Abstract
Background
Sodium bicarbonate therapy (SBT) is currently indicated for the management of a variety of acute drug poisonings. However, SBT effects on serum potassium concentrations may lead to delayed QTc prolongation (DQTP), and subsequent risk of adverse cardiovascular events (ACVE), including death. Emergency department (ED)–based studies evaluating associations between SBT and ACVE are limited; thus, we aimed to investigate the association between antidotal SBT, ECG changes, and ACVE.
Methods
This was a secondary data analysis of a consecutive cohort of ED patients with acute drug overdose over 3 years. Demographic and clinical data as well as SBT bolus dosage and infusion duration were collected, and outcomes were compared with an unmatched consecutive cohort of patients with potential indications for SBT but who did not receive SBT. The primary outcome was the occurrence of ACVE, and secondary outcomes were delayed QTc (Bazett) prolongation (DQTP), and death. Propensity score and multivariable adjusted analyses were conducted to evaluate associations between adverse outcomes and SBT administration. Planned subgroup analysis was performed for salicylates, wide QRS (> 100 ms), and acidosis (pH < 7.2).
Results
Out of 2365 patients screened, 369 patients had potential indications for SBT, of whom 31 (8.4%) actually received SBT. In adjusted analyses, SBT was found to be a significant predictor of ACVE (aOR 9.35, CI 3.6–24.1), DQTP (aOR 126.7, CI 9.8–1646.2), and death (aOR 11.9, CI 2.4–58.9). Using a propensity score model, SBT administration was associated with ACVE (OR 5.07, CI 1.8–14.0). Associations between SBT and ACVE were maintained in subgroup analyses of specific indications for sodium channel blockade (OR 21.03, CI 7.16–61.77) and metabolic acidosis (OR: 6.42, 95% CI: 1.20, 34.19).
Conclusion
In ED patients with acute drug overdose and potential indications for SBT, administration of SBT as part of routine clinical care was an independent, dose-dependent, predictor of ACVE, DQTP, and death. This study was not designed to determine whether the SBT or acute overdose itself was causative of ACVE; however, these data suggest that poisoned patients receiving antidotal SBT require close cardiovascular monitoring.
Keywords: Overdose, Sodium bicarbonate, Adverse cardiovascular events
Background
Sodium bicarbonate therapy (SBT) is commonly used in the management of a variety of drug overdoses, including those from salicylates and tricyclic antidepressants (TCAs) [1–3]. SBT is also often administered to treat QRS widening and acidosis [1, 4, 5]; SBT is additionally used frequently in conjunction with other therapies in the context of cardiac arrest [1, 6]. Through its change to serum pH, SBT administration leads to an intracellular shift of potassium [7, 8]. This acute reduction in serum potassium may lead to delayed QTc prolongation and thus increased risk for dysrhythmias as well as other adverse cardiovascular events (ACVEs) [1, 7–10].
Currently, this potential risk from SBT is a theoretical consideration and neither efficacy nor safety profiles of antidotal therapy with SBT in humans have been described in the medical toxicology literature. To date, the majority of evidence supporting use of SBT in the use of multiple indications is based on animal studies, case reports and consensus opinions [1, 4, 7, 11]. Human studies supporting the use of SBT are sparse, uncontrolled, and retrospective in design [12]. However, given the frequent use of SBT for a variety of indications and the continued morbidity and mortality associated with poisonings [13, 14], investigation of adverse outcomes associated with SBT is of paramount importance. The present study aims to investigate two specific hypotheses: (1) there will be a positive correlation between dose/duration of SBT administration and the occurrence of delayed QTc prolongation, ACVE, and/or death and (2) patients given SBT for a variety of indications, including salicylate overdose, TCA overdose, widened QRS, acidosis, or cardiac arrest will have higher rates of delayed QTc prolongation, ACVE, and death when compared with patients with similar clinical presentations who did not receive SBT.
Methods
Study Design and Hospital Setting
This was a secondary data analysis of a prospective cohort study of consecutive ED patients presenting to two academic, urban tertiary care centers from March 1, 2015, to December 30, 2018. The ED at one site has annual patient volumes of approximately 145,000 visits; the second site has approximately 106,000 ED visits annually. IRB approval was obtained with waiver of consent prior to data collection at the study institutions.
Study Population
Consecutive ED patients over the age of 18 with acute overdose or poisoning, and potential indications for SBT, were analyzed from an ongoing cohort which has previously been described [9]. Briefly, ED patients with suspected acute drug overdose were screened prospectively by trained research assistants based on presenting chief complaint. Patients were enrolled consecutively 24 h a day over the study period. Exclusion criteria from the cohort were as follows: age under 18, an alternative diagnosis, non-drug exposure, dermal exposure (due to the self-limiting nature of these exposures), chronic toxicity, prisoner status, or missing data. Consecutive patients within the cohort who had indications for SBT (see below for definitions) were identified for analysis in the present study. Subsequently, administration of SBT as part of routine clinical care was ascertained via chart review. Thus, all patients in the present study had at least one potential indication for SBT, and analyses below compared those who received SBT (SBT group) versus those who did not receive SBT (control group).
SBT Indications
Indications for SBT in patients meeting study criteria were defined as any one of the following four criteria: (A) salicylate overdose, (B) sodium channel blockade, (C) severe metabolic acidosis, and (D) overdose-related cardiac arrest. Salicylate overdose (A) was defined as suspected acute salicylate overdose (from either aspirin [ASA] or any other salicylates) with a positive serum salicylate concentration. Sodium channel blockade (B) was defined using the surrogate of a widened QRS interval (defined as QRS > 100 ms [15]) on any ECG within 6 h of ED arrival. Severe metabolic acidosis (C) was defined as serum pH less than 7.20 at any time, in the absence of a seizure. Finally, overdose-related cardiac arrest was defined as loss of pulses requiring CPR in any patient meeting study criteria. For instances of overlap between indication groups in subgroup analyses, patients with overlapping indications were included within analysis for each relevant subgroup.
Data Collection
Data was abstracted from the medical chart by several trained research assistants. Data collection from the medical chart occurred in accordance with accepted guidelines for valid medical chart abstraction, including training of abstractors and 95% agreement of a random sampling of ten test charts prior to mass data abstraction [16]. Abstractors were blind to study hypotheses.
Demographics, exposure information, toxin identification, initial mental status, prior cardiovascular disease, toxicology screen results, antidote administration, antidote dose and infusion duration, and cardiac biomarker concentrations were initially collected. Enrolled subjects were then prospectively followed to hospital discharge for occurrence of the study outcomes (below).
SBT Administration
SBT administration, as part of routine clinical care, was ascertained via chart review by trained research assistants. SBT infusion dose, total dose (bolus and infusion dose) as well as duration were dichotomized based on what were determined a priori to be clinically meaningful cutoff points by consensus of the study investigators. SBT infusion dose was dichotomized at a cutoff point of 140 mEq, total dose at a cutoff of 280 mEq and infusion duration at a cutoff point of 12 h.
Study Outcomes
The primary study outcome, adverse cardiovascular events (ACVE), was defined as the composite occurrence of any of the following: myocardial injury (serum troponin elevation > 0.10 ng/mL), ventricular dysrhythmias (defined as ventricular tachycardia (VT), ventricular fibrillation (VF) or Torsades des Pointes (TdP), cardiac arrest (loss of pulse requiring CPR) and shock (hypotension requiring administration of vasopressors). This definition has been previously defined and validated [17, 18].
The secondary study outcomes were delayed QTc prolongation (DQTP) and death. DQTP was defined as an initial QTc < 500 ms in the ED combined with a subsequent ECG in-hospital demonstrating QTc ≥ 500 ms. Based on prior literature [10], the computer-generated corrected QT interval (Bazett’s corrected QTc, QT/√RR)was used as it has been previously validated for this purpose. Death was defined as the occurrence of all-cause in-hospital mortality.
Main Analysis
Descriptive statistics examining patient demographics and clinical characteristics were calculated. Two sample t tests, Mann-Whitney U tests, and chi-squared tests were employed to compare demographic and clinical characteristics between groups. Chi-squared test and Fischer’s exact test were used to analyze differences in outcomes among groups by indication, and to analyze differences in outcomes by SBT bolus dose and infusion duration. Bolus dose and infusion duration were dichotomized by median values. We subsequently fit a multivariable logistic regression in order to assess SBT as a predictor of outcomes. Relevant demographic and clinical covariates including age, race, sex, number of ingestions, and clinical site were selected a priori for inclusion in the model. Sample size was determined a priori to ensure adequate power of the main data analysis (i.e., to find an association between SBT and the primary outcome in the dataset); assuming 10% administration of SBT in those with potential indications (based on clinical experience of the investigators), and 15% occurrence of the primary outcome (based on prior literature) [17], we calculated the need to enroll 300 patients (30 SBT, 270 control) to detect a 3-fold increased risk of the study outcome in the SBT group with 95% power and 5% alpha. Analyses were conducted using SAS University Edition v.9.4 (SAS Institute, Cary, NC) and SPSS v. 24 (IBM, Armonk, NY).
Propensity Score Analysis
Propensity score analysis was utilized to control for varying propensity to administer SBT based on each separate indication. First, a propensity score model was fitted using binary logistic regression where the outcome was sodium bicarbonate treatment with the following predictor variables: salicylate overdose (indication A), sodium channel blockade (indication B), severe metabolic acidosis (indication C), and overdose-related cardiac arrest (indication D). In this analysis the generated propensity scores were used as a variable in a new prediction model. Second, because there was no rationale to believe the propensity score would predict the outcome linearly, propensity scores were divided into quartiles yielding a four-category variable. Given the small number of observations in some categories, a binary variable was reconciled by comparing the highest score category against the three other categories combined. Third, a final prediction model was fitted using binary logistic regression to model the composite outcome (ACVE) as a function of treatment (SBT), the binary propensity score variable, and additional covariates such as age, sex, race, number of drugs, and site.
Subgroup Analyses for Each SBT Indication
Salicylate (indication A) subgroup analysis was performed to assess for differences in the outcomes of interest for only those with salicylate overdose. Those with salicylate overdose and a second concurrent indication for SBT were excluded from subgroup analysis. Fischer’s exact test was employed to examine unadjusted differences between those with salicylate overdose who received SBT and those who did not. In order to account for severity of overdose, we subsequently employed stratified analysis, examining the outcomes of interest based on serum salicylate concentration. Initial salicylate level was stratified into three groups based loosely on current recommendations [3, 19]: serum concentration < 15 mg/dL (group 1), 15–29 mg/dL (group 2), and 30+ mg/dL (group 3).
Subgroup analysis for sodium channel blockade (indication B) was performed to compare differences in the occurrence of the study outcomes among those with wide QRS. Fisher’s exact test was employed to examine unadjusted differences in outcomes between those who received SBT vs. those who did not. We subsequently conducted adjusted, stratified analysis to account for severity of QRS widening. QRS interval was stratified into three groups based on prior literature [2, 4, 15, 20]: 100–114 ms (group 1), 115–129 ms (group 2), and ≥ 130 ms (group 3).
Finally, subgroup analysis was performed for patients with metabolic acidosis (indication C) as the indication for SBT. Those with acidosis and a second, concurrent indication for SBT were excluded from this subgroup. Fisher’s exact test was employed to examine unadjusted differences in the occurrence of study outcomes among patients with acidosis who received SBT and those who did not. In order to account for the potential effect of severity of acidosis, we subsequently employed stratified analysis into three groups based on prior literature [5, 21]: pH < 7.0 (group 1), pH 7.0–7.1 (group 2), and pH > 7.1 (group 3).
Due to small numbers of overdose-related cardiac arrest (indication D), no stratified analysis was performed for this subgroup.
Results
Enrollment and Demographics
Out of 2365 patients who were screened, and after application of enrollment criteria, there were 31 SBT patients and 338 non-SBT patients for analysis. Enrollment with application of inclusion/exclusion criteria is summarized in Fig. 1. Patient demographics and clinical characteristics are summarized in Table 1. The SBT and non-SBT groups were similar in terms of clinical characteristics, aside from race/ethnicity, pH and indication.
Fig. 1.
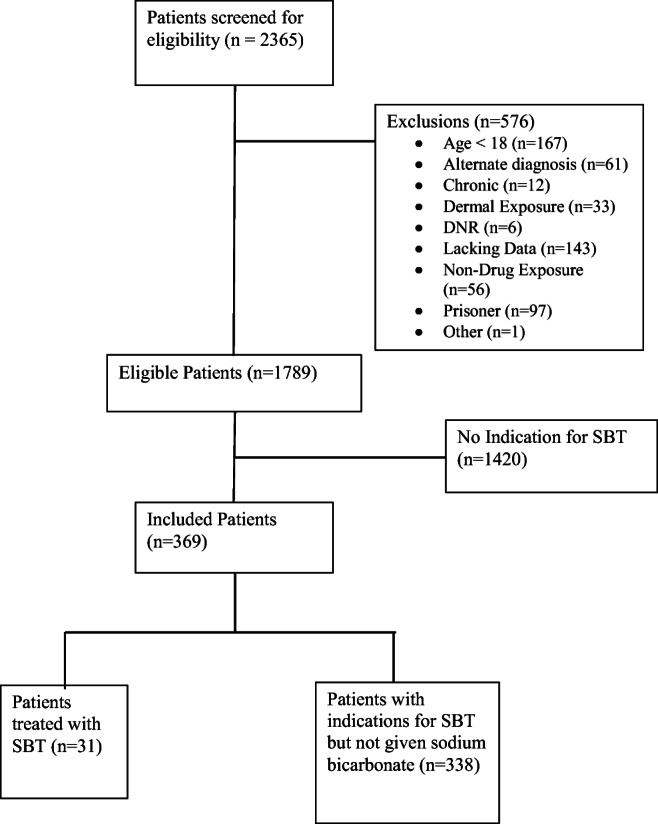
Study enrollment and inclusion/exclusion criteria. Summary of study enrollment and patient inclusion/exclusion. SBT, sodium bicarbonate therapy
Table 1.
Patient demographics and clinical characteristics
| SBT (N = 31) | No SBT (N = 338) | |
|---|---|---|
| Age | ||
| Mean (range) | 44.4 (20–88 years) | 43.5 (18–92 years) |
| Sex N (%) | ||
| Male | 20 (64.52%) | 247 (73.08%) |
| Race/ethnicity* N (%) | ||
| White | 12 (38.71%) | 108 (31.95%) |
| Black | 1 (3.23%) | 28 (8.28%) |
| Asian | 3 (9.68%) | 24 (7.10%) |
| Hispanic | 5 (21.74%) | 92 (31.83%) |
| Unknown | 8 (25.81%) | 27 (7.99%) |
| Indication for SBT N (%) | ||
| Indication A | 6 (19.35%) | 15 (4.44%) |
| Indication B | 17 (54.84%) | 283 (83.73%) |
| Indication C | 9 (29.03%) | 51 (15.09%) |
| Indication D | 9 (29.03%) | 16 (4.73%) |
| Number of ingestions (median) | 2.00 | 2.00 |
| pH (median)* | 7.32 | 7.35 |
| QRS (median) | 112 ms | 104 ms |
*= p < 0.05
SBT sodium bicarbonate therapy
Indication A = salicylate overdose
Indication B = widened QRS (QRS ≥ 100 ms)
Indication C = acidosis (pH < 7.2)
Indication D = cardiac arrest
Main Outcomes
In univariate analysis for all patients, the SBT group had significantly higher rates of ACVE (60.7% versus 12.4%, p < 0.0001), DQTP (22.8% versus 1.48%, p < 0.0001), and death (25% versus 2.1%, p < 0.0001). Among SBT patients, median time to peak QTc was 21 h (IQR: 93) from ED arrival. In the non-SBT group, median time to peak QTc was 33 h (IQR: 67). The median time to peak QTc in those with DQTP was 16.5 h (IQR 4.5, 35). Overall unadjusted outcomes and breakdown of ACVE are summarized in Table 2 and Table 3 respectively.
Table 2.
Overall outcomes by treatment group (unadjusted and adjusted+ analyses)
| SBT N (%) | No SBT N (%) | Unadjusted OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| ACVE | 18 (58.06%) | 42 (11.38%) | 9.76 (4.46, 21.35)* | 9.35 (3.63, 24.11)* |
| DQTP | 6 (20%) | 5 (1.48%) | 16.65 (1.03, 1.47)* | 126.68 (9.75, 1646.17)* |
| Death | 7 (22.58%) | 7 (2.08%) | 13.71 (4.44, 42.29)* | 11.86 (2.39, 58.92)* |
*= p < 0.05
+Model covariates: age, race, sex, number of ingestions and clinical site
SBT sodium bicarbonate therapy, ACVE adverse cardiovascular event, DQTP delayed QTc prolongation
Table 3.
Adverse cardiovascular events
| SBT N (%) | No SBT N (%) | |
|---|---|---|
| Shock | 15 (48.39%) | 12 (3.55%) |
| Myocardial injury | 9 (29.03%) | 30 (8.88%) |
| Arrhythmia | 5 (16.13%) | 3 (0.89%) |
| Cardiac arrest | 9 (29.03%) | 16 (4.73%) |
Using multivariate logistic regression adjusting for age, race, sex, number of ingestions, and clinical site, SBT was found to be a significant predictor of ACVE (aOR 9.35, CI 3.63–24.11, p < 0.0001), DQTP (aOR 126.68, CI 9.75–1646.17, p < 0.0001), and death (aOR 11.86, CI 2.39–58.92, p = 0.003). The adjusted model is summarized in Table 2.
Propensity Score Analysis
The propensity score model demonstrated that SBT administration was associated with a significantly higher likelihood of ACVE (OR 5.07, CI 1.83–14.02). All components of the model (including propensity scores and covariates) are summarized in Table 4. In this model, the only other significant association was age; each yearly increase in age contributed a 3.6% higher likelihood for ACVE (OR 1.036, CI 1.02–1.06). The prediction model had a ROC area under the curve of 0.89.
Table 4.
Propensity score model of SBT as a predictor of ACVE
| Clinical factor: | aOR for ACVE: | 95% CI: |
|---|---|---|
| SBT | 5.07 | 1.83–14.03 |
| Propensity quartile | 0.05 | 0.02–0.12 |
| Age* | 1.04 | 1.02–1.06 |
| Male sex | 1.59 | 0.75–3.38 |
| Race | ||
| White | Reference | Reference |
| Black | 0.99 | 0.28–3.57 |
| Hispanic/Latino | 0.62 | 0.10–4.05 |
| Asian | 0.93 | 0.42–2.06 |
| Other | 0.39 | 0.11–1.33 |
| Number Drugs* | 1.04 | 0.84–1.29 |
| Site† | ||
ACVE adverse cardiovascular events, aOR adjusted odds ratio, CI 95% confidence interval, SBT sodium bicarbonate therapy
Italic numbers indicate p < 0.01
*Odds ratios for age and number of drugs apply for each additional year and each additional drug, respectively
†Odds for site use Mount Sinai Hospital for comparison
Dose-Response Analysis
A total of 19 patients were placed on a bicarbonate infusion. As infusion dosage and duration were dichotomized by median values, 9 patients were in the low infusion dosage and short duration group while 10 patients were in the high infusion dosage and long duration group. Thirteen patients were in the low total dose group while 17 patients were in the high total dose group. There was a significantly higher rate of ACVE in the high infusion dose, high total dose, and long duration groups (both p < 0.05). All patients in the high total dose group (p = 0.0033) and the long duration group (p = 0.0031) experienced an ACVE; 81.82% of patients in the high infusion dose group experienced an ACVE (p = 0.0055). The full dose-response analysis is shown in Table 5.
Table 5.
Adverse cardiovascular events by infusion dose and duration
| Infusion duration | Infusion dose | Total dose (infusion + bolus) | |||
|---|---|---|---|---|---|
| Short (< 12 h) %, 95% CI (N) |
Long (> 12 h) %, 95% CI (N) |
Low (< 140 mEq) %, 95% CI (N) |
High (> 140 mEq) %, 95% CI (N) |
Low (< 280 mEq) %, 95% CI (N) |
High (> 280 mEq) %, 95% CI (N) |
| 25% CI: 5.5%, 57.2% (N = 3) | 100%, CI: 59%, 100% (N = 7) | 12.5%, CI: 0.3%, 52.7% (N = 1) | 81.82%, CI: 48.2%, 97.7% (N = 9) |
38.10%, CI: 18.1%, 61.6% (N = 8) |
100%, CI: 66.4%, 100% (N = 9) |
|
p = 0.0031 OR: ∞ |
p = 0.0055 OR: 31.5 (2.35, 422.30) |
p = 0.0033 OR: ∞ |
|||
Salicylate Subgroup Analysis
There was a total of 6 patients with salicylate overdose who received SBT and 15 who did not. Among those receiving SBT, 33.3% had ACVE (95% CI: 4.3%, 77.7%), and 33.3% had DQTP (95% CI: 4.3%, 77.7%), compared with 0% for both outcomes in the non-SBT group. There were no deaths in either treatment group. However, the analysis was underpowered to reach statistical significance (Table 6). Stratified analysis was similarly underpowered to detect differences in outcomes for any subgroup (Table 7).
Table 6.
Outcomes among treatment groups by indication for SBT
| SBT indication | ACVE | DQTP | Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SBT N (%, 95% CI) |
No SBT N (%, 95% CI) |
p value | SBT N (%, 95% CI) |
No SBT N (%, 95% CI) |
p value | SBT N (%, 95% CI) |
No SBT N (%, 95% CI) |
p value | |
| Widened QRS | 10 (58.8% CI: 32.9%, 81.6%) | 18 (6.4% CI: 3.8%, 9.9%) | < 0.0001 | 4 (23.5% CI: 6.8%, 49.9%) | 2 (0.71% CI: 0.1%, 2.5%) | < 0.0001 | 4 (23.5%CI: 6.8%, 49.9%) | 1 (0.36% CI: 0%, 2.0%) | < 0.0001 |
| Salicylate overdose | 2 (33.3% CI: 4.3%, 77.7%) | 0 | 0.071 | 2 (33.3% CI: 4.3%, 77.7%) | 0 | 0.071 | N/A* | N/A* | N/A* |
| Acidosis | 7 (77.8% CI: 40%, 97.2%) | 18 (35.3% CI: 22.4%, 49.9%) | 0.027 | 1 (11.1% CI: 0.3%, 48.2%) | 2 (3.9% CI: 0.5%, 13.5%) | 0.391 | 2 (22.2% CI: 2.8%, 60%) | 3 (6% CI: 1.3%, 16.5%) | 0.163 |
| Cardiac arrest | N/A+ | N/A+ | N/A+ | N/A | N/A | N/A | 6 (66.7% CI: 29.9%, 92.5%) | 7 (43.8% CI: 19.8%, 70.1%) | 0.411 |
*No events in either group
+Not applicable as cardiac arrest automatically led to all patients in both groups being classified as having an ACVE
Table 7.
Outcomes among treatment groups by indication for SBT: stratified results by severity of indication
| ACVE | DQTP | Death | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
SBT N (%, 95% CI) |
No SBT N (%, 95% CI) |
p value |
SBT N (%, 95% CI) |
No SBT N (%, 95% CI) |
p value |
SBT N (%, 95% CI) |
No SBT N (%, 95% CI) |
p value | ||
| QRS (ms) | 100–114 | 3 (50% CI: 11.8%, 88.2%) | 9 (4.6% CI: 2.1%, 8.6%) | 0.0030 | 2 (33.3% CI: 4.3%, 77.7%) | 1(0.52% CI: 0%, 2.8%) | 0.0022 | 2(33.33% CI: 4.3%, 77.7%) | 0 | 0.0008 |
| 115–129 | 4 (66.7% CI: 22.3%, 95.7%) | 2 (6.7% CI: 0.8%, 22.1%) | 0.0034 | 2 (33.3% CI: 4.3%, 77.7%) | 1 (3.3% CI: 0.1%, 17.2%) | 0.0658 | 1 (16.7% CI: 0.4%, 64.1%) | 0 | 0.1714 | |
| 130+ | 4 (44.4% CI: 13.7%, 78.8%) | 7 (11.9% CI: 4.9%, 22.9%) | 0.0320 | 2 (22.2% CI: 2.8%, 60%) | 0 | 0.0158 | 1(11.1% CI: 0.3%, 48.2%) | 1 (1.7% CI: 0%, 9.1%) | 0.2489 | |
|
Salicylate concentration (mg/dL) |
< 14 | None | None | N/A | None | None | N/A | None | 0 | N/A |
| 15–29 | None | None | N/A | None | None | N/A | None | 0 | N/A | |
| 30+ | 2 (33.3% CI: 4.3%, 77.7%) | 0 | 1.0000 | 2 (33.3% CI: 4.3%, 77.7%) | 0 | 1.000 | None | None | N/A | |
| pH | < 7.0 | 6 (85.7% CI: 42.1%, 99.6%) | 2 (100% CI: 5.8%, 100%) | 1.000 | 1 (14.29% CI: 0.4%, 57.9%) | 0 | 1.000 | 2 (28.6% CI: 3.7%, 71%) | 0 | 1.000 |
| 7.0–7.1 | 1 (100% CI: 2.5%, 100%) | 7 (53.9% CI: 25.1%, 80.8%) | 1.000 | 0 | 1 (7.7% CI: 0.2%, 36%) | 1.000 | 0 | 1 (7.7% CI: 0.2%, 36%) | 1.000 | |
| 7.1–7.2 | 0 | 9 (25% CI: 12.1%, 42.2%) | 1.000 | 0 | 1 (2.8% CI: 0.1%, 14.5%) | 1.000 | 0 | 2 (5.7% CI: 0.7%, 19.2%) | 1.000 | |
Sodium Channel Blockade Subgroup Analysis
There was a total of 17 patients who received SBT versus 283 who did not. There was a significantly increased ACVE rate in those receiving SBT (58.8%, 95%CI 32.9–81.6) compared with those not receiving SBT (6.4%, CI 3.8–9.9) with over 20-fold increased odds of the primary outcome (OR 21.03, CI 7.16–61.77, p < 0.0001). There was also an over 40-fold increased odds of DQTP in the SBT group (OR 43.2, CI 7.2–257.9, p = 0.0001). Similarly, a statistically significant increase in death was observed with 23.5% (95% CI: 6.8%, 49.9%) of SBT patients experiencing death compared with 0.36% (95% CI: 0%, 2.0%) of non-SBT patients (p < 0.0001, OR: 86.15 95% CI: 8.98, 826.25). Results are summarized in Table 6. Using stratified analysis, increased odds of the primary and both secondary study outcomes remained associated with SBT (see Table 7).
Metabolic Acidosis Subgroup Analysis
There was a total of 9 patients with acidosis who received SBT versus 51 who did not. We identified a statistically significant increase in the occurrence of ACVE among those receiving SBT with 77.8% (95% CI: 40%, 97.2%) experiencing an ACVE compared with 35.3% (95% CI: 22.4%, 49.9%) of non-SBT patients (p = 0.027, OR: 6.42 95% CI: 1.20, 34.19). Among patients receiving SBT, 11.1% (95% CI: 0.3%, 48.2%) experienced DQPT compared with 3.9% (95% CI: 0.5%, 13.5%) of non-SBT patients and 22.2% (95% CI: 2.8%, 60%) of SBT patients experienced death as compared with 6% (95% CI: 1.3%, 16.5%) of non-SBT patients; these results were not statistically significant. Results are presented in Table 6. The stratified analysis was underpowered to detect significant differences in outcomes (Table 7).
Discussion
The main finding in this analysis of ED patients with acute drug overdose who had potential indications for SBT was that administration of SBT was associated with a clinically significant and dose-dependent increased odds of ACVE, DQTP, and death overall. When adjusting for potential demographic, clinical confounders, and propensity scores, administration of SBT remained an independent predictor of study outcomes. This association was evident most strongly for two important indications: sodium channel blockade and metabolic acidosis. Conversely, the salicylate and cardiac arrest subgroup analyses did not demonstrate any association with study outcomes. Furthermore, because the study was not designed to determine whether the SBT or acute overdose itself was causative of ACVE, practitioners should weigh the potential risks of occurrence of cardiovascular events versus perceived clinical antidotal benefit of SBT. Based on these results, a more heightened caution to the approach to SBT may be warranted, and poisoned patients receiving antidotal SBT should receive close cardiovascular inpatient monitoring.
One of the more striking aspects of the present analysis is the dose-response effect in the association between SBT and outcomes (DQTP and ACVE). Indeed, dose-response is a key consideration for determination of causality in clinical toxicology investigations [22]. There was significantly increased odds of adverse outcomes with higher dose and longer duration of therapy. The significant association between adverse outcomes and both increased dose as well as longer duration are suggestive of causation between SBT and ACVE although this cannot be definitively demonstrated on the basis of the present study’s findings.
There are a variety of mechanistic explanations for the increased rate of ACVE across multiple indications for SBT in this study. First, SBT has effects on acutely lowering serum potassium concentrations [23] which may result in DQTP and subsequent risk of dysrhythmia [9]. In addition, further side effects of SBT include other electrolyte disturbances (such as hypocalcemia or hypernatremia), progression of vascular calcifications, rebound metabolic alkalosis, and potentially increased lactate production [24]. Another potentially deleterious side effect is the generation of carbon dioxide from bicarbonate, which may lead to hypercapnia especially in patients with acute drug overdose and impending respiratory failure [25]. Another potential mechanism is decreased cardiac output coupled with inability to compensate adequately via hyperventilation effects on cardiac contractility [26]. The above mechanistic concerns add credence to causation as the explanation for the present study’s findings with respect to the association between SBT and ACVE. Therefore, clinicians should weigh the potential risks of adverse events from SBT versus its potential clinical benefits.
When outcomes were analyzed specifically among those with salicylate overdose (indication A), we did not identify statistically significant differences in study outcomes; this may be partly because our subgroup analysis was underpowered to show significance. Thus, these results do not identify conclusive evidence of harm in those with salicylate overdose managed with SBT. Indeed, animal models demonstrate substantial benefits of SBT in salicylate poisoning [27, 28], though human studies are limited. Thus, further randomized trials with sufficient sample sizes are needed to better characterize the nature of this association between SBT and ACVE in salicylate-poisoned patients.
In the subgroup of those with sodium channel blockade (indication B), the present study identified a clinically and statistically significant association between SBT and the primary and secondary study outcomes in unadjusted analysis. Following stratification by QRS width, there continued to be a significant effect of SBT administration on the occurrence of adverse outcomes for each stratum. Our results suggest that the association between SBT and adverse outcomes among those with widened QRS is independent of QRS duration. However, it should be noted that this subgroup has perhaps the highest risk of confounding, since severe sodium channel blockade causes ACVE, especially shock, ventricular dysrhythmia, and cardiac arrest [15, 29, 30]. Therefore, the association between SBT and adverse events in the subgroup of patients with sodium channel blockade should be interpreted with extreme caution. Despite prior uncontrolled human studies suggesting benefit of SBT in patients with sodium channel blockade due to tricyclic antidepressant overdose [12, 31], the present data suggest that further studies are urgently needed to ascertain the optimal indications, doses, and durations for SBT in ED patients with overdose-related sodium channel blockade.
For the subgroup of patients with metabolic acidosis (indication C), there was significantly increased occurrence of ACVE in the SBT group compared with the control group; however, statistically significant differences in DQTP and death were not identified. Our results indicate a possible association between the occurrence of adverse events and SBT in patients with acidosis but further studies with a larger sample size are needed to better characterize this association and to account for the impact of severity of acidosis.
We found increased rates of death among patients undergoing CPR who received SBT (indication D) when compared with those not receiving SBT; these results did not reach statistical significance. However, the study sample size for patients undergoing CPR and receiving SBT was small limiting the study’s power to detect a meaningful difference between groups. These results should therefore not sway providers from using bicarbonate for this patient population who undergoes CPR.
Interestingly, we found that of patients with indications for SBT, only a small proportion (8.4%) actually received SBT. This may be due to a variety of reasons, some of which may include the following: evolving practice patterns with regard to routine administration of SBT for these clinical indications, provider experience/preference, level/type of provider training, or the degree to which medical toxicology consultation was involved with patient care. The small number of patients who received SBT contributed to our study’s limited ability to detect meaningful differences in various subgroup analyses and may have added an element of bias, as those patients who actually received SBT may have been more clinically ill or otherwise systematically different from the population with indications who did not receive SBT. Future studies exploring patient and provider/institution level reasons for SBT administration in these patient populations is warranted.
Future randomized studies with larger numbers of patients are warranted to further evaluate, validate and characterize the association between SBT and ACVE. Studies at multiple geographic sites and at different care settings are also warranted to improve generalizability. Additional studies examining the impact of potassium administration on mitigating adverse outcomes due to SBT are also warranted.
Limitations
The present study has several important limitations, most important of which is the observational, non-randomized, nature of the study. There may be a significant component of practice variation among clinicians which informed administration of SBT for specific indications. The lack of SBT randomization may have led to causal bias with respect to overdose severity (i.e., the alternate explanation that the overdose itself caused the ACVE); however, we performed propensity score analysis to specifically address this limitation, and our findings remained clinically and statistically significant. The dose-response findings noted above may also be complicated by this bias as patients with a more severe overdose may be more likely to receive a higher dose or longer duration of SBT. Another limitation was small sample sizes for each subgroup analysis, which decreased statistical power to detect meaningful differences for some indications and did not permit for extensive subgroup adjusted analysis. There were also limitations with regard to inability to evaluate the role of serial potassium concentrations on outcomes because follow-up potassium concentrations were not routinely collected—collection and analysis of serum potassium data in association with SBT outcomes is a potential direction for future studies. We additionally did not examine old ECGs to compare the study QRS duration to baseline ECG data. The majority of patients had multi-drug overdoses, which may have further confounded the occurrence of adverse events. We additionally did not collect data on patient use of QTc prolonging medications which may have contributed to delayed QTc prolongation [32]. Finally, the primary outcome was ACVE, which may be less important than overall mortality; it is theoretically possible for a therapy to improve mortality despite increasing rates of ACVE.
Conclusions
In ED patients with acute drug overdose and potential indications for sodium bicarbonate therapy (SBT), administration of SBT as part of routine clinical care was an independent, dose-dependent, predictor of ACVE, DQTP, and death. This study was not designed to determine whether the SBT or the acute overdose itself was causative of ACVE. However, emergency practitioners should cautiously approach use of SBT for acute drug overdose, particularly in the setting of sodium channel blockade and severe metabolic acidosis. These data suggest that poisoned patients receiving antidotal SBT require close cardiovascular monitoring. Future, randomized studies are needed to improve generalizability, validate these findings, and better characterize SBT indications and regimens.
Authors’ Contributions
SS performed data analysis and drafted the manuscript. JE assisted with data collection and analysis. RV assisted with data analysis. LDR provided assistance with funding and data collection. AM conceived the study, obtained funding, and oversaw data collection, analysis, and manuscript preparation. All authors helped edit the manuscript and approved the final version of the manuscript.
Funding Information
The study was made possible, in part, by grant DA037317 (PI: Manini) from the National Institute on Drug Abuse of the National Institutes of Health. Dr. Shastry is supported by an institutional training grant, 1T32 HL129974-01 (PI: Richardson), from the National Heart, Lung & Blood Institute of the National Institutes of Health. Dr. Manini is currently supported by grant R01DA048009 from the National Institute on Drug Abuse of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
IRB approval was obtained with waiver of consent prior to data collection at the study institutions.
Conflict of Interest
None.
Footnotes
Presentations
Data from this study were presented at the American College of Medical Toxicology (ACMT) Annual Scientific Meeting in San Francisco, CA in April 2019, at the Society for Academic Emergency Medicine (SAEM) Annual Meeting in Las Vegas, NV in May 2019, and at the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) Congress in Naples, Italy in May 2019.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wax PM. Antidotes in depth (A5): sodium bicarbonate. In: Nelson L, Lewin N, Howland M, editors. Goldfranks toxicological emergencies. New York: McGraw Hill; 2010. pp. 528–535. [Google Scholar]
- 2.Bradberry SM, Thanacoody HKR, Watt BE, Thomas SHL, Vale JA. Management of the cardiovascular complications of tricyclic antidepressant poisoning: role of sodium bicarbonate. Toxicol Rev. 2005;24:195–204. doi: 10.2165/00139709-200524030-00012. [DOI] [PubMed] [Google Scholar]
- 3.Boyer EW, Weibrecht KW. Salicylate (aspirin) poisoning in adults. In: Traub SJ, editor. UpToDate. Waltham: UpToDate; 2017. [Google Scholar]
- 4.Bruccoleri RE, Burns MM. A literature review of the use of sodium bicarbonate for the treatment of QRS widening. J Med Toxicol. 2016;12(1):121–129. doi: 10.1007/s13181-015-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garella S, Dana CL, Chazan JA. Severity of metabolic acidosis as a determinant of bicarbonate requirements. N Engl J Med. 1973;289:121–126. doi: 10.1056/NEJM197307192890303. [DOI] [PubMed] [Google Scholar]
- 6.Velissaris D, Karamouzos V, Pierrakos C, Koniari I, Apostolopoulou C, Karanikolas M. Use of sodium bicarbonate in cardiac arrest: current guidelines and literature review. J Clin Med Res. 2016;8(4):277–283. doi: 10.14740/jocmr2456w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mark NH, Leung JM, Arieff AI, Mangano DT. Safety of low-dose intraoperative bicarbonate therapy: a prospective, double-blind, randomized study. The Study of Perioperative Ischemia (SPI) Research Group. Crit Care Med. 1993;21:659–665. [PubMed] [Google Scholar]
- 8.Hoste EA, Colpaert K, Vanholder RC, Lameire NH, De Waele JJ, Blot SI, et al. Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis. J Nephrol. 2005;18:303–307. [PubMed] [Google Scholar]
- 9.Manini AF, Nair AP, Vedanthan R, Vlahov D, Hoffman RS. Validation of the prognostic utility of the electrocardiogram for acute drug overdose. J Am Heart Assoc. 2017;6(2):e004320. doi: 10.1161/JAHA.116.004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manini AF, Nelson LS, Skolnick AH, Slater W, Hoffman RS. Electrocardiographic predictors of adverse cardiovascular events in suspected poisoning. J Med Toxicol. 2010;6(2):106–115. doi: 10.1007/s13181-010-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackman K, Brown SG, Wilkes GJ. Plasma alkalinization for tricyclic antidepressant toxicity: a systematic review. Emerg Med (Fremantle) 2001;13:204–210. doi: 10.1046/j.1442-2026.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman JR, Votey SR, Bayer M, Silver L. Effect of hypertonic sodium bicarbonate in the treatment of moderate-to-severe cyclic antidepressant overdose. Am J Emerg Med. 1993;11(4):336–341. doi: 10.1016/0735-6757(93)90163-6. [DOI] [PubMed] [Google Scholar]
- 13.Gummin DD, Mowry JB, Spyker DA, Brooks DE, Osterthaler KM, et al. Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila) 2017;2018:1–202. doi: 10.1080/15563650.2018.1533727. [DOI] [PubMed] [Google Scholar]
- 14.Hedegaard H, Miniño AM, Warner M. NCHS data brief, no 329. Hyattsville: National Center for Health Statistics; 1999. Drug overdose deaths in the United States; p. 2018. [Google Scholar]
- 15.Boehnert MT, Lovejoy FH. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313:474–479. doi: 10.1056/NEJM198508223130804. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta D, Steiner J. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27:305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 17.Manini AF, Nelson LS, Stimmel B, Vlahov D, Hoffman RS. Incidence of adverse cardiovascular events in adults following drug overdose. Acad Emerg Med. 2012;19(7):843–849. doi: 10.1111/j.1553-2712.2012.01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manini AF, Hoffman RS, Stimmel B, Vlahov D. Clinical risk factors for in-hospital adverse cardiovascular events after acute drug overdose. Acad Emerg Med. 2015;22(5):499–507. doi: 10.1111/acem.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugassy DM. Salicylates. In: Nelson LS, Howland MA, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, editors. Goldfrank's Toxicologic Emergencies. New York: McGraw-Hill; 2019. [Google Scholar]
- 20.Groleau G, Jotte R, Barish R. The electrocardiographic manifestations of cyclic antidepressant therapy and overdose: a review. J Emerg Med. 1990;8(5):597–605. doi: 10.1016/0736-4679(90)90457-7. [DOI] [PubMed] [Google Scholar]
- 21.Ghauri SK, Javaeed A, Mustafa KJ, Podlasek A, Khan AS. Bicarbonate therapy for critically ill patients with metabolic acidosis: a systematic review. Cureus. 2019;11(3):e4297. doi: 10.7759/cureus.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H, Barton-Maclaren TS. Increasing scientific confidence in adverse outcome pathways: application of tailored Bradford-Hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol. 2015;72(3):514–537. doi: 10.1016/j.yrtph.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Lawson AA, Proudfoot AT, Brown SS, MacDonald RH, Fraser AG, Cameron JC, Matthew H. Forced diuresis in the treatment of acute salicylate poisoning in adults. Q J Med. 1969;38(149):31–48. [PubMed] [Google Scholar]
- 24.Hindman BJ. Sodium bicarbonate in the treatment of subtypes of acute lactic acidosis: physiologic considerations. Anesthesiology. 1990;72(6):1064–1076. [PubMed] [Google Scholar]
- 25.Adeva-Andany MM, Fernandez C, Mourino-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. Sci World J. 2014;2014:1–10. doi: 10.1155/2014/627673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espejo MS, Orlowski A, Ibañez AM, Di Mattía RA, Velásquez FC, Rossetti NS, et al. The functional association between the sodium/bicarbonate cotransporter (NBC) and the soluble adenylyl cyclase (sAC) modulates cardiac contractility. Pflugers Arch. 2019;22 [Epub ahead of print]. [DOI] [PubMed]
- 27.Buchanan N, Kundig H, Eyberg C. Experimental salicylate intoxication in young baboons. A preliminary report. J Pediatr. 1975;86(2):225–232. doi: 10.1016/s0022-3476(75)80473-2. [DOI] [PubMed] [Google Scholar]
- 28.Hill JB. Experimental salicylate poisoning: observations on the effects of altering blood pH on tissue and plasma salicylate concentrations. Pediatrics. 1971;47(4):658–665. [PubMed] [Google Scholar]
- 29.Kerr GW, McGuffie AC, Wilkie S. Tricyclic antidepressant overdose: a review. Emerg Med J. 2001;18:236–241. doi: 10.1136/emj.18.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abeyaratne DD, Liyanapathirana C, Gamage A, Karunarathne P, Botheju M, Indrakumar J. Survival after severe amitriptyline poisoning with prolonged ventricular tachycardia and cardiac arrest. BMC Res Notes. 2016;9:167. doi: 10.1186/s13104-016-1963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman JR, McElroy CR. Bicarbonate therapy for dysrhythmia and hypotension in tricyclic antidepressant overdose. West J Med. 1981;134(1):60–64. [PMC free article] [PubMed] [Google Scholar]
- 32.Campleman SL, Brent J, Pizon AF, et al. Drug-specific risk of severe QT prolongation following acute drug overdose. Clin Toxicol (Phila). 2020; 10.1080/15563650.2020.1746330. [DOI] [PMC free article] [PubMed]


