Highlights
-
•
Injury to major white matter pathways during language-area associated glioma surgery often results in permanent aphasia.
-
•
DTI-based tractography of language pathways allows to correlate individual tract injury profiles with functional outcome.
-
•
Infiltration of the AF is particularly associated with functional deterioration.
-
•
The temporo-parieto-occipital junction and the temporal stem were confirmed as pivotal functional nodes.
-
•
Standardized DTI-based tractography can help to determine the individual aphasia risk profile before surgery.
Keywords: Glioma, DTI, Risk stratification, Aphasia, Tractography, Language pathways, Arcuate fasciculus, Brain tumor
Abstract
Objectives
Injury to major white matter pathways during language-area associated glioma surgery often leads to permanent loss of neurological function. The aim was to establish standardized tractography of language pathways as a predictor of language outcome in clinical neurosurgery.
Methods
We prospectively analyzed 50 surgical cases of patients with left perisylvian, diffuse gliomas. Standardized preoperative Diffusion-Tensor-Imaging (DTI)-based tractography of the 5 main language tracts (Arcuate Fasciculus [AF], Frontal Aslant Tract [FAT], Inferior Fronto-Occipital Fasciculus [IFOF], Inferior Longitudinal Fasciculus [ILF], Uncinate Fasciculus [UF]) and spatial analysis of tumor and tracts was performed. Postoperative imaging and the resulting resection map were analyzed for potential surgical injury of tracts. The language status was assessed preoperatively, postoperatively and after 3 months using the Aachen Aphasia Test and Berlin Aphasia Score. Correlation analyses, two-step cluster analysis and binary logistic regression were used to analyze associations of tractography results with language outcome after surgery.
Results
In 14 out of 50 patients (28%), new aphasic symptoms were detected 3 months after surgery. The preoperative infiltration of the AF was associated with functional worsening (cc = 0.314; p = 0.019). Cluster analysis of tract injury profiles revealed two areas particularly related to aphasia: the temporo-parieto-occipital junction (TPO; temporo-parietal AF, middle IFOF, middle ILF) and the temporal stem/peri-insular white matter (middle IFOF, anterior ILF, temporal UF, temporal AF). Injury to these areas (TPO: OR: 23.04; CI: 4.11 – 129.06; temporal stem: OR: 21.96; CI: 2.93 – 164.41) was associated with a higher-risk of persisting aphasia.
Conclusions
Tractography of language pathways can help to determine the individual aphasia risk profile pre-surgically. The TPO and temporal stem/peri-insular white matter were confirmed as functional nodes particularly sensitive to surgical injuries.
1. Introduction
Injury to major white matter pathways during language-area associated glioma surgery often leads to permanent loss of neurological function (Duffau, 2014c, Meyer et al., 2017). While the gold standard to identify language eloquent brain areas is direct electrical stimulation (DES) during awake craniotomy, this procedure cannot be offered to every patient (Sanai et al., 2008). Diffusion-Tensor-Imaging (DTI)-based tractography allows visualization of language pathways non-invasively before surgery (Mori et al., 1999), making this technology a potentially valuable asset for presurgical planning (Caverzasi et al., 2016, Raffa et al., 2017, Sollmann et al.,, Sollmann et al., 2020).
In the contemporary model, the cerebral language network is organized in two parallel processing streams (Chang et al., 2015, Dick et al., 2014, Hickok and Poeppel, 2007). The dorsal stream is associated with the sensorimotor integration of language and includes the Arcuate Fasciculus (AF) as the main connection (Catani et al., 2005, Saur et al., 2008). The ventral stream relates to semantic processing and is comprised of the Inferior Fronto-Occipital Fasciculus (IFOF), the Inferior Longitudinal Fasciculus (ILF) and the Uncinate Fasciculus (UF) (Almairac et al., 2015, Duffau et al., 2009, Mandonnet et al., 2007). The Frontal Aslant Tract (FAT) is a short frontal association tract relevant for the processing of articulation (Catani et al., 2013). While the functionality of these tracts has been described in multiple awake mapping and functional imaging studies, there is limited data available about their functional relevance in case of surgical injury.
The AF, in particular the temporo-parietal junction, and the IFOF have been previously described to be of particular significance regarding preservation of language function (Caverzasi et al., 2016, Ius et al., 2011). Recently more evidence derived from intraoperative stimulation mapping and MEG connectivity studies was published on the outstanding importance of certain functional nodes within the cortico-subcortical networks to preserve neurological functioning (Lee et al., 2020, Sarubbo et al., 2020).
Here we set out to investigate whether standardized DTI-based language tractography – following a recently proposed protocol validated for brain tumor patients (Fekonja et al., 2019) - and its correlation with intraoperative tract injury and aphasia would allow us to replicate these recent findings and provide a clinically feasible method to non-invasively identify the functional nodes in individual glioma patients.
2. Methods
2.1. Ethics
The experimental protocol was approved by the local ethics committee of the Charité university hospital Berlin in accordance with the declaration of Helsinki (EA1/016/19). Written and informed consent for all medical evaluation and treatment was obtained from all patients. The study and this publication were conducted according to the STROBE-Guidelines (von Elm et al., 2007).
2.2. Patients and study design
This is a prospective, observational study including a series of 50 patients, who underwent surgical resection for a language area associated glioma from November 2014 to September 2019.
The following inclusion criteria were used: the presence of a left-hemispheric, diffuse glioma in proximity to presumed language related brain areas (typically the perisylvian cortex as well as subcortical fiber tracts, e.g. the arcuate fasciculus), German as a native language and age ≥ 18 years.
The exclusion criteria were general contraindications for MRI, such as metal implants and pregnancy. No subject had to be excluded in the course of the study.
2.3. Clinical examination of language status and aphasia grading
The language status was assessed preoperatively, postoperatively (within the first 7 days) and after 3 months. Every patient was examined preoperatively and postoperatively using both the Aachen Aphasia Test (AAT) (Huber et al., 1984) as well as the clinical Berlin Aphasia Score (BAS), which is adapted to the AAT (Picht et al., 2013). The AAT consists of different subtests assessing distinct linguistic qualities each - including: Token-Test (language comprehension and recognition of shapes and colors), object naming and word repetition. Due to organizational reasons concerning the 3 months follow up examinations, the AAT could only be performed in 26% (13 out of 50) of patients after 3 months. If an AAT-score was unavailable, the BAS alone was used to define the aphasia grade. The language examination results were used to categorize patients into a 4-level aphasia score: 0 = no aphasia (≥90% AAT-score / BAS = 0); 1 = mild aphasia (89% – 75% AAT-score / BAS = 1); 2 = moderate aphasia (74% – 55% AAT-score / BAS = 2); 3 = severe aphasia (<55% AAT-score / BAS = 3). Based on the difference of aphasia grade between the preoperative, postoperative and follow-up examinations, the functional outcome was defined: unchanged vs. transient new / worsened aphasia vs. permanent new / worsened aphasia.
The handedness of each patient was evaluated using the Edinburgh Handedness Inventory (Oldfield, 1971).
2.4. Image acquisition
MRI data were acquired on a Siemens Skyra 3T scanner (Erlangen, Germany) equipped with a 32-channel receiver head coil. These data consisted of a high-resolution T1-weighted structural sequence (TR/TE/TI 2300/2.32/900 m s, 9° flip angle, 256 × 256 matrix, 1 mm isotropic voxels, 192 slices, acquisition time: 5 min) and a single shell diffusion MRI acquisition for tractography (TR/TE 7500/95 m s, 2 × 2 × 2 mm3 voxels, 128 × 128 matrix, 60 slices, 3b 0 volumes), acquired at b 0, 1000 s/mm2 with 5 and 40 volumes respectively. T2-weighted, 3D fluid attenuated inversion recovery (FLAIR) and subtraction sequences were additionally performed.
Preoperatively, T1-weighted gadolinium enhanced (for high-grade tumors) and T2-weighted/FLAIR (for low-grade tumors) datasets were used to manually segment the tumor and calculate the volume using the Brainlab software Elements (BRAINLAB AG, Munich, Germany).
Postoperatively, T1- and T2-weighted datasets were used to identify and manually segment the resection cavity. Diffusion-weighted datasets were additionally analyzed to rule out ischemic events.
The extent of tumor resection was assessed by calculating the residual tumor volume using subtraction sequences for high-grade and FLAIR sequences for low-grade gliomas. If no residual tumor was found, a gross total resection (GTR) was documented. In case of a residual tumor volume of < 15 ml, a subtotal resection (STR) and in case of a volume of > 15 ml, a partial resection (PR) was documented.
2.5. Tractography
We used deterministic algorithms (FACT and TEND) (Mori et al., 1999) provided within the Brainlab Elements software to perform the DTI-based language tractography following a recently proposed standardized and user-friendly protocol (Fekonja et al., 2019). Using the patient-individual, anatomical T1-weighted image as a template, regions of interests (ROIs) were placed at predefined anatomic landmarks within the obligatory pathways of the desired tracts (AF, FAT, ILF, IFOF, UF). The ROIs were previously validated for successful use in a clinical setting in brain tumor patients, also in cases of displaced, disrupted or infiltrated fiber tracts (Fekonja et al., 2019). If tumors were located directly within or near anatomical landmarks used to define ROIs, individual ROI placement in the peritumoral white matter within the tractś obligatory pathways was performed. Spatial relations of the tumor and peritumoral streamlines can then be observed (displacement, disruption or infiltration) and further dissection is continued accordingly. An additional step-by-step protocol for performing tractography in an exemplary case with unfavorable tumor location is presented in the Supplementary data (Suppl.1). With this approach towards a common way of ROI placement, largely standardized and nearly user-independent tractography can be performed in a clinical neurosurgical setting despite the possible presence of infiltrating tumors and peritumoral edema.
Standard thresholds of the fractional anisotropy (FA) value (0.15), the minimum fiber length (50 mm) as well as the maximum angulation (30 degrees) were applied to perform tractography. ROIs were circular shaped with a diameter of 12 mm. In case of tumor displacing or infiltrating the predefined ROI location, the size and shape of the respective ROI were adjusted accordingly.
For the exact location of the ROIs used to visualize the respective tracts and a more detailed description of the tractography protocol, we refer to our previously published manual (Fekonja et al., 2019).
2.6. Image analysis
Preoperatively, the tumor-to-tract distance (TTD) was measured by defining the shortest distance of the tumor to the tract in all 3 radiological planes. In case of a TTD of 0 mm (=tract infiltration), the extent of contact between the tumor and the tract was approximated by calculating the surface intersection of both.
The preoperatively defined tracts were carefully fused with the postoperative images to analyze the potential surgery-related injury of tracts. Distortion correction was applied to minimize misalignments caused by brain shift (Gerhardt et al., 2019). In case of an overlap of a tract and resection cavity (=tract injury), the respective intersection volume was calculated (Fig. 1A). For a detailed localization of the lesion, each tract was parcellated into 3 subsegments using the following anatomical landmarks (Fig. 1B):
Fig. 1.
Aleft: Language tractography in a patient with a left, temporo-parietal glioblastoma (color coding, see B); AF (blue) shows direct contact to tumor (highlighted in red circle). Preoperative tractography is overlaid with postoperative MRI and corrected for local distortion. Overlap of the AF and resection cavity (outlined in white) suggests potential injury during surgery. The patient developed a postoperative aphasia. right: Tractography in a patient with a left, frontal glioblastoma shows contact of FAT (red) to tumor (highlighted in red circle). Resection map analysis reveals overlap of FAT and resection cavity (outlined in white) – suggesting surgical injury. The patient́s language function remained unchanged. B Tract Segmentation: AF(blue): frontal segment (A) –central sulcus – temporo-parietal segment (B) – perpendicular line from posterior insular point – temporal segment (C) FAT(red): medial segment (A) – superior frontal sulcus – middle segment (B) – middle frontal sulcus – lateral segment (C) IFOF(green): frontal segment (A) – circular sulcus of insula – middle segment (B) – parieto-occipital sulcus – occipital segment (C) ILF(purple): anterior segment (A) – limen insulae – middle segment (B) – parieto-occipital sulcus– occipital segment (C) UF(orange): frontal segment (A) –circular sulcus of insula – middle segment (B) – limen insulae – temporal segment (C). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
AF:
-
-
frontal segment – central sulcus – temporo-parietal segment – perpendicular line from posterior insular point – temporal segment
FAT:
-
-
medial segment – superior frontal sulcus – middle segment – middle frontal sulcus – lateral segment
IFOF:
-
-
frontal segment – circular sulcus of insula – middle segment – parieto-occipital sulcus – occipital segment
ILF:
-
-
anterior segment – limen insulae – middle segment – parieto-occipital sulcus – occipital segment
UF:
-
-
frontal segment – circular sulcus of insula – middle segment – limen insulae – temporal segment
2.7. Surgery
In all cases, neuro-navigation was used to aid tumor resection. Additionally, in 25 cases an intraoperative MRI (ioMRI) and in 23 cases awake craniotomy with language mapping by DES was performed. Awake craniotomy was performed under local anaesthesia with additional low-dose systemic analgesia and sedation (Bährend et al., 2020). An object naming task using a set of preoperatively selected line-drawings of objects (after 3 consecutive baseline test runs) was employed to intraoperatively assess the patientś language function as it́s disturbance is a common feature shared by most forms of aphasia (Kohn & Goodglass, 1985).
2.8. Statistics
Descriptive analyses were performed for each variable (sex, age, tumor volume, tumor characteristics, extent of resection, ioMRI / no ioMRI, awake / asleep surgery) using absolute and relative frequencies for nominal or ordinal data, median and interquartile range (IQR) or mean and standard deviation (SD) for continuous variables depending on the distribution.
Chi-squared-test was used to compare the number of infiltrated (TTD = 0 mm) and injured (overlap of tract and resection cavity according to resection map analysis) tracts among outcome groups. The contingency coefficient (cc) was calculated to estimate the effect size for the association of nominal variables.
Non-parametric Kruskal-Wallis-Test was used to compare the infiltrated and injured volumes (overlap volume of tract with tumor / resection cavity) of tracts and their subsegments between the outcome groups. Correlation analyses (Spearman-Rho, ρ) were additionally performed to provide effect size estimates. Association of early postoperative scores of AAT subtests (Token-Test, word repetition, object naming) with injury of tracts / tract segments was additionally analyzed using non-parametric Mann-Whitney-U-Test comparing test scores between patients with and without respective tract injury (injury = overlap of tract and resection cavity; no injury = distance of tract and resection cavity > 0 mm). Correlation analyses (η coefficients) were additionally performed to provide effect size estimates.
A two-step, explorative cluster analysis on the injury profiles of the patients using thirteen dichotomized variables on information about all tract injury patterns - AF (frontal / temporo-parietal / temporal), ILF (anterior / middle), IFOF (frontal / middle), UF (frontal / middle / temporal), FAT (medial / middle / lateral) - was performed and resulted in 4 distinct clusters of injury patterns. For each cluster probability of new aphasia at 3 months was assessed to identify high-risk injury profiles.
Additionally, different binary logistic regression-based models were analyzed to model the relation between 3 distinct sets of independent variables and new aphasia at 3 months. As independent variables for these models a) number of tract injuries per patient, b) clusters from the cluster analysis, and c) specific high-risk profiles based on resection map and cluster analysis were used. Models were compared with regard to model fit using the coefficient of determination (Pseudo-R2), area under the curve (AUC, and 95%CI), as well as the Bayesian information criterion (BIC). SPSS Statistics (IBM Corp. 2017, Version 24.0) was used for most statistical analyses. Stata IC version 15 (StataCorp, 2017) was used for regression modelling. A two-sided significance level of α = 0.05 was used. No adjustment for multiple testing was applied in this exploratory study. Therefore, p-values have to be interpreted with caution.
3. Results
3.1. Characteristics of patients and tumors
Twenty-four women and 26 men with a median age of 49 (25–78) years were included. Tumors – mostly high-grade gliomas (WHO grade III and IV) – were most frequently located within the frontal and temporal lobe. Every patient was right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). A detailed overview of patient and tumor characteristics is presented in Table 1.
Table 1.
Patient characteristics and surgical outcome of all patients (n = 50) as well as of those with new / worsened aphasia at 7 days and of those with persisting aphasia 3 months postoperatively (SMD: standardized mean difference, effect size for differences for those with/without aphasia).
| Variable | Total | Of those: New aphasia (7 days) | SMD | Of those: New aphasia (3 months) | SMD |
|---|---|---|---|---|---|
| No. of Patients | 50 | 23 | 14 | ||
| Age - yrs | 0.13 | 0.02 | |||
| mean (SD) | 50 (14) | 49 (15) | 50 (15) | ||
| median | 49 | 46 | 50 | ||
| range | 25–78 | 28–77 | 32–77 | ||
| Sex – no. (%) | 0.32 | 0.47 | |||
| male | 26 (52.0%) | 10 (43.5%) | 5 (35.7%) | ||
| female | 24 (48.0%) | 13 (56.5%) | 9 (64.3%) | ||
| Tumor location (left hemisphere) – no. (%) | 0.77 | 1.00 | |||
| frontal | 15 (30.0%) | 4 (17.4%) | 1 (7.1%) | ||
| temporal | 18 (36.0%) | 9 (39.1%) | 5 (35.7%) | ||
| parietal | 8 (16.0%) | 3 (13.0%) | 3 (21.4%) | ||
| fronto-temporal | 3 (6.0%) | 2 (8.7%) | 1 (7.1%) | ||
| fronto-parietal | 1 (2.0%) | 1 (4.3%) | 1 (7.1%) | ||
| temporo-parietal | 5 (10.0%) | 4 (17.4%) | 3 (21.4%) | ||
| Tumor volume – ml | 0.30 | 0.28 | |||
| median | 34 | 36 | 31 | ||
| interquartile range | 15 – 59 | 14 – 77 | 12 – 82 | ||
| WHO tumor grade – no. (%) | 0.21 | 0.14 | |||
| II | 9 (18.0%) | 4 (17.4%) | 2 (14.3%) | ||
| III | 17 (34.0%) | 9 (39.1%) | 5 (35.7%) | ||
| IV | 24 (48.0%) | 10 (43.5%) | 7 (50.0%) | ||
| Extent of resection – no. (%) | 0.40 | 0.16 | |||
| GTR (gross total resection) | 26 (52.0%) | 11 (47.8%) | 8 (57.1%) | ||
| STR (subtotal resection) | 15 (30.0%) | 9 (39.1%) | 4 (28.6%) | ||
| PR (partial resection) | 9 (18.0%) | 3 (13.0%) | 2 (14.3%) | ||
| Surgery | 0.40 | 0.11 | |||
| awake | 23 (46.0%) | 13 (56.5%) | 7 (50.0%) | ||
| asleep | 27 (54.0%) | 10 (43.5%) | 7 (50.0%) | ||
| Intraoperative MRI | 0.08 | 0.20 | |||
| yes | 25 (50.0%) | 11 (47.8%) | 8 (57.1%) | ||
| no | 25 (50.0%) | 12 (52.2%) | 6 (42.9%) | ||
There were no substantial differences regarding age, sex, tumor location, tumor volume and extent of resection between unchanged and aphasic patients in both the 7 days and 3 months outcome (Table 1)
Additionally, there were no substantial differences regarding functional outcome and extent of resection between cases with and without ioMRI. Patients who underwent awake craniotomy had a lower residual tumor volume (η = 0.379; p = 0.016). Detailed information regarding awake craniotomy and intraoperative MRI are presented in the Supplementary data (Suppl.2).
3.2. Functional outcome
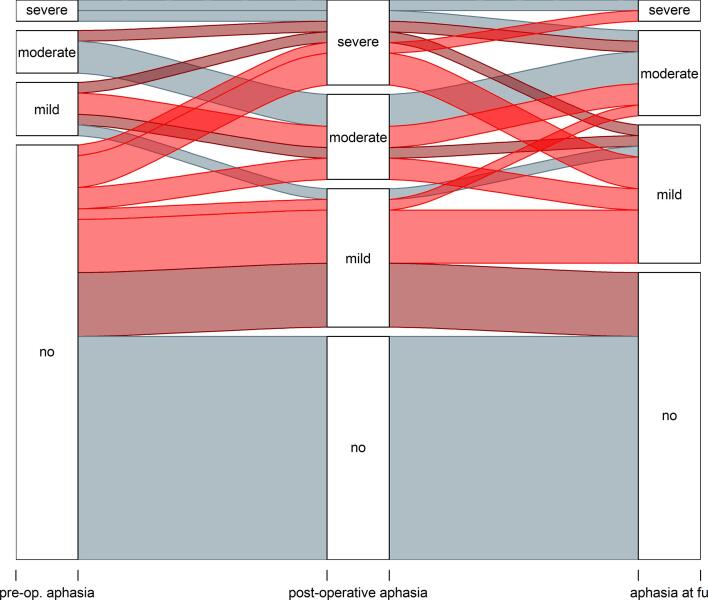
Eleven patients had an aphasia prior to surgery (22%) while 39 patients (78%) showed no preoperative language deficit. Postoperatively 23 patients (46%) developed a new aphasia or worsening of preexisting aphasia. Of those, 14 (28%) patients displayed persisting new aphasic symptoms after 3 months, while the other 9 patients recovered from their early postoperative deficit (Fig. 2). None of the patients with pre-surgical aphasia showed an improvement in the postoperative course. In none of the cases with functional deterioration the analysis of early postoperative MRI revealed ischemic events.
Fig. 2.
Language status preoperatively, postoperatively and after 3 months. Light red represents permanent functional worsening, dark red represents transient worsening. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Preoperative tract infiltration and outcome
The visualization of all tracts with their respective subsegments was feasible in every patient. The number of preoperatively infiltrated tracts (TTD = 0 mm) for each outcome group are presented in Table 2. The infiltration of the AF had the strongest association to postoperative functional worsening (36% with AF infiltration had new / worsened aphasia after 3 months vs. none of those without AF infiltration, cc = 0.314, p = 0.019). Additionally, the bigger the overlap of the AF and the tumor was (infiltrated volume), the higher was the incidence of new aphasia (Mdnaphasia = 0.26 ml; Mdnunchanged = 0.075 ml; = 0.354; p = 0.005). The infiltration of the other tracts was not substantially associated with functional worsening (Table 2).
Table 2.
Group comparison and association of preoperative infiltration of tracts to outcome after 7 days (new transient aphasia) and 3 months (new permanent aphasia). P-values (chi-squared-test) and contingency coefficient are presented.
| Association of tract injury and permanent aphasia |
||||||
|---|---|---|---|---|---|---|
| Tract | n | New transient aphasia (n = 9) | New permanent aphasia (n = 14) | P-value | Correlation: contingency coefficient | |
| AF | yes | 39 | 7 (17.9%) | 14 (35.9%) | 0.019 | 0.314 |
| no | 11 | 2 (18.1%) | – | |||
| FAT | yes | 19 | 3 (15.8%) | 3 (15.8%) | 0.132 | 0.208 |
| no | 31 | 6 (19.3%) | 11 (35.5%) | |||
| IFOF | yes | 33 | 7 (21.2%) | 10 (30.3%) | 0.613 | 0.071 |
| no | 17 | 2 (11.8%) | 4 (23.5%) | |||
| ILF | yes | 25 | 5 (20%) | 8 (32.0%) | 0.529 | 0.089 |
| no | 25 | 4 (16%) | 6 (24.0%) | |||
| UF | yes | 24 | 4 (16.7%) | 6 (25.0%) | 0.650 | 0.064 |
| no | 26 | 5 (19.2%) | 8 (30.8%) | |||
3.4. Postoperative resection map analysis
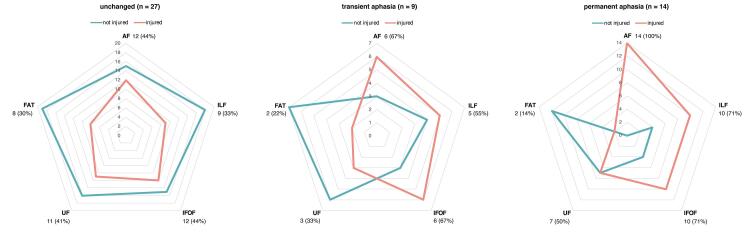
The number of injured tracts among the outcome groups is presented in Fig. 3. The AF was injured in every patient (n = 14) with new permanent aphasia after 3 months, while only 29.6% of patients (8 out of 27) without any new language deficit and 55.6% of patients (5 out of 9) with a transient deficit showed an injury to the AF (cc = 0.519; p < 0.001). The IFOF and ILF were also more often injured in patients with new permanent aphasia (IFOF injury: 40.7% of unchanged, 55.6% of transient, 71.4% of permanent, cc = 0.257; p = 0.171 / ILF injury: 18.5% of unchanged, 33.3% of transient, 71.4% of permanent, cc = 0.428; p = 0.004). On the contrary, injury of the UF and the FAT did not differ largely between outcome groups (UF injury: 40.7% of unchanged, 33.3% of transient, 50.0% of permanent, cc = 0.114; p = 0.718; FAT injury: 14.8% of unchanged, 22.2% of transient, 14.3% of permanent, cc = 0.080; p = 0.853, Fig. 3).
Fig. 3.
Comparison of tract injuries between unchanged patients (left), patients with transient (middle) and with permanent new aphasia (right) after 3 months; contingency coefficients (cc) and p-values (chi-squared test): AF: cc = 0.519 (p < 0.001); ILF: cc = 0.428 (p = 0.004); IFOF: cc = 0.257 (p = 0.171); UF: cc = 0.114 (p = 0.718); FAT: cc = 0.080 (p = 0.853).
3.5. Resection map analysis of subsegments of tracts
Injured volumes of individual subsegments of tracts and their association to the outcome are presented in Table 3. The injured volume of the temporo-parietal segment of the AF ( = 0.495; p = 0.002) and the middle segment of the IFOF ( = 0.483; p = 0.002) was higher in patients with new permanent aphasia compared with patients without new aphasia. In contrast, injury to the frontal segments of the respective tracts (AF, IFOF) had no distinct relation to aphasia (Table 3). Further, injury of the middle segment of the ILF was statistically significantly associated with new permanent aphasia ( = 0.385; p = 0.026), while injury to the subsegments of the UF and FAT showed no substantial relation to functional worsening (Table 3).
Table 3.
Comparison of injured volume of each tract segment between the outcome groups (unchanged, transient aphasia, permanent aphasia); p-values (Kruskal-Wallis-Test) and correlation coefficients (Spearman-Rho;) are presented. Bold faced values highlight tract segments with particular correlation. * median and interquartile range
| Tract | Unchanged: injured volume* – in ml (N = 27) | Transient aphasia: injured volume* – in ml (N = 9) | Permanent aphasia: injured volume* – in ml (N = 14) | p-value | Spearman’s ρ |
|---|---|---|---|---|---|
| AF | |||||
| frontal | 0 (0) | 0 (0 – 0.17) | 0 (0) | 0.779 | − 0.008 |
| temporo-parietal | 0 (0) | 0 (0 – 0.36) | 0.16 (0–2.40) | 0.002 | 0.495 |
| temporal | 0 (0) | 0 (0) | 0 (0 – 0.05) | 0.161 | 0.256 |
| FAT | |||||
| medial | 0 (0) | 0 (0) | 0 (0) | 0.503 | − 0.107 |
| middle | 0 (0) | 0 (0) | 0 (0) | 0.155 | 0.272 |
| lateral | 0 (0) | 0 (0) | 0 (0) | 0.419 | − 0.180 |
| IFOF | |||||
| frontal | 0 (0) | 0 (0 – 0.01) | 0 (0) | 0.491 | − 0.146 |
| middle | 0 (0) | 0 (0–0.22) | 0.20 (0 – 1.02) | 0.002 | 0.483 |
| occipital | – | – | – | – | – |
| ILF | |||||
| anterior | 0 (0) | 0 (0) | 0 (0 – 3.36) | 0.248 | 0.157 |
| middle | 0 (0) | 0 (0 – 0.62) | 0 (0 – 0.24) | 0.027 | 0.385 |
| occipital | – | – | – | – | – |
| UF | |||||
| frontal | 0 (0) | 0 (0–0.10) | 0 (0) | 0.595 | − 0.066 |
| middle | 0 (0) | 0 (0 – 0) | 0 (0 – 0.01) | 0.206 | 0.158 |
| temporal | 0 (0) | 0 (0) | 0 (0 – 0.28) | 0.683 | 0.097 |
3.6. Association of tract injuries with distinct linguistic qualities
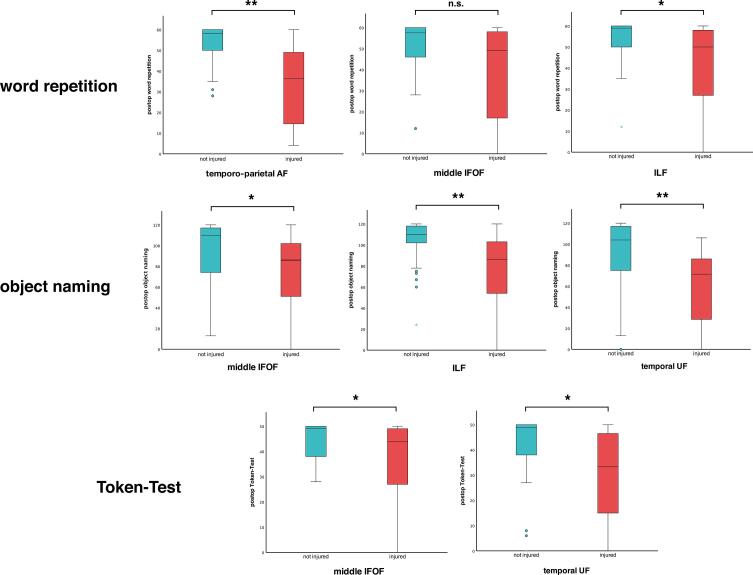
Associations of tract injuries with early postoperative performance in individual subtests of the AAT are presented in Fig. 4.
Fig. 4.
Boxplots comparing early postoperative scores of AAT subtests between patients with (red) and without (blue) tract injuries. Tracts / tract segments with particular association of injury with lower scores of the respective subtests are presented. Correlation coefficients (η) and p-values (Mann-Whitney-U-Test): word repetition (temporo-parietal AF: η = 0.433; p = 0.009; middle IFOF: η = 0.370; p = 0.066; ILF: η = 0.331; p = 0.031); object naming (middle IFOF: η = 0.314; p = 0.019; ILF: η = 0.383; p = 0.003; temporal UF: η = 0.363; p = 0.009); Token-Test (middle IFOF: η = 0.256; p = 0.05; temporal UF: η = 0.271; p = 0.042). * = p < 0.05; ** = p < 0.01; n.s. = non-significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Injury to the temporo-parietal segment of the AF (Mdninjury = 36.5; Mdnno injury = 58; η = 0.433; p = 0.009) was significantly associated with worsened word repetition test performance postoperatively. In addition, injury to the middle IFOF (Mdninjury = 49; Mdnno injury = 57.5; η = 0.370; p = 0.066) and ILF (Mdninjury = 48; Mdnno injury = 59; η = 0.331; p = 0.031) was associated with lower postoperative word repetition scores. Regarding object naming, injury to the middle IFOF (Mdninjury = 86; Mdnno injury = 110; η = 0.314; p = 0.019), ILF (Mdninjury = 86; Mdnno injury = 110; η = 0.383; p = 0.003) and temporal UF (Mdninjury = 71.5; Mdnno injury = 104; η = 0.363; p = 0.009) was notably associated with lower postoperative test scores. Injury to the middle IFOF and temporal UF was associated with worse performances in the Token-Test postoperatively (middle IFOF: Mdninjury = 44; Mdnno injury = 49; η = 0.256; p = 0.05; temporal UF: Mdninjury = 33.5; Mdnno injury = 49; η = 0.271; p = 0.042; Fig. 4).
3.7. Cluster analysis of tract injuries
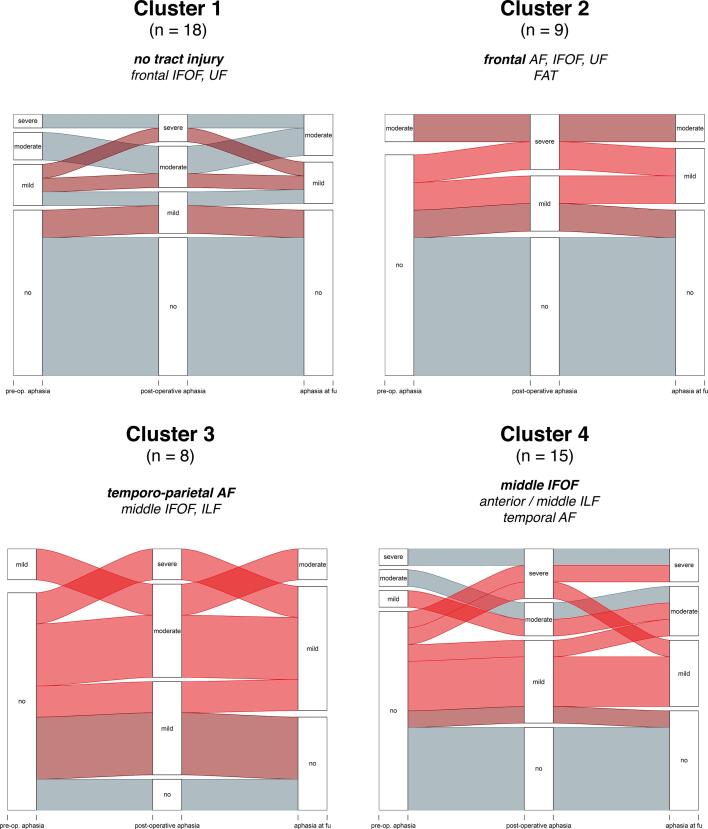
Two-step cluster analysis of individual injury profiles of tract segments among all patients (segments being simultaneously injured) resulted in 4 specific clusters, which are presented in Fig. 5.
Fig. 5.
Clusters of tract injury profiles revealed by two-step cluster analysis. For each cluster, the main injury profile as well as the language status of the patients preoperatively, postoperatively and after 3 months is presented. Light red represents permanent functional worsening, dark red represents transient worsening.
Cluster 1 (n = 18) mainly includes patients with no tract injury (n = 10) as well as patients with injury to the frontal segments of the IFOF and UF and segments of the FAT (n = 8). None of the patients in this cluster suffered from a persisting new aphasia. Cluster 2 (n = 9) only includes patients with injuries to frontal tracts / tract segments (frontal AF, IFOF, UF and the FAT). In this cluster, 2 patients developed a persisting new aphasia (22.2%).
Cluster 3 (n = 8) mainly includes patients with injury to the temporo-parietal AF as well as the middle IFOF and ILF. Among those, 62.5% of patients had persisting aphasic symptoms (n = 5). Cluster 4 (n = 15) predominantly includes patients with injuries to the temporal AF, middle IFOF, anterior / middle ILF and temporal / middle UF. In this cluster, 46.7% of patients (n = 7) had persisting aphasic symptoms (Fig. 4). Detailed descriptions of tract injuries within each cluster are presented in the Supplementary data (Suppl.3).
3.8. Analysis of anatomical location of tract injuries and their association with the outcome
The different clusters and their individual injury profiles can each be assigned to distinct anatomical locations in the cerebral white matter. Cluster 1 and in particular Cluster 2 mostly represent the frontal white matter – including the frontal segment of the AF, IFOF, UF and the entirety of the FAT. In these “frontal” clusters, the incidence of persisting language deficits is comparably low (see 3.7).
In contrast, injury profiles of clusters 3 and 4 are more strongly associated with the development of persisting aphasic symptoms and can be assigned to the following regions (visualized in Fig. 6):
-
-
the subcortical temporo-parieto-occipital junction (TPO), which contains the temporo-parietal segment of the AF as well as the middle IFOF and ILF (Cluster 3)
-
-
the temporal stem and peri-insular white matter, which contains the middle segment of the IFOF as well as the anterior / middle ILF, temporal / middle UF and the temporal AF (Cluster 4).
Fig. 6.
Tractography of the language network: highlighted areas in the red circles indicate subcortical functional nodes particularly sensitive to surgical injury: the temporo-parieto-occipital (TPO) junction (temporo-parietal AF, middle IFOF and ILF) and the temporal stem / peri-insular white matter (middle IFOF, ILF, UF and temporal AF). This figure was created using MRtrix3 and TractSeg (Tournier et al., 2019, Wasserthal et al., 2018). To highlight the areas of interest, Adobe® Photoshop® software was used.
3.9. Regression-based models of the association of tract injury and outcome
Three different logistic regression-based models for analyzing the association of tract injury and the outcome are presented in Table 4. Model 1 showed that the higher the number of tract injuries per patient was, the higher was the risk of postoperative deficits (OR: 2.31; CI: 1.37 – 3.88). In Model 2, the associations between the different clusters of tract injuries from the cluster analysis (Fig. 5) and the outcome are analyzed. Cluster 3 (TPO; OR: 58.14; CI: 2.59 – 1306.66) and Cluster 4 (temporal stem; OR: 32.65; CI: 1.67 – 639.98) were particularly associated with a higher risk of aphasia, while Cluster 2 (frontal white matter; OR: 12.33; CI: 0.53 – 288.58) did not amount to a statistically significant higher risk of functional worsening.
Table 4.
Odds ratios (95%CI) and model fit parameters for 3 different logistic regression-based models analyzing associations of tract injuries and aphasia after 3 months (n = 50 patients, 14 with permanent aphasia). tp-AF = temporo-parietal AF; a regression-coefficients are based on firthlogit command (Stata version 15 IC) that uses penalized maximum likelihood estimation.
| Model 1 | Model 2a | Model 3a | |
|---|---|---|---|
| Number of tract injuries per patient | 2.31 (1.37–3.88) | – | – |
| Cluster | – | – | |
| 1 | reference | ||
| 2 | 12.33 (0.53–288.58) | ||
| 3 | 58.14 (2.59–1306.66) | ||
| 4 | 32.65 (1.67–639.98) | ||
| Injury to tp-AF | – | – | 23.04 (4.11–129.06) |
| Injury to middle IFOF, anterior / middle ILF, temporal AF | – | – | 21.96 (2.93–164.41) |
| Pseudo-R2 | 0.19 | 0.31 | 0.36 |
| AUC (95%CI) | 0.79 (0.66–0.92) | 0.83 (0.73–0.94) | 0.85 (0.73–0.96) |
| Degrees of freedom (df) | 2 | 4 | 3 |
| BIC | 55.7 | 56.5 | 49.6 |
Model 3 directly analyzed the association of distinct, potential high-risk injury profiles (based on the preceding resection map and cluster analyses) with the outcome. Injury to the temporo-parietal AF (OR: 23.04; CI: 4.11 – 129.06) or injury to the middle IFOF, anterior / middle ILF and temporal AF (OR: 21.96; CI: 2.93 – 164.41) was associated with a higher risk of persisting aphasia.
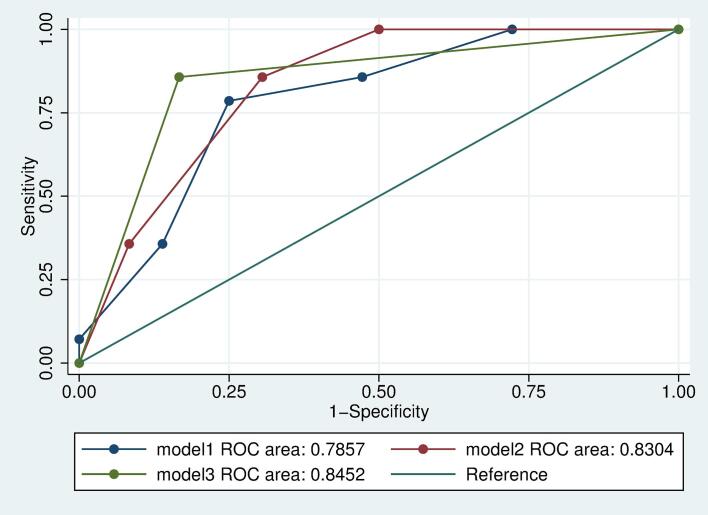
The comparison of the three different models in regard to model fit is visualized in Fig. 6 (ROC curves). As can be seen in the figure and according to the model parameters, model 3 - injury to the temporo-parietal AF (TPO) or injury to the middle IFOF, anterior / middle ILF and temporal AF (temporal stem) - provides the best model fit with lowest BIC value and highest R2 value with 0.36 and AUC (0.85 [0.73–0.96], Table 4, Fig. 7).
Fig. 7.
Receiver Operating Characteristic (ROC) curves for 3 different logistic regression-based models analyzing relations of tract injuries to the outcome after 3 months utilizing different independent variables. Model 1: number of tract injuries per patient; Model 2: clusters from two-step cluster analysis of tract injury profiles; Model 3: specific high-risk tract injury profiles.
4. Discussion
The present study showed a clear association of tractography-based results with the functional outcome and confirmed previous findings on the relevance of individual tracts. While previous studies could already show an association between language outcome and DTI-based tractography in brain tumor patients (Hayashi et al., 2012, Ille et al., 2018, Negwer et al., 2018, Sollmann et al., 2020), these studies were either limited by relatively small sample sizes or did not evaluate individual tracts in detail.
Our data show that in particular injury to the temporo-parieto-occipital junction (temporo-parietal AF, middle IFOF, ILF) and the temporal stem / peri-insular white matter (middle IFOF, ILF, UF and temporal AF) is associated with a higher risk of aphasia. Thus, standardized DTI-based tractography of language pathways provides a clinical tool to determine the individual aphasia risk profile pre-surgically.
4.1. Language pathways and their association with aphasia
4.1.1. The Arcuate Fasciculus and the dorsal language stream
The left AF has been previously described as the main subcortical component of the dorsal language stream (Catani et al., 2005, Dick et al., 2014, Saur et al., 2008). DES of the AF during awake craniotomy reliably evokes complete speech arrests and phonological paraphasia (Ries et al., 2019). Our results further reinforce these findings, as the preoperative tumorous infiltration as well as the surgical injury of the AF are associated with a greater risk of aphasia. In addition, injury to the (temporo-parietal) AF was particularly associated with worsening of early postoperative word repetition – reinforcing the classical concept of “conduction aphasia”, which is characterized by impaired word repetition and phonological paraphasia following damage to the AF (Bernal & Ardila, 2009).
In line with earlier findings (Caverzasi et al., 2016, Southwell et al., 2017), the temporo-parietal segment of the AF had the strongest association to the development of permanent aphasia when injured. It can be hypothesized that a lesion to the middle segment of an association tract, where the majority of axonal bundles converge, is functionally more debilitating than a lesion closer to the cortical surface, because it leads to a wider disconnection of the cortex (Catani & Mesulam, 2008).
4.1.2. The Inferior Fronto-Occipital Fasciculus and the ventral language stream
The left IFOF is increasingly considered to be an essential pathway for semantic processing as part of the ventral language stream (Almairac et al., 2015, Duffau, 2005). In the present study, the surgical injury to the (middle) IFOF revealed to be particularly associated with the development of persistent aphasia. Similar to the AF, the middle segment of the IFOF showed the strongest association with aphasia when injured – thus further verifying the functional relevance of the middle segment of an association tract.
While injury to the ILF (in particular the middle segment) showed mild association to the outcome in resection map analysis, the UF was not notably associated with functional worsening.
This further suggests - as previously hypothesized - the existence of a direct (IFOF) and indirect pathway (ILF, UF) of the ventral language stream. According to this, lesions to the direct pathway (IFOF) are more likely to lead to persisting language deficits than lesions to the indirect pathway (ILF and UF) (Duffau et al., 2013, Mandonnet et al., 2007). Furthermore, the proposed core function of the ventral language stream regarding semantic processing is further reinforced by the present study as injury to ventral tracts (middle IFOF, ILF and temporal UF) is notably associated with worse early postoperative performance in tasks involving object recognition and semantic retrieval (object naming and Token-Test).
4.1.3. The Frontal Aslant Tract: A relevant pathway for language?
The FAT was previously described to play a role in the planning of the articulation process (Catani et al., 2013, Dick et al., 2019). In the present study, the tumorous infiltration or surgical injury was not associated with permanent aphasia. This could be due to the fact that deficits caused by FAT lesions are often transient in nature (Dick et al., 2019). Further, speech-related deficits such as reduced verbal fluency need to be clearly distinguished from “core” language deficits and are not separately accounted for in this study (Finkl et al., 2020).
4.2. Functional nodes and the “Minimal Common Brain”
The potential of the adult brain for postlesional plastic reorganization is substantial, yet limited by the integrity of the subcortical connectivity (Duffau, 2014c). Thus, a “minimal common brain” is hypothesized to exist for each functional network - composed of critical functional areas in the cortex and white matter representing the essential connectivity needed to enable postlesional functional rehabilitation by brain plasticity (Ius et al., 2011, Lee et al., 2020, Müller et al., 2020, Sarubbo et al., 2020).
Our results confirm the existence of such functional nodes in the cerebral white matter, where the majority of connections of a functional system converge densely and surgical injury is most likely to result in a permanent deficit.
In the case of language function, these functional nodes may be represented by the TPO – mainly containing the temporo-parietal segment of the AF - for the dorsal language stream (De Benedictis et al., 2014, Southwell et al., 2017) and the temporal stem / peri-insular white matter – mainly containing the middle segment of the IFOF (direct pathway) as well as the ILF and UF (indirect pathway) - for the ventral language stream (Martino et al., 2010).
Similar functional properties as well as compensatory mechanisms in case of injury were described for both language streams (Hula et al., 2020, López-Barroso et al., 2013). The AF and IFOF are located in close proximity to each other in both the TPO and the peri-insular white matter – leading to possible simultaneous disconnection of the dorsal and ventral pathways. This is further reinforced by the observed association of injury to the middle IFOF and ILF with impaired word repetition along with the AF and worsening in semantic tasks along with the UF (object naming, Token-Test). If both the AF and IFOF were to be injured in these functional nodes, possible compensatory mechanisms can no longer be effective – resulting in a high probability of a new postoperative language deficit.
4.3. Towards a tractography-based risk stratification
While DTI-based tractography generally provides an accessible tool for presurgical planning and risk assessment in glioma surgery (Costabile et al., 2019), its routine use in clinical practice is not yet established (Duffau, 2014a, Duffau, 2014b).
The present study showed that the functionally critical interface between tumor and white matter can be reliably localized preoperatively by the means of standardized tractography and used for surgical planning. Aided by functional neuro-navigation these functional nodes can then be specifically spared during surgery to maintain the essential connectivity of the language network. If applicable, cortical and subcortical stimulation mapping during awake craniotomy for surveillance of language function should be performed in these cases.
4.4. Limitations
4.4.1. Study design
The patient number (n = 50) used in this exploratory study limits the logistic regression analysis, resulting in large confidence intervals in the multivariable predictor models. Bigger sample sizes are needed to calculate more robust statistical models. Nevertheless, our analysis reveals a significant association between postoperative aphasia and an injury of certain functional nodes.
Furthermore, we solely analyzed the white matter and correlated its infiltration or injury with the functional outcome. Deficits that may have occurred due to cortical lesions or combined cortico-subcortical lesions were not separately analyzed.
While every patient was a German native speaker, bilingualism or further spoken languages were not separately taken into account. Additionally, the hemispheric language dominance was not separately evaluated in this study. As the hemispheric dominance ranges even among right-handed subjects, this has to be considered as a potential limitation.
The incidence of persistent aphasic symptoms (28%) after surgery is relatively high compared to previous findings. This may be explained by the detailed language assessment through the AAT applied for this study. The effect of a reduced AAT score on individual quality of life is not clear and was not assessed in our study. This aspect needs to be further investigated in future studies. Moreover, the AAT was originally designed for assessing post-stroke aphasia and is therefore not yet fully validated for brain tumor patients (Huber et al., 1984).
Another limiting factor may be that the AAT was only performed in 26% of the 3 months follow-up examinations while the BAS was used in the other patients. Despite the BAS being adapted to the AAT (Picht et al., 2013), potential differences between the two testś sensitivity to detect language impairment cannot be ruled out.
4.4.2. Awake craniotomy, intraoperative MRI and surgical outcome
Patients undergoing awake craniotomy must meet certain inclusion criteria such as anaesthesiological, cognitive and psychological eligibility. In the awake group the amount of residual disease was significantly lower than in the asleep group while the functional outcome did not differ. No significant differences in the extent of resection were observed between cases with and without ioMRI. This is probably because ioMRI was performed more often in cases of higher complexity.
However, in order to analyze the effects of ioMRI and awake craniotomy on surgical outcome in detail, larger study cohorts and careful case separation and classification need to be considered.
4.4.3. Technical limitations
The analyses in our study are based exclusively on imaging studies, correlated with the postoperative language status. Despite efforts to avoid misalignments during fusion using distortion correction, errors due to brain shift or variable image acquisition protocols cannot be ruled out.
Tractography itself suffers from a variety of limitations that render its routine use difficult (Schilling et al., 2019). It is known that tractography results contain false positive (Maier-Hein et al., 2017) and false negative (Aydogan et al., 2018) streamlines, which may also terminate improperly (Tournier, 2019).
4.5. Conclusions
Our results show a clear association between TPO (temporo-parietal AF, middle IFOF, ILF) and temporal stem / peri-insular white matter (middle IFOF, ILF, UF and temporal AF) injury with postoperative aphasia and further confirm the functionality of individual tracts. In contrast, FAT injury was not associated with persisting aphasia. These results indicate that standardized language tractography can help to non-invasively identify critical functional nodes and further individualize the preoperative risk assessment in patients suffering from language area associated gliomas.
Funding
This work was supported by the DFG (EXC 2025). The views expressed are those of the author(s) and not necessarily those of the DFG.
CRediT authorship contribution statement
Mehmet Salih Tuncer: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - review & editing, Visualization. Luca Francesco Salvati: Investigation. Ulrike Grittner: Methodology, Formal analysis. Juliane Hardt: Methodology, Formal analysis. Ralph Schilling: Formal analysis. Ina Luca Bährend Leandro Silva: Investigation, Investigation. Lucius S. Fekonja: Visualization, Writing - review & editing. Katharina Faust: Investigation, Resources. Peter Vajkoczy: Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition. Tizian Rosenstock: Conceptualization, Methodology, Validation, Formal analysis, Writing - review & editing, Visualization, Supervision, Project administration. Thomas Picht: Conceptualization, Methodology, Validation, Formal analysis, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Heike Schneider for her assistance in the planning and execution of patient examinations. Dr. Rosenstock is participant in the BIH-Charité Junior Digital Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102541.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Almairac F., Herbet G., Moritz-Gasser S., de Champfleur N.M., Duffau H. The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Struct. Funct. 2015;220(4):1983–1995. doi: 10.1007/s00429-014-0773-1. [DOI] [PubMed] [Google Scholar]
- Aydogan D.B., Jacobs R., Dulawa S., Thompson S.L., Francois M.C., Toga A.W. When tractography meets tracer injections: a systematic study of trends and variation sources of diffusion-based connectivity. Brain Struct. Funct. 2018;223(6):2841–2858. doi: 10.1007/s00429-018-1663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bährend, I., Muench, M. R., Schneider, H., Moshourab, R., Dreyer, F. R., Vajkoczy, P., et al., 2020. Incidence and linguistic quality of speech errors: a comparison of preoperative transcranial magnetic stimulation and intraoperative direct cortex stimulation. Journal of Neurosurgery, 1-10. DOI:10.3171/2020.3.JNS193085. [DOI] [PubMed]
- Bernal B., Ardila A. The role of the arcuate fasciculus in conduction aphasia. Brain. 2009;132(9):2309–2316. doi: 10.1093/brain/awp206. [DOI] [PubMed] [Google Scholar]
- Catani, M., Mesulam, M. M., Jakobsen, E., Malik, F., Martersteck, A., Wieneke, C., et al., 2013. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain, 136(8), 2619-2628. DOI:10.1093/brain/awt163. [DOI] [PMC free article] [PubMed]
- Catani M., Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Jones D.K., ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Caverzasi E., Hervey-Jumper S.L., Jordan K.M., Lobach I.V., Li J., Panara V. Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas. JNS. 2016;125(1):33–45. doi: 10.3171/2015.6.JNS142203. [DOI] [PubMed] [Google Scholar]
- Chang E.F., Raygor K.P., Berger M.S. Contemporary model of language organization: an overview for neurosurgeons. JNS. 2015;122(2):250–261. doi: 10.3171/2014.10.JNS132647. [DOI] [PubMed] [Google Scholar]
- Costabile J.D., Alaswad E., D’Souza S., Thompson J.A., Ormond D.R. Current applications of diffusion tensor imaging and tractography in intracranial tumor resection. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis A., Duffau H., Paradiso B., Grandi E., Balbi S., Granieri E. Anatomo-functional study of the temporo-parieto-occipital region: dissection, tractographic and brain mapping evidence from a neurosurgical perspective. J. Anat. 2014;225(2):132–151. doi: 10.1111/joa.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Bernal B., Tremblay P. The language connectome: new pathways, new concepts. Neuroscientist. 2014;20(5):453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Dick A.S., Garic D., Graziano P., Tremblay P. The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex. 2019;111:148–163. doi: 10.1016/j.cortex.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128(4):797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H. The dangers of magnetic resonance imaging diffusion tensor tractography in brain surgery. World Neurosur. 2014;81(1):56–58. doi: 10.1016/j.wneu.2013.01.116. [DOI] [PubMed] [Google Scholar]
- Duffau H. Diffusion tensor imaging is a research and educational tool, but not yet a clinical tool. World Neurosurg. 2014;82(1-2):e43–e45. doi: 10.1016/j.wneu.2013.08.054. [DOI] [PubMed] [Google Scholar]
- Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi: 10.1016/j.cortex.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Duffau H., Gatignol P., Moritz-Gasser S., Mandonnet E. Is the left uncinate fasciculus essential for language? A cerebral stimulation study. J. Neurol. 2009;256(3):382–389. doi: 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Duffau H., Herbet G., Moritz-Gasser S. Toward a pluri-component, multimodal, and dynamic organization of the ventral semantic stream in humans: lessons from stimulation mapping in awake patients. Front. Syst. Neurosci. 2013;7 doi: 10.3389/fnsys.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekonja L., Wang Z., Bährend I., Rosenstock T., Rösler J., Wallmeroth L. Manual for clinical language tractography. Acta Neurochir. 2019;161(6):1125–1137. doi: 10.1007/s00701-019-03899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkl, T., Hahne, A., Friederici, A. D., Gerber, J., Mürbe, D., & Anwander, A. Language Without Speech: Segregating Distinct Circuits in the Human Brain. Cerebral Cortex. DOI:10.1093/cercor/bhz128. [DOI] [PubMed]
- Gerhardt J., Sollmann N., Hiepe P., Kirschke J.S., Meyer B., Krieg S.M., Ringel F. Retrospective distortion correction of diffusion tensor imaging data by semi-elastic image fusion – evaluation by means of anatomical landmarks. Clin. Neurol. Neurosurg. 2019;183:105387. doi: 10.1016/j.clineuro.2019.105387. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Kinoshita M., Nakada M., Hamada J.-I. Correlation between language function and the left arcuate fasciculus detected by diffusion tensor imaging tractography after brain tumor surgery: Clinical article. JNS. 2012;117(5):839–843. doi: 10.3171/2012.8.JNS12348. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Huber W., Poeck K., Willmes K. The Aachen Aphasia Test. Adv. Neurol. 1984;42:291–303. http://www.ncbi.nlm.nih.gov/pubmed/6209953 Retrieved from. [PubMed] [Google Scholar]
- Hula, W. D., Panesar, S., Gravier, M. L., Yeh, F.-C., Dresang, H. C., Dickey, M. W., & Fernandez-Miranda, J. C. Structural white matter connectometry of word production in aphasia: an observational study. Brain. DOI:10.1093/brain/awaa193. [DOI] [PMC free article] [PubMed]
- Ille S., Engel L., Kelm A., Meyer B., Krieg S.M. Language-eloquent white matter pathway tractography and the course of language function in glioma patients. Front. Oncol. 2018;8:572. doi: 10.3389/fonc.2018.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ius T., Angelini E., Thiebaut de Schotten M., Mandonnet E., Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: Towards a “minimal common brain”. NeuroImage. 2011;56(3):992–1000. doi: 10.1016/j.neuroimage.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Kohn S.E., Goodglass H. Picture-naming in aphasia. Brain Lang. 1985;24(2):266–283. doi: 10.1016/0093-934X(85)90135-X. [DOI] [PubMed] [Google Scholar]
- Lee, A. T., Faltermeier, C., Morshed, R. A., Young, J. S., Kakaizada, S., Valdivia, C., et al., 2020. The impact of high functional connectivity network hub resection on language task performance in adult low- and high-grade glioma. Journal of Neurosurgery, -1(aop), 1-11. DOI:10.3171/2020.1.JNS192267. [DOI] [PMC free article] [PubMed]
- López-Barroso D., Catani M., Ripollés P., Dell'Acqua F., Rodríguez-Fornells A., Diego-Balaguer R. Word learning is mediated by the left arcuate fasciculus. Proc. Natl. Acad. Sci. 2013;110(32):13168–13173. doi: 10.1073/pnas.1301696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein Klaus H., Neher Peter F., Houde Jean-Christophe, Côté Marc-Alexandre, Garyfallidis Eleftherios, Zhong Jidan. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017;8(1) doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E., Nouet A., Gatignol P., Capelle L., Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130(3):623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Martino, J., Vergani, F., Robles, S. G., & Duffau, H., 2010. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery, 66(3 Suppl Operative), 4-12. DOI:10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed]
- Meyer, E. J., Gaggl, W., Gilloon, B., Swan, B., Greenstein, M., Voss, J., et al., 2017. The Impact of Intracranial Tumor Proximity to White Matter Tracts on Morbidity and Mortality: A Retrospective Diffusion Tensor Imaging Study. Neurosurgery, 80(2), 193-200. DOI:10.1093/neuros/nyw040. [DOI] [PMC free article] [PubMed]
- Mori Susumu, Crain Barbara J., Chacko V.P., Van Zijl Peter C.M. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. http://www.ncbi.nlm.nih.gov/pubmed/9989633 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Müller, D. M. J., Robe, P. A., Ardon, H., Barkhof, F., Bello, L., Berger, M. S., et al., 2020. Quantifying eloquent locations for glioblastoma surgery using resection probability maps. Journal of Neurosurgery, -1(aop), 1-11. DOI:10.3171/2020.1.JNS193049. [DOI] [PubMed]
- Negwer Chiara, Beurskens Eva, Sollmann Nico, Maurer Stefanie, Ille Sebastian, Giglhuber Katrin. Loss of subcortical language pathways correlates with surgery-related aphasia in patients with brain tumor: an investigation via repetitive navigated transcranial magnetic stimulation–based diffusion tensor imaging fiber tracking. World Neurosurg. 2018;111:e806–e818. doi: 10.1016/j.wneu.2017.12.163. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Picht, T., Krieg, S. M., Sollmann, N., Rösler, J., Niraula, B., Neuvonen, T., et al., 2013. A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery, 72(5), 808-819. DOI:10.1227/NEU.0b013e3182889e01. [DOI] [PubMed]
- Raffa G., Conti A., Scibilia A., Sindorio C., Quattropani M.C., Visocchi M. Functional reconstruction of motor and language pathways based on navigated transcranial magnetic stimulation and DTI Fiber tracking for the preoperative planning of low grade glioma surgery: a new tool for preservation and restoration of eloquent networks. Acta Neurochirurgica Supplement. 2017;124:251–261. doi: 10.1007/978-3-319-39546-3_37. [DOI] [PubMed] [Google Scholar]
- Ries S.K., Piai V., Perry D., Griffin S., Jordan K., Henry R. Roles of ventral versus dorsal pathways in language production: an awake language mapping study. Brain Lang. 2019;191:17–27. doi: 10.1016/j.bandl.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai Nader, Mirzadeh Zaman, Berger Mitchel S. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 2008;358(1):18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Sarubbo Silvio, Tate Matthew, De Benedictis Alessandro, Merler Stefano, Moritz-Gasser Sylvie, Herbet Guillaume, Duffau Hugues. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. NeuroImage. 2020;205:116237. doi: 10.1016/j.neuroimage.2019.116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur Dorothee, Kreher Björn W., Schnell Susanne, Kümmerer Dorothee, Kellmeyer Philipp, Vry Magnus-Sebastian. Ventral and dorsal pathways for language. PNAS. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling Kurt G., Nath Vishwesh, Hansen Colin, Parvathaneni Prasanna, Blaber Justin, Gao Yurui. Limits to anatomical accuracy of diffusion tractography using modern approaches. NeuroImage. 2019;185:1–11. doi: 10.1016/j.neuroimage.2018.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann, N., Zhang, H., Fratini, A., Wildschuetz, N., Ille, S., Schröder, A., et al., 2020. Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors. Cancers, 12(5), 1264. DOI:10.3390/cancers12051264. [DOI] [PMC free article] [PubMed]
- Sollmann, N., Fratini, A., Zhang, H., Zimmer, C., Meyer, B., & Krieg, S. M., 2019. Associations between clinical outcome and tractography based on navigated transcranial magnetic stimulation in patients with language-eloquent brain lesions. Journal of Neurosurgery, 1-10. DOI:10.3171/2018.12.JNS182988. [DOI] [PubMed]
- Southwell, D. G., Riva, M., Jordan, K., Caverzasi, E., Li, J., Perry, D. W., et al., 2017. Language outcomes after resection of dominant inferior parietal lobule gliomas. Journal of Neurosurgery, 127(4), 781-789. DOI:10.3171/2016.8.JNS16443. [DOI] [PubMed]
- Tournier J-Donald. Diffusion MRI in the brain – theory and concepts. Prog. Nucl. Magn. Reson. Spectrosc. 2019;112-113:1–16. doi: 10.1016/j.pnmrs.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Tournier J-Donald, Smith Robert, Raffelt David, Tabbara Rami, Dhollander Thijs, Pietsch Maximilian. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019;202:116137. doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Wasserthal Jakob, Neher Peter, Maier-Hein Klaus H. TractSeg - Fast and accurate white matter tract segmentation. NeuroImage. 2018;183:239–253. doi: 10.1016/j.neuroimage.2018.07.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.