Highlights
-
•
Shikonin (SHK) induced apoptosis in Primary effusion lymphoma (PEL).
-
•
SHK treatment rabidly generated ROS and activated JNK and p38.
-
•
SHK inhibited ascites formation in xenograft mice.
Keywords: Primary effusion lymphoma (PEL), Shikonin (SHK), Reactive oxygen species (ROS), Apoptosis, Mouse model
Abstract
Primary effusion lymphoma (PEL) is an incurable non-Hodgkin's lymphoma and novel biology-based treatments are urgently needed in clinical settings. Shikonin (SHK), a napthoquinone derivative, has been used for the treatment of solid tumors. Here, we report that SHK is an effective agent for the treatment of PEL. Treatment with SHK results in significant reduction of proliferation in PEL cells and their rapid apoptosis in vitro. SHK-induced apoptosis of PEL cells is accompanied by the generation of reactive oxygen species (ROS), loss of mitochondrial membrane potential (Δψm), an activation of c-Jun-N-terminal kinase (JNK), p38, as well as caspase-3, -8, and -9. Scavenging of ROS in the presence of N-acetylcysteine (NAC) almost blocks the loss of mitochondrial membrane Δψm, activation of JNK, cleavage of caspase-3, -9, and an induction of apoptosis in SHK treated PEL cells. SP600125, a specific inhibitor of JNK, also rescues a proportion of cells from the apoptotic effect of SHK. In addition, inhibition of caspase activation in the presence of pan-caspase inhibitor, Q-VD-OPh, blocks the SHK-inducing apoptosis, but doesn't completely inhibit SHK-mediated JNK activation. Therefore, ROS is an upstream trigger of SHK-induced caspase dependent apoptosis of PEL cells through disruption of mitochondrial membrane Δψm in an intrinsic pathway and an activation of JNK in an extrinsic pathway. In a PEL xenografted mouse model, SHK treatment suppresses PEL-mediated ascites formation without showing any significant adverse toxicity. These results suggested that SHK could be a potent anti-tumor agent for the treatment of PEL.
Introduction
Primary effusion lymphoma (PEL) is a subtype of non-Hodgkin's B-cell lymphoma (NHL) that is universally associated with HHV-8/KSHV and frequently occurs in an immunodeficient state in HIV/AIDS patients [1,2]. HIV infected AIDS patients are at a 60 to 200 times higher risk of developing NHL than HIV negative patients and PEL is accounted approximately 4% of all HIV infected NHL [3,4]. Although an application of combined antiretroviral therapy improved the therapeutic outcome of AIDS related lymphoma, PEL is an aggressive tumor with a median survival of 6.2 months and treatment with the usual chemotherapeutic regimens achieved a complete remission rate of only 50% [5]. Actively proliferating PEL used many signals including the NF-κB, JAK/STAT, and PI3/AKT pathways for their survival [6], [7], [8], and an effective treatment for PEL hasn't been discovered yet. Therefore, the development of an effective and less toxic chemotherapy for PEL is urgently needed.
Shikonin (SHK), a naphthoquinone compound of Lithospermum erythrorhizon root extract, has been used for wound healing, anti-inflammation, and anti-fungal activity [9] [10]. Recently, SHK has been shown to be a potent anti-tumor agent by inducing cancer cell death [11] and an in vivo context, it is less toxic with good bioavailability [12]. It has been reported that SHK induced apoptosis through DNA fragmentation as well as caspase-3 cleavage and inhibited angiogenesis in treated HL60 cell tumors [13]. Several other studies have also been focused on other specific molecular targets of SHK such as NF-κB [14], proteasome [15], mitogen activated protein kinase family (MAPK) [11,16], IL-6 induced STAT3 activity [17], and PI3K/AKT to inhibit various types of cancer cells growth [9,18].
Most recently, it has been shown that SHK induced intracellular reactive oxygen species (ROS) with disturbance of mitochondrial membrane potential (Δψm) during an early apoptotic phase in human glioma U87MG and Hs683 cells [19]. SHK also induced cell cycle arrest and necroptosis (programmed necrosis) in a broad spectrum of human cancers including cervical, colon and hepatocellular carcinoma [12]. Induction of apoptosis and necroptosis by SHK has also been reported in human hematological malignant multiple myeloma cell lines [20].
However, despite these promising results, no study has examined the SHK-mediated induction of apoptosis in PEL cells. Whether SHK can induce apoptosis in PEL cells is in need of examination, as well. The present study provides us with an insight into a molecular mechanism underlying SHK-mediated PEL cell apoptosis in vitro. This study also demonstrates an in vivo screening system for the evaluation of SHK as an anti-PEL agent.
Materials and methods
Cell lines
Five human PEL cell lines including BCBL-1, BC-1, BC-3, TY-1, and GTO were obtained [21], [22], [23], [24], [25] and cultured in an exponential growth phase in RPMI containing 10% (v/v) fetal bovine serum (Thermo Fisher Scientific, MA) supplemented with 100 U/ml penicillin (Wako, Osaka, Japan) and 100 μg/ml streptomycin (Wako) in a 5% CO2 atmosphere at 37 °C.
Reagents
Pan-caspase inhibitor (Q-VD-OPh, Tonbo, CA), JNK inhibitor (SP600125, Santa Cruz, CA), N-Acetyl-L-cysteine (NAC) (Sigma-Aldrich, MO), and JC-1 (5,5´,6,6´-tetrachloro-1,1´,3,3´-tetraethyl-benzimidazolyl-carbocyanine-iodide) (Cell Technology, CA) were dissolved in DMSO. SHK was purchased from Sigma-Aldrich and dissolved in water.
MTT assays
PEL cell cytotoxicity was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) (Sigma-Aldrich, USA) assay [26]. Briefly, PEL (4 × 105 cells/ml) cells in an exponential phase were incubated with different doses of SHK in 96-well plate at a volume of 0.1 ml for 24 h and then MTT (0.5 mg/ml as a final concentration in each wells) in a volume of 0.01 ml was added for an additional 4 h in each well to develop formazan crystal in growing cells in 5% CO2 atmosphere at 37 °C. Subsequently, an equal volume of 0.04 N HCl in isopropanol was added in each well to dissolve formazan crystals and the absorbance was measured at 570 nm using iMark microplate reader (Bio-Rad Laboratories, CA).
Annexin V binding assay
Annexin V binding assay was performed to determine an early and late apoptotic GTO cells by using an Annexin V-FITC apoptotic detection kits (eBiosciences, CA) [27]. Briefly, GTO (4 × 105 cells/ml) cells in an exponential phase were treated with different doses of SHK for 12 h in 5% CO2 atmosphere at 37 °C. Cells were harvested, washed with Annexin V binding buffer and incubated in dark with Annexin V-FITC for 20 min at room temperature. Subsequently, propidium iodide (PI) at a concentration of 1.0 μg/ml in PBS was added in Annexin V-FITC stained cells before being analyzed by flow cytometry (BD-LSRII, CA). The flow cytometric data was analyzed on FlowJo software version 10 (TreeStar, CA).
Cell viability assay
Cell viability in cultured PEL cells was determined by staining with PI [28]. Briefly, PEL (4 × 105 cells/ml) cells in an exponential phase were pre-incubated with or without 20 μM pan-caspase inhibitor (Q-VD-OPh) / 5 mM ROS scavenger (NAC) / 30 μM JNK inhibitor (SP600125) for 2 h, subsequently treated with different doses of SHK in 6-well cultured plates for 12 h in 5% CO2 atmosphere at 37 °C. The harvested cells were stained with PI at a concentration of 1.0 μg/ml in PBS before being analyzed with LSR II. The flow cytometric data was analyzed on FlowJo software ver. 10.
Measurement of intracellular ROS
Intracellular reactive oxygen species (ROS) in GTO cells were evaluated by using DCFH-DA (2´-7´-dichlorodihydrofluorescein diacetate) redox-sensitive fluorescent probe (Sekisui Medical, Japan) [29]. Briefly, GTO (4 × 105 cells/ml) cells in an exponential phase were pre-incubated in the presence or absence of 5 mM NAC for 2 h followed by the addition of 10 μM DCFH-DA for an additional 1 h, subsequently, the pre-incubated cells were treated with or without 2 µM SHK for 0 to 6 h or 0 to 2 µM SHK for 1 h in 5% CO2 atmosphere at 37 °C, and the induction of ROS was analyzed with LSR II. The flow cytometric data was analyzed on FlowJo software version v10 (TreeStar, CA).
Measurement of intracellular Δψ
The mitochondrial membrane potential (Δψ) in the treated and untreated PEL cells were measured by staining with JC-1 [30]. Briefly, PEL (4 × 105 cells/ml) cells in an exponential phase were pre-incubated with or without 5 mM NAC for 2 h and subsequently treated with or without SHK in a concentration of 2 µM for an additional 0 to 12 h. The treated and untreated cells were harvested and incubated with 0.5 ml of 1 × JC-1 reagent solution in RPMI-1640 for 15 min in 5% CO2 atmosphere at 37 °C before being analyzed by flow cytometry with green (JC-1 monomers) versus red (JC-1 aggregates) channel. The flow cytometric data was analyzed on FlowJo software version 10 (TreeStar, CA).
Protein extraction and western blotting
Activation of proteins in SHK treated PEL cells were determined by using western blot as described previously [26]. Briefly, PEL (4 × 105 cells/ml) cells in an exponential phase were treated with or without SHK for a specified dose and time interval in 5% CO2 atmosphere at 37 °C. The cells were harvested, pelleted, and lysed in whole cell lysis buffer in the presence of protease inhibitor cocktail (Nacalai Tesque, Japan) for 1 h on ice. The protein concentration was measured using a bicinchoninic acid (BCA) protein assay (Thermo Scientific, USA). Protein extracts were separated on SDS-PAGE and transferred and blotted onto a polyvinylidene difluoride (PVDF) membrane (GE Healthcare, UK). The membrane was blocked using a 5% skim milk in wash buffer and incubated with primary followed by secondary antibody conjugates. Chemi-Lumi one super reagents (Nacalai Tesque) were added on the protein-antibody conjugates containing membranes and the images of protein band were quantified using an ImageQuant LAS 4000 system (GE Healthcare, USA). The following antibodies and reagents were used for western blots: cleaved caspase-3 (Asp175) (5A1E), cleaved caspase-8 (D391) (18C8), cleaved caspase-9 (Asp330) (D2D4), JNK (56G8), phospho-JNK (Thr183/Tyr185) (81E11), p38 (D13E1), phospho-p38 (Thr180/Tyr182) (D3F9), ERK 1/2 (137F5), phospho-ERK 1/2 (Thr202/Tyr204) (D13.14.4E), phospho-NFκB-p65 (S536) (93H1), Akt (C67E7), phospho-Akt (T-308) (D25E6), phospho-Akt (S-473) (D9E), phospho-MLKL (Ser358) (D6H3V), and HRP-conjugated anti-mouse and anti-rabbit IgG were from Cell Signaling Technology; while actin (C-2) (sc-8432), NF-κB-p65 (C-20) (sc372), STAT3 (F-2) (sc-8019), and phospho-STAT3 (Tyr705)-R (sc-7993-R) were from Santa Cruz.
Animal experiments
GTO cells (1 × 107cells/mouse) in 200 μl PBS were intraperitoneally injected into the abdominal regions of Nude Rag2/Jak3 doubly deficient (Nude-RJ) mice on day zero [31]. On day 3, the mice were randomly divided into two groups where the treated group received SHK in a concentration of 2.5 mg/kg and the control group received only PBS twice a week for twenty-five days. Tumor growth was determined by measuring the spleen weight and the volume of ascites at the end of experiment as described[32], [33], [34]. Mouse behavior and appearance were carefully observed every day at Kumamoto University animal center. Animal experiments were performed according to the rules of the Kumamoto University committee for animal care.
Statistical analysis
The statistical significance between the treated and untreated groups with one variable were analyzed by an ordinary one-way ANOVA followed by Tukey's post hoc tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. All error bars represent the mean ± the standard deviation of the mean (SD).
Results
SHK had a prompt cytotoxic effect on PEL cell lines
SHK has been shown to exert its cytotoxic effects on various cancer cells such as human pre-myelocytic leukemia, multiple myeloma, and oral squamous cell carcinoma [13,14,20]. To determine its effect on PEL, we performed cytotoxicity assays and showed that SHK treatment for 24 h dose dependently inhibited PEL cell proliferation where GTO cells showed highest and BC-1 cells showed lowest sensitivity in comparison with other PEL cell lines (Fig. 1A). The CC50 value of BCBL-1, GTO, BC-1, BC-3, and TY-1 were 2.43, 1.38, 3.31, 2.04, and 2.11 µM, respectively, after a 24 h treatment with SHK (Fig. 1B). Thus, these data suggested that SHK had a potent cytotoxic effect on PEL cells.
Fig. 1.
Anti-proliferative effect of SHK in PEL cells. (A) Human PEL cells treated with or without SHK for 24 h were incubated with MTT for an additional 4 h for measurement of their proliferation (mean ± SD, n=3) by automatic microplate reader. (B) CC50 values of SHK-treated PEL cells. One representative result of five independent experiments is shown.
Caspase-mediated apoptosis in SHK-treated PEL cell lines
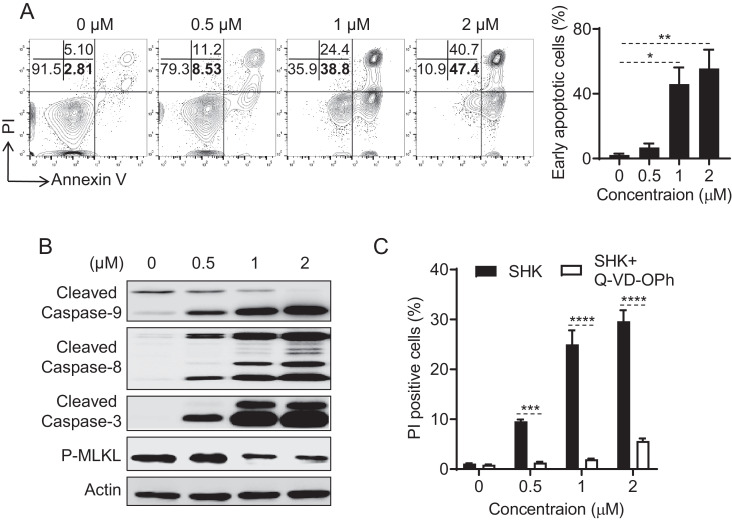
We next tried to determine the mechanisms of SHK-mediated cytotoxic effects on PEL using GTO, BCBL-1, and BC-3 cell lines. It has been reported that SHK induced both apoptosis and/or necroptosis pathways to kill cancer cells including osteosarcoma and multiple myeloma [20,35]. Therefore, we first analyzed SHK-mediated apoptotic and/or necroptotic pathways [27] on the killing of GTO cells and found that SHK treatment for 12 h dose-dependently increased the percentages of both early apoptotic (annexin V+/PI-) (2.81 to 47.4%) and late apoptotic (annexin V+/PI+) (5.10 to 40.7%) cells (Fig. 2A). Thus, SHK may use both apoptotic and necroptotic pathways to kill GTO cells. To further explore the specific mode of action of this SHK-induced killing effect on GTO cells, we have investigated apoptosis and necroptosis related proteins in response to SHK treatment. We have found that SHK-treatment for 6 h in GTO cells dose-dependently increased the activation of caspase-3, -8, and -9 and decreased the activation of phospho-MLKL, further indicating an apoptotic mode of cell death (Fig. 2B). Additionally, activation of caspase-8 and deactivation of phospho-mixed linage kinase domain-like protein (MLKL) in SHK treated cancer cells indicated an omission of necroptosis pathways [20,36]. Indeed, a pan caspase inhibitor, (Q-VD-OPh) [37] effectively blocked the SHK-induced GTO, BCBL-1, and BC-3 cells death (Fig. 2C, S1A-B), confirming the apoptosis mode of cell death. Thus, the results of these experiments demonstrate that SHK-inducing PEL cells death was due to caspase dependent apoptosis.
Fig. 2.
SHK induced caspase-3-dependent apoptosis in GTO cells. (A) Human GTO cells treated with or without SHK for 12 h were stained for measurement of early and late apoptotic cells (Dot-blot) by flow cytometry. (B) Human GTO cells treated with or without SHK for 6 h were processed for measurement of protein activation by western blot. (C) Human GTO cells pre-incubated with or without Q-VD-OPh in a concentration of 20 μM for 2 h, subsequently treated with or without SHK for 12 h were stained for measurement of apoptotic cells (mean ± SD, n=3) by flow cytometry. Asterisks (*) denote statistical significance (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001) using one-way ANOVA followed by Tukey's post hoc test. One representative result of three independent experiments is shown.
ROS was required for SHK-induced apoptosis in PEL cells
It has been reported that SHK induced the apoptosis of cancer cells through the generation of ROS [38]. We therefore tried to determine whether SHK has any effect on ROS generation in PEL using GTO cell lines. A probe DCFH-DA was applied to SHK treated GTO cells that could readily be oxidized to a fluorescent probe, DCF, by intracellular ROS. We have found that intracellular ROS was maximally accumulated (around 3-fold) within 1 h in 2 μM SHK treated GTO cells in comparison with control treated cells (Fig. 3A) in a manner that was dose dependent (Fig. 3B). Importantly, the ROS production could be blocked by pre-treatment with a free radical scavenger, NAC, in SHK treated GTO cells (Fig. 3A-B). These data demonstrated that SHK induced the generation of ROS in GTO cell lines. We next investigated whether SHK inducing production of ROS is involved in the apoptosis of PEL using GTO, BCBL-1, and BC-3 cells. We found that SHK induced apoptosis of PEL cells was completely reversed by pre-treatment of cells with the antioxidant NAC, indicating that ROS is an important mediator of apoptosis (Fig. 3C, S2A-B). We then tried to determine whether the generation of ROS is the preceding event of caspase-3 activation. We have found that antioxidant NAC significantly reduced the levels of caspase-3 activation in SHK treated PEL cells in protein level further indicating that ROS was an upstream event of caspase-3 activation for the induction of SHK-induced apoptosis in PEL cells (Fig. 3C, S2C-D). We have found that antioxidant NAC significantly reduced the levels of caspase-3 activation in SHK treated GTO cells in protein level further indicating that ROS was an upstream event of caspase-3 activation for the induction of SHK-induced apoptosis in GTO cells (Fig. 3D). These observations suggest that SHK induced apoptosis occurs through the production of ROS, which subsequently initiates the caspase-3 dependent apoptotic pathways in PEL cell lines.
Fig. 3.
SHK induced ROS mediated apoptosis in GTO cells. Human GTO cells pre-incubated with or without NAC in a concentration of 5 mM for 2 h, subsequently added DCFH-DA in a concentration of 10 µM for an additional 1 h and then treated with or without SHK at a concentration of 2 µM (A) for 0 to 6 h or 0 to 2 µM (B) for 1 h more were processed for measurement of ROS by flow cytometry (DCF = Dichlorofluorescein, MFI = Mean fluorescent intensity). (C) Human GTO cells pre-incubated with or without NAC in a concentration of 5 mM for 2 h, subsequently treated with or without SHK for 12 h were stained for measurement of apoptotic cells (mean ± SD, n=3) by flow cytometry. (D) Human GTO cells pre-incubated with or without NAC in a concentration of 5 mM or Q-VD-OPh in a concentration of 20 μM for 2 h, subsequently treated with or without SHK in a concentration of 2 μM for 6 h were processed for measurement of protein activation by western blot. Asterisks (*) denote statistical significance (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001) using one-way ANOVA followed by Tukey's post hoc test. One representative result of three independent experiments in (B), (C) and (D) is shown.
JNK activation is required for SHK-induced apoptosis in PEL cell lines
Previous studies had reported that SHK induces MAPK pathways [11,16] similar to other anticancer compounds for inducing apoptosis of cancer cells [39], [40], [41]. We therefore further investigated the SHK effects on MAPK signaling pathways in PEL using GTO, BCBL-1, and BC-3 cell lines. Indeed, SHK treatment for 6 h activated JNK, p38, and ERK pathways in PEL cells in a manner that was concentration dependent (Fig. 4A, S3A-B). Therefore, MAPK may be involved in SHK induced apoptosis of PEL cells.
Fig. 4.
JNK activation contributes to SHK-induced apoptosis of GTO cells. Human GTO cells treated with or without SHK for 6 h (A) or in a concentration of 2 μM (B) were processed for measurement of protein activation by western blot. (C) Human GTO cells pre-incubated with or without SP600125 in a concentration of 30 μM for 2 h, subsequently treated with or without SHK in a concentration of 2 μM for 12 h were stained for measurement of apoptotic cells (mean ± SD, n=3) by flow cytometry. (D) Human GTO cells pre-incubated with or without NAC in a concentration of 5 mM or Q-VD-OPh in a concentration of 20 μM for 2 h, subsequently treated with or without SHK in a concentration of 2 μM for 6 h were processed for measurement of protein activation by western blot. Asterisks (*) denote statistical significance (**p<0.01) using one-way ANOVA followed by Tukey's post hoc test. One representative result of three independent experiments is shown.
It has also previously been reported that constitutive activation of NF-κB, JAK/STAT, and PI3K/AKT signaling pathways are important for the survival of PEL, and the inhibition of these pathways may be involved in PEL cell death [6], [7], [8]. We therefore examined whether suppression of these pathways was necessary for SHK-induced apoptosis of PEL using GTO cell lines. We found that the expression level of phospho-NF-κB p65, Akt-T308, and Akt-S473 were not affected in GTO cells when SHK concentration was increased for 6 h. At the initial low concentration of SHK (0–1 µM), cells were still activating STAT3 but the activation of STAT3 pathways was rapidly suppressed in 2 µM SHK treated GTO cells (Fig. S4). Furthermore, time dependent (30 min - 6 h) experiments clearly demonstrated the activation of JNK prior to p38 activation and/or STAT3 suppression in 2 µM SHK treated GTO cells (Fig. 4B). JNK was activated within 30 minutes of 2 µM SHK treated GTO cells. We next investigated the functional roles of JNK signaling in SHK treated GTO cells. We show that a specific JNK inhibitor, SP600125, could partly rescue the GTO cells from the apoptotic effect of SHK (Fig. 4C). These results indicate that JNK activation was also partly involved in inducing apoptosis of SHK treated PEL cells. We next tried to determine the events upstream of JNK pathways in SHK induced GTO cell death. Our data indicates that the ROS inhibitor, NAC, completely reduced the activation of JNK, but Q-VD-OPh did not significantly modify the activation of JNK induced by SHK at the protein level in GTO cells (Fig. 4D). Altogether, these data indicated that SHK induced apoptosis of GTO cell death was partly dependent on ROS-mediated JNK activation and was independent of the p38, MAPK, NF-κB, JAK/STAT, and PI3K pathways.
SHK triggered mitochondrial-dependent apoptosis in PEL cell lines
A previous study has reported that SHK disruption of mitochondrial membrane Δψm [42] is associated with the generation of ROS as well as an activation of JNK signaling pathways [43,44]. Mitochondrial membrane Δψm is a central intermediate in an oxidative energy metabolism in living cells. We therefore investigated whether SHK treatment resulted in the disruption of mitochondrial membrane Δψm in PEL using GTO and BCBL-1 cell lines. In our experiment, we used JC-1 dye to detect an intact versus disrupted mitochondrial membrane Δψm in SHK treated PEL cells. JC-1 dye could either form J-aggregates with an emission of red fluorescence indicating a presence of high levels of mitochondrial membrane Δψm of intact mitochondria, or persist as J-monomers with an emission of green fluorescence indicating low levels of mitochondrial membrane Δψm with disrupted mitochondria in living cells. Indeed, our data demonstrated that SHK in a concentration of 2 µM treatment in PEL cells time dependently (0 to 12 h) resulted in increased percentage of cells (2.09, 4.46, 12.9 and 64.7%) with disrupted mitochondrial membrane Δψm (Fig. 5A-B, S5). Moreover, ROS inhibitor NAC rescued the cells (54.9 to 5.99%) from the loss of mitochondrial membrane Δψm at 12 h of SHK (2 μM) treated GTO cells, indicating that ROS is an upstream event of mitochondrial mediated apoptosis (Fig. 5C-D). Additionally, JNK inhibitor SP600125 did not rescue the cells (data not shown) from the loss of mitochondrial membrane Δψm, indicating that SHK-mediated JNK activation in treated GTO cells committed to apoptosis independent of mitochondrial membrane Δψm. We next tried to identify downstream mitochondrial mediated apoptotic pathways and found that caspase-9 was activated in SHK treated GTO cells (Fig. 5E). Additionally, we have showed that both the ROS inhibitor, NAC, and pan caspase inhibitor, Q-VD-OPh, significantly reduced the levels of caspase-9 activation at the protein level in SHK induced GTO cells (Fig. 5F). Thus, these results demonstrated that SHK-induced apoptosis of PEL cells was also mediated by pathways involved in the loss of mitochondrial membrane Δψm and followed by an activation of caspase-9.
Fig. 5.
SHK induced apoptosis through ROS dependent mitochondrial pathways in GTO cells. (A) Human GTO cells treated with or without SHK in a concentration of 2 μM were stained with fluorochrome dye JC-1 for measurement of Δψm (Dot-blot) by flow cytometry (JC-1 green positive cells indicate live with disrupt Δψm). (B) % of cells with low Δψm (mean ± SD, n=3) in panel (A). (C) Human GTO cells pre-incubated with or without NAC in a concentration of 5 mM for 2 h, and subsequently treated with or without SHK in a concentration of 2 μM for 12 h, were stained for measurement of Δψm (Dot-blot) by flow cytometry (JC-1 green positive cells indicate live with disrupt Δψm). (D) % of cells with low Δψm (mean ± SD, n=3) in panel (C). (E) Human GTO cells treated with or without SHK in a concentration of 2 μM were processed for measurement of protein activation by western blot. (F) Human GTO cells pre-incubated with or without NAC in a concentration of 5 mM or Q-VD-OPh in a concentration of 20 μM for 2 h, and subsequently treated with or without SHK in a concentration of 2 μM for 6 h, were processed for measurement of protein activation by western blot. Asterisks (*) denote statistical significance (**p<0.01, ****p<0.0001) using one-way ANOVA followed by Tukey's post hoc test. One representative result of three independent experiments is shown.
SHK completely suppressed PEL cell growth in a PEL-xenografted mouse model
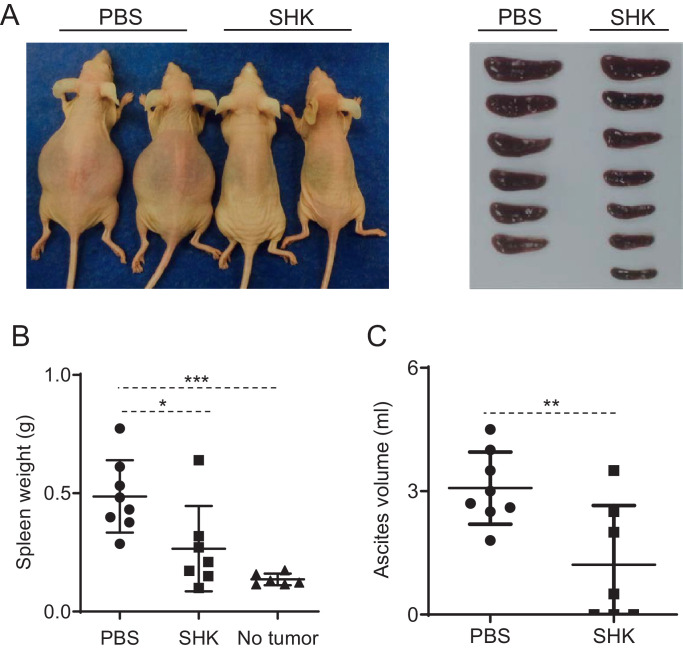
We next investigated whether SHK has any anti-PEL activity in vivo. We therefore established a PEL xenografted Nude-RJ mouse model [31] by intraperitoneal injection of 1 × 107 GTO cells. GTO-xenografted mice were randomly divided into two groups on day three and treated with either SHK in a concentration of 2.5 mg/kg SHK or PBS (control) twice a week for twenty-five days. The applied dose of SHK in this study was much lower than the dose used to treat other cancer cell lines, including hepatocellular carcinoma Huh7 cells, murine hepatoma H22 cells, human prostate cancer PC-3 cells, and murine leukemia P388 cells [15,45]. Hence, the dosage of SHK in our experiment was expected to be safe, and SHK-treated mice appeared stay healthy during treated. We have shown that control mice with PBS treatment at the end of twenty-five days had a swollen abdomen with lymphoma compared with SHK treated mice where lymphoma was completely suppressed (Fig. 6A). As size and weight of spleen and volume of ascites represent the progress of PEL in vivo [32], [33], [34], we weighed the mouse spleen and demonstrated that SHK-treated mouse spleen size was similar to normal mice with no lymphoma. Control mice exhibited a mean spleen weight of 486.6 ± 152.8 mg, compared to 265.7 ± 180.6 mg for mice treated with SHK (Fig. 6B). Finally, we measured the ascites volume of mice and showed that the growth of GTO cells in ascites of mice treated with SHK was significantly lower than in control mice (Fig. 6C). The volume of ascites in control mice was 3.07 ± 0.87 ml, compared to 1.21 ± 1.43 ml for mice treated with SHK, indicating that SHK treatment results in anti-PEL proliferation in vivo.
Fig. 6.
Treatment of GTO-xenografted Nude-RJ mice with SHK completely suppressed the growth of KSHV-associated lymphoma in mouse model. Human GTO cells in PBS were intraperitoneally injected into the abdominal cavity of Nude-RJ mice for three days before treatment with or without SHK in a concentration of 2.5 mg/kg twice a week for twenty-five days. (A) A photograph of GTO-xenografted mice and their spleens. Untreated mice have lymphoma while SHK treated mice lack lymphoma. (B) Spleen weights and (C) ascites volumes at the end of experiment were measured. Asterisks (*) denote statistical significance *p<0.05, **p<0.01, ***p<0.001 using one-way ANOVA followed by Tukey's post hoc test. Shown is the result of one (mean ± SD) representative out of two experiments.
Discussion
Over the past few years, SHK has been shown to be a potent anticancer agent on cancer cells [13,46,47]. No report is yet published on the role of SHK in the treatment of PEL. This study provides the first evidence to demonstrate that SHK treatment completely suppresses PEL cell growth in a PEL-xenografted mouse model.
How does SHK induce the cell death of PEL? We have shown that SHK induces apoptotic pathways when killing PEL cells. Treatment with SHK dose dependently increased both early and late apoptotic PEL cells, providing evidence of either an apoptosis and/or necroptosis effect. Several reports have also suggested that SHK could use either apoptosis or necroptosis, or both pathways, for the death of cancer cells [11,13,14,20] . Necroptosis is the phenomenon of necrosis induced through the receptor interaction of protein kinase 1 and 3, thereby inhibiting the activation of caspase-8 in caspase independent cell death and/or activating a pseudo-kinase of necroptosis initiation, phospho-MLKL [20,35,36]. Indeed, we have shown that caspase-8 was activated and phospho-MLKL was inhibited in SHK treated PEL cells. This finding ruled out the possibility of necroptosis rather than an induction of apoptosis as the driver of this effect. In addition, an apoptosis inducing protein, caspase-3, was activated in SHK treated PEL cells and inhibition of this protein by pre-incubating with pan-caspase inhibitor, Q-VD-OPh [37], completely rescued the cells from apoptosis. This provided us with further evidence that the PEL cell death mode by SHK was due to the induction of apoptosis. Activation of caspase-8 in PEL cells is partly a consequence of the loss of mitochondrial Δψm [48,49] and it is reported that SHK induction of apoptosis in cancer cells was dependent on impaired mitochondrial functions [11]. Our data also showed that SHK disrupts mitochondrial function, resulting in the loss of Δψm and the activation of caspase-9 in PEL cells. Thus, the PEL cell death induced by SHK could be considered caspase dependent apoptosis with the activation of both intrinsic (mitochondrial) as well as extrinsic (caspase-8) pathways.
The other possible extrinsic means of causing apoptosis of PEL cells is based on the JNK signaling pathway [11]. JNK is a member of the MAPK family [50] proteins and SHK induced significant elevation of phospho-p38, phospho-ERK, and phospho-JNK in PEL cells. JNK activation was an earlier event than p38 or ERK activation in 2 µM SHK induction of apoptosis in GTO cells. Moreover, we noted that a specific inhibitor, SP600125, targeted on JNK could rescue a portion of the cells from the apoptotic effect of SHK. This suggests that SHK also promoted GTO cell death via the JNK signaling pathway. We ruled out the other three important PEL surviving signaling pathways, NF-κB, PI3K and STAT3, that could be implicated in SHK-induced killing of GTO cells [6], [7], [8]. We showed that SHK neither activated nor inhibited the NF-κB and PI3K pathways. Protein levels of STAT3 activation were significantly inhibited in 2 µM SHK-treated GTO cells. Time dependency western blot data demonstrated that JNK activation occurred before the inhibition of STAT3 activation in stress induced SHK treated GTO cells. Thus, SHK also induced PEL cell death through the JNK mediated extrinsic apoptosis pathway.
The next question is to find out the upstream role of events in SHK induced apoptosis of PEL cells. SHK is a ROS inducer and an accumulation of ROS in cancer cells plays an upstream role in apoptosis pathways [38]. Moreover, oxidative stress in cancer cells is activated by pro-death signals through ROS-dependent JNK [11]. Thus, ROS could be able to augment JNK activation during SHK-induced apoptosis of PEL cells. In addition, it has also been reported that ROS induction in cancer cells by SHK may be generated from futile mitochondrial redox cycling [49]. During an induction of apoptosis of cancer cells, the semiquinone moieties of SHK are metabolized by a mitochondrial NADH-ubiquinone oxidoreductase to form unstable semiquinone radicals that enter into the redox cycle, leading to the reformation of quinones moieties in the presence of molecular oxygen. As a result, excessive ROS can be generated through the reaction with dioxygen and semiquinone radicals [42]. Indeed, our results show that an intracellular ROS accumulation was generated in the course of SHK induced PEL cell death that became attenuated by the antioxidant, NAC. Furthermore, although an inhibition of ROS by NAC reduced SHK-induced activation of both JNK, and caspase-3 and -9, an inhibition of caspase activation by Q-VD-OPh only remarkably reduced the activation of caspase-3 and -9, and did not show any significant impact on the inhibition of JNK activation at the protein level. Thus, ROS generation was a proximal and an initiating event of JNK as well as caspase-3 activation in SHK-induced apoptosis of PEL cells. Moreover, pre-incubation of PEL cells with NAC almost completely decreased an apoptotic cell number indicating that manipulating ROS levels is an important factor for specifically killing cancer cells without causing damage to normal cells. Additionally, while NAC completely rescued the cells from a loss of Δψm, SP600125 could not (data not shown), indicating that JNK activated induction of apoptosis of PEL cells was not entirely dependent on mitochondrial mediated pathways. Taken together, these mechanistic results suggest that SHK selectively induces the apoptosis of PEL cells in vitro and this should highlight SHK involvement in the suppression of PEL cell growth in vivo.
In conclusion, we have provided both in vitro and in vivo evidence that SHK efficiently induces the apoptosis of PEL cells and suppresses their growth in a PEL xenografted mouse model. The mechanism of SHK inducing apoptosis was achieved via a ROS-mediated intrinsic and extrinsic pathway, including an activation of JNK, caspase-9, and caspase-8, and a dissipation of Δψm (Fig. 7). Co-operation of these pathways induced by SHK converge on either an activation of caspase-3 or by directly inducing apoptosis of PEL cells. Thus, this study provides a basis for a novel strategy using SHK in the treatment of PEL.
Fig. 7.
The signaling pathways proposed for SHK-induced apoptosis of PEL cells. SHK induced the generation of ROS in PEL cells. One part of ROS leads to the disruption of mitochondrial membrane potential (Δψm) with the activation of caspase-9 to promote intrinsic apoptotic, while another part of ROS leads to the activation of caspase-8 with an early activation of JNK to promote extrinsic apoptotic pathways.
Credit authorship contribution statement
Md Masud Alam: Formal analysis, Investigation, Writing - Original Draft, Ryusho Kariya: Conceptualization, Methodology, Validation, Investigation, Writing - Original Draft, Piyanard Boonnate: Formal analysis, Investigation, Azusa Kawaguchi: Formal analysis, Investigation, Seiji Okada: Conceptualization, Validation, Resources, Writing - Review & Editing, Supervision, Project administration, Funding acquisition
Declaration of Competing Interest
The authors have declared that no conflicts of interest exist.
Acknowledgments
Acknowledgements
We thank to Ms. S. Fujikawa for technical assistance and Ms. Y. Kanagawa for secretarial assistance. We thank T.J. Meyer for editorial assistance.
Funding
This work was supported by the Research program on HIV/AIDS from Japan Agency for Medical Research and development (AMED) (Grant No. 18fk0410008h0003) and Grants-in-Aid for Science Research (Grant No. 16K08742) from the Ministry of Education, Science, Sports, and Culture of Japan.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.101006.
Appendix. Supplementary materials
References
- 1.Nador R.G., Cesarman E., Chadburn A., Dawson D.B., Ansari M.Q., Sald J., Knowles D.M. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 2.Chen Y.B., Rahemtullah A., Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569–576. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- 3.Beral V., Peterman T., Berkelman R., Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;337:805–809. doi: 10.1016/0140-6736(91)92513-2. [DOI] [PubMed] [Google Scholar]
- 4.Ota Y., Hishima T., Mochizuki M., Kodama Y., Moritani S., Oyaizu N., Mine S., Ajisawa A., Tanuma J., Uehira T., Hagiwara S., Yajima K., Koizumi Y., Shirasaka T., Kojima Y., Nagai H., Yokomaku Y., Shiozawa Y., Koibuchi T., Iwamoto A., Oka S., Hasegawa H., Okada S., Katano H. Classification of AIDS-related lymphoma cases between 1987 and 2012 in Japan based on the WHO classification of lymphomas, Cancer Med. 2014;3:143–153. doi: 10.1002/cam4.178. fourth edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulanger E., Gerard L., Gabarre J., Molina J.M., Rapp C., Abino J.F., Cadranel J., Chevret S., Oksenhendler E. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J. Clin. Oncol. 2005;23:4372–4380. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 6.Keller S.A., Schattner E.J., Cesarman E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–2542. [PubMed] [Google Scholar]
- 7.Aoki Y., Feldman G.M., Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 8.Uddin S., Hussain A.R., Al-Hussein K.A., Manogaran P.S., Wickrema A., Gutierrez M.I., Bhatia K.G. Inhibition of phosphatidylinositol 3′-kinase/AKT signaling promotes apoptosis of primary effusion lymphoma cells. Clin. Cancer Res. 2005;11:3102–3108. doi: 10.1158/1078-0432.CCR-04-1857. [DOI] [PubMed] [Google Scholar]
- 9.Ahn J., Won M., Choi J.H., Kim Y.S., Jung C.R., Im D.S., Kyun M.L., Lee K., Song K.B., Chung K.S. Reactive oxygen species-mediated activation of the Akt/ASK1/p38 signaling cascade and p21(Cip1) downregulation are required for shikonin-induced apoptosis. Apoptosis. 2013;18:870–881. doi: 10.1007/s10495-013-0835-5. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka S., Tajima M., Tsukada M., Tabata M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J. Nat. Prod. 1986;49:466–469. doi: 10.1021/np50045a014. [DOI] [PubMed] [Google Scholar]
- 11.Mao X., Yu C.R., Li W.H., Li W.X. Induction of apoptosis by Shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008;18:879–888. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]
- 12.Andujar I., Rios J.L., Giner R.M., Recio M.C. Pharmacological properties of shikonin - a review of literature since 2002. Planta Med. 2013;79:1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- 13.Yoon Y., Kim Y.O., Lim N.Y., Jeon W.K., Sung H.J. Shikonin, an ingredient of lithospermum erythrorhizon induced apoptosis in HL60 human premyelocytic leukemia cell line. Planta Med. 1999;65:532–535. doi: 10.1055/s-1999-14010. [DOI] [PubMed] [Google Scholar]
- 14.Min R., Tong J., Wenjun Y., Wenhu D., Xiaojian Z., Jiacai H., Jian Z., Wantao C., Chenping Z. Growth inhibition and induction of apoptosis in human oral squamous cell carcinoma Tca-8113 cell lines by Shikonin was partly through the inactivation of NF-kappaB pathway. Phytother Res. 2008;22:407–415. doi: 10.1002/ptr.2340. [DOI] [PubMed] [Google Scholar]
- 15.Yang H., Zhou P., Huang H., Chen D., Ma N., Cui Q.C., Shen S., Dong W., Zhang X., Lian W., Wang X., Dou Q.P., Liu J. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int. J. Cancer. 2009;124:2450–2459. doi: 10.1002/ijc.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh F., Gao D., Lebwohl M.G., Wei H. Shikonin modulates cell proliferation by inhibiting epidermal growth factor receptor signaling in human epidermoid carcinoma cells. Cancer Lett. 2003;200:115–121. doi: 10.1016/s0304-3835(03)00239-8. [DOI] [PubMed] [Google Scholar]
- 17.Thakur R., Trivedi R., Rastogi N., Singh M., Mishra D.P. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci. Rep. 2015;5:10194. doi: 10.1038/srep10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu D., Qian J., Li W., Feng Q., Pan S., Zhang S. beta-hydroxyisovaleryl-shikonin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling. Oncol. Lett. 2015;10:3434–3442. doi: 10.3892/ol.2015.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J.T., Li Z.L., Wu J.Y., Lu F.J., Chen C.H. An oxidative stress mechanism of shikonin in human glioma cells. PLoS One. 2014;9:e94180. doi: 10.1371/journal.pone.0094180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada N., Kawano Y., Fujiwara S., Kikukawa Y., Okuno Y., Tasaki M., Ueda M., Ando Y., Yoshinaga K., Ri M., Iida S., Nakashima T., Shiotsu Y., Mitsuya H., Hata H. Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int. J. Oncol. 2015;46:963–972. doi: 10.3892/ijo.2014.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renne R., Zhong W., Herndier B., McGrath M., Abbey N., Kedes D., Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 22.Cesarman E., Moore P.S., Rao P.H., Inghirami G., Knowles D.M., Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 23.Arvanitakis L., Mesri E.A., Nador R.G., Said J.W., Asch A.S., Knowles D.M., Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 24.Katano H., Hoshino Y., Morishita Y., Nakamura T., Satoh H., Iwamoto A., Herndier B., Mori S. Establishing and characterizing a CD30-positive cell line harboring HHV-8 from a primary effusion lymphoma. J Med Virol. 1999;58:394–401. [PubMed] [Google Scholar]
- 25.Goto H., Kojima Y., Nagai H., Okada S. Establishment of a CD4-positive cell line from an AIDS-related primary effusion lymphoma. Int. J. Hematol. 2013;97:624–633. doi: 10.1007/s12185-013-1339-3. [DOI] [PubMed] [Google Scholar]
- 26.Masud Alam M., Kariya R., Kawaguchi A., Matsuda K., Kudo E., Okada S. Inhibition of autophagy by chloroquine induces apoptosis in primary effusion lymphoma in vitro and in vivo through induction of endoplasmic reticulum stress. Apoptosis. 2016;21:1191–1201. doi: 10.1007/s10495-016-1277-7. [DOI] [PubMed] [Google Scholar]
- 27.Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 28.Gottschalk A.R., Boise L.H., Thompson C.B., Quintans J. Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc. Natl. Acad. Sci USA. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aranda A., Sequedo L., Tolosa L., Quintas G., Burello E., Castell J.V., Gombau L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro. 2013;27:954–963. doi: 10.1016/j.tiv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Perelman A., Wachtel C., Cohen M., Haupt S., Shapiro H., Tzur A. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e430. doi: 10.1038/cddis.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kariya R., Matsuda K., Gotoh K., Vaeteewoottacharn K., Hattori S., Okada S. Establishment of nude mice with complete loss of lymphocytes and NK cells and application for in vivo bio-imaging. In Vivo. 2014;28:779–784. [PubMed] [Google Scholar]
- 32.Dai L., Trillo-Tinoco J., Bai L., Kang B., Xu Z., Wen X., Del Valle L., Qin Z. Systematic analysis of a xenograft mice model for KSHV+ primary effusion lymphoma (PEL) PLoS One. 2014;9:e90349. doi: 10.1371/journal.pone.0090349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto H., Matsuda K., Srikoon P., Kariya R., Hattori S., Taura M., Katano H., Okada S. Potent antitumor activity of zoledronic acid-induced Vgamma9Vdelta2 T cells against primary effusion lymphoma. Cancer Lett. 2013;331:174–182. doi: 10.1016/j.canlet.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Matsuno T., Kariya R., Yano S., Morino-Koga S., Taura M., Suico M.A., Shimauchi Y., Matsuyama S., Okamoto Y., Shuto T., Kai H., Okada S. Diethyldithiocarbamate induces apoptosis in HHV-8-infected primary effusion lymphoma cells via inhibition of the NF-kappaB pathway. Int. J. Oncol. 2012;40:1071–1078. doi: 10.3892/ijo.2011.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Z., Deng B., Liao Y., Shan L., Yin F., Wang Z., Zeng H., Zuo D., Hua Y., Cai Z. The anti-tumor effect of shikonin on osteosarcoma by inducing RIP1 and RIP3 dependent necroptosis. BMC Cancer. 2013;13:580. doi: 10.1186/1471-2407-13-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Li W., Ren J., Huang D., He W.T., Song Y., Yang C., Li W., Zheng X., Chen P., Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caserta T.M., Smith A.N., Gultice A.D., Reedy M.A., Brown T.L. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 38.Liang W., Cui J., Zhang K., Xi H., Cai A., Li J., Gao Y., Hu C., Liu Y., Lu Y., Wang N., Wu X., Wei B., Chen L. Shikonin induces ROS-based mitochondria-mediated apoptosis in colon cancer. Oncotarget. 2017;8:109094–109106. doi: 10.18632/oncotarget.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appierto V., Tiberio P., Villani M.G., Cavadini E., Formelli F. PLAB induction in fenretinide-induced apoptosis of ovarian cancer cells occurs via a ROS-dependent mechanism involving ER stress and JNK activation. Carcinogenesis. 2009;30:824–831. doi: 10.1093/carcin/bgp067. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., He Q.Y., Che C.M., Tsao S.W., Sun R.W., Chiu J.F. Modulation of gold(III) porphyrin 1a-induced apoptosis by mitogen-activated protein kinase signaling pathways. Biochem. Pharmacol. 2008;75:1282–1291. doi: 10.1016/j.bcp.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 41.Wang C.C., Chiang Y.M., Sung S.C., Hsu Y.L., Chang J.K., Kuo P.L. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;259:82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Chen C.H., Lin M.L., Ong P.L., Yang J.T. Novel multiple apoptotic mechanism of shikonin in human glioma cells. Ann. Surg. Oncol. 2012;19:3097–3106. doi: 10.1245/s10434-012-2324-4. [DOI] [PubMed] [Google Scholar]
- 43.Chauhan D., Li G., Sattler M., Podar K., Mitsiades C., Mitsiades N., Munshi N., Hideshima T., Anderson K.C. Superoxide-dependent and -independent mitochondrial signaling during apoptosis in multiple myeloma cells. Oncogene. 2003;22:6296–6300. doi: 10.1038/sj.onc.1206734. [DOI] [PubMed] [Google Scholar]
- 44.Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong K., Li W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: a potential new treatment for hepatocellular carcinoma. Free Radic. Biol. Med. 2011;51:2259–2271. doi: 10.1016/j.freeradbiomed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Wu Z., Wu L., Li L., Tashiro S., Onodera S., Ikejima T. p53-mediated cell cycle arrest and apoptosis induced by shikonin via a caspase-9-dependent mechanism in human malignant melanoma A375-S2 cells. J. Pharmacol. Sci. 2004;94:166–176. doi: 10.1254/jphs.94.166. [DOI] [PubMed] [Google Scholar]
- 47.Hsu P.C., Huang Y.T., Tsai M.L., Wang Y.J., Lin J.K., Pan M.H. Induction of apoptosis by shikonin through coordinative modulation of the Bcl-2 family, p27, and p53, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J. Agric. Food Chem. 2004;52:6330–6337. doi: 10.1021/jf0495993. [DOI] [PubMed] [Google Scholar]
- 48.Mehmet H. Caspases find a new place to hide. Nature. 2000;403:29–30. doi: 10.1038/47377. [DOI] [PubMed] [Google Scholar]
- 49.Henry-Mowatt J., Dive C., Martinou J.C., James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–2860. doi: 10.1038/sj.onc.1207534. [DOI] [PubMed] [Google Scholar]
- 50.Raman M., Chen W., Cobb M.H. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.