Abstract
Objective
Although hemodialysis is recommended for patients with severe metformin-associated lactic acidosis (MALA), the amount of metformin removed by hemodialysis is poorly documented. We analyzed endogenous clearance and hemodialysis clearance in a patient with MALA.
Methods
A 62-year-old man with a history of type II diabetes mellitus presented after several days of vomiting and diarrhea and was found to have acute kidney injury (AKI) and severe acidemia. Initial serum metformin concentration was 315.34 μmol/L (40.73 μg/mL) (typical therapeutic concentrations 1–2 μg/mL). He underwent 6 h of hemodialysis. We collected hourly whole blood, serum, urine, and dialysate metformin concentrations. Blood, urine, and dialysate samples were analyzed, and clearances were determined using standard pharmacokinetic calculations.
Results
The total amount of metformin removed by 6 h of hemodialysis was 888 mg, approximately equivalent to one therapeutic dose. Approximately 142 mg of metformin was cleared in the urine during this time. His acid-base status and creatinine improved over the following days. No further hemodialysis was required.
Conclusion
We report a case of MALA likely secondary to AKI and severe volume depletion. The patient improved with supportive care, sodium bicarbonate, and hemodialysis. Analysis of whole blood, serum, urine, and dialysate concentrations showed limited efficacy of hemodialysis in the removal of metformin from blood, contrary to previously published data. Despite evidence of acute kidney injury, a relatively large amount of metformin was eliminated in the urine while the patient was undergoing hemodialysis. These data suggest that clinical improvement is likely due to factors besides removal of metformin.
Electronic supplementary material
The online version of this article (10.1007/s13181-020-00802-7) contains supplementary material, which is available to authorized users.
Keywords: Metformin, Biguanides, Lactic acidosis, Hemodialysis, Drug clearance
Introduction
Metformin is an oral biguanide used in the management of patients with diabetes mellitus. In 2015, there were over 83 million prescriptions of the medication, making it the fourth most commonly prescribed medication in the USA [1]. Mechanistically, it works by inhibiting gluconeogenesis to decrease hepatic production of glucose while also augmenting uptake of glucose in peripheral tissues. Additionally, metformin enhances glucose transporters in muscle tissue, increases intestinal glucose utilization, and decreases fatty acid oxidation.
In severe toxicity and in the setting of therapeutic use with acute kidney injury, patients develop a metabolic acidosis with hyperlactatemia, more commonly referred to as metformin-associated lactic acidosis (MALA). While supportive care alone is beneficial for many patients, and sodium bicarbonate is frequently used in the case of severe acidemia, hemodialysis (HD) is ultimately the treatment of choice for patients with severe MALA. [2] Although some of the characteristics of metformin (low molecular weight [165 Da] and low protein binding [1.1%–2.8%]) are properties that make it seem amenable to HD, these benefits are outweighed by its large volume of distribution (in adults, 654 ± 358 L) and the partitioning of metformin into erythrocytes over time [3, 4]. While some studies suggest a benefit of HD in MALA patients, rigorous pharmacokinetic data analyzing the removal of metformin and comparing it with endogenous clearance has not yet been reported. We report a patient receiving therapeutic dosing of metformin who developed MALA and was treated with sodium bicarbonate and HD with a favorable outcome. Our analysis includes blood, urine, and dialysate metformin concentrations to help characterize the clearance of metformin both endogenously and by HD.
Case Report
A 62-year-old incarcerated man with a history of type II diabetes mellitus presented to the ED after several days of abdominal pain with vomiting and diarrhea. He was receiving metformin by the prison infirmary (metformin 500 mg twice daily) and was unlikely to have acutely overdosed. On arrival, his vital signs were BP, 132/91 mmHg; HR, 94/min; RR, 40/min; O2 Sat, 100% on room air; and temperature, 97 °F. His weight (only measured after HD) was 73.8 kg. Shortly thereafter, on bedside physical examination, he appeared uncomfortable, was tachycardic and tachypneic, and had diffused abdominal tenderness to palpation. He was found to have acute kidney injury (AKI) with a serum creatinine of 10.5 mg/dL (significantly higher than his baseline serum creatinine of 1.1 mg/dL). Severe acidemia was also present: pH 6.818 (reference range 7.32–7.41), PCO2 25.7 mmHg (reference range 42–53 mmHg), bicarbonate 5.9 mEq/L (reference range 24–28 mEq/L), and lactate > 20 mmol/L (reference range 0.7–2.1 mmol/L). Full laboratory studies are reported in Table 1. His initial serum metformin concentration in the ED was 40.73 μg/mL (315.34 μmol/L). For comparison, typical dosing regimens result in steady-state metformin concentrations of less than 1 μg/mL, with peak concentrations ranging from 2.01 μg/mL (healthy nondiabetic adults) to 4.12 μg/mL (severe renal impairment) [4]. In the emergency department, he received 3 ampules (150 mEq) of sodium bicarbonate as an intravenous bolus followed by an infusion of 6 ampules (300 mEq) of sodium bicarbonate in 2 l of normal saline. His heart rate and abdominal pain improved with this intervention. However, the decision was made to perform HD given the degree of acidemia, hyperlactatemia, and kidney injury. The team placed a HD catheter in the right femoral vein. On transfer to the ICU, he underwent 6 h of HD (Fresenius 2008 k dialysis machine, Optiflux Advanced filter, 250 mL/min blood flow rate, 600 mL/min dialysate flow rate, HCT 25.5%). We collected and analyzed hourly whole blood, serum, urine, and dialysate metformin concentrations before, during, and after HD to quantify the efficiency of HD in the removal of metformin. Consent for publication of this case was obtained from the patient in accordance with JMT policy.
Table 1.
Initial emergency department laboratory results
| Venous blood gas | |
| pH | 6.818 |
| PCO2 | 25.7 mmHg |
| PO2 | 65.7 mmHg |
| Bicarbonate | 3.9 mEq/L |
| Lactate | > 20 mmol/L |
| Complete blood count | |
| White blood cell count | 8800/nL |
| Hemoglobin | 8.9 g/dL |
| Hematocrit | 27.5% |
| Chemistry | |
| Sodium | 146 mmol/L |
| Potassium | 5.9 mmol/L |
| Chloride | 89 mmol/L |
| CO2 | < 10 mmol/L |
| Blood urea nitrogen | 88 mg/dL |
| Creatinine | 10.5 mg/dL |
| Glucose | 335 mg/dL |
| Calcium | 8.5 mg/dL |
| AST | 27 U/L |
| ALT | 13 U/L |
Methods
Hourly blood, urine, and dialysate samples were collected at bedside beginning 2 h prior to HD, proceeding through HD, and ending 1 h following completion of HD. Blood samples were drawn from the patient’s IV line (pre-HD) and from the return line from the HD machine (post-HD). Urine samples were collected from the Foley catheter bag, followed by emptying the bag until the next sample. Dialysate samples were obtained from the HD machine drainage line. Blood samples were centrifuged by the hospital laboratory, so each blood sample had both whole blood and serum samples obtained for analysis. Both samples were obtained and analyzed to evaluate the amount of metformin redistributed into red blood cells. The samples were then analyzed using LC-MS/MS (Pinpoint Testing, LLC; Little Rock, AR). Due to initial metformin concentrations being above the upper limit of detection, a 1:100 dilution was required to obtain meaningful concentrations in blood, serum, urine, and dialysate. The test results were then analyzed using Microsoft Excel (Redmond, WA). Urine clearance, HD removal rates, and total amounts cleared/removed were determined using standard pharmacokinetic calculations (Table 2) [5].
Table 2.
Pharmacokinetic calculations used
| Hemodialytic clearance (CLHD) = (QD x CE) | |
| Whole Blood clearance (CLWB) = ER x QB | |
| Serum clearance (CLS) = ER x [1 - (Hematocrit/100)] x QB | |
| ER = Extraction ratio = (CACCESS - CRETURN)/CACCESS | |
| (calculated for both whole blood and serum) | |
| Renal clearance (CLR) = (CU x QU)/CP | |
| Legend | |
| Concentrations (C) | |
| CACCESS Whole blood or plasma sampled pre-HD | |
| CRETURN Whole blood or plasma sampled post-HD | |
| CE Effluent | |
| CU Urine | |
| Q Flows | |
| QB Blood | |
| QD Dialysance | |
| QU Urine |
Results
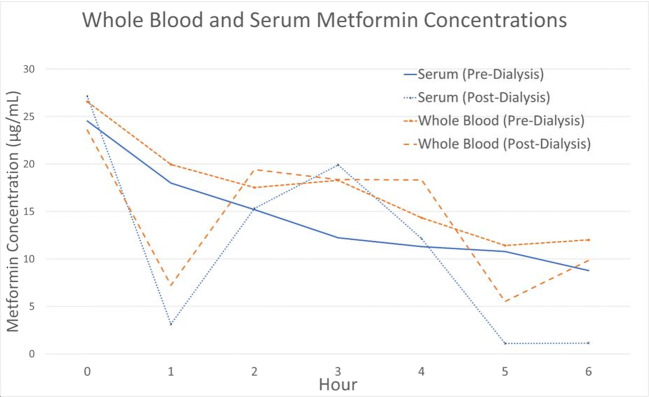
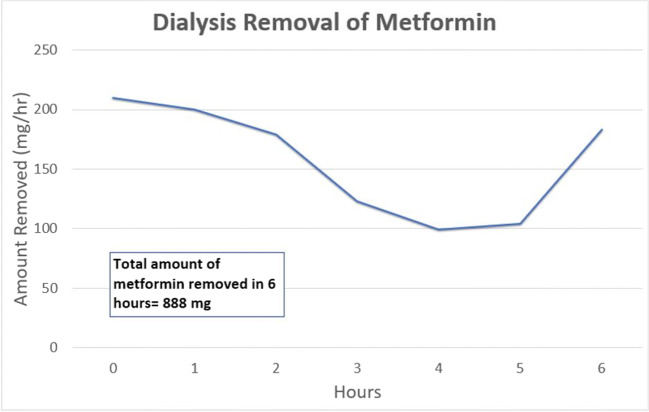
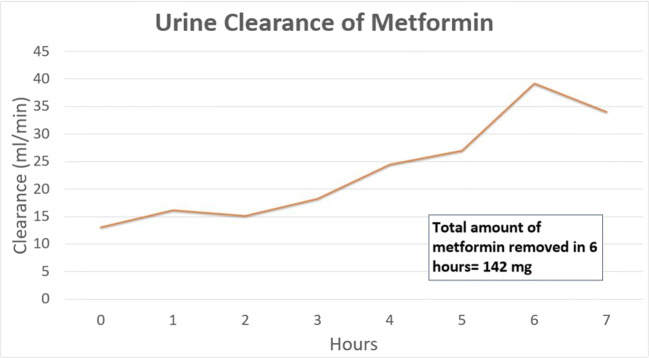
The average whole blood clearance of metformin during HD was 37.74 mL/min, while the average plasma clearance of metformin was 47.27 mL/min (Fig. 1). The average whole blood extraction ratio was 0.15. While on HD, the average urine clearance of metformin was 21.88 mL/min, which improved to 34.01 mL/min upon completion of HD. The total amount of metformin removed by 6 h of HD was only 888 mg, which is approximately one therapeutic dose (Fig. 2). In comparison, approximately 142 mg of metformin was cleared in the patient’s urine over the same 6 h (Fig. 3). No further HD was required, and the patient was discharged several days later after improvement in acid-base status and kidney function.
Fig. 1.
Whole blood and serum metformin concentrations during hemodialysis. (Patient placed on HD at hour 0)
Fig. 2.
HD removal of metformin during hemodialysis. Analysis total metformin removed does not include time point 0, given that was when HD was started
Fig. 3.
Urinary clearance of metformin during hemodialysis
Discussion
The data obtained from this patient represents, to our knowledge, the most thorough analysis of metformin concentrations during HD that is currently available in that we analyzed serial serum and whole blood metformin concentrations prior to, during, and following HD, and in the patient’s dialysate and urine metformin concentrations. The patient presented with typical chronic metformin toxicity associated with volume depletion or illness. As such, our data pertains to chronic toxicity as opposed to acute toxicity. Given the time-dependent partitioning of metformin into red blood cells, over time, metformin becomes less available in the serum for rapid removal via hemodialysis. Conversely, in acute overdose more of the medication is available in the serum compartment prior to redistribution, making it more available for removal by HD. While it is possible for incarcerated patients to acutely overdose on a substance and this possibility cannot be absolutely excluded in this case, the patient insisted on only therapeutic dosing of his medication and had no history of depression or suicidality. Current guidelines for use of HD in MALA state that HD is recommended for a lactic acid concentration > 20 mmol/L, pH ≤ 7.0, shock, failure of standard supportive measures, or decreased level of consciousness [2]. In line with this, he received HD based on his severe metabolic acidemia and hyperlactatemia, with the dual goal of improving his acid-base status and removing metformin. Upon review of the data analyzed, however, it became evident that the amount of metformin removed (slightly more than one therapeutic dose—Fig. 2) made it unlikely to be the primary driving force behind the patient’s clinical improvement. It is also interesting to note that, as the patient improved clinically (hemodynamics stabilized, acidemia improved, and serum lactate concentration slowly decreased), the amount of metformin cleared in the urine increased, further suggesting that aggressive supportive care is helpful in these clinical situations (see Fig. 3). Furthermore, we provide data for both whole blood and serum metformin concentrations, quantifying the effects of potential partitioning of drug into the red blood cells. These results are somewhat consistent with amounts reportedly removed by HD in previous papers In contrast, however, other authors have used an extraction ratio to predict clearance (and as a result showed much higher serum clearance rates), whereas our results are directly quantified not only from blood and dialysate sampling but also urine [6]. As a result, previous conclusions that HD is efficacious in removal of HD may be overstated; they do not take into account endogenous urinary clearance once the acid-base and volume status of the patient is corrected. Furthermore, previously documented successful use of high flow HD that showed precipitous decreases in metformin concentration were reported in cases of acute metformin ingestion, rather than cases of chronic ingestion complicated by acute kidney injury as with our patient [7]. Given other data that suggests metformin is well-removed from the plasma, in conjunction with data showing partitioning of metformin into deeper components (notably erythrocytes and other tissues), it would be reasonable to assume this partitioning is responsible for the decreased metformin removal seen in this case [8, 9]. Redistribution from these compartments may also explain the rebound metformin concentrations seen at hour 4 in our patient. Once all elimination mechanisms are taken into account, the amount of metformin removed with HD, while higher than endogenous urinary clearance is far less than anticipated. This suggests that, once acid-base status has been addressed with HD, less-invasive supportive care measures can be utilized to promote further metformin elimination via endogenous excretion.
Limitations
As with all case-based publications, this specific case represents the pattern of elimination of metformin in one single instance of toxicity. Unfortunately, the patient’s other comorbidities, his baseline creatinine, and subsequent laboratory studies and therapeutics following this admission were unavailable. Variations between this report and others may relate to human variability or differences in HD technique such as blood flow rate, dialysate flow rate, or filter. There also appear to be notable differences between MALA secondary to chronic accumulation vs acute ingestions (in which HD may remove more metformin as it is still mainly in the plasma). However, given the extensive amount of data obtained in our case including blood from both before and after HD, as well as urine and dialysate, we believe that these data can be applied to manage similar patients with chronic metformin toxicity. Likewise, our results are on the order of magnitude reported by others [6]. It is difficult to definitively explain the rebound pattern seen in the blood metformin concentrations at hour 4. We hypothesize this rebound may be related to either redistribution from a secondary compartment, hemolysis in the HD circuit, or laboratory error, although labeling and analysis were performed rigorously to avoid any confusion of samples.
Conclusions
Metformin associated lactic acidosis represents the most severe complication that occurs with therapeutic use and overdose of metformin. Given the severe derangements in metabolic status, the cornerstones of patient care are aggressive supportive care and, in extreme cases, HD. Supportive care and hemodialysis improved urinary excretion of metformin in line with improving clinical status, while hemodialysis removed less metformin than previously reported. While the clinical efficacy of HD in improving patient outcomes seems clear, the data presented here questions the mechanism by which HD seems to benefit metformin-toxic patients— correction of acid-base status rather than metformin removal.
Electronic supplementary material
(DOCX 1303 kb)
Compliance with Ethical Standards
Consent for publication of this case was obtained from the patient in accordance with JMT. policy.
Conflict of Interest
The authors report no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kane, SP. Clincalc.com [Internet]. ClinCalc LLC; 2020 [updated 2016 May 09; cited 2020 Jun 11]. Available from: https://clincalc.com/DrugStats/
- 2.Callelo DP, et al. Extracorporeal treatment for metformin poisoning: systematic review and recommendations from the extracorporeal treatments in poisoning workgroup. Crit Care Med. 2015;43(8):1716–1730. doi: 10.1097/CCM.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 3.Regolisti G, Antoniotti R, Fani F, Greco P, Fiaccadori E. Treatment of metformin intoxication complicated by lactic acidosis and acute kidney injury: the role of prolonged intermittent hemodialysis. Am J Kidney Dis. 2017;70(2):290–296. doi: 10.1053/j.ajkd.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Glucophage [Packet Insert]. Bristol-Myers Squibb Company, NJ; 2018.
- 5.Hernandez SH, Howland M, Schiano TD, Hoffman RS. The pharmacokinetics and extracorporeal removal of N-acetylcysteine during renal replacement therapies. Clin Toxicol (Phila) 2015;53(10):941–949. doi: 10.3109/15563650.2015.1100305. [DOI] [PubMed] [Google Scholar]
- 6.Ayoub P, Hétu PO, Cormier M, Benoit A, Palumbo A, Dubé MC, Gosselin S,Ghannoum M. Toxicokinetics of Metformin During Hemodialysis. Kidney Int Rep. 2017;2(4):759–762. doi: 10.1016/j.ekir.2017.02.017. eCollection 2017 Jul. [DOI] [PMC free article] [PubMed]
- 7.Suzuki K, Okada H, Yoshida S, Okamoto H, Suzuki A, Suzuki K, Yamada Y, Hayashi H, Yasuda R, Fukuta T, Kitagawa Y, Miyake T, Kawaguchi T, Watanabe T, Doi T, Kumada K, Ushikoshi H, Sugiyama T, Itoh Y, Ogura S. Effect of high-flow high-volume-intermittent hemodiafiltration on metformin-associated lactic acidosis with circulatory failure: a case report. J Med Case Rep. 2018;12:280. doi: 10.1186/s13256-018-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith FC, Kumar SS, Furlong TJ, Gangaram SV, Greenfield JR, Stocker SL, Graham GG, Williams KM, Day RO. Pharmacokinetics of metformin in patients receiving regular hemodiafiltration. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;68:990–992. doi: 10.1053/j.ajkd.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Andrejak M, Lalau JD. Unexpectedly long half-life of metformin elimination in cases of metformin accumulation. Diabetic Medicine. 2016;33:105–110. doi: 10.1111/dme.12959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1303 kb)