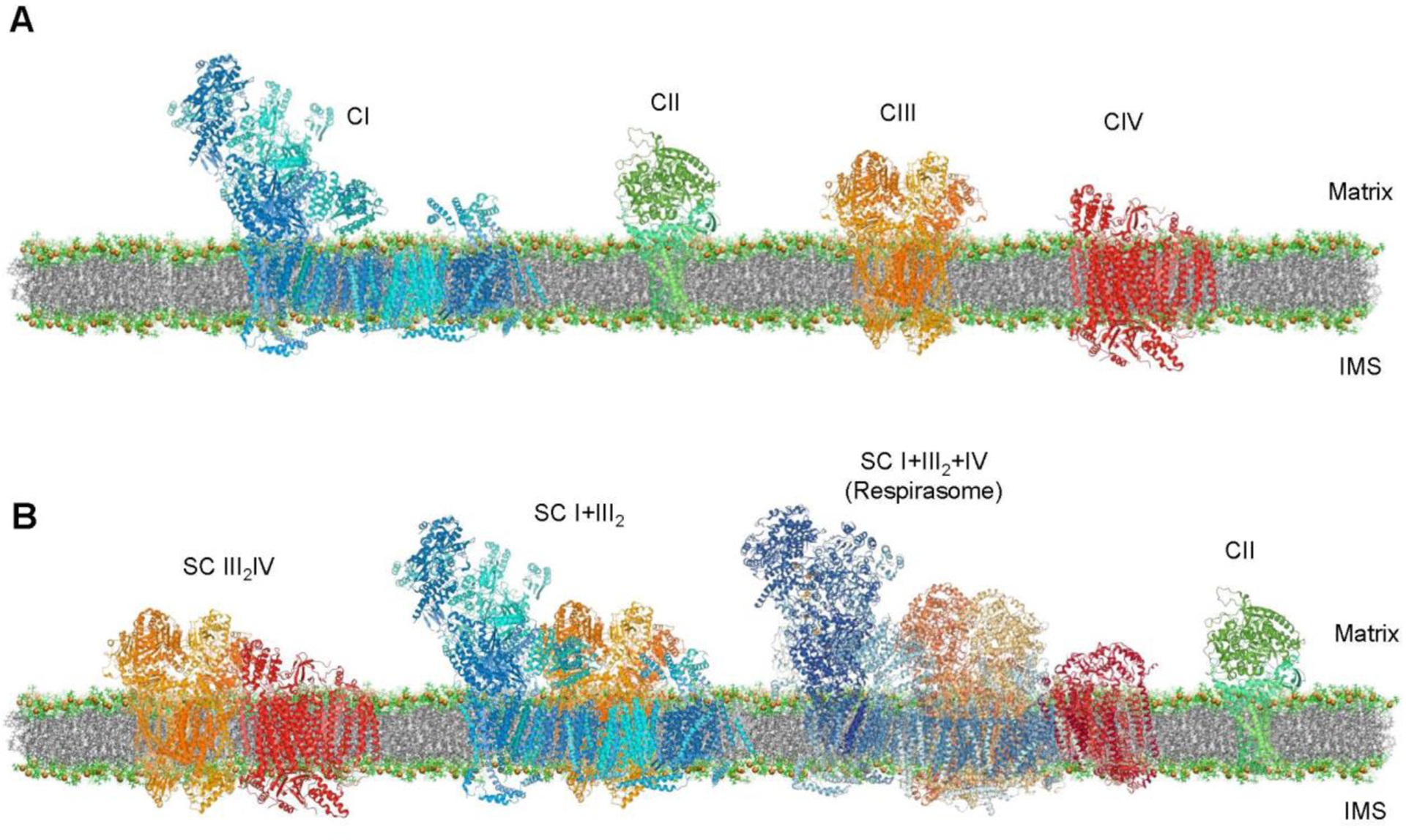
Structural organization of ETC complexes: fluid-state (or random-collision) model (A) and solid-state model (B). Molecule Images created using RCSB PDB ID 4UQ8 (CI, blue), 1ZOY (CII, green), 1BGY (CIII, orange), 3ASO (CIV, red) and 5J4Z (respirasome) and Mol* software [147]. The membrane created from the images shown in the study by [148].
Significant achievements in the understanding of the structural organization of SCs initiated new studies to understand the role of SCs in mitochondria in health and disease [17]. A large number of studies using cell/animal models of human diseases, and biological samples from patients demonstrated disintegration of SCs during cardiovascular [18, 19], and neurodegenerative [20, 21] disorders, Barth syndrome [22, 23], diabetes [24], and aging [25], whereas exercise was found to stimulate SCs assembly [26, 27]. In contrast, several studies were not able to find an associative link between diseases and SCs. Besides, the roles of SCs in substrate channeling and ROS prevention have been challenged [28, 29]. These controversies raised the question of whether SCs play any physiological and/or pathophysiological role in the cell, and are they involved in the pathogenesis of human diseases.
In this review, we summarize and discuss the structural assembly of SCs and their possible functional advantages in the regulation of mitochondrial bioenergetics. Detailed analysis of the structure and potential mechanisms of assembly and stabilization of SCs have been reviewed previously [30–32]. Therefore, we will briefly discuss the structural organization of SCs, and mostly focus on whether SCs disassembly contributes to a mitochondria-mediated cell or organ dysfunction in response to pathological stimuli.
Structural organization and regulation of assembly and stability of respiratory SCs
Structure of mammalian respiratory SCs
Pioneer studies using BN-PAGE identified respiratory SCs containing CI, CIII, and CIV in various stoichiometry, such as CI+CIII2, CIII2+CIV, and CI+CIII2+CIV in bovine heart mitochondria [4, 33]. These studies revealed that approximately 80% of CI, 65% of CIII, and 15% of CIV are involved in SCs. The main SC is the respirasome that consists of CI, CIII, and CIV (SC CIn+CIII2+CIVn). Assembly of these complexes is termed the respirasome because it, together with mobile electron carriers Q and cytochrome c, can operate as a single functional unit that transfers electrons through the ETC to oxygen while simultaneously developing the electrochemical gradient across the IMM. Downregulation of adenine nucleotide translocase (ANT), the most abundant protein of the IMM that exchanges the matrix ATP for ADP in the intermembrane space, was associated with reduced levels of the respirasome [34]. In this study, protein levels of individual ETC complexes were not analyzed, although the enzymatic activity of the complexes remained unchanged in ANT1 knockdown cells. It is not clear whether the decreases in SC levels observed in ANT deficit mitochondria are due to SC instability or defects in SC formation. These findings suggest a crosstalk between respirasome and synthasome, another mitochondrial SC containing F1FO-ATP synthase, ANT, and inorganic phosphate carrier (PiC) that links mitochondrial ATP production with energy transfer [35, 36]. In addition to respirasome, BN-PAGE analysis revealed the SCs containing CI and CIII2 (SC CI+CIII2) [33] as well as CIII2 and CIV (SC CIII2+CIV) [4, 37]. The level and ratio of respiratory SCs can vary in different tissue and mammalian species [4]. Recent mass-spectrometric studies revealed that SC stability and assembly also varies among vertebrates; SCs are unstable in endotherms (e.g., mammals and birds) and highly stable in ectotherms such as reptiles [38]. Interestingly, the stability of SCs does not affect mitochondrial ROS (mtROS) production and respiration rates in the examined vertebrates. Due to its importance in mitochondrial energetics, structural and functional properties of the respirasome are broadly discussed, and this review will be mainly concentrated on the respirasome. ETC complexes I, III, and IV are assembled at different stoichiometry and therefore, appear on the BN-PAGE gel as different bands depending on the molecular weight.
The BN-PAGE analysis did not detect CII in the respirasome and other SCs [4], although structural studies by cryo-EM suggested an interaction of CII with respirasome to form the respiratory megacomplex containing all ETC complexes (I2+II2+III2+IV2) [39]. A significant portion of CII was found to associate with other ETC complexes to form SCs, and a putative SC containing CII, CIII, and CIV was found in mouse mitochondria by BN-PAGE analysis [6]. Genetic silencing of the membrane-anchored SDHC subunit of CII did not affect the respirasome levels in cardioblast cells and isolated cardiac mitochondria [40]. However, inhibition of CII enzymatic activity in this study reduced the respirasome levels, suggesting that even though CII is not involved in the SCs, it can play a regulatory role in respirasome assembly and/or be engaged in the maintenance of its stability. Also, CII and other potential assembly proteins could be involved in the architecture of respirasomes by weak interactions but are easily lost due to a quick dilution-induced dissociation during the preparation of protein samples for BN-PAGE or cryo-EM analysis [41].
Detailed structural organization of SCs in mammalian (bovine, ovine, and porcine) mitochondria was elucidated recently using single-particle cryo-EM analysis at high (4–9 Å) resolution [9–12]. These studies reported the heterogeneity in SC structure amongst mammalian species. Two distinct arrangements of the respirasome, a major ‘tight’ and a minor ‘loose’ forms, were identified in ovine mitochondria [10]. These conformations may represent different stages in assembly or disassembly of the respirasome. Furthermore, structural biology studies proposed high-resolution models of respirasome, wherein specific interactions between individual subunits of the ETC complexes maintain the SC structural integrity [9–12]. Accordingly, CIII dimer interacts with CIV and the membrane arm of CI at specific sites in the mammalian respirasome. Two main interactions between CI and CIII occur at the following sites: i) NDUFA11 and NDUFB4 (CI subunits) interact with UQCRQ (CIII subunit), and ii) NDUFB4 and NDUFB9 (CI subunits) interact with UQCRFS1 (Rieske protein) and UQCRC1 (CIII subunits) [9, 10, 12]. In mammalian mitochondria, CI consists of 44 subunits, including 14 highly conserved core subunits and 30 supernumerary or accessory subunits acquired during the evolution of eukaryotes. The core subunits are responsible for the activity of the complex, whereas supernumerary subunits are involved in the assembly and structural stability of complex I [42, 43], but the precise role of all CI subunits is not fully understood. All three CI subunits (NDUFA11, NDUFB4, NDUFB9) involved in the CI-CIII interaction are the supernumerary subunits. The sites responsible for CI-CIII2 and CIII2-CIV interactions vary in different mammalian species. Interactions between CIII dimer and CIV in ovine mitochondria, COX7A, a CIV subunit, has been shown to interact with UQCR1 and UQCR11 (CIII subunits) [10].
Thus, advanced biochemical and electron microscopy techniques identified the structural identity of respiratory SCs, which include SCs CI+CIII2, CIII2+CIV, and CIn+CIII2+CIVn or respirasome. Individual ETC complexes are assembled in the SCs in different stoichiometric ratios through the interaction at specific binding sites of CI, CIII, and CIV. The precise mechanisms of SC assembly have not yet been elucidated and require further studies.
Regulation of biogenesis, assembly, and stability of respiratory SCs
The IMM contains two regions, the inner boundary membrane, and the cristae membrane. The inner boundary membrane is localized beneath the outer mitochondrial membrane and is comprised of numerous carrier proteins that transport ions and metabolites across the IMM. The cristae membrane are invaginations of the inner boundary membrane with narrow crista junctions extended into the matrix. The cristae contact the outer mitochondrial membrane by the mitochondrial contact site and cristae organizing (MICOS) system that consists of one soluble and five membrane proteins [44]. ETC and OXPHOS complexes are localized in the cristae membrane, where dimers of the F1FO-ATP synthase organize the structure of crista rims [45–47]. Furthermore, SCs have been found to concentrate in the cristae membrane [46, 48].
Different phospholipid species have opposite effects on the SC stability to regulate the balance between SCs and individual complexes. Phosphatidylethanolamine stimulates the disintegration of SCs, whereas cardiolipin stabilizes their assembly [49]. Cardiolipin, a signature phospholipid exclusively found in mitochondria, accounts for ~20% of total lipids in the IMM and 3–4% in the outer mitochondrial membrane [50, 51]. Two lines of evidence confirm the role of cardiolipin in SC stability. First, CIII and CIV reconstituted into liposomes can form SCs only in the presence of cardiolipin [52]. Second, the downregulation of cardiolipin synthesis is associated with SC disintegration. In favor of this, a genetic deficiency of tafazzin (a mitochondrial phospholipid-lysophospholipid acyltransferase), one of three enzymes that catalyze remodeling (maturation) of cardiolipin, induces degradation of SCs in patients with Barth syndrome [22] and tafazzin knockdown mice [23]. Notably, respiratory SCs also play a role in cardiolipin stability since a loss of SCs enhances access of phospholipases to cardiolipin, thereby inducing its degradation in patients with Barth syndrome [53].
Recent studies identified several proteins that are involved indirectly or directly in the regulation of SCs formation and stability. Stomatin-like protein 2 (SLP2) is widely expressed in the IMM, where it interacts with prohibitin 1 and 2 and binds to cardiolipin. In mammalian cells, the upregulation of SLP2 increases cardiolipin levels associated with activation of IMM metabolism and mitochondrial biogenesis [54]. SLP2 regulates mitochondrial cardiolipin content by creating cardiolipin-enriched microdomains in the IMM. In T cells, the deficiency of SLP2 decreased levels and activities of SC I+III2 and respirasome with no effects on the assembly of individual ETC complexes [55]. Likely, the effect of SLP2 on the formation of SCs is secondary to the cardiolipin-regulatory role of this protein. As an alternative mechanism, SLP2 can be involved in the structural organization of SCs by regulation of the optic atrophy 1 protein (OPA1), a dynamin-like GTPase localized exclusively in the IMM [56]. In addition to the role of fusion of mitochondria, OPA1 is important for the maintenance of the IMM topography and cristae structure [57, 58]. Proteolytic cleavage of long-OPA1 (L-OPA1) is catalyzed by two distinctly regulated IMM proteases, OMA1 and YME1L, that produce short-OPA1 (S-OPA1) [59, 60]. SLP2 has been shown to anchor a proteolytic hub in mitochondria containing the i-AAA protease, YME1L [61], and change its enzymatic activity. As a result, the balance between L-OPA1 and S-OPA1 could regulate SC assembly. OPA1 ablation in mouse embryonic fibroblasts induced disintegration of SCs, and conversely, fibroblasts isolated from OPA1 transgenic mice contained increased levels of SCs [62]. Likewise, OPA1 deficiency impaired the SC assembly and diminished ETC activity and OXPHOS in HEK-293 cells [63].
Interestingly, SLP2 and other scaffold proteins, such as prohibitins, are membrane proteins involved in the SPFH protein superfamily (stomatin, prohibitin, flotillin, and HflK/C) that hold an evolutionarily conserved SPFH domain [64]. Like SLP2, prohibitins 1 and 2 have been shown to interact with AAA proteins, particularly mAAA protease [65]. Deficiency in prohibitins 1 and 2 in HeLa cells impaired the assembly of SCs, particularly SC III2+IV, without altering the structural integrity of CI, CIII, and CIV [66]. These studies suggest that proteolytic cleavage of L-OPA1 could mediate the effects of both SLP2 and prohibitins to regulate SCs assembly through changes in the cristae morphology. Another mitochondrial cardiolipin-binding protein C11orf83, also known as ubiquinol-cytochrome c reductase complex assembly factor 3 (UQCC3), has a high affinity for CIII dimer, and is involved in stabilization of CIII-containing SCs, especially the SC CIII2+CIV in H9c2 cells [67]. C11orf83 depletion impaired cristae morphology associated with a decreased mitochondrial respiration and ATP production and significant changes in cardiolipin composition. Apparently, SLP2 and C11orf83 participate in SC assembly indirectly; they regulate synthesis and compartmentalization of cardiolipin and thus, maintain the cristae structure and the integrity of IMM proteins.
Respiratory complex factors 1 and 2 (Rcf1 and Rcf2) in yeast [13, 68–70] and the mammalian orthologs of Rcf1, HIGD1A and HIGD2A [14, 71, 72], have been shown to participate in the assembly and stabilization of CIV and thus, indirectly involved in the formation of CIV-containing SCs, particularly SC CIII+CIV. These factors stably and independently interact with both CIII and CIV to maintain the structural and functional integrity of these complexes. Most recent studies demonstrated that HIGD1A and HIGD2A participate in the biogenesis of individual CIII2 and CIV as well as their association to form respiratory SCs [71] suggesting that these factors can directly modulate the assembly of SCs. MCJ/DnaJC15, a cochaperone that localizes in the IMM and interacts preferentially with CI, can be involved in SC formation [73]. Analysis of heart mitochondria isolated from MCJ knockout mice revealed increased levels of SCs compared to WT counterparts, suggesting that MCJ acts as a negative regulator of SC assembly. Increased formation of SCs in MCJ knockout mitochondria should reduce the levels of individual CI, CIII, and CIV. Although the loss of MCJ increased CI activity and ATP production, the levels of monomeric CI and CIV, and dimer CIII were not affected in these mitochondria [73]. Therefore, the observed effects of MCJ deficiency on SC assembly might be indirect through increasing CI activity; however, no changes in the levels of individual ETC complexes undermine the role of MCJ in SC formation.
The COX7A2-like protein (COX7A2L), also known as COX7RP (COX7AR) or SCAF1 (supercomplex assembly factor 1), is a specific regulatory protein that is directly involved in SC formation. Initial studies using skeletal muscle mitochondria of COX7A2L knockout mice [15, 74] revealed the ability of COX7A2L to stimulate the interaction between CIII and CIV and thus, enhance the structural integrity of SC III2+IV and respirasome. The regulatory role of COX7A2L depends on the expression levels of short and long isoforms of the protein. Studies on mouse strains containing short (C57BL/6J and C57BL/6N mice) and long (CD1 mice) COX7a2l gene isoforms showed that the long isoform, but not short COX7A2L, is required for the SC III2+IV assembly [16]. The most recent studies detected specifically associated with respirasome and SC CIII2+CIV where it confers the interaction between III2 and IV and hence, maintains the assembly and kinetic properties of the SCs [75]. Human COX7A2L was able to regulate biogenesis of CIII and stimulate remodeling of SCs although the COX7A2L-dependent ETC reorganization was not essential to maintain mitochondrial bioenergetics [76]. Heart and liver mitochondria isolated from mice with different Cox7a2l isoforms exhibited no differences in respiratory chain activity and respirasome formation and assembly. Results of this and other studies [77, 78] concluded that COX7A2L stabilizes the SC III2+IV, but has no effect on the formation of respirasome. It has been suggested that a homologous region of COX7A2L interacts with CIV by replacing with COX7A that leads to stabilization of SC III2+IV. In mutagenesis studies with mouse COX7A2L, His73 in the COX7A homologous region was identified to promote the interaction between CIII2 and CIV [37]. These studies revealed that the two residues Pro71 and Ile72 adjacent to His73 play a critical role in the CIII2-CIV interaction, and the lack of these residues in the short COX7A2L isoform found in various cells/tissues prevents these complexes from interacting with each other.
Notably, structural biology techniques such as cryo-EM were not able to identify any SC assembly protein that can be explained by its low resolution to detect all the protein densities. Besides, the interactions of assembly proteins with SCs can be transient and weak and get lost during the processing of mitochondrial samples and isolation of SCs. On the other hand, assembly factors could not be detected following the processing of mitochondria, because they are not the SC core components and not involved in the structure of SCs.
Potential advantages of respiratory SCs assembly
Since the discovery of respiratory SCs, a large number of studies proposed several structural or functional advantages of these multimolecular structures; however, the role of SCs in mitochondria remains unknown. SCs, particularly the respirasome has been suggested to i) improve the efficiency of electron transport through the ETC and thus, facilitate substrate channeling [15, 79, 80], ii) prevent electron leakage and reduce mtROS generation [81, 82], iii) maintain the structural integrity of individual ETC complexes [83–85], and iv) avoid protein aggregation in the IMM containing a heavily populated protein environment [28, 86].
Substrate channeling.
Cryo-EM studies using bovine heart mitochondria proposed that a solid-state structure can function as a functional unit that encloses Q cycle and cytochrome c to provide efficient electron transfer from CI to CIII and from CIII to CIV in a single entity [11, 79]. Flux control analysis of bovine mitochondria revealed substrate channeling between CI and CIII via ubiquinone, but not between CIII and CIV via cytochrome c [80]. Functional analysis of enriched respirasome fractions in combination with quantitative data-independent proteomics and 2D BN-PAGE revealed that the structural attachment facilitated by SCAF1 between III2 and IV in the respirasome stimulated NADH-dependent respiration and reduced mtROS production [75]. This study shows that SCAF1 can be involved in SCs as a structural component and the assembly of CI and CIII into the SC CI+CIII can confer retention of CoQ and as a result, partial segregation of CoQ pool enables substrate channeling. However, numerous structural biology studies challenged the role of substrate channeling and distance between individual complexes in respirasome [10, 87]. The respirasome does not contain any barriers to prevent free diffusion of electrons, thus arguing against the substrate-channeling concept. Active sites of complexes within the respirasome may activate oxidation-reduction reactions, and the lack of channels and barriers allows free random diffusion of Q cycle components and cytochrome c. The role of the distance between complexes in the diffusion of electron carriers is not clear because it is difficult to quantify the rate of diffusion that depends on the physicochemical characteristics of the IMM. Spectroscopic and kinetic experiments on mitochondrial membrane preparations containing all SCs demonstrated that NADH and succinate oxidation occurs through a single Q-pool without any partitioning or channeling [28, 29]. Furthermore, CoQ trapping in the isolated SC I+III2 has been shown to limit CI turnover [88]. Therefore, substrate channeling could limit CoQ oxidation due to unequal access of CoQ to the active sites of CIII2 and, thus, diminish mitochondrial respiration. Altogether, both structural and kinetic studies mostly support the free-diffusion theory indicating a free exchange of both Q pool and cytochrome c and, thus, contend against the existence of a physical channel for substrate transfer across the respirasome.
Prevention of mtROS generation:
First evidence on the possible role of SCs in the prevention of mtROS production came from in vitro studies that utilized two models: i) disintegration of SCs in bovine heart mitochondria or liposome-reconstituted SC I+III, and ii) reconstitution of CI and CIII, the major sources of mtROS production, into liposomes with high phospholipids to protein ratio [81]. Both models demonstrated that ROS production is strongly increased upon disruption of respiratory SCs, suggesting that the association between CI and CIII can reduce superoxide generation by these complexes. Likewise, high levels of free CI found in astrocytes were associated with low mitochondrial respiration and high ROS, whereas CI mostly embedded into SCs in neurons was concomitant with a high respiratory function and low mtROS levels [82]. It is still not clear whether SCs are responsible for the differences in respiratory function and ROS levels between neurons and astrocytes and whether changes in CI and CIII assembly in the SC can modulate ROS production. Notably, the flavin site of CI is important for superoxide anion production [89, 90], and cryo-EM studies demonstrate that the CI flavin site (a hydrophilic arm of NADH:ubiquinone oxidoreductase) is exposed outside the respirasome and not hindered by the adjacent CIII and CIV [9, 10, 12] (Fig. 2). Recent studies found no correlation between SC stability and ROS production in different vertebrates. It has been suggested that SCs can play an adaptive role in the regulation of body temperature without affecting the ETC activity and ROS production [38]. Further studies are required to establish a cause-and-effect relationship between SC assembly and ROS production in mitochondria.
Figure 2.
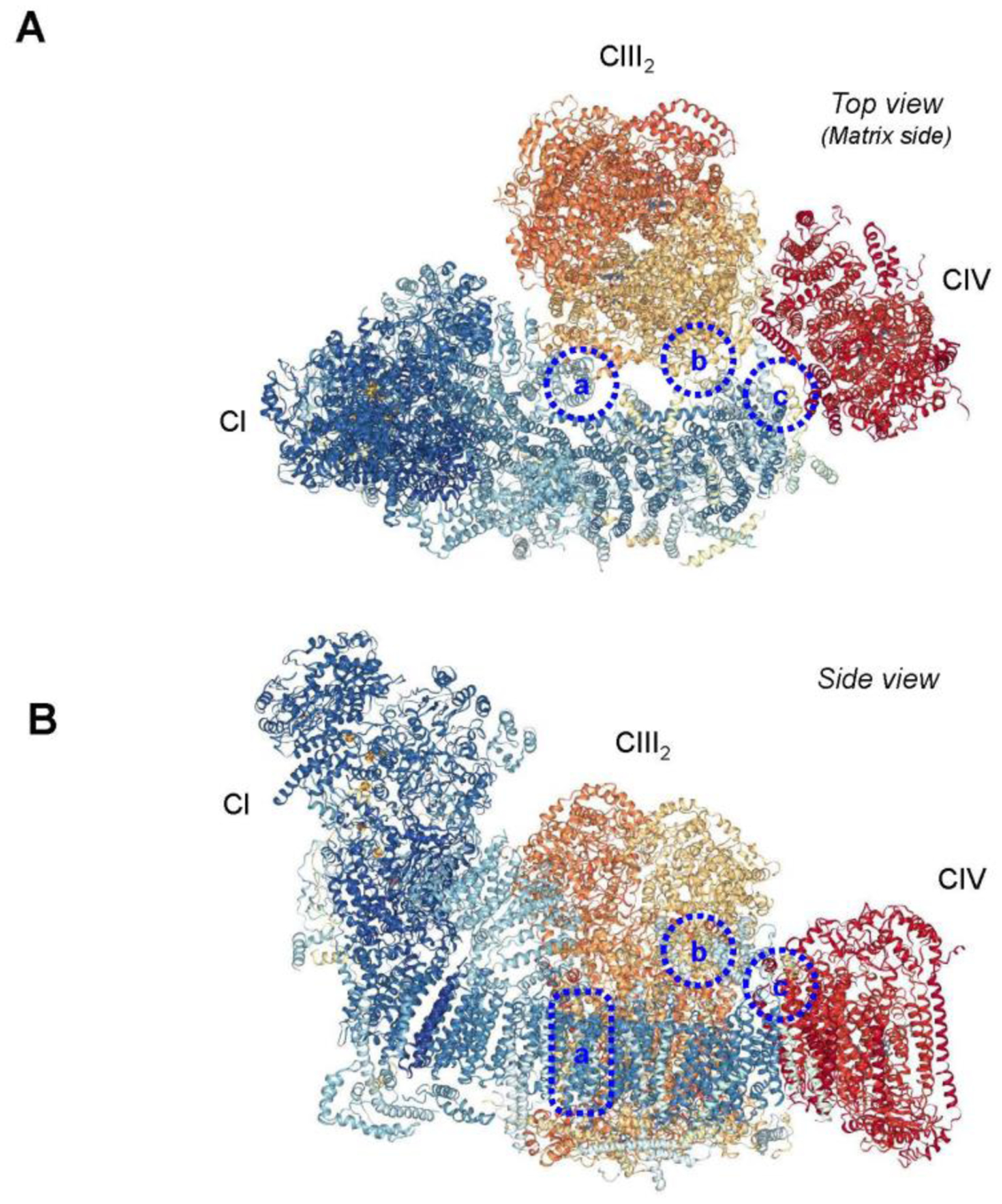
Interaction sites within the respirasome. Top view (A) and side view (B) are shown. (a) Interactions in the membrane between the CI subunit B14.7 (NDUFA11) and adjacent CIII2 subunits UQCRB, UQCRQ, and UQCRH. (b) Interactions in the mitochondrial matrix between the subunits B15 (NDUFB4) and B22 (NDUFB9) of CI and the UQCR1 (UQCRC1) subunit of CIII2. (c) Possible interaction sites between the ND5 subunit of CI and the COX7C subunit of CIV. Images were created with the PDB ID 5J4Z and NGL Viewer [149] and RCSB PDB. Interaction sites are based on the study by [30].
Maintenance of the structural integrity of individual ETC complexes:
Several studies reported structural crosstalk between ETC complexes and SCs, and SCs have been proposed to stabilize the structural integrity of individual ETC complexes, particularly, CI, CIII, and CIV. Mutations in the NDUFS4 gene in patients were associated with combined CI/CIII deficiency [91]. The authors suggested that alterations in SC assembly induced by the truncated NDUFS4 may be responsible for a combined deficiency of CI and CIII. NDUFS4 knockout decreased the activity and stability of CI in different mouse tissues; however, despite the deficiency in the subunit, SCs still allowed the formation of active and fully assembled CI, indicating the importance of supramolecular interactions for both stabilization and assembly of CI [85]. Likewise, genetic deficiency in CIII assembly in patients, as well as in mouse and human cultured cell models, prevented respirasome formation and caused a secondary loss of CI that resulted in a combined CI/CIII deficiency [83, 84]. ROS-associated decreases in CI and CIV and SC disassembly were observed in mouse lung fibroblasts lacking the Rieske iron-sulfur protein, one of the catalytic subunits of CIII [92]. Mammalian fibroblasts with genetic ablation of specific subunits of individual CI, CIII, or CIV showed that knockout of one complex subunit of any one complex disrupted that complex as well as the other complexes assembled into the same SC [93]. CI was found degraded in the mutant cells with CIII or CIV deficiency, although the structural integrity of CI proteins in CIII knockout cells was preserved better than in CIV knockout cells, suggesting that CI is more stable in the presence of CIV. Analysis of the structural rearrangements of ETC complexes in human cell lines depleted of the catalytic CIV subunit COX1 or COX2 demonstrated that incorporation of an atypical COX1-HIGD2A submodule attenuates the turnover rate of SC CI+CIII2 [94]. These findings indicate the importance of CIV subunits in SC stabilization as well as the structural dependence of CI on CIV in the biogenesis of SCs.
Thus, the combined defects in SCs induced by deficiency in a single complex suggest structural interactions between ETC complexes assembled in the SCs. However, it is not clear whether SCs play a causative role or are directly involved in the assembly and stability of individual complexes. Structural and functional defects in one ETC complex can trigger dysfunction or instability of other complexes in the SC-independent mechanisms. For example, deficiencies in ETC activity inhibit oxidation of reducing equivalents, and reduced pools of NAD+/NADH, succinate, and ubiquinone/ubiquinol (Q/QH2), which enhance ROS production [90]. Consequently, high ROS due to dysfunctional ETC can alter the stability of CI and other complexes (CIII, CIV), leading to the disintegration of the SC. Likewise, CI assembly was found impaired in the absence of CIII suggesting the importance of the CIII for maintenance of ETC activity and SC formation [95]. Thus, the stability of individual complexes plays a crucial role in the structural and functional integrity of SCs, and defects in complexes can affect the formation of SCs.
Prevention of protein aggregation in the IMM.
The IMM is one of the most protein-rich membranes with a protein-to-lipid mass ratio of 80:20 [96]. Phospholipids, particularly cardiolipin, play an essential role in maintaining the structural organization and stability of the ETC complexes and other IMM proteins. Low phospholipid levels and the high protein-to-lipid ratio in the IMM significantly increase the probability of random aggregation of proteins. The random and uncontrolled aggregation can diminish the functional activity of proteins and alter the topography of the IMM. Structural studies of respirasomes were not able to detect strong contact sites in CI, CIII, and CIV interactions [79, 87]. Respiratory SCs may be a result of evolutionary adaptation of ETC complexes to a highly protein-rich environment to avoid random/disorganized aggregation in the IMM of eukaryotic cells. It has been proposed that [28] SCs maintain the structural integrity of proteins across the IMM and prevent their nucleation and random aggregation. The IMM in a highly dynamic membrane; its topography and function are sensitive to changes in the matrix volume [97, 98]. Weak and transient (reversible) interactions in SCs could prevent the strong interactions between proteins that could occur due to aggregation and affect the functional activity of individual complexes and IMM structure. Both in vivo and in vitro studies demonstrated the relationship between IMM morphology and SC assembly [62]. Thus, SCs are involved in maintaining the membrane topography and protein packaging in mitochondria.
Thus, a growing number of studies provide evidence that respiratory SCs can play potential functional and structural roles in mitochondria however, several studies were not able to confirm the role of SCs in facilitating mitochondrial bioenergetics and reducing ROS production. Besides, the structural role of SCs in the IMM has not been proven yet and requires further studies.
Respiratory SCs and human diseases
A growing number of studies using different animal models and human tissue samples suggested associative links between the disintegration of SCs, particularly the respirasome and organ dysfunction. Several studies reported that cardiovascular and neurodegenerative diseases, Barth syndrome, cancer, and aging are associated with respirasome disassembly. A representative image showing the destabilization of respiratory SCs is shown in Figure 3. However, a number of other studies failed to find an associative link between these diseases and the respirasome thus counteracting the argument for the functional role of SCs in the pathogenesis of human diseases.
Figure 3.
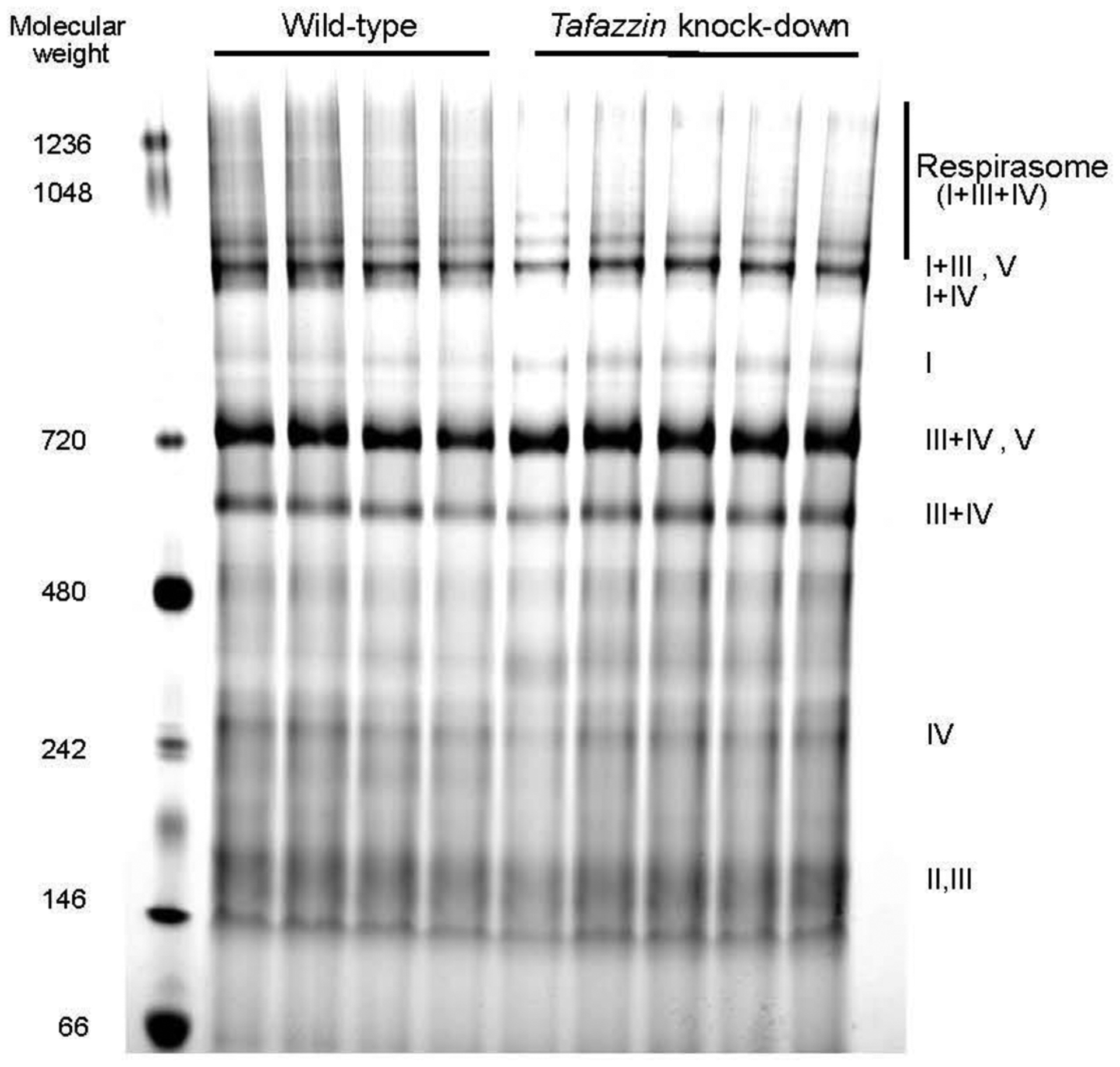
Representative image of Coomassie-stained BN-PAGE gel of mitochondria isolated from wild-type and tafazzin knockdown mice. ETC complexes were identified after the second-dimension of PAGE followed by immunoblotting using the Total OXPHOS Rodent WB Antibody Cocktail (Abcam). The image is adapted from our previous publication [19] with permission from Mary Ann Liebert, Inc.
Myocardial infarction and heart failure
The first studies using a canine model of heart failure demonstrated that a dramatic decrease of OXPHOS was associated with a significant reduction of respiratory SCs in subsarcolemmal and intermyofibrillar mitochondria [18]. The authors proposed that the defects in OXPHOS were caused by the disintegration of respirasome. Likewise, respirasome levels and the activity of CI, CIII, and CIV were remarkably reduced in the isolated and perfused guinea pig [99] and rat [100] hearts subjected to ischemia-reperfusion (IR). Cardioprotective effects of different pharmacological and conditional approaches used in these studies prevented respirasome degradation and normalized the activity of the ETC complexes. Post-infarction heart failure induced by ligating the left coronary artery in mice for 4 weeks decreased respirasome and CIII2+IV levels in cardiac mitochondria [101]. Interestingly, treatment with linoleic acid significantly improved mitochondrial respiration and cardiac function without any noticeable improvement in respirasome formation. Mitochondrial respiratory function was impaired concurrent with reduced SC controlled coupling factor, a mathematical construct to determine SC influence on respiratory measurement in freshly isolated mitochondria from failing human hearts compared to non-failing hearts [102]. The study proposed that mitochondria-targeted drugs can improve mitochondria function through the prevention of SC disassembly in the failing hearts. However, only the in-gel activity of SCs was analyzed in this study, and the conclusion on the reduction of SC levels was made by reductions of the SC coupling control factor ratio calculated from respiration rates for CI and CII.
Studies from several other groups reported no changes or even increases of SCs during myocardial infarction and heart failure. Pressure overload by transverse aortic constriction caused heart failure in rats after 20 weeks, which was associated with contractile dysfunction and a substantial decrease of mitochondrial respiration in gastrocnemius and soleus muscles [103]. Although the activity of all ETC complexes and protein expression levels of CI or CIII was significantly reduced, no differences in SC assembly were found in these muscles at 20 weeks of pressure overload compared to control. Coronary artery occlusion for 30 min, followed by 4-h reperfusion in mice, induced no significant changes in SC levels [104]. Cardiac IR injury induced by global 25-min ischemia followed by 60-min reperfusion in the Langendorff perfused rat hearts resulted in a ~5% decrease in respirasome levels, despite severe cardiac and mitochondrial dysfunction as evidenced by reduced post-ischemic recovery, high mtROS production, and low respiration rates [19]. Interestingly, oxidative stress induced by tert-butyl hydroperoxide (TBH) did not affect SC assemblies, while excess Ca2+ (1 mM) disintegrated SCs in cardiac mitochondria in vitro [105]. No remarkable changes were observed in the enzymatic activity of most individual ETC complexes, except CIII decreased by 19% at the end of reperfusion. In vivo myocardial infarction induced by coronary artery occlusion with or without subsequent reperfusion for 2 and 28 days in female rats significantly reduced cardiac function but had no effect on the respirasome integrity [106]. Analysis of mitochondria isolated from IR and ischemic preconditioned mouse hearts revealed no differences in the levels of SCs, and CIII and CIV between these groups, even though preconditioning significantly improved cardiac and mitochondrial function [107]. An enriched mitochondrial fraction isolated from the frozen heart tissue of patients with coronary artery disease (CAD) contained more respirasomes and less CI activity compared to non-CAD patients [108]. The high respirasome levels were associated with increased protein expression of all ETC complexes and CV in mitochondria obtained from CAD patients. In our recent studies, the disintegration of SC induced by ethanol and isopropanol was not associated with cardiac dysfunction, arguing against the causative role of SC disassembly in cardiac dysfunction [109]. Ethanol and isopropanol stimulated respirasome disassembly in Langendorff-perfused rat hearts without significant effects on heart contractility and mitochondrial respiration. We proposed that mitochondrial bioenergetics and cardiac function are not susceptible to moderate disassembly of respiratory SCs observed in our studies; however, severe disassembly of the respirasome beyond a critical threshold may affect both mitochondria and heart function.
Existing contradictory data on the effects of cardiac IR injury on respirasome assembly can be explained by differences in severity and duration of the oxidative stress. High mitochondrial Ca2+ and ROS in response to cardiac IR [110] causes opening of permeability transition pores (PTP) in the IMM, which, in turn, increases colloid-osmotic pressure and swelling of the mitochondrial matrix. As a result, the matrix swelling enhances the stretching stress on the IMM and change its topography [97, 111]. It is tempting to speculate that mild mechanical stretching (e.g., during ischemic preconditioning) of the membrane due to transient or mild increases in the matrix volume may increase SC formation to overcome the osmotic pressure on the membrane and prevent protein nucleation and aggregation. However, severe oxidative stress accompanied by excessive swelling of the matrix may not affect or disintegrate the SCs (Fig. 4). In favor of this hypothesis, inhibition of mitochondrial swelling by the PTP blocker sanglifehrin A (inhibitor of cyclophilin D, a major PTP regulator) prevented respirasome disassembly induced by cardiac IR injury [19]. Besides, moderate and severe oxidative stress has been shown to affect the SC assembly differently in brain mitochondria of in the neuron-specific Rieske iron-sulfur protein and COX10 knockout mice; moderate oxidative stress significantly increased whereas high levels of oxidative stress reduced SC levels [20] suggesting that mild oxidative stress can enhance the structural integrity of the IMM by stimulating SC assemblies to overcome possible negative effects on the membrane.
Figure 4.
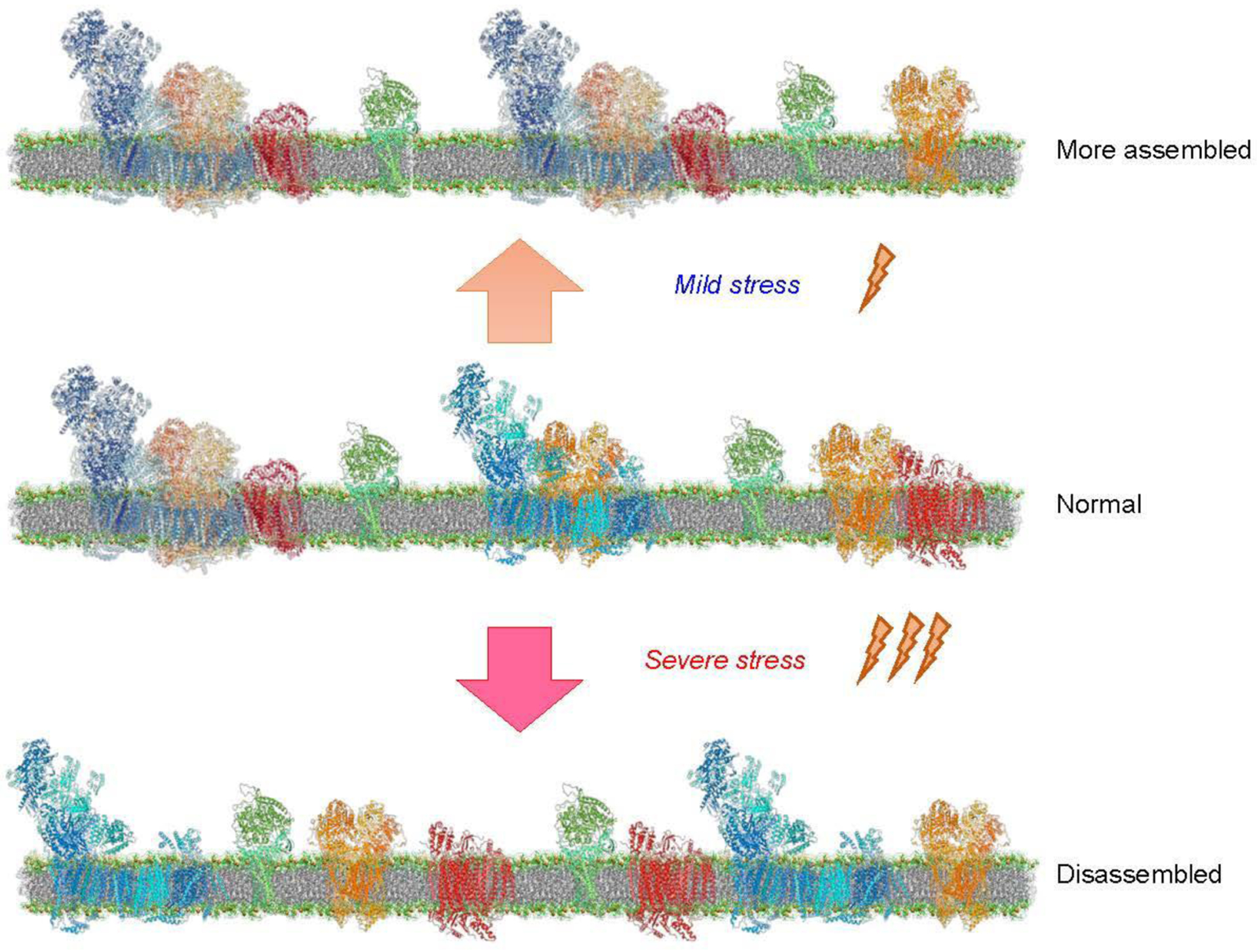
The effects of mild and severe stresses on the assembly of respiratory SCs. Mild stress stimulates the formation of SCs (top), whereas severe stress induces disassembly of SCs (bottom), suggesting that assembly/disassembly of SCs compared to the normal state (middle) depends on the severity of stress stimuli.
Cancer
Energy production is indispensable for tumorigenesis, and it is expected that SC assembly could facilitate electron transport, reduce ROS production, and enhance ATP synthesis. However, the role of SCs in tumorigenesis remains to be elucidated. Recently, several studies provide strong evidence that respiratory SC assembly can be affected in different types of cancer. COX7AR, also known as COX7A2L, a stress-inducible protein that is incorporated in the COX subunit, participates in mitochondrial SC assembly and respiratory activity [15, 77]. It is highly expressed in aggressive forms of human breast cancer, and its upregulation increased energy production and cell proliferation in the cancer cells [112]. These findings suggest that respiratory SCs stimulated by COX7AR might have an underlying role in improving mitochondrial bioenergetics, although SCs were not analyzed in this study. Analysis of human and mice breast cancer cell lines showed that the cells with high HER2 (human epidermal growth factor receptor 2) expression contained increased SC levels [113]. Treatment with MitoFam, the mitochondrial-specific tamoxifen that blocks the action of estrogen, prevented SC assembly and inhibited respiration associated with increased ROS production and cell death that prevented tumor growth in a high HER2 mouse breast cancer model [113]. Conversely, tamoxifen-resistant cells contained reduced SC levels and diminished mitochondrial respiration. The cells shifted their metabolism towards a glycolytic phenotype associated with increased glycolysis, higher ROS production, and fragmented mitochondria [114]. It was concluded that reduced mitochondrial respiratory function and high ROS production due to SC disassembly contribute to tamoxifen resistance in breast cancer cells.
In addition to SCs, regulatory factors of SC assembly were found affected in cancer cells. Nearly half of patients with acute myeloid leukemia displayed overexpression of neurolysin, a zinc metallopeptidase [115]. Neurolysin was found to interact with ETC complexes, and chemical and genetic inhibition of neurolysin disrupted SCs assembly and OXPHOS. Also, neurolysin was shown to interact with LETM1, a SC regulator [115], suggesting that like breast cancer cells, inhibition of SC assembly could serve as a promising therapeutic target against acute myeloid leukemia. COX7RP or SCAF1, a critical regulator in the formation of respiratory SCs, was found abundantly expressed in clinical breast and endometrial cancers, and its overexpression associated with the prognosis of breast cancer in patients [116]. In breast and endometrial cancer cells, SCAF1 overexpression promoted tumorigenesis and enhanced hypoxia tolerance associated with the stabilization of SC assembly and modulation of the metabolic profile. Likewise, overexpression of SCAF1 enhanced growth and metastasis of hepatocellular carcinoma. Downregulation of miR-130a-3p in human hepatoma cell lines led to an overexpression of SCAF1 [117]. The authors suggested that the defects in SC assembly may play a crucial role in hepatocellular carcinogenesis, although SC levels were not analyzed in this study. Dihydropyrimidinase like 4 (DPYSL4) protein that localizes in mitochondria and interacts with respiratory SCs, was found overexpressed in cancer cells and preadipocytes. The downregulation of DPYSL4 had a negative effect on tumor growth and metastasis, therefore creating a potential link between SCs, energy production, and cancer [118]. These findings suggest a potential role of SCs in metabolic reprogramming during carcinogenesis.
Thus, studies using biological samples of patients and different animal and cell models demonstrate that respiratory SC assembly is affected by cancer and, targeting SCs may have therapeutic potential for inhibition of cancer development. Further studies are required to establish the precise role of SCs in regulating mitochondrial metabolic pathways and energy production in cancer cells and the beneficial effects of SC disassembly in the prevention of cancer.
Barth syndrome
As mentioned above, cardiolipin plays an essential role in maintaining the structural and functional activity of respiratory complexes and SCs. Therefore, a reduction in cardiolipin levels and its oxidation is expected to play a causative role in the disintegration of SCs and alteration of the ETC activity. Alterations in the synthesis of mature cardiolipin induced by X-linked mutations in the tafazzin gene result in Barth syndrome, a genetic multi-system disorder associated with dilated cardiomyopathy, skeletal myopathy, immunodeficiency, and growth delay [119]. Tafazzin knockdown mice develop cardiac and skeletal muscle abnormalities and other defects similar to patients with Barth syndrome [120, 121]. Mitochondria isolated from patients with Barth syndrome, and tafazzin knockdown mice exhibited structural and functional abnormalities, decreased levels of mature cardiolipin, and accumulation of the intermediate monolysocardiolipins. Analysis of respiratory SCs in mitochondria isolated from lymphoblasts of patients with Barth syndrome [22] and the heart of tafazzin knockdown mice [19, 23] showed destabilization of SCs that was associated with changes in the activity and protein levels of individual ETC complexes. Protein levels of cyclophilin D, a PTP regulator, was higher in tafazzin knockdown mitochondria that demonstrated high mitochondrial swelling due to increased PTP opening in these hearts [19]. Hence, SC disassembly in tafazzin knockdown mice could be explained by cardiolipin deficiency and enhanced PTP-induced swelling. Despite 40% of respirasome levels in cardiac mitochondria isolated from tafazzin knockdown mice, there was no difference in ROS production rates between wild-type and tafazzin knockdown animals [19]. In addition to in vitro studies [29], these findings challenge the role of respirasome in the reduction of mtROS production.
Neurodegenerative diseases
Comparative analysis of mitochondria in neurons and astrocytes revealed an associative link between SC assembly and mitochondrial respiration and mtROS [82]. Astrocytes contained a high amount of free CI associated with high ROS production by these cells, whereas CI was found predominantly assembled into SCs in neurons that produced substantially less mtROS compared to astrocytes. In neurons, the CI subunit NDUFS1 was more abundant than in astrocytes, and NDUFS1 knockdown in neurons diminished incorporation of CI into SCs that was associated with impaired mitochondrial respiration and increased mtROS production. In contrast, overexpression of NDUFS1 in astrocytes increased CI assembling into SCs and reduced ROS production [82]. The amount of CI assembled in SCs has been proposed to explain the differences in mitochondrial bioenergetics and mtROS production between neurons and astrocytes. Fragile X-associated tremor/ataxia syndrome (FXTAS) is an inherited neurodegenerative disorder that affects primarily male carriers of a premutation expansion (55–200 CGG repeats) of the fragile X mental retardation 1 gene (FMR1). Expression of CGG premutation in SH-SY5Y and HEK-293 cells induced SC disassembly associated with diminished activity and expression of ETC complexes, and low ATP production [122].
Mitochondria isolated from patients with Parkinson’s disease (PD) and animal models of parkinsonism were shown to contain reduced CI activity and other abnormalities of the ETC [123, 124]. Composition and abundance of individual ETC complexes and SCs in striatal mitochondria were found unaffected in rats with early PD [125]. However, analysis of fibroblasts from patients with PD-relevant Pink1 mutations, as well as in cultured neurons and forebrain samples from of Pink1−/− and PD-related Dj1−/− mice showed that CI was disassembled from SCs and partially lost [126]. The loss of CIV was even more remarkable than CI. Apparently, diminished mitochondrial respiration due to CI damage concomitant with SC disassembly leads to energy deficiency and mtROS overproduction observed in PD patients. Alzheimer’s disease is characterized by the accumulation of β-amyloid peptides due to mutations in the catalytic subunits of γ-secretase, presenilin-1 (PSEN1) and presenilin-2 (PSEN2), and the amyloid precursor protein (APP). The activity and protein levels of respirasome, SCI+III, and SCIII+IV were found reduced in PSEN1 and PSEN2 double knockout mouse embryonic fibroblasts [127]. Chemical inhibition of γ-secretase showed similar effects on SC assembly and activity. Mitochondria from the postmortem prefrontal cortex of patients with Alzheimer’s disease revealed significant reductions in CII, CIII, and CV and no changes in CI levels [128]. Interestingly, despite the defects in ETC and OXPHOS, respiratory SCs remained well preserved in these patients, and no differences were found between the patients and healthy individuals.
Aging
A growing number of studies examined the role of mitochondria in cellular senescence and aging as a major source of ROS. According to the mitochondrial free radical theory of aging proposed in 1956 [129], ROS accumulation due to age-related alterations in ETC activity and OXPHOS induces genomic damage to nuclear and mitochondrial DNA [130] and accelerate telomere shortening [131] acting as the central drivers of aging and major regulators of signaling network necessary for cellular senescence. However, the precise and primary role of mitochondria in cellular senescence and aging remains unclear. In addition to increased mtROS production, low antioxidant capacity [132], diminished redox system [133], increased susceptibility to oxidative stress [134], and non-mtROS sources [135] also play an important role in aging.
ETC activity and OXPHOS decline with aging that is associated with mitochondrial uncoupling and low ATP due to diminished activity of individual complexes [136, 137]. Therefore, it is expected that the organization of respirasome can be altered in the aged mitochondria. In favor of this, SC levels were significantly less in heart mitochondria isolated from 24-month old rats compared to young (5-month old) counterparts [25]. Mostly, SCs with the highest molecular masses, such as respirasome, were severely diminished. Analysis of SCs in gastrocnemius mitochondria isolated from 3-month and 24-month old rats revealed significant reductions in SCs, particularly SC I1+III2, in old animals that contained the respirasome as a major SC among other supramolecular complexes [138]. Likewise, a 25% decrease in SCs was observed in gastrocnemius mitochondria isolated from aged rats (29-month old) compared with their adult (5-month old) counterparts [139]. Cerebral cortex mitochondria of 30-month old rats contained significantly lower levels of CI-associated SCs than 5-month old counterparts [140]. The SC III2+IV1 was reduced slightly (only 1.5%) in the aged cortex mitochondria.
In contrast, no significant differences in the protein levels of SCs were found in kidney mitochondria isolated from young (6-month old) and old (24-month old) rats [141]. Analysis of primary fibroblasts isolated from the skin of young (~27 years) and older (~75 years) and old individuals (about 100 years old) showed low protein expression levels of CI and CIV, and more CV dimers and oligomers in old individuals compared to other two groups [142]. Although SCs were not compared between the groups, no significant differences in the distribution of CI, CIII, and CIV in SCs were found in young, older, and old individuals. Interestingly, exercise was shown to increase SC levels in skeletal muscle mitochondria of aged (65 years) individuals [26].
Low levels of SCs, particularly respirasome, in mitochondria of aged cells may be a consequence of age-related alterations in the structural and functional organization of individual ETC complexes. Two models were proposed to explain CI-containing SCs, particularly, SC I+III2 and respirasome [32]. According to the first model, CI is required to be fully assembled before SC formation [143], suggesting that SC assembly occurs by direct interaction of individual preassembled complexes. We have shown that pharmacological inhibition of CI and CIII, but not CIV induced disintegration of respirasome in mitochondria isolated from rat hearts [40]. Individual knockout of the accessory subunits of CI disrupted CI activity and SC assembly in human HEK293T cells, whereas assembly of CIII and CIV in the SC was not affected [144]. Likewise, genetic silencing of the CI subunit NDUFA11 disrupted the respirasome and reduced the CI and CIII activity in H9c2 cardioblasts [40]. The second model proposes that preassembly of individual complexes is not required for SC assembly. In favor of this, SC assembly was observed in Neurospora crassa in the absence of full pre-assembly of individual CIs [145]. Also, addition of new COX subunits to mitochondria stimulated SC formation in patients with reduced CIV levels [146]. Although currently, both models of SC assembly are equally considerable, we suggest that age-related post-translational changes (e.g., acetylation) and low enzymatic activity of ETC complexes along with other structural and functional changes in the IMM can hamper the assembly of the respirasome and other SCs in aged cells.
Concluding remarks
A plethora of biochemical and structural studies provide strong evidence that individual ETC complexes, particularly, CI, CIII, and/or CIV are assembled into respiratory SCs in various stoichiometric ratios. The main SC is the respirasome containing all three complexes. Although several studies proposed a possible role of SCs in the maintenance of the structural organization of individual ETC complexes, reduction of electron leakage and ROS production, facilitating substrate channeling, and prevention of protein nucleation and aggregation, other studies challenged these findings. A large number of studies from different groups found an associative link between SC disintegration and organ/tissue dysfunction during cardiovascular and neurodegenerative diseases, cancer, Barth syndrome, and aging, suggesting a possible contribution of SCs in the pathogenesis of human diseases and aging. However, a cause-and-effect relationship between SC disassembly and human diseases remains unresolved. Furthermore, several studies demonstrated no change, even increases in SC levels in response to pathological stimuli suggesting that: i) disintegration of SCs depends on the severity and type of diseases, and ii) SCs may play a structural rather than functional role in mitochondria. Contradictory data on the changes of SC levels in various human diseases and animal disease models can be explained by technical challenges in the analysis of respiratory SCs. Mostly, conclusions are based on Coomassie staining, and in-gel activity/immunoblotting using the first dimension BN-PAGE gels, which are not the most accurate quantitative techniques, and many factors including the solubilization of the mitochondria can influence the results. Also, in most of these reports, the quality of the gels and the images are questionable. These technical difficulties could be the reason why some of the results on the SC stability/disassembly are contradictory. Further studies are required to clarify the role of SCs in physiological conditions, as well as in response to pathological stimuli.
Acknowledgments
The authors apologize to all colleagues whose important studies were not cited due to space restriction.
Funding
This study was supported by the National Institute of General Medical Sciences (Grant SC1GM128210 to Sa.J.) and the National Heart, Lung, and Blood Institute (Grant R01 HL-131673 to A.K.S.C.) of the National Institutes of Health, National Science Foundation (Grant 2006477 to Sa.J), and Advancing A Healthier Wisconsin (AHW; Grant 5520444 to A.K.S.C.).
Abbreviations
- ANT
adenine nucleotide translocase
- BN-PAGE
blue native polyacrylamide gel electrophoresis
- CI
complex I
- CII
complex II
- CIII
complex III
- CIV
complex IV
- CV
complex V
- cryo-EM
cryogenic electron microscopy
- ETC
electron transport chain
- IMM
inner mitochondrial membrane
- IR
ischemia-reperfusion
- mtROS
mitochondrial ROS
- OPA1
optic atrophy 1 protein
- OXPHOS
oxidative phosphorylation
- PTP
permeability transition pore
- ROS
reactive oxygen species
- SC
supercomplex
- SCAF1
supercomplex assembly factor 1
- SLP2
stomatin-like protein 2
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest
The authors declare no conflict of interest.
Ethics approval Not applicable
Availability of data and materials Not applicable
Code availability Not applicable
References
- 1.Chance B, Estabrook RW, Lee CP (1963) Electron Transport in the Oxysome. Science 140: 379–380. DOI 10.1126/science.140.3565.379-c [DOI] [PubMed] [Google Scholar]
- 2.Hatefi Y, Haavik AG, Griffiths DE (1962) Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem 237: 1676–1680 [PubMed] [Google Scholar]
- 3.Hackenbrock CR, Chazotte B, Gupte SS (1986) The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. Journal of bioenergetics and biomembranes 18: 331–368. DOI 10.1007/bf00743010 [DOI] [PubMed] [Google Scholar]
- 4.Schagger H, Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. The EMBO journal 19: 1777–1783. DOI 10.1093/emboj/19.8.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies KM, Strauss M, Daum B, Kief JH, Osiewacz HD, Rycovska A, Zickermann V, Kuhlbrandt W (2011) Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc Natl Acad Sci U S A 108: 14121–14126. DOI 10.1073/pnas.1103621108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Molecular cell 32: 529–539. DOI 10.1016/j.molcel.2008.10.021 [DOI] [PubMed] [Google Scholar]
- 7.Chavez JD, Lee CF, Caudal A, Keller A, Tian R, Bruce JE (2018) Chemical Crosslinking Mass Spectrometry Analysis of Protein Conformations and Supercomplexes in Heart Tissue. Cell Syst 6: 136–141 e135. DOI 10.1016/j.cels.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu F, Lossl P, Rabbitts BM, Balaban RS, Heck AJR (2018) The interactome of intact mitochondria by cross-linking mass spectrometry provides evidence for coexisting respiratory supercomplexes. Molecular & cellular proteomics : MCP 17: 216–232. DOI 10.1074/mcp.RA117.000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu J, Wu M, Guo R, Yan K, Lei J, Gao N, Yang M (2016) The architecture of the mammalian respirasome. Nature 537: 639–643. DOI 10.1038/nature19359 [DOI] [PubMed] [Google Scholar]
- 10.Letts JA, Fiedorczuk K, Sazanov LA (2016) The architecture of respiratory supercomplexes. Nature 537: 644–648. DOI 10.1038/nature19774 [DOI] [PubMed] [Google Scholar]
- 11.Sousa JS, Mills DJ, Vonck J, Kuhlbrandt W (2016) Functional asymmetry and electron flow in the bovine respirasome. eLife 5 DOI 10.7554/eLife.21290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M, Gu J, Guo R, Huang Y, Yang M (2016) Structure of Mammalian Respiratory Supercomplex I1III2IV1. Cell 167: 1598–1609 e1510. DOI 10.1016/j.cell.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 13.Strogolova V, Furness A, Robb-McGrath M, Garlich J, Stuart RA (2012) Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Mol Cell Biol 32: 1363–1373. DOI 10.1128/MCB.06369-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YC, Taylor EB, Dephoure N, Heo JM, Tonhato A, Papandreou I, Nath N, Denko NC, Gygi SP, Rutter J (2012) Identification of a protein mediating respiratory supercomplex stability. Cell Metab 15: 348–360. DOI 10.1016/j.cmet.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapuente-Brun E, Moreno-Loshuertos R, Acin-Perez R, Latorre-Pellicer A, Colas C, Balsa E, Perales-Clemente E, Quiros PM, Calvo E, Rodriguez-Hernandez MA, et al. (2013) Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340: 1567–1570. DOI 10.1126/science.1230381 [DOI] [PubMed] [Google Scholar]
- 16.Mourier A, Matic S, Ruzzenente B, Larsson NG, Milenkovic D (2014) The respiratory chain supercomplex organization is independent of COX7a2l isoforms. Cell Metab 20: 1069–1075. DOI 10.1016/j.cmet.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javadov S, Kozlov AV, Camara AKS (2020) Mitochondria in Health and Diseases. Cells 9 DOI 10.3390/cells9051177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL (2008) Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovascular research 80: 30–39. DOI 10.1093/cvr/cvn184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang S, Lewis TS, Powers C, Khuchua Z, Baines CP, Wipf P, Javadov S (2017) Elucidating Mitochondrial Electron Transport Chain Supercomplexes in the Heart During Ischemia-Reperfusion. Antioxidants & redox signaling 27: 57–69. DOI 10.1089/ars.2016.6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anwar MR, Saldana-Caboverde A, Garcia S, Diaz F (2018) The Organization of Mitochondrial Supercomplexes is Modulated by Oxidative Stress In Vivo in Mouse Models of Mitochondrial Encephalopathy. International journal of molecular sciences 19 DOI 10.3390/ijms19061582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arthur CR, Morton SL, Dunham LD, Keeney PM, Bennett JP Jr. (2009) Parkinson’s disease brain mitochondria have impaired respirasome assembly, age-related increases in distribution of oxidative damage to mtDNA and no differences in heteroplasmic mtDNA mutation abundance. Molecular neurodegeneration 4: 37 DOI 10.1186/1750-1326-4-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie M, Lazarou M, Thorburn DR, Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. Journal of molecular biology 361: 462–469. DOI 10.1016/j.jmb.2006.06.057 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Powers C, Madala SK, Greis KD, Haffey WD, Towbin JA, Purevjav E, Javadov S, Strauss AW, Khuchua Z (2015) Cardiac metabolic pathways affected in the mouse model of barth syndrome. PloS one 10: e0128561 DOI 10.1371/journal.pone.0128561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoun G, McMurray F, Thrush AB, Patten DA, Peixoto AC, Slack RS, McPherson R, Dent R, Harper ME (2015) Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 58: 2861–2866. DOI 10.1007/s00125-015-3772-8 [DOI] [PubMed] [Google Scholar]
- 25.Gomez LA, Monette JS, Chavez JD, Maier CS, Hagen TM (2009) Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Archives of biochemistry and biophysics 490: 30–35. DOI 10.1016/j.abb.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greggio C, Jha P, Kulkarni SS, Lagarrigue S, Broskey NT, Boutant M, Wang X, Conde Alonso S, Ofori E, Auwerx J, et al. (2017) Enhanced Respiratory Chain Supercomplex Formation in Response to Exercise in Human Skeletal Muscle. Cell Metab 25: 301–311. DOI 10.1016/j.cmet.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 27.Casuso RA, Al-Fazazi S, Hidalgo-Gutierrez A, Lopez LC, Plaza-Diaz J, Rueda-Robles A, Huertas JR (2019) Hydroxytyrosol influences exercise-induced mitochondrial respiratory complex assembly into supercomplexes in rats. Free Radic Biol Med 134: 304–310. DOI 10.1016/j.freeradbiomed.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 28.Blaza JN, Serreli R, Jones AJ, Mohammed K, Hirst J (2014) Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc Natl Acad Sci U S A 111: 15735–15740. DOI 10.1073/pnas.1413855111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedor JG, Hirst J (2018) Mitochondrial Supercomplexes Do Not Enhance Catalysis by Quinone Channeling. Cell Metab 28: 525–531 e524. DOI 10.1016/j.cmet.2018.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letts JA, Sazanov LA (2017) Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nature structural & molecular biology 24: 800–808. DOI 10.1038/nsmb.3460 [DOI] [PubMed] [Google Scholar]
- 31.Milenkovic D, Blaza JN, Larsson NG, Hirst J (2017) The Enigma of the Respiratory Chain Supercomplex. Cell Metab 25: 765–776. DOI 10.1016/j.cmet.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 32.Lobo-Jarne T, Ugalde C (2018) Respiratory chain supercomplexes: Structures, function and biogenesis. Semin Cell Dev Biol 76: 179–190. DOI 10.1016/j.semcdb.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schagger H, Pfeiffer K (2001) The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J Biol Chem 276: 37861–37867. DOI 10.1074/jbc.M106474200 [DOI] [PubMed] [Google Scholar]
- 34.R MP-R, Chapa-Dubocq X, Guzman-Hernandez R, Jang S, C AT-R, Ayala-Pena S, Javadov S (2019) The Role of Adenine Nucleotide Translocase in the Assembly of Respiratory Supercomplexes in Cardiac Cells. Cells 8 DOI 10.3390/cells8101247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko YH, Delannoy M, Hullihen J, Chiu W, Pedersen PL (2003) Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J Biol Chem 278: 12305–12309. DOI 10.1074/jbc.C200703200 [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, Pedersen PL (2004) Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J Biol Chem 279: 31761–31768. DOI 10.1074/jbc.M401353200 [DOI] [PubMed] [Google Scholar]
- 37.Cogliati S, Calvo E, Loureiro M, Guaras AM, Nieto-Arellano R, Garcia-Poyatos C, Ezkurdia I, Mercader N, Vazquez J, Enriquez JA (2016) Mechanism of super-assembly of respiratory complexes III and IV. Nature 539: 579–582. DOI 10.1038/nature20157 [DOI] [PubMed] [Google Scholar]
- 38.Bundgaard A, James AM, Harbour ME, Murphy MP, Fago A (2020) Stable mitochondrial CICIII2 supercomplex interactions in reptiles versus homeothermic vertebrates. J Exp Biol 223 DOI 10.1242/jeb.223776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo R, Zong S, Wu M, Gu J, Yang M (2017) Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell 170: 1247–1257 e1212. DOI 10.1016/j.cell.2017.07.050 [DOI] [PubMed] [Google Scholar]
- 40.Jang S, Javadov S (2018) Elucidating the contribution of ETC complexes I and II to the respirasome formation in cardiac mitochondria. Sci Rep 8: 17732 DOI 10.1038/s41598-018-36040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang S, Javadov S (2018) Current Challenges in Elucidating Respiratory Supercomplexes in Mitochondria: Methodological Obstacles. Front Physiol 9: 238 DOI 10.3389/fphys.2018.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clason T, Ruiz T, Schagger H, Peng G, Zickermann V, Brandt U, Michel H, Radermacher M (2010) The structure of eukaryotic and prokaryotic complex I. Journal of structural biology 169: 81–88. DOI 10.1016/j.jsb.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinothkumar KR, Zhu J, Hirst J (2014) Architecture of mammalian respiratory complex I. Nature 515: 80–84. DOI 10.1038/nature13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfanner N, van der Laan M, Amati P, Capaldi RA, Caudy AA, Chacinska A, Darshi M, Deckers M, Hoppins S, Icho T, et al. (2014) Uniform nomenclature for the mitochondrial contact site and cristae organizing system. J Cell Biol 204: 1083–1086. DOI 10.1083/jcb.201401006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilkerson RW, Selker JM, Capaldi RA (2003) The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett 546: 355–358. DOI 10.1016/s0014-5793(03)00633-1 [DOI] [PubMed] [Google Scholar]
- 46.Vogel F, Bornhovd C, Neupert W, Reichert AS (2006) Dynamic subcompartmentalization of the mitochondrial inner membrane. J Cell Biol 175: 237–247. DOI 10.1083/jcb.200605138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rampelt H, Zerbes RM, van der Laan M, Pfanner N (2017) Role of the mitochondrial contact site and cristae organizing system in membrane architecture and dynamics. Biochim Biophys Acta Mol Cell Res 1864: 737–746. DOI 10.1016/j.bbamcr.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 48.Wilkens V, Kohl W, Busch K (2013) Restricted diffusion of OXPHOS complexes in dynamic mitochondria delays their exchange between cristae and engenders a transitory mosaic distribution. J Cell Sci 126: 103–116. DOI 10.1242/jcs.108852 [DOI] [PubMed] [Google Scholar]
- 49.Bottinger L, Horvath SE, Kleinschroth T, Hunte C, Daum G, Pfanner N, Becker T (2012) Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. Journal of molecular biology 423: 677–686. DOI 10.1016/j.jmb.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B (1997) Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim Biophys Acta 1325: 108–116. DOI 10.1016/s0005-2736(96)00240-4 [DOI] [PubMed] [Google Scholar]
- 51.Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, et al. (2009) Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol 19: 2133–2139. DOI 10.1016/j.cub.2009.10.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bazan S, Mileykovskaya E, Mallampalli VK, Heacock P, Sparagna GC, Dowhan W (2013) Cardiolipin-dependent reconstitution of respiratory supercomplexes from purified Saccharomyces cerevisiae complexes III and IV. J Biol Chem 288: 401–411. DOI 10.1074/jbc.M112.425876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Y, Phoon CK, Berno B, D’Souza K, Hoedt E, Zhang G, Neubert TA, Epand RM, Ren M, Schlame M (2016) Loss of protein association causes cardiolipin degradation in Barth syndrome. Nat Chem Biol 12: 641–647. DOI 10.1038/nchembio.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christie DA, Lemke CD, Elias IM, Chau LA, Kirchhof MG, Li B, Ball EH, Dunn SD, Hatch GM, Madrenas J (2011) Stomatin-like protein 2 binds cardiolipin and regulates mitochondrial biogenesis and function. Mol Cell Biol 31: 3845–3856. DOI 10.1128/MCB.05393-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitsopoulos P, Chang YH, Wai T, Konig T, Dunn SD, Langer T, Madrenas J (2015) Stomatin-like protein 2 is required for in vivo mitochondrial respiratory chain supercomplex formation and optimal cell function. Mol Cell Biol 35: 1838–1847. DOI 10.1128/MCB.00047-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A 101: 15927–15932. DOI 10.1073/pnas.0407043101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, et al. (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189. DOI 10.1016/j.cell.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 58.Patten DA, Wong J, Khacho M, Soubannier V, Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM, Trinkle-Mulcahy L, et al. (2014) OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. The EMBO journal 33: 2676–2691. DOI 10.15252/embj.201488349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaser M, Kambacheld M, Kisters-Woike B, Langer T (2003) Oma1, a novel membrane-bound metallopeptidase in mitochondria with activities overlapping with the m-AAA protease. J Biol Chem 278: 46414–46423. DOI 10.1074/jbc.M305584200 [DOI] [PubMed] [Google Scholar]
- 60.Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. The EMBO journal 25: 2966–2977. DOI 10.1038/sj.emboj.7601184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wai T, Saita S, Nolte H, Muller S, Konig T, Richter-Dennerlein R, Sprenger HG, Madrenas J, Muhlmeister M, Brandt U, et al. (2016) The membrane scaffold SLP2 anchors a proteolytic hub in mitochondria containing PARL and the i-AAA protease YME1L. EMBO Rep 17: 1844–1856. DOI 10.15252/embr.201642698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, et al. (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155: 160–171. DOI 10.1016/j.cell.2013.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jang S, Javadov S (2020) OPA1 regulates respiratory supercomplexes assembly: The role of mitochondrial swelling. Mitochondrion 51: 30–39. DOI 10.1016/j.mito.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tavernarakis N, Driscoll M, Kyrpides NC (1999) The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem Sci 24: 425–427. DOI 10.1016/s0968-0004(99)01467-x [DOI] [PubMed] [Google Scholar]
- 65.Osman C, Merkwirth C, Langer T (2009) Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci 122: 3823–3830. DOI 10.1242/jcs.037655 [DOI] [PubMed] [Google Scholar]
- 66.Jian C, Xu F, Hou T, Sun T, Li J, Cheng H, Wang X (2017) Deficiency of PHB complex impairs respiratory supercomplex formation and activates mitochondrial flashes. J Cell Sci 130: 2620–2630. DOI 10.1242/jcs.198523 [DOI] [PubMed] [Google Scholar]
- 67.Desmurs M, Foti M, Raemy E, Vaz FM, Martinou JC, Bairoch A, Lane L (2015) C11orf83, a mitochondrial cardiolipin-binding protein involved in bc1 complex assembly and supercomplex stabilization. Mol Cell Biol 35: 1139–1156. DOI 10.1128/MCB.01047-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vukotic M, Oeljeklaus S, Wiese S, Vogtle FN, Meisinger C, Meyer HE, Zieseniss A, Katschinski DM, Jans DC, Jakobs S, et al. (2012) Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab 15: 336–347. DOI 10.1016/j.cmet.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 69.Strogolova V, Hoang NH, Hosler J, Stuart RA (2019) The yeast mitochondrial proteins Rcf1 and Rcf2 support the enzymology of the cytochrome c oxidase complex and generation of the proton motive force. J Biol Chem 294: 4867–4877. DOI 10.1074/jbc.RA118.006888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dawitz H, Schafer J, Schaart JM, Magits W, Brzezinski P, Ott M (2019) Rcf1 Modulates Cytochrome c Oxidase Activity Especially Under Energy-Demanding Conditions. Front Physiol 10: 1555 DOI 10.3389/fphys.2019.01555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Timon-Gomez A, Garlich J, Stuart RA, Ugalde C, Barrientos A (2020) Distinct Roles of Mitochondrial HIGD1A and HIGD2A in Respiratory Complex and Supercomplex Biogenesis. Cell Rep 31: 107607 DOI 10.1016/j.celrep.2020.107607 [DOI] [PubMed] [Google Scholar]
- 72.Hock DH, Reljic B, Ang CS, Muellner-Wong L, Mountford HS, Compton AG, Ryan MT, Thorburn DR, Stroud DA (2020) HIGD2A is Required for Assembly of the COX3 Module of Human Mitochondrial Complex IV. Molecular & cellular proteomics : MCP 19: 1145–1160. DOI 10.1074/mcp.RA120.002076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatle KM, Gummadidala P, Navasa N, Bernardo E, Dodge J, Silverstrim B, Fortner K, Burg E, Suratt BT, Hammer J, et al. (2013) MCJ/DnaJC15, an endogenous mitochondrial repressor of the respiratory chain that controls metabolic alterations. Mol Cell Biol 33: 2302–2314. DOI 10.1128/MCB.00189-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda K, Shiba S, Horie-Inoue K, Shimokata K, Inoue S (2013) A stabilizing factor for mitochondrial respiratory supercomplex assembly regulates energy metabolism in muscle. Nat Commun 4: 2147 DOI 10.1038/ncomms3147 [DOI] [PubMed] [Google Scholar]
- 75.Calvo E, Cogliati S, Hernansanz-Agustin P, Loureiro-Lopez M, Guaras A, Casuso RA, Garcia-Marques F, Acin-Perez R, Marti-Mateos Y, Silla-Castro JC, et al. (2020) Functional role of respiratory supercomplexes in mice: SCAF1 relevance and segmentation of the Qpool. Sci Adv 6: eaba7509 DOI 10.1126/sciadv.aba7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lobo-Jarne T, Nyvltova E, Perez-Perez R, Timon-Gomez A, Molinie T, Choi A, Mourier A, Fontanesi F, Ugalde C, Barrientos A (2018) Human COX7A2L Regulates Complex III Biogenesis and Promotes Supercomplex Organization Remodeling without Affecting Mitochondrial Bioenergetics. Cell Rep 25: 1786–1799 e1784. DOI 10.1016/j.celrep.2018.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez-Perez R, Lobo-Jarne T, Milenkovic D, Mourier A, Bratic A, Garcia-Bartolome A, Fernandez-Vizarra E, Cadenas S, Delmiro A, Garcia-Consuegra I, et al. (2016) COX7A2L Is a Mitochondrial Complex III Binding Protein that Stabilizes the III2+IV Supercomplex without Affecting Respirasome Formation. Cell Rep 16: 2387–2398. DOI 10.1016/j.celrep.2016.07.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams EG, Wu Y, Jha P, Dubuis S, Blattmann P, Argmann CA, Houten SM, Amariuta T, Wolski W, Zamboni N, et al. (2016) Systems proteomics of liver mitochondria function. Science 352: aad0189 DOI 10.1126/science.aad0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Althoff T, Mills DJ, Popot JL, Kuhlbrandt W (2011) Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. The EMBO journal 30: 4652–4664. DOI 10.1038/emboj.2011.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bianchi C, Genova ML, Parenti Castelli G, Lenaz G (2004) The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem 279: 36562–36569. DOI 10.1074/jbc.M405135200 [DOI] [PubMed] [Google Scholar]
- 81.Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML (2013) Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxidants & redox signaling 19: 1469–1480. DOI 10.1089/ars.2012.4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolanos JP (2016) Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci U S A 113: 13063–13068. DOI 10.1073/pnas.1613701113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acin-Perez R, Bayona-Bafaluy MP, Fernandez-Silva P, Moreno-Loshuertos R, Perez-Martos A, Bruno C, Moraes CT, Enriquez JA (2004) Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell 13: 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schagger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U (2004) Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem 279: 36349–36353. DOI 10.1074/jbc.M404033200 [DOI] [PubMed] [Google Scholar]
- 85.Calvaruso MA, Willems P, van den Brand M, Valsecchi F, Kruse S, Palmiter R, Smeitink J, Nijtmans L (2012) Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Human molecular genetics 21: 115–120. DOI 10.1093/hmg/ddr446 [DOI] [PubMed] [Google Scholar]
- 86.Hirst J (2018) Open questions: respiratory chain supercomplexes-why are they there and what do they do? BMC Biol 16: 111 DOI 10.1186/s12915-018-0577-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dudkina NV, Kudryashev M, Stahlberg H, Boekema EJ (2011) Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proc Natl Acad Sci U S A 108: 15196–15200. DOI 10.1073/pnas.1107819108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Letts JA, Fiedorczuk K, Degliesposti G, Skehel M, Sazanov LA (2019) Structures of Respiratory Supercomplex I+III2 Reveal Functional and Conformational Crosstalk. Mol Cell 75: 1131–1146 e1136. DOI 10.1016/j.molcel.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kushnareva Y, Murphy AN, Andreyev A (2002) Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J 368: 545–553. DOI 10.1042/BJ20021121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kussmaul L, Hirst J (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A 103: 7607–7612. DOI 10.1073/pnas.0510977103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Budde SM, van den Heuvel LP, Janssen AJ, Smeets RJ, Buskens CA, DeMeirleir L, Van Coster R, Baethmann M, Voit T, Trijbels JM, et al. (2000) Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochem Biophys Res Commun 275: 63–68. DOI 10.1006/bbrc.2000.3257 [DOI] [PubMed] [Google Scholar]
- 92.Diaz F, Enriquez JA, Moraes CT (2012) Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV. Mol Cell Biol 32: 415–429. DOI 10.1128/MCB.06051-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balsa E, Marco R, Perales-Clemente E, Szklarczyk R, Calvo E, Landazuri MO, Enriquez JA (2012) NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab 16: 378–386. DOI 10.1016/j.cmet.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 94.Lobo-Jarne T, Perez-Perez R, Fontanesi F, Timon-Gomez A, Wittig I, Penas A, Serrano-Lorenzo P, Garcia-Consuegra I, Arenas J, Martin MA, et al. (2020) Multiple pathways coordinate assembly of human mitochondrial complex IV and stabilization of respiratory supercomplexes. The EMBO journal 39: e103912 DOI 10.15252/embj.2019103912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Protasoni M, Perez-Perez R, Lobo-Jarne T, Harbour ME, Ding S, Penas A, Diaz F, Moraes CT, Fearnley IM, Zeviani M, et al. (2020) Respiratory supercomplexes act as a platform for complex III-mediated maturation of human mitochondrial complexes I and IV. The EMBO journal 39: e102817 DOI 10.15252/embj.2019102817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Comte J, Maisterrena B, Gautheron DC (1976) Lipid composition and protein profiles of outer and inner membranes from pig heart mitochondria. Comparison with microsomes. Biochim Biophys Acta 419: 271–284. DOI 10.1016/0005-2736(76)90353-9 [DOI] [PubMed] [Google Scholar]
- 97.Kaasik A, Safiulina D, Zharkovsky A, Veksler V (2007) Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol 292: C157–163. DOI 10.1152/ajpcell.00272.2006 [DOI] [PubMed] [Google Scholar]
- 98.Javadov S, Chapa-Dubocq X, Makarov V (2018) Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion 38: 58–70. DOI 10.1016/j.mito.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gadicherla AK, Stowe DF, Antholine WE, Yang M, Camara AK (2012) Damage to mitochondrial complex I during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine. Biochim Biophys Acta 1817: 419–429. DOI 10.1016/j.bbabio.2011.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramirez-Camacho I, Correa F, El Hafidi M, Silva-Palacios A, Ostolga-Chavarria M, Esparza-Perusquia M, Olvera-Sanchez S, Flores-Herrera O, Zazueta C (2018) Cardioprotective strategies preserve the stability of respiratory chain supercomplexes and reduce oxidative stress in reperfused ischemic hearts. Free Radic Biol Med 129: 407–417. DOI 10.1016/j.freeradbiomed.2018.09.047 [DOI] [PubMed] [Google Scholar]
- 101.Maekawa S, Takada S, Nambu H, Furihata T, Kakutani N, Setoyama D, Ueyanagi Y, Kang D, Sabe H, Kinugawa S (2019) Linoleic acid improves assembly of the CII subunit and CIII2/CIV complex of the mitochondrial oxidative phosphorylation system in heart failure. Cell Commun Signal 17: 128 DOI 10.1186/s12964-019-0445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chatfield KC, Sparagna GC, Chau S, Phillips EK, Ambardekar AV, Aftab M, Mitchell MB, Sucharov CC, Miyamoto SD, Stauffer BL (2019) Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl Sci 4: 147–157. DOI 10.1016/j.jacbts.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schrepper A, Schwarzer M, Schope M, Amorim PA, Doenst T (2012) Biphasic response of skeletal muscle mitochondria to chronic cardiac pressure overload - role of respiratory chain complex activity. J Mol Cell Cardiol 52: 125–135. DOI 10.1016/j.yjmcc.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 104.Lotz C, Zhang J, Fang C, Liem D, Ping P (2015) Isoflurane protects the myocardium against ischemic injury via the preservation of mitochondrial respiration and its supramolecular organization. Anesth Analg 120: 265–274. DOI 10.1213/ANE.0000000000000494 [DOI] [PubMed] [Google Scholar]
- 105.Jang S, Javadov S (2017) Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Archives of biochemistry and biophysics 630: 1–8. DOI 10.1016/j.abb.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parodi-Rullan RM, Soto-Prado J, Vega-Lugo J, Chapa-Dubocq X, Diaz-Cordero SI, Javadov S (2018) Divergent Effects of Cyclophilin-D Inhibition on the Female Rat Heart: Acute Versus Chronic Post-Myocardial Infarction. Cell Physiol Biochem 50: 288–303. DOI 10.1159/000494006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong R, Aponte AM, Steenbergen C, Murphy E (2010) Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol 298: H75–91. DOI 10.1152/ajpheart.00515.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ait-Aissa K, Blaszak SC, Beutner G, Tsaih SW, Morgan G, Santos JH, Flister MJ, Joyce DL, Camara AKS, Gutterman DD, et al. (2019) Mitochondrial Oxidative Phosphorylation defect in the Heart of Subjects with Coronary Artery Disease. Sci Rep 9: 7623 DOI 10.1038/s41598-019-43761-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chapa-Dubocq XR, Rodriguez-Graciani KM, Guzman-Hernandez RA, Jang S, Brookes PS, Javadov S (2020) Cardiac Function is not Susceptible to Moderate Disassembly of Mitochondrial Respiratory Supercomplexes. International journal of molecular sciences 21 DOI 10.3390/ijms21051555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovascular research 61: 372–385. DOI 10.1016/S0008-6363(03)00533-9 [DOI] [PubMed] [Google Scholar]
- 111.Nowikovsky K, Schweyen RJ, Bernardi P (2009) Pathophysiology of mitochondrial volume homeostasis: potassium transport and permeability transition. Biochim Biophys Acta 1787: 345–350. DOI 10.1016/j.bbabio.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 112.Zhang K, Wang G, Zhang X, Huttemann PP, Qiu Y, Liu J, Mitchell A, Lee I, Zhang C, Lee JS, et al. (2016) COX7AR is a Stress-inducible Mitochondrial COX Subunit that Promotes Breast Cancer Malignancy. Sci Rep 6: 31742 DOI 10.1038/srep31742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rohlenova K, Sachaphibulkij K, Stursa J, Bezawork-Geleta A, Blecha J, Endaya B, Werner L, Cerny J, Zobalova R, Goodwin J, et al. (2017) Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2(high) Breast Cancer. Antioxidants & redox signaling 26: 84–103. DOI 10.1089/ars.2016.6677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tomkova V, Sandoval-Acuna C, Torrealba N, Truksa J (2019) Mitochondrial fragmentation, elevated mitochondrial superoxide and respiratory supercomplexes disassembly is connected with the tamoxifen-resistant phenotype of breast cancer cells. Free Radic Biol Med 143: 510–521. DOI 10.1016/j.freeradbiomed.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 115.Mirali S, Botham A, Voisin V, Xu C, St-Germain J, Sharon D, Hoff FW, Qiu Y, Hurren R, Gronda M, et al. (2020) The mitochondrial peptidase, neurolysin, regulates respiratory chain supercomplex formation and is necessary for AML viability. Sci Transl Med 12 DOI 10.1126/scitranslmed.aaz8264 [DOI] [PubMed] [Google Scholar]
- 116.Ikeda K, Horie-Inoue K, Suzuki T, Hobo R, Nakasato N, Takeda S, Inoue S (2019) Mitochondrial supercomplex assembly promotes breast and endometrial tumorigenesis by metabolic alterations and enhanced hypoxia tolerance. Nat Commun 10: 4108 DOI 10.1038/s41467-019-12124-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang G, Popovic B, Tao J, Jiang A (2020) Overexpression of COX7RP promotes tumor growth and metastasis by inducing ROS production in hepatocellular carcinoma cells. Am J Cancer Res 10: 1366–1383 [PMC free article] [PubMed] [Google Scholar]
- 118.Nagano H, Hashimoto N, Nakayama A, Suzuki S, Miyabayashi Y, Yamato A, Higuchi S, Fujimoto M, Sakuma I, Beppu M, et al. (2018) p53-inducible DPYSL4 associates with mitochondrial supercomplexes and regulates energy metabolism in adipocytes and cancer cells. Proc Natl Acad Sci U S A 115: 8370–8375. DOI 10.1073/pnas.1804243115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barth PG, Scholte HR, Berden JA, Van der Klei-Van Moorsel JM, Luyt-Houwen IE, Van ‘t Veer-Korthof ET, Van der Harten JJ, Sobotka-Plojhar MA (1983) An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci 62: 327–355. DOI 10.1016/0022-510x(83)90209-5 [DOI] [PubMed] [Google Scholar]
- 120.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, et al. (2011) Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286: 899–908. DOI 10.1074/jbc.M110.171439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soustek MS, Falk DJ, Mah CS, Toth MJ, Schlame M, Lewin AS, Byrne BJ (2011) Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum Gene Ther 22: 865–871. DOI 10.1089/hum.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gohel D, Sripada L, Prajapati P, Singh K, Roy M, Kotadia D, Tassone F, Charlet-Berguerand N, Singh R (2019) FMRpolyG alters mitochondrial transcripts level and respiratory chain complex assembly in Fragile X associated tremor/ataxia syndrome [FXTAS]. Biochim Biophys Acta Mol Basis Dis 1865: 1379–1388. DOI 10.1016/j.bbadis.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 123.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54: 823–827. DOI 10.1111/j.1471-4159.1990.tb02325.x [DOI] [PubMed] [Google Scholar]
- 124.Bindoff LA, Birch-Machin MA, Cartlidge NE, Parker WD Jr., Turnbull DM (1991) Respiratory chain abnormalities in skeletal muscle from patients with Parkinson’s disease. J Neurol Sci 104: 203–208. DOI 10.1016/0022-510x(91)90311-t [DOI] [PubMed] [Google Scholar]
- 125.Wernicke C, Hellmann J, Zieba B, Kuter K, Ossowska K, Frenzel M, Dencher NA, Rommelspacher H (2010) 9-Methyl-beta-carboline has restorative effects in an animal model of Parkinson’s disease. Pharmacol Rep 62: 35–53. DOI 10.1016/s1734-1140(10)70241-3 [DOI] [PubMed] [Google Scholar]
- 126.Lopez-Fabuel I, Martin-Martin L, Resch-Beusher M, Azkona G, Sanchez-Pernaute R, Bolanos JP (2017) Mitochondrial respiratory chain disorganization in Parkinson’s disease-relevant PINK1 and DJ1 mutants. Neurochem Int 109: 101–105. DOI 10.1016/j.neuint.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 127.Pera M, Larrea D, Guardia-Laguarta C, Montesinos J, Velasco KR, Agrawal RR, Xu Y, Chan RB, Di Paolo G, Mehler MF, et al. (2017) Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease. The EMBO journal 36: 3356–3371. DOI 10.15252/embj.201796797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kenney PM, Bennett JP Jr. (2019) Alzheimer’s Disease Frontal Cortex Mitochondria Show a Loss of Individual Respiratory Proteins but Preservation of Respiratory Supercomplexes. Int J Alzheimers Dis 2019: 4814783 DOI 10.1155/2019/4814783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11: 298–300. DOI 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 130.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5: 741–747. DOI 10.1038/ncb1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344. DOI 10.1016/s0968-0004(02)02110-2 [DOI] [PubMed] [Google Scholar]
- 132.Blander G, de Oliveira RM, Conboy CM, Haigis M, Guarente L (2003) Superoxide dismutase 1 knock-down induces senescence in human fibroblasts. J Biol Chem 278: 38966–38969. DOI 10.1074/jbc.M307146200 [DOI] [PubMed] [Google Scholar]
- 133.Sohal RS, Orr WC (2012) The redox stress hypothesis of aging. Free Radic Biol Med 52: 539–555. DOI 10.1016/j.freeradbiomed.2011.10.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Darr D, Fridovich I (1995) Adaptation to oxidative stress in young, but not in mature or old, Caenorhabditis elegans. Free Radic Biol Med 18: 195–201. DOI 10.1016/0891-5849(94)00118-4 [DOI] [PubMed] [Google Scholar]
- 135.Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, Hara E (2006) Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol 8: 1291–1297. DOI 10.1038/ncb1491 [DOI] [PubMed] [Google Scholar]
- 136.Kwong LK, Sohal RS (2000) Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Archives of biochemistry and biophysics 373: 16–22. DOI 10.1006/abbi.1999.1495 [DOI] [PubMed] [Google Scholar]
- 137.Lesnefsky EJ, Chen Q, Hoppel CL (2016) Mitochondrial Metabolism in Aging Heart. Circ Res 118: 1593–1611. DOI 10.1161/CIRCRESAHA.116.307505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lombardi A, Silvestri E, Cioffi F, Senese R, Lanni A, Goglia F, de Lange P, Moreno M (2009) Defining the transcriptomic and proteomic profiles of rat ageing skeletal muscle by the use of a cDNA array, 2D- and Blue native-PAGE approach. J Proteomics 72: 708–721. DOI 10.1016/j.jprot.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 139.Javadov S, Jang S, Rodriguez-Reyes N, Rodriguez-Zayas AE, Soto Hernandez J, Krainz T, Wipf P, Frontera W (2015) Mitochondria-targeted antioxidant preserves contractile properties and mitochondrial function of skeletal muscle in aged rats. Oncotarget 6: 39469–39481. DOI 10.18632/oncotarget.5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frenzel M, Rommelspacher H, Sugawa MD, Dencher NA (2010) Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Experimental gerontology 45: 563–572. DOI 10.1016/j.exger.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 141.O’Toole JF, Patel HV, Naples CJ, Fujioka H, Hoppel CL (2010) Decreased cytochrome c mediates an age-related decline of oxidative phosphorylation in rat kidney mitochondria. Biochem J 427: 105–112. DOI 10.1042/BJ20091373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sgarbi G, Matarrese P, Pinti M, Lanzarini C, Ascione B, Gibellini L, Dika E, Patrizi A, Tommasino C, Capri M, et al. (2014) Mitochondria hyperfusion and elevated autophagic activity are key mechanisms for cellular bioenergetic preservation in centenarians. Aging (Albany NY) 6: 296–310. DOI 10.18632/aging.100654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guerrero-Castillo S, Baertling F, Kownatzki D, Wessels HJ, Arnold S, Brandt U, Nijtmans L (2017) The Assembly Pathway of Mitochondrial Respiratory Chain Complex I. Cell Metab 25: 128–139. DOI 10.1016/j.cmet.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 144.Stroud DA, Surgenor EE, Formosa LE, Reljic B, Frazier AE, Dibley MG, Osellame LD, Stait T, Beilharz TH, Thorburn DR, et al. (2016) Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 538: 123–126. DOI 10.1038/nature19754 [DOI] [PubMed] [Google Scholar]
- 145.Marques I, Dencher NA, Videira A, Krause F (2007) Supramolecular organization of the respiratory chain in Neurospora crassa mitochondria. Eukaryot Cell 6: 2391–2405. DOI 10.1128/EC.00149-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lazarou M, Smith SM, Thorburn DR, Ryan MT, McKenzie M (2009) Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J 276: 6701–6713. DOI 10.1111/j.1742-4658.2009.07384.x [DOI] [PubMed] [Google Scholar]
- 147.Sehnal D, Rose AS, Kovca J, Burley SK, S. V (2018) Mol*: Towards a common library and tools for web molecular graphics MolVA/EuroVis Proceedings. [Google Scholar]
- 148.Dickson CJ, Madej BD, Skjevik AA, Betz RM, Teigen K, Gould IR, Walker RC (2014) Lipid14: The Amber Lipid Force Field. J Chem Theory Comput 10: 865–879. DOI 10.1021/ct4010307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rose AS, Bradley AR, Valasatava Y, Duarte JM, Prlic A, Rose PW (2018) NGL viewer: web-based molecular graphics for large complexes. Bioinformatics 34: 3755–3758. DOI 10.1093/bioinformatics/bty419 [DOI] [PMC free article] [PubMed] [Google Scholar]


