Abstract
Introduction
Acceleration of radiotherapy in 5 fractions for breast cancer can reduce the burden of treatment. We report on acute toxicity after whole-breast irradiation with a simultaneous integrated boost in 5 fractions over 10–12 days.
Material and methods
Acute toxicity and health-related quality of life (HRQoL) of 200 patients, randomized between a 15- or 5-fractions schedule, were collected, using the CTCAE toxicity scoring system, the Multidimensional Fatigue Inventory, EORTC QLQ-C30 and BR23 and the BREAST-Q questionnaire. The prescribed dose to the breast was either 15∗2.67 Gy (40.05 Gy) or 5∗5.7 Gy (28.5 Gy). 90% of patients received a SIB to a cumulative dose of 46.8 Gy (15∗3.12 Gy) or 31 Gy (5∗6.2 Gy).
Results
Physician-assessed toxicity was lower for the 5-fractions group. A significant difference was observed for breast pain (p = 0.002), fatigue (p < 0.0001), breast edema (p = 0.001) and dermatitis (p = 0.003). Patients treated in 5 fractions reported better mean HRQoL scores for breast symptoms (p = 0.001) and physical well-being (p = 0.001). A clinically important deterioration in HRQoL of 10 points or more was also less frequently observed in the latter group for physical functioning (p = 0.0005), social functioning (p = 0.0007), fatigue (p = 0.003), breast symptoms (p = 0.0002) and physical well-being (p = 0.002).
Conclusion
In this single institute study, acute toxicity of accelerated breast radiotherapy in 5 fractions over 10–12 days seems to compare favourably to hypofractionated breast radiotherapy in 15 fractions. Less breast edema, dermatitis, desquamation, breast pain and fatigue are seen. Social and physical functioning are also less disturbed and patients have a better future perspective.
Keywords: Breast cancer, Radiotherapy, Acceleration
Highlights
-
•
Patients treated in 5 fractions show less physician-assessed and less patient-relatedcacute toxicity.
-
•
Patients treated in 5 fractions show less physician-assessed and patient-related pain and fatigue.
-
•
Patients treated in 5 fractions show less deterioration for physical and social functioning.
1. Introduction
Adjuvant radiotherapy (RT) after breast conserving surgery (BCS) for breast cancer reduces the locoregional recurrence rate and improves overall survival [1,2]. Hypofractionation with 15 or 16 fractions instead of 25 fractions has become the standard of care for whole breast irradiation without nodal RT. Equal or less toxicity was seen with the hypofractionated schedules in the Canadian hypofractionation trial and the UK START trials [[3], [4], [5]]. In the START-B trial, reducing the overall treatment time from 5 weeks to 3 weeks led to improved disease free survival and overall survival rates, probably due to less distant relapses [3].
Acceleration in 5 fractions over 5 weeks is feasible in the elderly population without an increase in relapse rates and with acceptable toxicity [[6], [7], [8], [9]]. The UK FAST trial randomized between a 5-fractions schedule, 1 fraction a week, and the 25-fractions schedule. In this study, only patients aged 50 years or older with node negative breast cancer were included. None of the patients received a boost, i.e. a higher dose to the tumour bed [10]. No differences were seen for late toxicity, locoregional or distant relapse, nor mortality [9]. From a radiobiological point of view as well as from a patient’s point of view, it is of interest to further shorten the overall treatment time. At Ghent University Hospital, a hypofractionation schedule of 5 fractions of 5.7 Gy over 10–12 days was introduced in patients agzed 65 yeats or older [11]. We chose the same prescription dose as the FAST trial to not compromise on tumour control but treatment on consecutive days was not allowed to allow repair of normal tissues in-between fractions. A simultaneous integrated boost (SIB) of 5 times 6.5 Gy was allowed. Using an alpha/beta of 4.6 Gy, this schedule was judged to be equivalent to the then standard fractionation schedule used at Ghent University Hospital: 15 fractions of 2.67 Gy with a sequential boost (SEB) of 4 times 2.5 Gy. Feasibility of this schedule was tested in the HAI-5 trial [11]. Matched-case analyses with patients treated in 15 fractions showed less acute [12] and less late [13] toxicity for 5 fractions except for fibrosis outside the tumour bed. Better health-related quality of life (HRQoL) was observed in the group treated in 5 fractions, both immediately after RT and after 1 year [14].
More recently, the FAST Forward trial was published, further limiting overall treatment time to 5 fractions of 5.2 Gy in 1 week [15]. Again, this schedule showed equivalent results for late toxicity, relapse and survival at 5 years after irradiation as a 15-fractions schedule of 2.67 Gy over 3 weeks. Only one fourth of patients received a boost, which was given sequentially. Such SEB of 5–8 fractions at least doubles the treatment time and increases the number of hospital visits, which is not the case with the HAI5 schedule using a SIB.
After showing feasibility of the HAI5 schedule in older patients, the YO-HAI5 (Young-Old Highly Accelerated Irradiation in 5 fractions) randomized controlled trial was introduced at Ghent University Hospital in patients of 18 years or older, receiving whole breast irradiation (WBI) ± SIB after BCS. Taken into account the reduced overall treatment time and fearing increased late toxicity, the SIB dose was reduced to 5 fractions of 6.2 Gy.
In the present interim analysis of the YO-HAI5 trial, physician-assessed acute toxicity and patient-related outcomes for HRQoL are reported in the first 200 randomized patients.
2. Materials and methods
2.1. Study design and -population
The YO-HAI5 study (NCT03677427, www.clinicaltrials.gov) is a randomized controlled trial in 400 breast cancer patients aged 18 years or older treated with BCS, requiring adjuvant WBI ± SIB according to the multidisciplinary tumour board. Informed consent was obtained, signed and dated for all patients before specific protocol procedures. Exclusion criteria are: lymph node metastases or distant metastases; bilateral breast irradiation; a history of radiation treatment to the same region, including radiation treatment to the contralateral breast; a life expectancy of less than 2 years; planned reconstructive surgery; conditions making toxicity evaluation difficult (e.g. skin disorders); inability to respect constraints on organs at risks and patients unlikely to comply with the protocol, e.g. unable to return for follow-up visits or unlikely to complete the study. The study protocol was approved by the hospital’s ethics board. The primary endpoint of the trial is breast retraction 2 years after RT. Secondary endpoints are acute breast toxicity, late toxicities other than breast retraction, fatigue, cosmesis, HRQoL, cost effectiveness, locoregional and distant tumour control and dose/volume parameters of target and organs of risk. In the present sub-analysis, acute toxicity and HRQoL are reported for the first 200 randomized patients. SAS Power and Sample Size software was used for the sample size calculation with α = 0.05 and β = 0.8. Dermatitis was the toxicity of main interest for acute toxicity, the sub-analysis was conducted in at least 170 patients, based on the matched-case analysis [12].
2.2. Randomization and radiotherapy
A computer-generated simple randomization was used to assign patients to a 15- or 5-fractions RT schedule. In the standard arm, treatment consists of 15 fractions of 2.67 Gy to the whole breast with a SIB to the tumour bed of 15∗3.12 Gy, if indicated. RT was delivered 5 days a week, not in the weekend. In the experimental arm, 5 fractions of 5.7 Gy were delivered with a SIB of 5∗6.2 Gy. RT was delivered on week-days with at least 40 h between consecutive treatment fractions, leading to an overall treatment time of 10–12 days.
All patients underwent computed tomography (CT) imaging without administration of iodine contrast for treatment planning. If possible, patients were treated in prone position, with supine position as alternative if prone position proved impossible. Left sided breast cancer patients were treated with deep inspiration breath hold to reduce heart dose when dose constraints were not achieved in free breathing. The clinical target volume (CTV) for WBI was delineated as proposed by the ESTRO guidelines [16,17] with the aid of a radio-opaque wire placed around the breast contour. Target objectives and dose constraints were added in appendix.
2.3. Assessment
Both physician-assessed toxicity and patient-reported outcome are evaluated. Physician-assessed acute toxicity is scored at baseline and at 2–4 weeks after treatment stop using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 toxicity scoring system. Fatigue is measured by the Multidimensional Fatigue Inventory (MFI-206).
HRQoL, collected at the same moments of physician-assessed toxicity, is evaluated using the European Organisation for Research and Treatment of Cancer (EORTC) 30-item Quality of Life Questionnaire (QLQ-C30), the breast cancer specific module of the EORTC QLQ (QLQ-BR23) and the BREAST-Q questionnaire [[18], [19], [20]]. From the QLQ-C30 and QLQ-BR23 questionnaires, only the items evaluating RT-related HRQoL were withheld: the global health scale, 2 functional scales (physical and social functioning) and 2 symptom scales (fatigue and pain) for QLQ-C30, and 2 symptom scales (arm- and breast symptoms) and one functional scale (future perspective) for QLQ-BR23. The questionnaires can be found in appendix B.
Study data were collected and managed anonymously using REDCap electronic data capture tools hosted at Ghent University Hospital.
2.4. Statistical analysis
The statistical package SPSS version 26 was used to analyse the data. RT-related toxicity was defined as any baseline toxicity that deteriorated during or after RT and any toxicity that arose during or after RT and was not present at baseline. Mild toxicity (grade 1), as well as moderate (grade 2) and marked (grade 3 or more) toxicity was taken into account. Results were analysed for both effective treatment and intention to treat (Table 2). A clinically relevant deterioration of HRQoL was defined as a difference in score of 10 points or more between baseline and 2–4 weeks after RT [21]. Differences in RT-related toxicity and clinically relevant deterioration of HRQoL between groups were analysed by performing a Chi-square test with a significance level of p < 0.05. For HRQoL, statistical differences between baseline scores and scores after 2–4 weeks were evaluated with the Mann Whitney U test. Due to the multiple tests for HRQoL, the Bonferroni correction was used to avoid type I errors which leads to an adjusted p-value of p < 0.005.
Table 2.
Physician-assessed acute toxicity for effective treatment and intention to treat.
| Acute toxicity for effective treatment |
Acute toxicity for intention to treat |
|||
|---|---|---|---|---|
| 5-fraction schedule | 15-fraction schedule | 5-fraction schedule | 15-fraction schedule | |
| N | 105 | 94 | 100 | 99 |
| Breast pain | 29 (27.6%) | 47 (50.0%) | 25 (25.0%) | 51 (51.5%) |
| Fatigue | 12 (11.4%) | 37 (39.4%) | 10 (10.0%) | 39 (39.4%) |
| Breast edema | 25 (23.8%) | 44 (46.8%) | 22 (22.0%) | 47 (47.5%) |
| Grade 1 | 24 (22.2%) | 40 (42.6%) | 21 (21.0%) | 43 (43.4%) |
| Grade 2 | 1 (1.0%) | 4 (4.3%) | 1 (1.0%) | 4 (4.0%) |
| Dermatitis | 61 (58.1%) | 73 (77.7%) | 57 (57.0%) | 77 (77.8%) |
| Grade 1 | 44 (40.7%) | 54 (57.4%) | 44 (44.0%) | 54 (54.5%) |
| Grade 2 | 17 (16.2%) | 19 (20.2%) | 13 (13.0%) | 23 (23.2%) |
| Desquamation | 15 (14.3%) | 22 (23.4%) | 12 (12.0%) | 25 (25.3%) |
| Grade 1 | 12 (11.1%) | 17 (18.1%) | 11 (11.0%) | 18 (18.2%) |
| Grade 2 | 3 (2.9%) | 5 (5.3%) | 1 (1.0%) | 7 (7.1%) |
3. Results
This sub-analysis of acute toxicity was performed on 200 patients, enrolled in the study between October 2017 and Februari 2020. Hundred and one patients were randomized to the 5-fractions schedule and 99 to the 15-fractions schedule. Five patients randomized to 15 fractions were actually treated in 5 fractions (protocol violation), which led to 106 patients effectively treated in 5 fractions and 94 patients in 15 fractions. Data of acute toxicity and HRQoL were lost in 1 case, since this patient refused follow-up after randomization to the 5 fractions arm. Table 1 describes the patient, tumour and treatment characteristics for both study groups.
Table 1.
Patient, tumour and treatment characteristics for effective treatment.
| 5-fractions schedule (N = 106) | 15-fractions schedule (N = 94) | Sign. | |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | p = 0.3 | ||
| Median | 59 | 62 | |
| Range | 37–83 | 26–84 | |
| <40 | 3 (2.8%) | 5 (5.3%) | |
| 40-49 | 10 (9.4%) | 10 (10.6%) | |
| 50-59 | 41 (38.7%) | 25 (26.6%) | |
| 60-69 | 34 (32.1%) | 40 (42.6%) | |
| 70-79 | 16 (15.1%) | 11 (11.7%) | |
| ≥80 | 2 (1.9%) | 3 (2.8%) | |
| Body Mass Index (BMI) | p = 0.05 | ||
| Median | 25.0 | 25.6 | |
| <20 | 6 (5.7%) | 12 (12.8%) | |
| 20-25 | 47 (44.3%) | 28 (29.8%) | |
| >25 | 53 (50%) | 54 (57.4%) | |
| Breast volume (mean) | 472.30 C C | 489.77 C C | p = 0.9 |
| Smoking behaviour | p = 0.6 | ||
| Current | 9 (8.5%) | 12 (12.8%) | |
| Former | 26 (24.5%) | 24 (25.5%) | |
| Never | 71 (67.0%) | 58 (61.7%) | |
| Alcohol use | p = 0.4 | ||
| Current | 72 (67.9%) | 71 (75.5%) | |
| Former | 6 (5.7%) | 6 (6.4%) | |
| Never | 28 (26.4%) | 17 (18.1%) | |
| Tumour characteristics | |||
| Tumour side | p = 0.5 | ||
| Left | 53 (50.0%) | 42 (44.7%) | |
| Right | 53 (50.0%) | 52 (55.3%) | |
| Tumour location | p = 0.6 | ||
| Lower-inner quadrant | 12 (12%) | 12 (13%) | |
| Lower-outer quadrant | 12 (12%) | 17 (18%) | |
| Upper-inner quadrant | 18 (17%) | 9 (9%) | |
| Upper-outer quadrant | 47 (44%) | 42 (44%) | |
| Central portion of the breast | 17 (16%) | 16 (17%) | |
| Histological grade | p = 0.6 | ||
| Poor | 23 (22%) | 21 (22%) | |
| Moderate | 65 (61%) | 52 (55%) | |
| Well | 18 (17%) | 21 (22%) | |
| Histological type | p = 0.6 | ||
| DCIS | 5 (5%) | 8 (8%) | |
| Infiltrating lobular | 10 (10%) | 8 (8%) | |
| Infiltrating ductal | 86 (81%) | 72 (77%) | |
| Tubular | 1 (1%) | 3 (3%) | |
| Other | 4 (4%) | 3 (3%) | |
| Pathological tumour size | p = 0.5 | ||
| Mean | 15 mm (SD 6.2) | 11 mm (SD 7.7) | |
| Range | 1–26 mm | 1–35 mm | |
| Ki 67 | p = 0.9 | ||
| <20% | 53 (50.0%) | 48 (51.1%) | |
| ≥20% | 41 (38.7%) | 34 (36.2%) | |
| Unknown | 12 (11.3%) | 12 (12.8%) | |
| HER-2 Immunohistochemistry | p = 0.5 | ||
| Negative | 43 (40.6%) | 34 (36.2%) | |
| Positive: 1+ | 21 (19.8%) | 28 (29.8%) | |
| Positive: 2+ | 17 (16.0%) | 15 (16.0%) | |
| Positive: 3+ | 7 (6.6%) | 3 (3.2%) | |
| Unknown | 18 (17.0%) | 14 (14.9%) | |
| FISH/SISH/CISH | p = 0.3 | ||
| Amplification | 11 (10.4%) | 6 (6.4%) | |
| No amplification | 41 (38.7%) | 46 (48.9%) | |
| Unknown | 54 (50.9%) | 42 (44.7%) | |
| ER status | p = 0.7 | ||
| Positive (>1%) | 75 (70.8%) | 63 (67.0%) | |
| Negative (≤1%) | 10 (9.4%) | 12 (12.8%) | |
| Unknown | 21 (19.8%) | 19 (20.2%) | |
| PR status | p = 1.0 | ||
| Positive (>5%) | 69 (65.1%) | 61 (64.9%) | |
| Negative (≤5%) | 16 (15.1%) | 14 (14.9%) | |
| Unknown | 21 (19.8%) | 19 (20.2%) | |
| cTNM | p = 0.5 | ||
| T1N0M0 | 90 (85%) | 78 (83%) | |
| T1N1M0 | 0 (0%) | 1 (1%) | |
| T2N0M0 | 11 (10%) | 7 (7%) | |
| TisN0M0 | 5 (5%) | 8 (9%) | |
| pTNM | p = 0.5 | ||
| T1N0M0 | 86 (81%) | 77 (82%) | |
| T1N1 (mi)M0 | 4 (4%) | 2 (2%) | |
| T2N0M0 | 11 (10%) | 7 (7%) | |
| TisN0M0 | 5 (5%) | 8 (9%) | |
| Treatment characteristics | |||
| Surgery | |||
| Type of surgery | p = 0.8 | ||
| Segmentectomy/quadrantectomy | 4 (3.8%) | 3 (3.2%) | |
| Wide local excision | 102 (96.2%) | 91 (96.8%) | |
| Axillary surgery | p = 0.09 | ||
| Axillary clearance | 0 (0.0%) | 1 (1.1%) | |
| Sentinel node biopsy | 94 (88.7%) | 87 (92.6%) | |
| No axillary surgery | 12 (11,3%) | 6 (6.4%) | |
| Postoperative haematoma | p = 05 | ||
| Yes | 12 (11.3%) | 11 (11.7%) | |
| No | 90 (84.9%) | 83 (88.3%) | |
| Unknown | 4 (3.8%) | 0 (0.0%) | |
| Postoperative edema | p = 0.9 | ||
| Yes | 5 (4.7%) | 4 (4.3%) | |
| No | 99 (93.4%) | 88 (93.6%) | |
| Unknown | 2 (1.9%) | 2 (2.1%) | |
| Postoperative infection | p = 0.6 | ||
| Yes | 5 (4.7%) | 6 (6.4%) | |
| No | 98 (92.5%) | 87 (92.6%) | |
| Unknown | 3 (2.8%) | 1 (1.1%) | |
| Delayed wound healing | p = 0.2 | ||
| Yes | 0 (0.0%) | 4 (4.3%) | |
| No | 103 (97.2%) | 89 (94.7%) | |
| Unknown | 3 (2.8%) | 1 (1.1%) | |
| Chemotherapy | |||
| Neoadjuvant chemotherapy | p = 0.9 | ||
| Yes | 4 (3.8%) | 4 (4.3%) | |
| No | 102 (96.2%) | 90 (95.7%) | |
| Adjuvant chemotherapy | p = 0.7 | ||
| Yes | 27 (25.5%) | 22 (23.4%) | |
| No | 79 (74.5%) | 72 (76.6%) | |
| Hormone therapy | p = 0.9 | ||
| Tamoxifen | 48 (45.3%) | 46 (48.9%) | |
| Aromatase inhibitor | 34 (32.1%) | 29 (30.9%) | |
| None | 24 (22.6%) | 19 (20.2) | |
| Targeted therapy with trastuzumab | p = 0.3 | ||
| Yes | 11 (10.4%) | 6 (6.4%) | |
| No | 95 (89.6%) | 88 (93.6%) | |
| Radiotherapy | |||
| Simultaneous integrated boost | 95 (90.5%) | 85 (90.4%) | p = 1.0 |
| DIBH | 2 (1.9%) | 2 (2.1%) | p = 0.9 |
| Position | p = 0.3 | ||
| Prone | 101 (95.3%) | 91 (96.8%) | |
| Supine | 5 (4.7%) | 3 (3.2%) | |
| Overall treatment time (mean) | 10.6 days (SD 0.8) | 21.6 days (SD 1.3) | |
BMI: body mass index, DCIS: ductal carcinoma in situ, HER2: human epidermal growth factor receptor 2.
ER: estrogen receptor, PR: progesterone receptor, DIBH: deep inspiration breath hold.
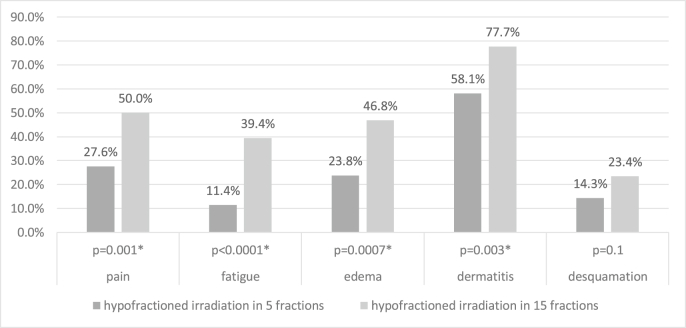
All physician-assessed RT-related acute toxicities of any grade are lower for the 5-fractions group, reaching statistical significance for breast pain (p = 0.002), fatigue (p < 0.0001), breast edema (p = 0.001) and dermatitis (p = 0.003) but not for desquamation (p = 0.1) (Fig. 1).
Fig. 1.
Physician-assessed acute toxicity for effective treatment (Chi-squared test,∗ Significance level: p < 0.05).
Grade 2-toxicity is rare for breast edema and desquamation, but not for dermatitis, with in both groups almost one fifth of patients developing grade 2 toxicity (16% in the 5-fractions group and 20% in the 15-fractions group). Grade ≥3 toxicity did not occur. As shown in Table 2, the intention to treat analysis did not change the results.
In Table 3, patient reported outcomes are shown. No significant differences between both groups are seen in HRQoL scores at baseline. Two to four weeks after RT, patients treated in 5 fractions experience significantly less breast symptoms and report a better score for physical well-being. Significantly less patients in the 5-fractions group experience a clinically relevant deterioration in HRQoL of 10 points or more for physical functioning, social functioning, fatigue, breast symptoms and physical well-being.
Table 3.
Health-related quality of life.
| Baseline |
2–4 weeks after RT |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean score (SD) |
p-valuea | Mean score (SD) |
p-valuea | Clinically important deterioration |
|||||
| 5-fraction schedule | 15-fraction schedule | 5-fraction schedule | 15-fraction schedule | 5-fraction schedule | 15-fraction schedule | p-valueb | |||
| N | 105 | 94 | 105 | 94 | 105 | 94 | |||
| EORT QLQ-C30/BR23 | |||||||||
| Global health status (QL)c | 71.9 (17.2) | 72.9 (19.2) | p = 0.8 | 73.9 (14.8) | 67.4 (20.5) | p = 0.01 | 16 (15%) | 30 (32%) | p = 0.005 |
| Functional scalesc | |||||||||
| Physical functioning (PF2) | 85.1 (16.4) | 89.4 (11.6) | p = 0.1 | 87.0 (15.2) | 86.8 (13.6) | p = 0.6 | 7 (7%) | 23 (24%) | p = 0.0005e |
| Social functioning (SF) | 85.1 (21.5) | 84.4 (22.5) | p = 0.9 | 87.6 (20.4) | 83.0 (21.9) | p = 0.03 | 12 (11%) | 29 (31%) | p = 0.0007e |
| Future perspective (BRFU) | 60.6 (26.9) | 63.1 (26.1) | p = 0.5 | 66.3 (22.9) | 59.9 (24.7) | p = 0.06 | 13 (12%) | 20 (21%) | p = 0.09 |
| Symptom scalesd | |||||||||
| Fatigue (FA) | 27.5 (19.6) | 26.7 (22.6) | p = 0.5 | 28.7 (22.2) | 35.2 (23.4) | p = 0.07 | 31 (30%) | 47 (50%) | p = 0.003e |
| Pain (PA) | 18.7 (21.3) | 15.4 (17.7) | p = 0.3 | 16.2 (18.1) | 20.2 (21.7) | p = 0.2 | 23 (22%) | 35 (37%) | p = 0.02 |
| Arm symptoms (BRAS) | 10.4 (13.7) | 10.4 (15.4) | p = 0.7 | 9.6 (13.2) | 9.1 (14.0) | p = 0.6 | 18 (17%) | 21 (22%) | p = 0.4 |
| Breast symptoms (BRBS) | 16.7 (15.6) | 15.8 (16.6) | p = 0.5 | 23.7 (17.6) | 32.8 (19.9) | p = 0.001e | 31 (30%) | 56 (60%) | p = 0.0002e |
| BREAST-Q | |||||||||
| Satisfaction with breast c | 74.1 (16.8) | 71.6 (17.7) | p = 0.5 | 72.9 (17.5) | 67.4 (17.5) | p = 0.04 | 19 (18%) | 30 (32%) | p = 0.02 |
| Physical well-being d | 14.2 (16.5) | 12.6 (14.1) | p = 0.5 | 18.4 (18.8) | 27.4 (20.3) | p = 0.001e | 28 (27%) | 45 (48%) | p = 0.002e |
Mann Whitney U test.
Chi squared test, For botha andb Bonferroni correction is applied (significance level: p < 0,005).
Higher score indicates better functioning.
Higher score indicates more symptoms.
Significance.
4. Discussion
Two randomized controlled trials investigating an accelerated RT schedule in 5 fractions have been published. In the FAST trial a schedule of 5 fractions over 5 weeks, 1 fraction a week, was compared with the former standard schedule of 25 fractions of 2 Gy. Tender or bright dermatitis with or without dry desquamation 2–8 weeks after RT was seen in 8.5% (28.5 Gy/5) vs. 35.5% (50 Gy/25) and patchy moist desquamation and moderate edema in 5.2% vs. 10.9% [10]. At 10 years follow-up, irradiation in 5 fractions was comparable to 25 fractions regarding toxicity. Five fractions of 5.7 Gy was significantly milder than 5 fractions of 6.0 Gy for adverse effects in the breast [9]. Later, in the FAST Forward trial, acceleration to 5 fractions in 1 week was investigated: 5∗5.2 Gy and 5∗5.4 Gy were compared to the current state of the art schedule of 15∗2.67 Gy. Moderate dermatitis was seen in 27% vs. 47%, moderate desquamation in 5% vs. 19% and moderate breast edema in 0% vs. 5%. Five-year results showed no statistically significant differences in late toxicity, nor in locoregional or distant relapse, breast-related events or all-cause mortality [15]. However, the application of these 5-fractions schedules in combination with a boost remains a topic for debate. In the FAST trial, patients were all over 50 years old and no boost was allowed. In the FAST-Forward trial, 25% of patients received a sequential boost of 5–8 fractions of 2 Gy. However, it is quite contradictory to treat the whole breast in fractions of over 5 Gy, while delivering the boost in fractions of 2 Gy. Moreover, 1–2 weeks of treatment are added which doubles the overall treatment time and goes against the principle of acceleration. Omission of the boost is justifiable in older patients since the benefit is limited and the risk of toxicity is higher [22]. However, in some cases a boost is still recommended. Use of a SIB allows to deliver a boost without increasing the number of treatment fractions.
In the present sub-analysis of the YO-HAI5 study, acute toxicity with a schedule of 5∗5.7 Gy to the whole breast over 10–12 days is evaluated. Over 90% of all patients received a SIB of 5∗6.2 Gy. None of the patients received lymph node irradiation (LNI). Physician-assessment showed more than 20% less breast edema (p = 0.0007), dermatitis (p = 0.003), pain (p = 0.001) and fatigue (p = 0.0001) with this schedule than with a moderate hypofractionation schedule of 15∗2.67 Gy (SIB of 15∗3.12Gy). The lower rates of acute toxicity were not unexpected since these effects are very sensitive to total dose, which is reduced using 5 fractions compared to 15 fractions, and not to dose per fraction. The FAST-Forward trial showed comparable results [15]. However, acute toxicity might not predict for late toxicity, which is more dependent on dose per fraction. In the FAST-Forward trial, late toxicity was similar despite the marked difference in acute toxicity [23].
Despite the use of a SIB, moderate dermatitis and desquamation (grade 2) were less frequent in our population than in the FAST Forward trial. An explanation might be the 40 h treatment interval between 2 fractions, but the same effect was observed for the 15-fractions group making it more likely that the prone treatment position [24] was responsible for the lower rates of toxicity than observed in the FAST Forward trial [23]. However, comparison between both trials is challenging due to the different timing of toxicity assessment. In the FAST Forward trial, acute toxicity was measured up to 4 weeks after RT, while in our trial mean follow-up time after completion of RT was 16.7 days ±6.0 days (16.6 days for irradiation in 15 fractions, 16.7 days for 5 fractions). Toxicity occurring after that time point could have been missed. However, in the 15 fractions group of FAST-Forward, maximum tocixity rates seemed to be reached at 2 weeks. This is consistent with our own data published on WBI ± SIB in 15 fractions [24]. In the 5 fractions group, most toxicity did not seem to aggravate beyond 2 weeks after RT, except for some very rare grade 3 toxicity, appearing at 4 weeks after RT, possibly explained by the use of a sequentional boost. Our data show that the frequency of toxicity was lower at the last follow-up visit than at the end of RT, suggesting that maximum toxicity occurred earlier than 2 weeks after RT. Patients were also asked to contact us in case toxicity got worse after the last follow-up visit.
Patient-reported outcomes showed that a deterioration in global health status is less frequent in the group with less treatment fractions (p = 0.005). This is not surprising since the number of patients experiencing a clinically relevant deterioration of fatigue, of breast symptoms in general and of physical well-being were reduced by respectively 20%, 30% and 21% in the 5-fractions group, compared to the 15-fractions group (p = 0.003, p = 0.0002, p = 0.002). The interfering factors such as surgery, chemotherapy, hormone therapy or RT technique were not significantly different between both groups, so it can be assumed that this is the effect of the acceleration, though other unknown variables can’t be excluded.
Less treatment fractions also result in better social functioning: 20% less patients experience a disturbance of family life and/or social activities in the 5-fractions group (p = 0.0007). Some of the physician-assessed toxicity endpoints are probably linked to items of patient-reported outcome, e.g. physical functioning examines the extent to which physical exertion is still possible, which is most likely linked to fatigue. Breast symptoms and physical well-being question pain, edema, skin problems, and how the breast feels and its appearance. For linked items, both physician assessed and patient reported outcome showed comparable results, i.e. superiority of 5 fractions over 15 fractions.
In conclusion, physician-assessed toxicity is lower and patient-reported outcome is better with an accelerated RT-schedule in 5 fractions over 10–12 days than with a hypofractionation schedule of 15 fractions.
Contributor Information
Hans Van Hulle, Email: hans.vanhulle@uzgent.be.
Vincent Vakaet, Email: vincent.vakaet@ugent.be.
Chris Monten, Email: chris.monten@ugent.be.
Pieter Deseyne, Email: pieter.deseyne@ugent.be.
Max Schoepen, Email: max.schoepen@ugent.be.
Cato Colman, Email: cato.colman@ugent.be.
Leen Paelinck, Email: leen.paelinck@uzgent.be.
Annick Van Greveling, Email: annick.vangreveling@uzgent.be.
Giselle Post, Email: giselle.post@ugent.be.
Bruno Speleers, Email: bruno.speleers@ugent.be.
Katrien Vandecasteele, Email: katrien.vandecasteele@ugent.be.
Marc Mareel, Email: marc.mareel@ugent.be.
Wilfried De Neve, Email: wilfried.deNeve@ugent.be.
Liv Veldeman, Email: liv.veldeman@ugent.be.
References
- 1.Early Breast Cancer Trialists’ Collaborative G., Darby S., McGale P., Correa C., Taylor C., Arriagada R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet (London, England) 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke M., Collins R., Darby S., Davies C., Elphinstone P., Evans V. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Group S.T., Bentzen S.M., Agrawal R.K., Aird E.G., Barrett J.M., Barrett-Lee P.J. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen J.R., Ashton A., Bliss J.M., Homewood J., Harper C., Hanson J. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 5.Whelan T.J., Pignol J.P., Levine M.N., Julian J.A., MacKenzie R., Parpia S. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 6.Kirova Y.M., Campana F., Savignoni A., Laki F., Muresan M., Dendale R. Breast-conserving treatment in the elderly: long-term results of adjuvant hypofractionated and normofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:76–81. doi: 10.1016/j.ijrobp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Ortholan C., Hannoun-Levi J.M., Ferrero J.M., Largillier R., Courdi A. Long-term results of adjuvant hypofractionated radiotherapy for breast cancer in elderly patients. Int J Radiat Oncol Biol Phys. 2005;61:154–162. doi: 10.1016/j.ijrobp.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 8.Rovea P., Fozza A., Franco P., De Colle C., Cannizzaro A., Di Dio A. Once-weekly hypofractionated whole-breast radiotherapy after breast-conserving surgery in older patients: a potential alternative treatment schedule to daily 3-week hypofractionation. Clin Breast Canc. 2015;15:270–276. doi: 10.1016/j.clbc.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Brunt A.M., Haviland J., Sydenham M., Algurafi H., Alhasso A., Bliss P. FAST phase III RCT of radiotherapy hypofractionation for treatment of early breast cancer: 10-year results (CRUKE/04/015) Int J Radiat Oncol. 2018;102:1603–1604. [Google Scholar]
- 10.group F.T., Agrawal R.K., Alhasso A., Barrett-Lee P.J., Bliss J.M., Bliss P. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015) Radiother Oncol. 2011;100:93–100. doi: 10.1016/j.radonc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Monten C., Lievens Y., Olteanu L.A.M., Paelinck L., Speleers B., Deseyne P. Highly accelerated irradiation in 5 fractions (HAI-5): feasibility in elderly women with early or locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 2017;98:922–930. doi: 10.1016/j.ijrobp.2017.01.229. [DOI] [PubMed] [Google Scholar]
- 12.Van Hulle H., Naudts D., Deschepper E., Vakaet V., Paelinck L., Post G. Accelerating adjuvant breast irradiation in women over 65 years: matched case analysis comparing a 5-fractions schedule with 15 fractions in early and locally advanced breast cancer. J Geriatr Oncol. 2019;10(6):987–989. doi: 10.1016/j.jgo.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Van Hulle H., Vakaet V., Deckmyn K., Monten C., Paelinck L., Van Greveling A. Two-year toxicity of hypofractionated breast cancer radiotherapy in five fractions. Acta Oncol. 2020:1–4. doi: 10.1080/0284186X.2020.1747638. [DOI] [PubMed] [Google Scholar]
- 14.Van Hulle H., Vakaet V., Bultijnck R., Deseyne P., Schoepen M., Van Greveling A. Health-related quality of life after accelerated breast irradiation in five fractions: a comparison with fifteen fractions. Radiother Oncol. 2020;151:47–55. doi: 10.1016/j.radonc.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Brunt A.H.J., Wheatley D., Sydenham M., Alhasso A., Bloomfield D. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Offersen B.V., Boersma L.J., Kirkove C., Hol S., Aznar M.C., Biete Sola A. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Verhoeven K., Weltens C., Remouchamps V., Mahjoubi K., Veldeman L., Lengele B. Vessel based delineation guidelines for the elective lymph node regions in breast cancer radiation therapy - PROCAB guidelines. Radiother Oncol. 2015;114:11–16. doi: 10.1016/j.radonc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen J., Popovic M., Chow E., Cella D., Beaumont J.L., Chu D. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: a literature review. J Comp Eff Res. 2015;4:157–166. doi: 10.2217/cer.14.76. [DOI] [PubMed] [Google Scholar]
- 19.Xia J., Tang Z., Wu P., Wang J.W., Yu J.M. Use of item response theory to develop a shortened version of the EORTC QLQ-BR23 scales. Sci Rep. 2019;9 doi: 10.1038/s41598-018-37965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusic A.L., Klassen A.F., Scott A.M., Klok J.A., Cordeiro P.G., Cano S.J. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 21.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 22.Bartelink H., Maingon, Poortmans P. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial (vol16, pg 47, 2015) Lancet Oncol. 2015;16 doi: 10.1016/S1470-2045(14)71156-8. E6-E. [DOI] [PubMed] [Google Scholar]
- 23.Brunt A.M., Wheatley D., Yarnold J., Somaiah N., Kelly S., Harnett A. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol. 2016;120:114–118. doi: 10.1016/j.radonc.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paelinck L., Gulyban A., Lakosi F., Vercauteren T., De Gersem W., Speleers B. Does an integrated boost increase acute toxicity in prone hypofractionated breast irradiation? A randomized controlled trial. Radiother Oncol. 2017;122:30–36. doi: 10.1016/j.radonc.2016.12.023. [DOI] [PubMed] [Google Scholar]