Abstract
Miracle fruit plant or Miracle berry plant (Synsepalum dulcificum) is a peculiar medicinal plant because of the unique taste-modifying property of its fruit which is due to the presence of the glycoprotein, miraculin. This property has been known for centuries to the people of tropical Western and Central Africa who also employ different parts of the plant in the management of various ailments. Scientific investigations have unravelled several pharmacological properties of the plant which include antidiabetic, blood cholesterol-lowering, anti-hyperuricaemia, antioxidant, anticonvulsant and anticancer properties. Also, subacute administration of the plant extract up to 200 mg/kg was not found to be toxic in rats. Apart from miraculin, other pharmacologically active compounds have been identified in the plant including alkaloids (dihydro-feruloyl-5-methoxytyramine, N-cis-caffeoyltyramine, N-cis-feruloyl-tyramine), lignins (+-syringaresinol, +-epi-syringaresinol), phytosterols, triterpenoids, phenolic acids, flavonoids, and amino acids. The plant has also been credited with notable nutritional benefits. Proper documentation of available information on folkloric use, biological activity, constituent phytocompounds, and nutritional benefits of ethnobotanicals will go a long way in affording optimal benefits from their therapeutic potentials. This can also aid in the conservation of species at risk of extinction. This work presents an up-to-date review of the ethnobotany, phytochemistry, biological and nutritional properties of Synsepalum dulcificum.
Keywords: Miracle fruit, Ethnobotanical use, Bioactivities, Phytochemicals, Nutritional benefits, Commercial applications
Miracle fruit; Ethnobotanical use; Bioactivities; Phytochemicals; Nutritional; Benefits; Commercial applications.
1. Introduction
Synsepalum dulcificum, commonly known as Miracle plant, Miracle berry plant or red berry, is an evergreen shrub belonging to the Sapotaceae family.
The miracle plant was first discovered in West and Central Africa, specifically in countries like Congo, Ghana and Nigeria (He et al., 2015; Sulaini and Sabran, 2018). The plant is known as Agbayun and Uni respectively among the Yoruba and Igbo people of southern Nigeria (Obafemi et al., 2019). Miracle plant grows to a height of about 3 m in cultivation, and 6.1 m in a native habitat. Leaves are 5–10 cm long and 2–3.7 cm wide. Flowers are cream-colored but turn dark-red or brown with maturity. The flowers are bisexual. The calyx is made up of four to five sepals, the corolla four to five petals, and the androecium five stamens. The gynoecium stands erect with an unspectacular stigma (Xingwei et al., 2016). The ripe fruit is red in color. The miracle berry is not as common as other berry fruits such as blueberry, blackberry, cranberry and grapes. The berry is about the size of a coffee bean, roughly 2 cm long and 1 cm wide. It has relatively large seed which is encapsulated by a translucent pulp that is covered by a thin skin. The sweetening effect is contained in the pulp. The fruits are usually clustered at the ends of the branches (Figure 1) (He et al., 2016).
Figure 1.
Miracle berry (Mangla and Kohli, 2018).
Miracle fruit can be used in either raw form or processed in the making of food and medicine or in the cosmetic industry as additives. Historically, the plant fruit is referred to as miracle plant because of its unique taste-modifying property that causes sour edibles to taste sweet after the mouth has been exposed to the fruits. There has been a growing interest in the potential usage of the plant for centuries by local people of West Africa where the fruit has been used to sweeten sour foods and beverages such as Koko and Kenkey made from fermented maize and millet, and palm wine (Akinmoladun, 2016). Miraculin, a glycoprotein in the fruit has been credited with this property. Miraculin binds to the sweet receptor cells of the tongue and represses the feedback of a sour taste from the brain (Du et al., 2014). S. dulcificum has been considered as an impending food colorant because it produces orange-red color when added to carbonated water and sugar solution. It was categorized as an additive by the US Food and Drug Administration (Encyclopedia Britannica, 2019; Swamy et al., 2014).
There has been continued interest in the pharmacological potentials of Synsepalum dulcificum in Africa where it is endemic, and other parts of the world where it is an exotic plant. Research investigations have revealed the plant as a potential source of pharmaceutical products. Miracle fruit is popular among patients with diabetes and obesity in Japan. A mixture of miracle fruit, carambola, pumpkin and papaya could boost the immune function of mice (Du et al., 2014). In view of the uniqueness of this plant which enjoys appreciable mention in scientific literature, the present study aims to present a holistic review that collates scientific information on its ethnomedicinal uses, phytochemical constituents, biological activities and nutritional benefits.
2. Taxonomic classification
The taxonomic classification of miracle fruit plant is presented in Table 1. The online plant database, The Plant List, includes 164 scientific plant names of species rank for the genus Synsepalum. Of these, 29 are accepted species names, including Synsepalum dulcificum (Schumach. & Thonn.) Daniell (The Plant List, 2020). Synsepalum dulcificum was formerly known as Richadella dulcifica and other synonyms (Bartoshuk et al., 1974; Ohkura et al., 2018).
Table 1.
Taxonomic classification of miracle berry plant (Synsepalum dulcificum).
| Taxonomic rank | Name |
|---|---|
| Kingdom | Plantae |
| Subkingdom | Viridiplantae |
| Infrakingdom | Streptophyta |
| Division | Tracheophyta |
| Infradivision | Angiospermae |
| Class | Magnoliopsida |
| Superorder | Asteranae |
| Order | Ericales |
| Family | Sapotaceae |
| Genus | Synsepalum |
| Species | Synsepalum dulcificum |
Source: (Gardens, 2014).
3. Geographical distribution of Synsepalum dulcificum
Synsepalum dulcificum was originally found in the tropical parts of Central and Western Africa but has subsequently been introduced to other continents (Gardens, 2014). S. dulcificum is produced and cultivated in large quantities in Taiwan and Japan because of the unique taste-modifying characteristic of the fruit (Xingwei et al., 2016).
Its habitat lies within and grows ideally in a warm environment with a climate that varies between 30-40 °C. The plant has been adapted to a slightly acidic soil pH 4.5–5.8 and produces edible fruits after three to four years of planting (Yamamoto et al., 2006). The plant has now been known to be propagated by the United States Department of Agriculture, and Federal Experiment Station in Puerto Rico. It best adapts to Jamaica, the south of U.S. (best in Florida) or Hawaii (Inglett & May, 1968). The fruit grows in a single season ranging from May to September, though berries are generated year-round. The plant grows slowly and eventually reaches six to fifteen feet in height when fully mature.
4. Nutritional and health benefits of S. dulcificum
Apart from the important pharmacological actions and potentials of the miracle fruit plant, there are reports on the nutritional quality of the fruit berries. The fruit of Synsepalum dulcificum has been the focus of nutritional evaluation of the plant. The pulp of the fruit is the only part of the fruit that contains miraculin and it is just 4.44% of the weight of the fresh fruit. Miracle fruit is a good source, not only for flavor and color, but also antioxidant activity for functional food applications (He et al., 2016; Inglett & May, 1968).
The berries reportedly contain appreciable amount of important vitamins required for healthy living. These include vitamins A involved in vision, healthy bone formation and immune system and vitamin C which is important to warding off infections. Others are vitamin E which is involved in vision health, fertility and maintenance of cellular integrity, and vitamin K which is key to blood clotting and bone health. The berries could also serve as good source of both essential (lysine, leucine, isoleucine, phenylalanine, threonine etc) and non-essential (glycine, proline, serine, tyrosine) amino acids (Njoku et al., 2015).
The repertoire of vitamins, proteins, lipids, and dietary phytochemicals present in Synsepalum dulcificum is a reflection of its nutritional and health benefits. The bright red colour of the fruit is indicative of the presence of beneficial, antioxidant flavonoids, especially anthocyanins. Flavonoids including anthocyanins have been reported as anticancer and chemopreventive agents, and as potential functional ingredients in beverages (Farombi et al., 2019; Grumezescu and Holban, 2019). Miracle fruit was reported to contain potent antioxidantive phytochemicals like epicatechin, rutin, quercetin, myricetin, kaempferol, gallic, ferulic, syringic acid, delphinidin glucoside, cyanidin galactoside and malvidin galactoside, a-tocotrienol, a- and c-tocopherol and lutein.
The proximate composition of the fruit has been reported. He et al. reported that no fat was present in the flesh while ten different fatty acids were identified in the seed oil with a total unsaturated fatty acid content of 52.7%. Vitamins A, C, D and K were reported present. Specifically, the fruit was reported to contain a large amount of vitamin C making it an important source of the vitamin (He et al., 2016; Nkwocha et al., 2014a).
The proximate mineral analysis of the fruit pulp showed the presence of Ca (100 ppm), Fe (24.20 ppm), Zn (9.49 ppm), Cu (6.22 ppm), Cr (0.01 ppm) and Co (0.01 ppm) with no lead detected (Nkwocha et al., 2014a). Corroborating findings of other workers, Nkwocha et al. (2014b) reported a high amount of flavonoids (57.01%) in Miracle fruit. Essential and non-essential amino acids were also reported present including tryptophan (8.06%), histidine (0.4%), isoleucine (0.7%), leucine (0.6%), lysine (0.6%), methionine (1.05%), phenylalanine (0.7%), threonine (1.1%), and valine (0.69%). The marker compound of the fruit ‘‘miraculin’’ consisted of sugars (glucosamine, mannose, fucose, xylose, and galactose), nitrogen, carbohydrates, and nearly 191 amino acid residues (Lim, 2012). Guney and Nawar (1977) studied the lipid contents of S. dulcificum seed and reported that there lipid types in the seed namely neutral lipids (90.8 %), glycolipids (7.3 %) and phospholipids (3.16 %) fractions. According to their findings, the phospholipid fraction consisted of phosphatidyl ethanolamine, phosphatidyl choline and phosphatidyl inositol. On the other hand, the glycolipids in the seed include monogalactosyl diglyceride, digalactosyl diglyceride and cerebrosides in different proportions. Also, the neutral lipids include triglycerides, diglycerides, monoglycerides, free fatty acids and an unsaponifiable material which was revealed using thin-layer chromatographic technique.
An important nutritional and health benefit of the plant is the taste-modifying property of the fruit, due to the presence of miraculin. Miraculin is now being explored as a low-calorie sweetener.
5. Miraculin: a sweetener
The taste-modifying property of S. dulcificum fruit has been considerably studied. Series of research works have shown that miraculin is the active compound responsible for the taste-modifying property of the fruit. The compound was first separated and purified in 1968 (Kurihara and Beidler, 1968) and named miraculin in relation to the miracle fruit. The sequence of miraculin was determined by in 1989 (Theerasilp et al., 1989).
-
(a)
Structural properties of miraculin
Miraculin is a single polypeptide glycoprotein consisting of two sugars linked to two amino acid residues and with molecular weight ranging from 24,000 to 45,000 Da (Lim, 2012). The two amino acid residues are held together by intramolecular disulfide bonds and are composed of 191 amino acid residues.
The sweetening effect takes place when miraculin binds to taste cell membranes near the sweet receptor site (Bartoshuk et al., 1974). Consequently, a conformational change occurs in the receptor membrane, allowing the carbohydrate portion of miraculin to bind to the sweet receptor site, and producing the sensation of sweetness. The presence of protons (H+) is required during the conformational change. That is, the property of miraculin to induce sweetness through conformational changes in the receptor membrane is pH-dependent. The taste modifying characteristic of miracle fruit is effective on all acids and lasts around 30 min (Bartoshuk et al., 1974; Yamamoto et al., 2006; Rodrigues et al., 2016).
-
(b)
Mechanism of action of miraculin
The mechanism of action of miraculin was evaluated on monkeys by (Brouwer et al., 1983). The study was conducted using five different acids to evaluate the acceptability rate. The result shows that acceptability rate increased when pretreated with 0.3–0.5 mg miraculin. The nerve response to acid was doubled as compared to sucrose response after pretreatment with miraculin. The duration of miraculin effect was established to be an hour and it vanishes gradually within 20 min. The result established that miraculin amplified the sweet-sensing cells sensitivity to acid. Another study investigated the brain mechanism of the sweet taste elicited from the consumption of miracle fruit that is, how the fruit converts the sour taste of acids to sweet and appetizing taste. Recording of human cerebral cortex using magnetic fields to monitor the sweet taste elicited by miracle fruit was performed. In the report, the preliminary taste responses were limited to the fronto-parietal cortex described as the brain site that received neuronal signal for taste. The response latency of sucrose and citric acid, after mastication of miracle fruit, was shown to be equivalent whereas there was a large difference (250–300 ms) between sucrose and citric acid in the absence of the fruit. The sour taste vanishes at the subcortical level while the sweet taste neuronal signal gets to the cortical level. In another study, the molecular mechanism of miraculin was unravelled by examining the main taste receptor affected by the compound. Miraculin binds to the epithelial plasma membrane of the receptor taste receptor type 1 member 3 (T1R2-T1R3) and acts as antagonist in any sour solution. The experiment shows that miraculin only exhibits the effect on its receptor in an acidic pH and shift between the two receptors (Swamy et al., 2014; Yamamoto et al., 2006).
6. Phytochemistry
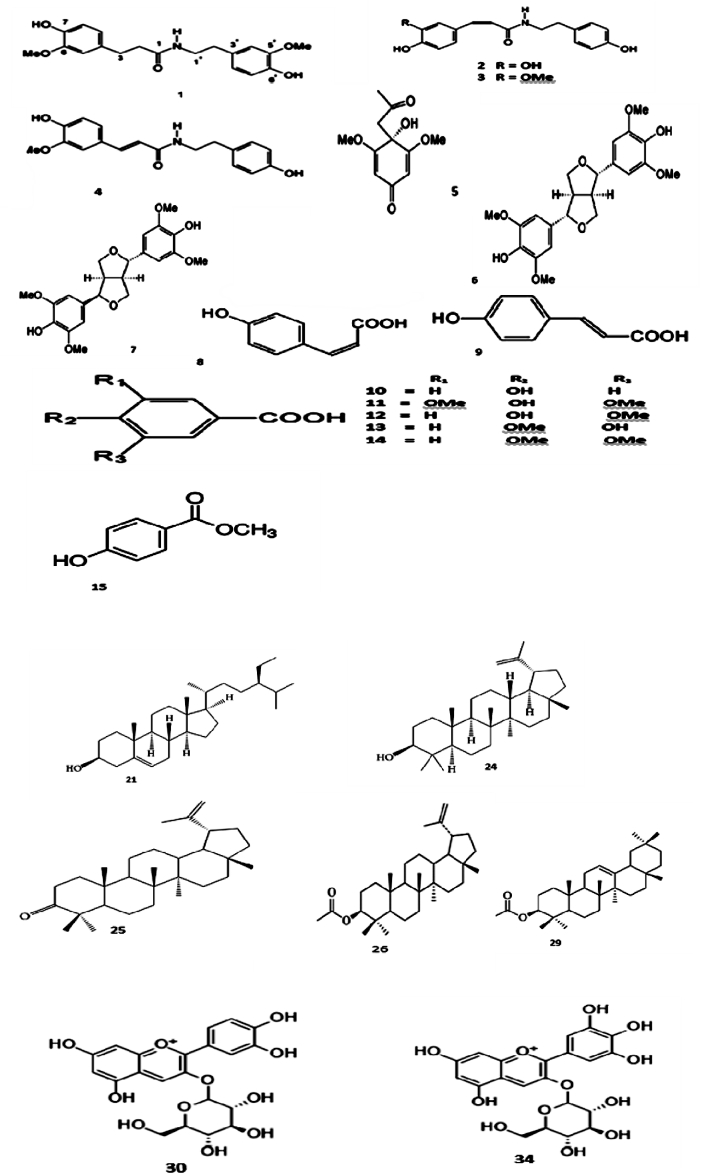
Phytochemicals such as alkaloids, glycosides, flavonoids, volatile oils, tannins, and resins have been employed for a wide range of industrial and commercial applications such as medicine, cosmetics, flavors, preservatives and natural pigment. Several reports on the phytochemicals of S. dulcificum have documented different compounds (Vikram et al., 2014). Thirteen bioconstituents were identified in the methanol extract of S. dulcificum stem using spectroscopic techniques (Wang et al., 2011). The compounds include dihydro-feruloyl-5-methoxytyramine; (+)-syringaresinol, (+)-epi-syringaresinol, 4-acetonyl-3,5- dimethoxy-p-quinol, cis-p-coumaric acid, trans-pcoumaric acid, p-hydroxybenzoic acid, syringic acid, vanillic acid, veratric acid, N-cis-feruloyltyramine, N-trans-feruloyltyramine, and N-cis-caffeoyltyramine (Figure 1). Similarly, (Cheng et al., 2015) reported six pure compounds namely, propane-1,2,3-triol; 2,5-dimethoxyphenol; 3,4,5-trimethoxybenzoic acid; nicotinic acid; β-sitosterol, and stigmasterol from the methanol extract of the plant stem. The isolated compounds, (+)-epi-syringaresinol and (+)-syringaresinol were reported to possess anti-aging and anti-oxidative activities and the potential to inhibit human skin cancer cells in vitro. Correspondingly, (+)-epi-syringaresinol, 4-acetonyl-3,5-dimethoxy-p-quinol, trans-p-coumaric acid, p-hydroxybenzoic acid, cis-p-coumaric acid, vanillic acid, and N-cis-feruloyltyramine reduce mushroom tyrosinase activity, hence have an unlimited potential to inhibit melanin synthesis and conversely used in hyper-pigmentation treatment. Likewise, β-sitosterol and stigmasterol exhibit anti-inflammatory, antineoplastic, antipyretic and immuno-modulating activity in animal (Sharma et al., 2014). Thus, constituents from S. dulcificum show potential applications in medical cosmetology and food supplementation.
In another report, a chromatographic separation of the methanol extract of S. dulcificum leaves revealed the presence of eight compounds including a mixture of β-sitosterol and stigmasterol, pheophytin-a, pheophytin-b, lupeol, lupenone, lupeol acetate, and a-tocopheryl quinone (Chen et al., 2010a). The triterpenes, lupenone and lupeol, have been reported to exhibit multiple bioactivities (Singh et al., 2014; Beserra et al., 2019). Also, a lupeol-based cream enhanced the healing process in hyperglycemic rats through anti-inflammatory mechanisms (Beserra et al., 2019). Using high performance liquid chromatography with a diode array detector (HPLC-DAD), Obafemi and colleagues also identified the bioactive phytochemicals, rutin, quercetin, isoquercitrin, quercitrin, kaempferol, ellagic acid, caffeic acid, chlorogenic acid, catechin, gallic acid, epicatechin, tocopherol, b-carotene and lycopene in a methanol leaf extract of S. dulcificum (Obafemi et al., 2017). The root of S. dulcificum was also investigated by (Chen et al., 2010b) who revealed the presence of nine chemical constituents in the methanolic extract. These include N-trans-feruloyltyramine, N-cis-feruloyltyramine, N-trans-feruloylmethoxytyramine, N-cis-feruloylmethoxytyramine, p-hydroxybenzoicacid, methylparaben, vanillic acid, isovanillic acid and syringic acid which were discovered for the first time in the plant.
Phytochemicals like phenolic compounds and flavonoids are widely distributed in plants and are known for their multiple biological effects including free radical scavenging and anti-inflammatory to mention but a few (Buckmire and Francis, 1976). There are lines of evidence that miracle fruit plant parts contain an appreciable number of polyphenols (Buckmire and Francis, 1976; Akinmoladun, 2016; Obafemi et al., 2019). Ficumone, (R∗)-4-hydroxy-2-oxetanone, has been isolated from the fruit (Cheng et al., 2010). Most importantly, the fruit contains miraculin, the taste-modifying glycoprotein which has found use in pharmaceutical industries (Giroux and Henkin, 1974). The glycoprotein is has a molecular weight ranging from 24,000 to 45,000 Da, consisting of sugars (glucosamine, mannose, fucose, xylose, and galactose), nitrogen, carbohydrates, and 191 amino acids (Lim, 2012). Table 2 and Figure 2 show some of the compounds present in this plant. A number of flavonoids have been characterized from Synsepalum dulcificum (Buckmire and Francis, 1976; Achigan-Dako et al., 2015). They include quercetin-3-monogalactoside, kaempferol-3-monoglucoside, myricetin-3- monogatactoside while the red pigment are cyanidin-3-monogalactoside, cyanidin-3- monoglucoside, cyanidin-3-monoarabinoside, delphinidin-3-monogalactoside and delphmidin-3-monoarabinoside.
Table 2.
Chemical compounds isolated and characterized from Srnsepalum dulcificum.
| Chemical Class | Compound Name | Part | Reference |
|---|---|---|---|
| Alkaloid | 1. dihydro-feruloyl-5-methoxytyramine 2. N-cis-Caffeoyltyramine 3. N-cis-Feruloyl-tyramine 4. N-trans-Feruloyl-tyramine 5. 4-acetonyl-3,5-dimethoxy-p-quinol |
Stem and root | Chen et al. (2010b); Wang et al. (2011) |
| Lignan | 6. (+)-syringaresinol 7. (+)-epi-syringaresinol |
Stem | Wang et al. (2011) |
| Phenolic acid | 8. cis-p-coumaric acid 9. trans-p-coumaric acid 10. p-hydroxybenzoic acid 11. syringic acid 12. vanillic acid 13. isovanillic 14. veratric acid 15. methylparaben |
Stem and root | Chen et al. (2010b); Wang et al. (2011) |
| Glycoprotein Oil substance |
16. Miraculin 17. 4-hydroxy-2- oxetanone 18. Anthocyanins |
Fruit Fruit |
Cheng et al. (2010) |
| Phytosterols Triterpenes |
19. β-sitosterol and stigmasterol 20. pheophytin-a 21. pheophytin-b 22. lupeol 23. lupenone 24. lupeol acetate 25. α -tocopheryl quinone 26. α –amyrin 27. β-amyrin acetate |
Leaf | Chen et al. (2010a); Ragasa et al. (2015) |
| Colour pigment Flavonoid |
28. cyanidin-3-O-glucoside 29. cyanidin-3-monogalactoside 30. delphinidin-3- monoarabinoside 31. cyanidin-3-monoarabinoside 32. delphinidin-3-O- glucoside 33. quercetin-3-monogalactoside 34. kaempferol-3- monoglucoside 35. myricetin-3-monogatactoside |
Leaf and fruit | Mangla and Kohli (2018) |
Source: (Achigan-Dako et al., 2015; Buckmire and Francis, 1976).
Figure 2.
Chemical structures of the major compounds isolated from Synsepalum dulcificum (Numbers correspond to names in Table 1) Source: (Achigan-Dako et al., 2015; Buckmire and Francis, 1976)
7. Ethnomedicinal uses of Synsepalum dulcificum
All parts of S. dulcificum are reputed to cure or manage various human diseases and ailments and different herbal preparations from the plant are used in traditional medicine.
In Benin, the root is used to treat sexual weakness, cough and tuberculosis. Likewise, the leaves are involved in the treatment of diabetes, malaria, hyperthermia and enuresis while the bark is employed in the treatment of prostate ailments. The branches are used as vegetable toothbrush for good dental and oral health (Oumorou et al., 2010). In Nigeria, the local people of Akwa Ibom State call it mkpantun where the root that is macerated in local gin or soda water is used for the cure of gonorrhoea (Ekpo et al., 2008). In Lagos (Nigeria), S. dulcificum leaves are used for the management of asthma, male infertility, diabetes, weight loss and cancer (Makinde et al., 2015). In South-Eastern and South-Western part of Nigeria, decoction or juice of leaves is used for the treatment of diabetes mellitus (Oyedemi et al., 2017). In other region of West Africa like Ghana, the fruit has been used to sweeten sour foods and beverages such as Koko and Kenkey made from fermented maize and millet, and palm wine.
In Congo, where it is known as bomonga, the bark is used as cure for erectile dysfunction (Fandohan et al., 2017). Literature documentation (Sulaini and Sabran, 2018; Wilfred et al., 2006) also revealed that crushed leaves of S. dulcificum are taken orally in Malaysia and Tanzania as ethnomedicinal remedy for postnatal care and also as appetizer and sweetener. In Japan, miracle fruit is popularly used by diabetic and obese patients (Du et al., 2014). The use of S. dulcificum as a multipurpose traditional medicine has been translated into several commercial applications and it is a highly valued plant in the pharmaceutical, natural health and food industries (Akinmoladun, 2016).
In traditional medicine, the leaves are the most useful plant parts (90%), followed by the root (7%), bark (1.5%), stem (1%) and fruit (0.5%). Information on ethnomedicinal uses of S. dulcificum documented in this review was obtained from literature spanning seven countries. It is worthy of note that the region with the highest number of ethnomedicinal uses of S. dulcificum is West Africa. Literature records also show high degree of consensus for at least two major categories of diseases, notably diabetes and sexually-related diseases, for which this plant is used.
8. Pharmacological activities
A number of pharmacological activities of S. dulcificum have been reported in literature, which justifies some of its ethnomedicinal uses. These pharmacological activities include antidiabetic, anticancer, anti- hyperuricaemia, and anticonvulsant.
-
a)
Antidiabetic activity
The effect of miracle fruit (Synsepalum dulcificum) on insulin resistance induced by fructose-rich chow in male Wistar rats was evaluated by Chen et al. (Chen et al., 2006). Treatment of insulin-resistant animals with 0.02, 0.04 and 0.2 mg/kg extract of miracle fruit ameliorated the effect of fructose-rich chow on glucose-insulin index and the time interval for response to tolbutamide (10.0 mg/kg, i.p.). There was also a significant increase in insulin sensitivity as a result of treatment with miracle fruit powder.
The hypoglycemic effects of S. dulcificum fruit (50% and 100%) and leaf (50% and 100%) extracts on the blood glucose level of alloxan-induced diabetic albino rats was reported by Dioso et al. (2016). Both the fruits and the leaves of S. dulcificum showed significant hypoglycaemic effect. It was suggested that the observed effect could be due to miraculin and other phytochemicals such as flavonoids and saponins present in the plant.
In the same vein, Obafemi and colleagues (Obafemi et al., 2017, 2019) evaluated the antidiabetic potential of both methanolic and flavonoid-rich leaf extracts of S. dulcificum on fructose-fed, streptozotocin-injected rats. Biochemical parameters, such as liver and kidney function tests, lipid profile, markers of lipid peroxidation, antioxidant enzymes as well as pathological changes in the type 2 diabetes model were assessed. Oral administration of the plant extract to rats for 21 days significantly improved the biochemical and pathological alterations in type 2 diabetes. Altered glycated hemoglobin and serum levels of interleukin-6 and tumor necrosis factor alpha in diabetic animals were corrected by treatment with the extract. Also, S. dulcificum fruit and leaf extracts showed significant α-amylase and α-glucosidase inhibitory activities. In addition, molecular docking analyses suggested that some polyphenolic component of the plant possess strong binding affinities with glucokinase.
Fazilah et al. also evaluated the anti-diabetic properties of S. dulcificum and its potential inclusion in functional yogurt (Fazilah et al., 2020). Aqueous extract of S. dulcificum pulp, seed and leaves in powder form were added to freshly prepared yogurts. In vitro antidiabetic analyses carried out showed potential inhibition of α-amylase and α-glucosidase enzymes following the addition of the extracts. Furthermore, pulp extract demonstrated superior α-amylase (IC50 9.66) and α-glucosidase (IC50 0.08) inhibitory activities compared to the seed, leaf and the standard drug, acarbose. These results indicate the potential of the plant in the treatment of diabetic patients and validate the folkloric use of S. dulcificum in the management of diabetes in Nigeria (Makinde et al., 2015).
-
b)
Anticancer activity
Bioconstituents from the stem of S. dulcificum were evaluated on mushroom tyrosinase and melanoma cells (A375.S2). Tyrosinase is an important enzyme that directly controls the production of melanin in the body system (Hirobe, 2005). Previous evaluation indicated that two of the compounds {(+)-syringaresinol and (+)-epi-syringaresinol} found in S. dulcificum have inhibitory effects on human skin cancer cells and significant antioxidant activity in vitro. Compounds like (+)-epi-syringaresinol, 4-acetonyl-3,5-dimethoxy-p-quinol, cis-p-Coumaric acid, trans-p-coumaric acid, p-hydroxybenzoic acid, vanillic acid and N-cis-feruloyl-tyramine inhibited mushroom tyrosinase activity. Tyrosinase inhibitors are employed medically to treat hyper-pigmentation and in cosmetic industry (Wang et al., 2011).
In another study, the effect of aqueous leaf extracts of miracle fruit on 2-aminoanthracene (2-AA) and 4-nitroquinoline-N-oxide (4-NQO)-induced mutation and oxidative damage was evaluated (Chen et al., 2015). The leaf extracts caused concentration-dependent inhibition of the mutagenicity of 2-AA (an indirect mutagen) and 4-NQO (a direct mutagen) toward Salmonella typhimurium TA 98 and TA 100. The antimutagenic activity was attributed to the phenolic constituents of S. dulcificum leaf compounds. Active phenolics present in the sample include p-hydroxybenzoic acid, vanillic acid, syringic acid, trans-p-coumaric acid, and veratric acid.
Cytotoxic activity of S. dulcificum berry and stem extracts on colorectal cancer cells (HCT-116, HT-29) and its effect on the expression of early apoptotic genes, c-fos and c-jun have also been investigated (Seong et al., 2018). The genes, c-fos and c-jun, encode for proteins called activator protein-1 (AP-1) that belong to the class of transcription factors associated with cell functions such as cell proliferation, differentiation and apoptosis (Shi et al., 2016). Both extracts significantly up-regulated the expression of c-fos and c-jun leading to a cytotoxic effect on colorectal cancer cells.
-
c)
Anti-hyperuricaemic activity
Hyperuricemia can lead to lead to several diseases (Benn et al., 2018). The anti-hyperuricaemia activity of S. dulcificum fruit extract in xanthine oxidase and monosodiumurate (MSU)-treated RAW264.7 macrophages has been reported (Shi et al., 2016). Xanthine oxidase mediates the production of uric acid. In mammals, uric acid resulting from the action of xanthine oxidase is catabolised to allantoin hennce inhibition of xanthine oxidase will directly inhibit uric acid formation. In the study conducted on potassium salt-induced hyperuricaemic mice, butanol extract of S. dulcificum fruits mitigated the elevated serum uric acid in induced animals. Butanol extract also inhibited xanthine oxidase activity in monosodium urate (MSU)-treated RAW264.7 macrophages (Shi et al., 2016).
-
d)
Anticonvulsant activity
Jeremiah et al. (2015) evaluated the anticonvulsant potential of S. ducificum seed aqueous fraction in mice subjected to pentylenetetrazole (PTZ)-, strychnine- and Maximal Electroshock (MES)-induced seizures. PTZ is a noncompetitive antagonist of gamma-aminobutyric acid type A (GABAA) receptor which binds to picrotoxin (PTX) site of the receptor. There was 33.33% defence against transience in PTZ- and strychnine-induced convulsion with significant reduction in the time of recovery from MES-induced seizure in animals pre-administered S. ducificum seed. According to the findings, specific phytochemicals in S. dulcificum seed extract possess anticonvulsant activity and the inhibitory neurotransmission was due to mild affinity for PTX binding site of the GABAA receptor.
9. Other health-promoting and cosmetic properties
Synsepalum dulcificum possesses health-promoting properties including boosting antioxidant capacity, lowering blood cholesterol and cosmetic functions like the improvement of hair breakage in women with damaged hair.
-
a)
Antioxidant activity
Antioxidants prevent deleterious that cause oxidative stress. Antioxidant phytochemicals like flavonoids are abundant in Miracle berry pulps (Du et al., 2014). In vitro antioxidant and radical scavenging activities of the methanol extracts of the flesh and seed of S. dulcificum were evaluated and confirmed by a few studies (Cheng et al., 2015; Hirobe, 2005; Shi et al., 2016). Methods employed included ABTS (2,2′-azino-bis(3-ethyl49 benzothiazoline-6-sulfonic acid) and DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activities as well as ferric-reducing antioxidant power, FRAP assays among others. Also, the stabilization of lipid oxidation by the extract was analysed using fish oil emulsion model. It was discovered that the sample reduced oxidation of polyunsaturated fatty acid in fish oil. Therefore, S.dulcificum plant could serve as an antioxidant-rich fruit capable of promoting health. The established antioxidant activities of S.dulcificum plant part extracts may be due to flavonoids and phenols that have been isolated from fruits, leaves, and roots (Cheng et al., 2015; Hirobe, 2005; Shi et al., 2016).
An evaluation of the effects of S. dulcificum methanolic fruit extract on oxidative stress and hepatotoxicity indices in rats following subacute administration of the extract (50–200 mg/kg) was carried out (Akinmoladun, 2016). The result showed a decrease in serum total bilirubin and hepatic malondialdehyde levels in treated groups level while glutathione-S-transferase activity was significantly (P < 0.05) increased in the liver. The findings suggested that extract at lower dose (<100 mg/kg) could boost the antioxidant defense and exert hepatoprotective properties.
-
b)
Cholesterol-lowering effect
Cholesterol-lowering activity of S. dulcificum was evaluated by (Huang et al., 2020) using a hamster's model under an experimental diet consisting of 2% of ethanolic and water extract of S. dulcificum seed, leaves and dry pulp. Experimental diets containing the seed extract of S. dulcificum reduced the plasma total cholesterol while lupeol acetate and β-amyrin acetate (triterpenoids isolated from the seed extract) decrease plasma total cholesterol by 15%–20% in hamsters. It was concluded that the ethanol extract of the seed decreased plasma total cholesterol which could be associated with active triterpenoids in the extract.
-
c)
Improvement of hair breakage in women with damaged hair
The efficacy of S. dulcificum seed oil and its effect on hair loss was also evaluated in healthy women with long hair but susceptible to excessive hair breakage. The hair length and hair mass index among other parameters as well as questionnaires were employed in the study. The findings revealed significant growth in the hair length, hair mass and hair index of women within 4 8 months of the seed oil usage. It was thus concluded that the hair oil product formulated from S. dulcificum seed might be a harmless and active choice for the treatment of women suffering from hair breakage and damaged hair (Del Campo et al., 2017).
10. Conclusion and future perspective
S. dulcificum is an extraordinary and outstanding shrub due to its outstanding pharmacological and nutritional values. Different parts of the plant are useful for industrial and therapeutic purposes. The plant is listed as one of commercially important African medicinal plants with high potential as a food and nutritional supplement (Lykke and Padonou, 2019; Small and Catling, 2006; Van Wyk, 2015). The miracle fruit plant has potential anticancer, antioxidant, anticovulsant, antihyperuricemia and cholesterol-lowering properties among others. In particular, miraculin has great nutritional and therapeutic benefits as a low-calorie sweetner and an andiabetic agent. Other significant components which are not yet detected may be present in the plant. This calls for continuing investigations. Known components should be standardized and proper safety evaluations performed to enhance acceptability of products from the plant in more countries. Drug development from the plant for the management of diabetes and diabetic complications are potentially viable areas of focus. In addition, synthesis of analogues of miraculin with comparable or better activity should be explored which can aid in the conservation of this species. The miracle plant offers a vast library of bioactive compounds and more pharmacological activities of the plant are likely to emerge in the coming years. For example, the plant is used ethnomedically to treat sexually-related diseases but pharmacological study along this line is absent or scanty. There is every reason to believe that the miracle fruit plant still has a yet untapped benefits for man.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Achigan-Dako E.G., Tchokponhoué D.A., N’Danikou S., Gebauer J., Vodouhè R.S. Current knowledge and breeding perspectives for the miracle plant Synsepalum dulcificum (Schum. et Thonn.) Daniell. Genet. Resour. Crop Evol. 2015;62(3):465–476. [Google Scholar]
- Akinmoladun A. Effect of Synsepalum dulcificum berry extract on oxidative stress and hepatotoxicity indices, following subacute administration in normal rats. FUTA J. Res. Sci. 2016;12:167–177. [Google Scholar]
- Bartoshuk L.M., Gentile R.L., Moskowitz H.R., Meiselman H.L. Sweet taste induced by miracle fruit (Synsepalum dulcificum) Physiol. Behav. 1974;12(3):449–456. doi: 10.1016/0031-9384(74)90122-x. [DOI] [PubMed] [Google Scholar]
- Benn C.L., Dua P., Gurrell R., Loudon P., Pike A., Storer R.I., Vangjeli C. Physiology of hyperuricemia and urate-lowering treatments. Front. Med. 2018;5 doi: 10.3389/fmed.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beserra F.P., Vieira A.J., Gushiken L.F.S., de Souza E.O., Hussni M.F., Hussni C.A.…de Azevedo Maia G.L. Lupeol, a dietary triterpene, enhances wound healing in streptozotocin-induced hyperglycemic rats with modulatory effects on inflammation, oxidative stress, and angiogenesis. Oxidat. Med. Cellular Longevity. 2019 doi: 10.1155/2019/3182627. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer J., Glaser D., af Segerstad C.H., Hellekant G., Ninomiya Y., Van der Wel H. The sweetness-inducing effect of miraculin; behavioural and neurophysiological experiments in the rhesus monkey Macaca mulatta. J. Physiol. 1983;337(1):221–240. doi: 10.1113/jphysiol.1983.sp014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire R., Francis F. Anthocyanins and flavonols of miracle fruit, Synsepalum dulcificum, Schum. J. Food Sci. 1976;41(6):1363–1365. [Google Scholar]
- Chen C., Wang Y., Wang H. Chemical constituents from the leaves of Synsepalum dulcificum. Chem. Nat. Compd. 2010;46(3):495. [Google Scholar]
- Chen C., Wang Y., Wang H. Chemical constituents from the roots of Synsepalum dulcificum. Chem. Nat. Compd. 2010;46(3):448–449. [Google Scholar]
- Chen C.C., Liu I.M., Cheng J.T. Improvement of insulin resistance by miracle fruit (Synsepalum dulcificum) in fructose-rich chow-fed rats. Phytother Res. 2006;20(11):987–992. doi: 10.1002/ptr.1919. [DOI] [PubMed] [Google Scholar]
- Chen T.-Y., Kang Z.-C., Yen M.-T., Huang M.-H., Wang B.-S. Inhibitory effect of aqueous extracts from Miracle Fruit leaves on mutation and oxidative damage. Food Chem. 2015;169:411–416. doi: 10.1016/j.foodchem.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Cheng F.-Y., Huang S.-T., Lin M.-L., Lai J.-T. Polyphenol measurement and antioxidant activity of miracle fruit. Int. J. Chem. Eng. Appl. 2015;6(3):211. [Google Scholar]
- Cheng M.-J., Lo W.-L., Huang L.-Y., Wang C.-J., Chen C.-Y. Isolation of a 2-oxetanone from the fruits of Synsepalum dulcificum. Nat. Prod. Res. 2010;24(19):1850–1853. doi: 10.1080/14786419.2010.482934. [DOI] [PubMed] [Google Scholar]
- Del Campo R., Zhang Y., Wakeford C. Effect of miracle fruit (Synsepalum dulcificum) seed oil (MFSO®) on the measurable improvement of hair breakage in women with damaged hair: a randomized, double-blind, placebo-controlled, eight-month trial. J. Clin. Aesthet. Dermatol. 2017;10(11):39. [PMC free article] [PubMed] [Google Scholar]
- Dioso M.K.M., Satsatin D.C.A., Ching J.A. Hypoglycemic effects of Synsepalum dulcificum (Schumach. & Thonn.) Daniell (Miracle Berry) fruit and leaf extracts on the blood glucose level of albino rats. Der Pharm. Lett. 2016;8(14):104–108. [Google Scholar]
- Du L., Shen Y., Zhang X., Prinyawiwatkul W., Xu Z. Antioxidant-rich phytochemicals in miracle berry. Food Chem. 2014;153:279–284. doi: 10.1016/j.foodchem.2013.12.072. [DOI] [PubMed] [Google Scholar]
- Ekpo B.A., Bala D.N., Essien E.E., Adesanya S.A. Ethnobotanical survey of Akwa Ibom state of Nigeria. J. Ethnopharmacol. 2008;115(3):387–408. doi: 10.1016/j.jep.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Encyclopedia Britannica . Encyclopedia Britannica; 2019. Miracle Fruit | Description, Effects, & Uses.https://www.britannica.com/plant/miracle-fruit [Google Scholar]
- Fandohan A.B., Gouwakinnou G.N., Tovissode C.F., Bonou A., Djonlonkou S.F.B., Houndelo L.F.…Assogbadjo A.E. Usages traditionnels et valeur économique de Synsepalum dulcificum au Sud-Bénin. Bois Forets Tropiques. 2017;332(332):17–30. [Google Scholar]
- Farombi E.O., Akinmoladun A.C., Owumi S.E. Anti-cancer foods: flavonoids. In: Melton L., Shahidi F., Varelis P., editors. Encyclopedia of Food Chemistry. Academic Press; 2019. pp. 224–236. [Google Scholar]
- Fazilah N., Ariff A., Khayat M., Halim M. Paper Presented at the IOP Conference Series: Materials Science and Engineering. 2020. Anti-diabetic properties of Synsepalum dulcificum and its potential inclusion in functional yogurt. [Google Scholar]
- Gardens R.B. Retrieved 2009-01-03. 2014. Kew world checklist of selected plant families. [Google Scholar]
- Giroux E.L., Henkin R.I. Purification and some properties of miraculin, a glycoprotein from Synsepalum dulcificum which provokes sweetness and blocks sourness. J. Agric. Food Chem. 1974;22(4):595–601. doi: 10.1021/jf60194a033. [DOI] [PubMed] [Google Scholar]
- Grumezescu A., Holban A.M. Elsevier; 2019. Value-added Ingredients and Enrichments of Beverages. [Google Scholar]
- Guney S., Nawar W. Seed lipids of the miracle fruit (Synsepalum dulcificum) J. Food Biochem. 1977;1(2):173–184. [Google Scholar]
- He Z., Tan J.S., Abbasiliasi S., Lai O.M., Tam Y.J., Ariff A.B. Phytochemicals, nutritionals and antioxidant properties of miracle fruit Synsepalum dulcificum. Ind. Crop. Prod. 2016;86:87–94. [Google Scholar]
- He Z., Tan J.S., Lai O.M., Ariff A.B. Optimization of conditions for the single step IMAC purification of miraculin from Synsepalum dulcificum. Food Chem. 2015;181:19–24. doi: 10.1016/j.foodchem.2014.11.166. [DOI] [PubMed] [Google Scholar]
- Hirobe T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigm. Cell Res. 2005;18(1):2–12. doi: 10.1111/j.1600-0749.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- Huang W., Chung H.Y., Xuan W., Wang G., Li Y. The cholesterol-lowering activity of miracle fruit (Synsepalum dulcificum) J. Food Biochem. 2020 doi: 10.1111/jfbc.13185. [DOI] [PubMed] [Google Scholar]
- Inglett G., May J.F. Tropical plants with unusual taste properties. Econ. Bot. 1968;22(4):326–331. [Google Scholar]
- Jeremiah O.J., Ilesanmi O.R., Ige M.M. Evaluation of the anticonvulsant potential of aqueous fraction of Synsepalum dulcificum seed extract in mice. Eur. J. Med. Plants. 2015:1–8. [Google Scholar]
- Kurihara K., Beidler L.M. Taste-modifying protein from miracle fruit. Science. 1968;161(3847):1241–1243. doi: 10.1126/science.161.3847.1241. [DOI] [PubMed] [Google Scholar]
- Lim T.K. Vol. 1. Springer; 2012. (Edible Medicinal and Non-medicinal Plants). [Google Scholar]
- Lykee M., Padonou E.A. Carbohydrates, proteins, fats and other essential components of food from native tress in West Africa. Heliyon. 2019;5(5):e01744. doi: 10.1016/j.heliyon.2019.e01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinde S., Ojekale A., Oshinaike T., Awusinu T. An ethnomedical and ethnobotanical survey of plants herbal therapy used for obesity, asthma, diabetes and fertility by the Badagry people of Lagos state, Nigeria. J. Med. Plants Stud. 2015;3(5):1–6. [Google Scholar]
- Mangla B., Kohli K. Pharmaceutical and therapeutic potential of miraculin and miracle berry. Trop. J. Nat. Prod. Res. 2018;2(1):12–17. [Google Scholar]
- Njoku N.E., Ubbaonu C.N., Alagbaoso S.O., Eluchie C.N., Umelo M.C. Amino acid profile and oxidizable vitamin content of Synsepalum dulcificum berry (miracle fruit) pulp. Food Sci. Nutr. 2015;3(3):252–256. doi: 10.1002/fsn3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkwocha C., Njoku O., Ekwueme F. Proximate and micronutrient analyses of Synsepalum dulcificum pulp. Sci. Res. J. 2014;2(1):2201–2796. [Google Scholar]
- Nkwocha C., Njoku O., Ekwueme F. Phytochemical, antinutrient and amino acid composition of Synsepalum dulcificum pulp. Int. J. Pharm. Biol. Sci. 2014;9:25–29. [Google Scholar]
- Obafemi T., Akinmoladun A., Olaleye M., Agboade S.O., Onasanya A.A. Antidiabetic potential of methanolic and flavonoid-rich leaf extracts of Synsepalum dulcificum in type 2 diabetic rats. J. Ayurveda Integr. Med. 2017;8(4):238–246. doi: 10.1016/j.jaim.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obafemi T.O., Olaleye M.T., Akinmoladun A.C. Antidiabetic property of miracle fruit plant (Synsepalum dulcificum Shumach. & Thonn. Daniell) leaf extracts in fructose-fed streptozotocin-injected rats via anti-inflammatory activity and inhibition of carbohydrate metabolizing enzymes. J. Ethnopharmacol. 2019;244:112124. doi: 10.1016/j.jep.2019.112124. [DOI] [PubMed] [Google Scholar]
- Ohkura S., Hori M., Saitoh K., Okuzawa T., Okamoto I., Furukawa N., Shimizu-Ibuka A. Structural and functional analysis of miraculin-like protein from Vitis vinifera. Biochim. Biophys. Acta Protein Proteonomics. 2018;1866(11):1125–1130. doi: 10.1016/j.bbapap.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Oumorou M., Dah Dovonon J., Aboh B., Hounsou Kaka M., Sinsin B. INRA, LEA, EPAC; 2010. Contribution à la conservation de Synsepalum dulcificum: régénération et importance socioéconomique dans le département de l’Ouémé (Bénin) [Google Scholar]
- Oyedemi S.O., Oyedemi B.O., Ijeh I.I., Ohanyerem P.E., Coopoosamy R.M., Aiyegoro O.A. Alpha-amylase inhibition and antioxidative capacity of some antidiabetic plants used by the traditional healers in Southeastern Nigeria. Sci. World J. 2017 doi: 10.1155/2017/3592491. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragasa C.Y., Cornelio K., Bauca T., Chua S., Shen C.-C. Chemical constituents of the leaves, stems, and fruits of Synsepalum dulcificum. Chem. Nat. Compd. 2015;51(3):588–589. [Google Scholar]
- Rodrigues J.F., da Silva Andrade R., Bastos S.C., Coelho S.B., Pinheiro A.C.M. Miracle fruit: an alternative sugar substitute in sour beverages. Appetite. 2016;107:645–653. doi: 10.1016/j.appet.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Seong J., Oyong G.G., Cabrera E.C. Synsepalum dulcificum extracts exhibit cytotoxic activity on human colorectal cancer cells and upregulate c-fos and c-jun early apoptotic gene expression. Asian Pac. J. Trop. Biomed. 2018;8(3):173. [Google Scholar]
- Sharma G., Prakash D., Gupta C. Phytochemicals of nutraceutical importance: do they defend against diseases. In: Prakash D., Sharma G., editors. Phytochemicals of Nutraceutical Importance. CABI Books; 2014. [Google Scholar]
- Shi Y.-C., Lin K.-S., Jhai Y.-F., Lee B.-H., Han Y., Cui Z.…Wu S.-C. Miracle fruit (Synsepalum dulcificum) exhibits as a novel anti-hyperuricaemia agent. Molecules. 2016;21(2):140. doi: 10.3390/molecules21020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Sharma S., Singh P. Phytochemicals of Nutraceutical Importance; 2014. 16 Antioxidants: Their Health Benefits and Plant Sources; p. 248. [Google Scholar]
- Small E., Catling P.M. Blossoming treasures of biodiversity. Biodiversity. 2006;7(2):21–26. [Google Scholar]
- Sulaini A.A., Sabran S.F. Paper Presented at the AIP Conference Proceedings. 2018. Edible and medicinal plants sold at selected local markets in Batu Pahat, Johor, Malaysia. [Google Scholar]
- Swamy K.B., Hadi S.A., Sekaran M., Pichika M.R. The clinical effects of Synsepalum dulcificum: a review. J. Med. Food. 2014;17(11):1165–1169. doi: 10.1089/jmf.2013.3084. [DOI] [PubMed] [Google Scholar]
- Theerasilp S., Hitotsuya H., Nakajo S., Nakaya K., Nakamura Y., Kurihara Y. Complete amino acid sequence and structure characterization of the taste-modifying protein, miraculin. J. Biol. Chem. 1989;264(12):6655–6659. [PubMed] [Google Scholar]
- The Plant List . 2020. Synsepalum.http://www.theplantlist.org/1.1/browse/A/Sapotaceae/Synsepalum [Google Scholar]
- Van Wyk B.-E. A review of commercially important African medicinal plants. J. Ethnopharmacol. 2015;176:118–134. doi: 10.1016/j.jep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Vikram P., Chiruvella K.K., Ripain I.H.A., Arifullah M. A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.) Asian Pac. J. Trop. Biomed. 2014;4(6):430–435. doi: 10.12980/APJTB.4.2014C1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-M., Chou Y.-T., Hong Z.-L., Chen H.-A., Chang Y.-C., Yang W.-L.…Chen C.-Y. Bioconstituents from stems of Synsepalum dulcificum Daniell (Sapotaceae) inhibit human melanoma proliferation, reduce mushroom tyrosinase activity and have antioxidant properties. J. Taiwan Inst. Chem. Eng. 2011;42(2):204–211. [Google Scholar]
- Wilfred P., Madoffe S.S., Luoga E.J. Indigenous plant uses and use values in Uluguru mountains, Morogoro, Tanzania. J. East Afr. Nat. Hist. 2006;95(2):235–240. [Google Scholar]
- Xingwei C., Abdullah T.L., Taheri S., Abdullah N.A.P., Hassan S.A. Flower ontogenesis and fruit development of Synsepalum dulcificum. Hortscience. 2016;51(6):697–702. [Google Scholar]
- Yamamoto C., Nagai H., Takahashi K., Nakagawa S., Yamaguchi M., Tonoike M., Yamamoto T. Cortical representation of taste-modifying action of miracle fruit in humans. Neuroimage. 2006;33(4):1145–1151. doi: 10.1016/j.neuroimage.2006.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.