Graphical abstract
Abbreviations: AgNPs, silver nanoparticles; Yle, the finnish broadcasting company; WHO, the World Health Organization; NOAEL, no observable adverse effect level; RfD, reference dose; U.S. EPA, the environmental protection agency of the USA; Fimea, the finnish medicines agency; Evira, the finnish food safety authority; Tukes, the finnish safety and chemicals agency; THL, the finnish institute for health and welfare; ROS, reactive oxygen species; APTT, active partial thromboplastin time; ATP, adenosine triphosphate; LDH, lactate dehydrogenase
Keywords: Silver nanoparticles, Colloidal silver, Pseudo-medicine, Quackery, Web-based advertising, Social media
Highlights
-
•
Silver nanoparticles and silver ions in colloidal silver are toxic.
-
•
Internet marketing of colloidal silver included false claims.
-
•
Internal use of colloidal silver was promoted by companies and users.
-
•
Distrust in authorities expressed in websites and Facebook nulled interventions.
Abstract
Aims
The aim was to investigate the marketing practices, beliefs and health claims regarding the use of colloidal silver in Finland. Silver nanoparticles (AgNPs) are potentially toxic due to their small size and Ag+-release capabilities, and the use of colloidal silver products containing AgNPs can cause a wide variety of adverse effects such as argyria.
Methods
Contents of three company websites selling colloidal silver were reviewed, and the claims used in the marketing of colloidal silver were compared to the scientific information about silver. In Facebook posts and discussion about colloidal silver were analyzed.
Results
In Finland, the marketing of colloidal silver products on websites selling the products did not follow the regulations of authorities; several scientifically unfounded claims about the efficacy and medical use of colloidal silver were found. After the Finnish Broadcasting Company (Yle) documentary and an intervention by authorities, contents of the websites were changed, but still questionable information and misleading claims could be found. In the analyzed Facebook groups attitudes towards medical use of colloidal silver were uncritically positive, internal use was highly promoted and the restrictions of use were considered unjustified.
Conclusions
The use of quackery products such as colloidal silver can be dangerous, and their use and marketing should be controlled and restricted.
1. Introduction
The popularity and use of quackery products peaks occasionally as a trend within the population. Quackery, also called pseudo-medicine and medical fraud, means advertisement and offering of services and products using unscientific promises about efficacy and safety [1,2]. Quackery is sometimes misleadingly called “alternative medicine” or “complementary therapies”, even though they have no connection to evidence-based medicine [1].
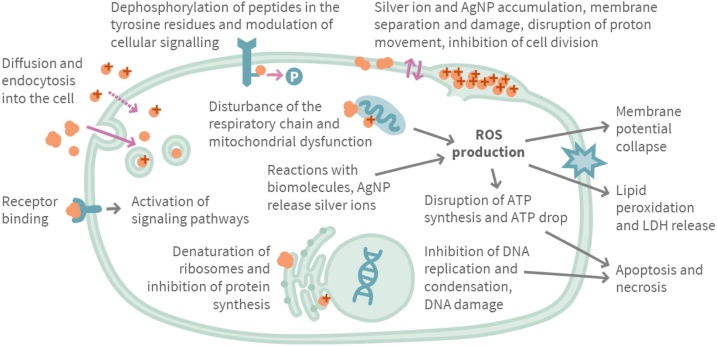
Silver has been used for ages to treat various diseases from common cold to cancer [3]. Due to its antimicrobial effects silver is still utilized in products for local antimicrobial treatment, e.g. in silver wound dressings. However, systemic use has been banned in the EU as non-effective and potentially toxic (EU Directive 2002/46/EC). Although silver nanoparticles (AgNPs) are effective against bacteria [4,5] as well as some fungi and viruses [6], bacteria can become resistant to silver [[7], [8], [9]]. Toxicity of AgNPs is caused by several mechanisms (Fig. 1), and has been reviewed in detail in a number of publications [[5], [6], [7],[9], [10], [11], [12], [13], [14]]. An important mechanism of the antimicrobial effects of AgNPs is the strong oxidative activity due to an abundant release of silver ions (Ag+), which is also the basis of its toxicity in various organs [6].
Fig. 1.
Mechanisms of toxicity of silver nanoparticles (AgNPs, brown particles) and silver ions, brown particles with a cross). ROS = reactive oxygen species; ATP = adenosine triphosphate; LDH = lactate dehydrogenase. The figure is based on information from the following references: [10,11], [5], [12], [7], [6,8,9,13,14].
After ingestion AgNPs are systemically available in small amounts [7]. Both AgNPs and the released Ag+ ions persistently accumulate in the body [15], and 2–4 % of the absorbed silver is retained in the tissues [16]. The most visible consequences of prolonged exposure to silver is argyria, irreversible bluish pigmentation of the skin due to accumulation of silver [12,15,16]. In addition to argyria, numerous studies have described multiple adverse effects and toxicity in various organs due to AgNP exposure (Supplementary Table A.1).
Colloidal silver consists of silver particles and silver ions in aqueous suspension [17]. In this mixture the particle size of silver usually varies between nanoparticles (≤100 nm) and microparticles (up to 1000 nm). The silver ions in colloidal silver products mediate toxic effects in the human body. Colloidal silver products have been misleadingly marketed as “essential” mineral supplements [3]. Although silver is found in small amounts in the human body, it has no physiological function and is not an essential element [12]. No scientific evidence has been found for positive health effects of internal use of colloidal silver products, and the marketing and internal use has been restricted, for example, in the USA [18], Australia [19], and in the EU (Directive 2002/46/EC). Still, this product fascinates people, and the use is popular in some parts of the world, including Finland.
The World Health Organization (WHO) has issued a No Observable Adverse Effect Level (NOAEL) of 6.5 μg/kg bodyweight/day (bw/d) for all exposure routes for the general population, and the U.S. Environmental Protection Agency (U.S. EPA) has given a Reference Dose (RfD) of 5 μg/kg bw/d for chronic oral silver exposure [7]. However, these guidance values and most of the risk assessments of silver are based on argyria development, which is not the most sensitive endpoint of toxicity. Guidance values of 2.6 μg/kg bw/d and 2.5 μg/kg bw/d based on embryotoxicity in rats [20] and silver nanoparticle induced cytokine response in mice [15], respectively, have been proposed. The draft assessment according to the European Biocides Directive (BPD, 98/8/EC) derived a guidance value of 0.3 μg/kg bw/d extrapolated from a 2-year toxicity study in rats. Most of the colloidal silver products on the market are claimed to contain 10–30 mg/l of silver: therefore the given guidance values can be readily exceeded when drinking colloidal silver. Just a few tablespoons (about 15 mL) of 10 mg/l colloidal silver contains about 300 μg of silver and exceeds the U.S. EPA RfD for a person of 60 kg.
Nowadays colloidal silver is categorized as a biocide in the EU and should only be used as an external disinfection product also in Finland. According to the EU biocide regulation the marketing of products cannot contain misleading information or give false information about the effects on human health or the environment (BPR, EU Regulation 528/2012). The advertisement of biocides should not underrate the risks concerning the use of the products. The labels of biocide products should not understate the possible risks concerning the use of the product, and phrases such as “non-toxic”, “harmless” or “natural” should not be used. Similar phrases should not be mentioned in the advertising of the products.
In late autumn 2017, in the county of Ostrobothnia on the Western coast of Finland, the use of colloidal silver products (“silver water”) had increased to the extent that medical doctors became concerned about potential health effects. Based on this, the Finnish Broadcasting Company (Yle) presented a documentary in the public TV [21]. The Finnish Medicines Agency (Fimea), the Finnish Food Safety Authority (Evira), the Finnish Institute for Health and Welfare (THL) and the Finnish Safety and Chemicals Agency (Tukes) took actively part in the conversation in the media and informed about the risks of colloidal silver, and Fimea intervened in the misleading marketing of the products by some companies.
In Finland, the safety of various consumer products is monitored by Tukes based on potential risks, meaning that market surveillance is targeted at products with the greatest potential safety risks and the biggest impact on public health. Products such as colloidal silver often fall outside these surveillance programs. If a consumer notices that products are recommended by the manufacturer or retailer for other uses than the accepted ones in a way that poses a safety risk to consumers, this can be reported to Tukes. This applies to recommendations given on the product packaging, website, social media, as well as private messages. If a product poses a safety risk, Tukes will oblige the manufacturer or retailer to take action and remove the risk. In rare cases, a penalty payment can be issued, in order to ensure that safety risks would not occur again. In the future, if companies continue unsafe advertising, Fimea can reclassify colloidal silver as a medical ingredient, which allows limitation or ending of both marketing and sales of colloidal silver products.
The aim of this study was to investigate the marketing practices, beliefs and health claims regarding the use of colloidal silver in Finland. The specific objectives were to assess the websites of companies marketing colloidal silver, to monitor the influence of the Yle documentary on the use of colloidal silver and on the content in the internet, as well as to analyze lay people’s attitudes in social media about the use of colloidal silver. Based on these observations it was further analyzed, how lay people handle information about silver products, and whether safety of consumers can be endangered due to the false information.
2. Materials and methods
2.1. Websites of companies
Websites of companies marketing colloidal silver were searched from the internet using the search terms “colloidal silver”, “colloidal silver manufacturer” and “colloidal silver sales”. The searches were made in the two official languages in Finland, Finnish and in Swedish. Contents of the identified websites were reviewed, and data were collected using a time frame of January 2016 to December 2018, ranging from one year before the year of the Yle documentary to one year after. The websites were analyzed in order to assess if there were enough data to conduct a comparison of contents between the different time points. The older versions of the websites were obtained from http://web.archive.org/, which is a digital library of older versions of websites. The websites of three companies marketing colloidal silver were selected based on the availability of contents on the sites from different time points and the popularity of products. Two of them were Finnish companies (Website 1 and Website 2) and one was a Swedish company (Website 3). Information about the analyzed websites are provided in the Supplementary Table B.1. Website 3 was included because the company’s colloidal silver product was mentioned in the Yle documentary and is commonly sold in Finland. In addition, the use of colloidal silver of this company is very common especially in the Swedish-speaking parts of the county of Ostrobothnia.
The claims presented in this study were translated into English from their original languages, Finnish or Swedish. The potentially most harmful claims as to health were selected and presented in the Tables. Because of the excessive amount of available data, not all found claims are presented. The claims found on the websites were documented in a tabular form. The validity of the provided information was analyzed by comparing the claims used in the marketing of colloidal silver to the scientific information about silver. Also, the indirect or suggestive impressions given by the claims were evaluated. Changes on the websites after the Yle documentary were analyzed by comparing the older versions of the websites to the new ones.
The claims were categorized into different groups using a method developed by Faerber and Kreling [22]. In their study Preston’s typology was used as a guiding framework for content analysis to categorize false and misleading claims in television advertising. The typology has five categories of claims, and each of them is increasingly misleading and potentially harmful for consumers; Objectively True Claims; Selected Facts Claims; Minimal Facts Claims; Nonfacts Claims; and False Claims. In our study, selected facts claims and nonfacts claims were categorized into one category of “potentially misleading” claims (Table 1). The category “minimal facts claims” was not applicable in this study, because it was about the case of clinical drugs: The claims collected from websites marketing colloidal silver were compared to the definitions for each selected category and placed into the most appropriate one.
Table 1.
Definitions of Objectively true, Misleading and False claims (applied from Faerber and Kreling [22].
| Objectively True | A claim that presented all information, both positive and negative, in order for a consumer to compare products on the advertised attribute |
| Potentially misleading | Selected facts |
| A claim that omitted important facts about the advertised product that would affect consumer evaluation of the advertised attribute. | |
| Nonfacts | |
| A claim that presented an intangible characteristic, but not about the product. Often these claims were in the form of product opinions or lifestyle claims. Opinions did not directly mention the product, but left the consumer to misinterpret the opinion as an objective product evaluation. Lifestyle claims associated the product with a target audience that the advertiser believed was likely to buy the product, in the absence of evidence to support beneficial effects. | |
| False | A claim that was objectively false by directly contradicting evidence, or lacking any evidence to support it. |
2.2. Social media
People’s attitudes towards colloidal silver products were observed by going through posts and analyzing discussions about the subject in the social media platform Facebook. Groups about colloidal silver were searched in Facebook using the search term “colloidal silver” in Finnish, Swedish and English. Identified groups were evaluated based on their activity, amount of content, whether they could be joined, and possible connections to Finland. The two most relevant Facebook groups were chosen for the analysis: one public Facebook group with approximately 800 members, and one private group with approximately 10 000 members. Information about these Facebook groups is provided in the Supplementary Table B.2. The groups were joined using a real personal Facebook account. All conversations about colloidal silver in the groups were reviewed and the topics of discussion were collected using the same timeframe of January 2016 to December 2018 that was used in comparing the contents of old and new websites. Data from the posts made on Facebook was collected completely anonymously. To protect individual privacy, no names or other personal information of the members, screenshots, copies or transcripts of the conversations were collected.
3. Results
3.1. Websites marketing colloidal silver
3.1.1. Claims
In all of the three analyzed websites, inaccurate information about the efficacy and safety of colloidal silver was found before the Yle documentary (Table 2, Table 3, Table 4). Health and medical claims about colloidal silver were found in the marketing of the products despite the EU biocide regulation (BPR, Regulation (EU) 528/2012), in which the use of such claims in the marketing of non-medicinal products is forbidden. Also recommendations for internal use of colloidal silver were found, even though the use of colloidal silver as a food supplement is forbidden in the EU (Directive 2002/46/EC). References used to support the claims were typically commercial websites, or other unscientific sources. In some cases, actual valid studies were cited but the results were interpreted incorrectly to support the statements used in the marketing.
Table 2.
Claims about colloidal silver presented on the Website 1.
| Claims | Comment | Category |
|---|---|---|
| “Colloidal silver works as the natures’ own antibiotic.” | There is no proof for the medical usefulness of colloidal silver as an antibiotic; colloidal silver is neither safe nor effective [18]. | False |
| “Colloidal silver might be the best protective compound against the feared biological weapons.” | The claim is not scientifically valid [18]. | False |
| “Unlike medical antibiotics, bacteria will never develop resistance to colloidal silver.” | Bacteria can develop resistance to AgNPs ([7], [8] [9],). | False |
| “Pharmaceutical industry is not interested in colloidal silver because silver is an element and can’t be patented, so it is worthless for it.” | There is no use for colloidal silver in the pharmaceutical industry, since its efficacy in oral use is not scientifically proven [18]. | False |
| “Excess silver is removed from the body through natural ways.” | Excess silver is not efficiently removed. Silver has a long elimination half-life and therefore it accumulates in the human body [15]. | Misleading |
| “Some tumors and warts can disappear with colloidal silver water.” | The claim is not scientifically valid [18]. | False |
Table 3.
Claims about colloidal silver presented on the Website 2.
| Claim | Comment | Category |
|---|---|---|
| “The silver in colloidal silver water is harmless for humans as pure colloidal metal and as ions. The salts of silver are dangerous. “ | Metallic silver is quite inert in the human body, but it can release silver ions through oxidation processes [9]. Silver ions are one of the main factors causing toxicity in soluble silver compounds. | False |
| “The human body tolerates colloidal silver extremely well making silver one of the safest preparations to be used for the treatment of diseases.” | Therapeutic effects of colloidal silver have not been scientifically proven [18]. AgNPs cause several adverse effects in the human body [15]. | False |
| “Colloidal silver water can be used for treating wounds, acne, burns, chafes, herpes, genital herpes, herpes zoster, insect stings and bites, rashes, psoriasis, fungal infections like athlete’s foot.” | The claim is not scientifically valid [18]. In some cases, silver is still utilized in today’s medicine due to its antimicrobial properties, but the use is limited to topical applications. If silver is inhaled or ingested, there is a risk of accumulation and toxicity [16]. | Misleading |
| “Colloidal silver can be used as a prophylactic against diseases when the immune system is impaired.” | The claim is not scientifically valid [18]. | False |
| “Silver seems to have some kind of a role in our immune system, and it may be possible that the people’s silver intake is insufficient.” | AgNPs are proposed to have immunomodulatory properties, both stimulating and suppressive effects (Lappas 2015). However, silver does not have a physiological role in human body [12]. | Misleading |
Table 4.
Claims about colloidal silver presented on the Website 3.
| Claim | Comment | Category |
|---|---|---|
| “There are no studies indicating that bacteria would become resistant to electrocolloidal silver, or that silver-resistant bacteria could develop into a serious problem. Everything is just based on speculation.” | Bacteria can become resistant to AgNPs, which are found in colloidal silver ([7], [8] [9],). | False |
| “The only side effect reported after far too high intake of a mostly wrongly produced silver product is a cosmetic effect, such as argyria.” | Argyria is a well-known side-effect of chronic silver exposure, but it also causes toxicity in several other ways, for example damage to kidneys and liver and irritation of eyes, skin and respiratory tract [7]. Method of production does not affect the effects of Ag+. | False |
| “It is not proven that silver is not used for anything in the body. Since silver is a mineral found in most of the body's tissues, exhibits broad effects on microorganisms and conducts electricity best of all minerals it must be used for something. Research of the subject is lacking as it cannot be justified economically. “ | Silver does not have a physiological role in the human body ([15] [12],). | Misleading |
| “According to the World Health Organization, one can consume 6 teaspoons of 10 ppm colloidal silver daily for 80 years without a risk of the only side effect of ingesting too much silver - argyria.” | WHO has issued a NOAEL of 10 g for a lifetime exposure to silver, which is based on argyria development. Colloidal silver is not mentioned [23]. The given NOAEL cannot fully guarantee avoiding argyria development, since the exact dose needed for argyria differs from case to case. It has been proposed that 1−30 g of ingested soluble silver salts can cause argyria [12]. Argyria is not the only and not the most sensitive toxic effect of silver. | Misleading |
Although objectively true claims occurred, for instance “silver may cause argyria in very high doses”, false and misleading claims were much more common (Table 2, Table 3, Table 4). Most of the claims selected for this study were categorized as False, based on lack of evidence or direct contradiction with scientific literature. For example, in two websites it was claimed that bacteria are not able to develop resistance against colloidal silver, even though this has been shown in several publications (Table 2, Table 4). There is also no evidence for the claim that colloidal silver would protect from biological weapons (Table 2). In addition, claims concerning the effects, uses and consequences were often not justified. It was suggested that the use of the products is completely safe when the manufacturers’ instructions are followed, and the possible adverse effects were understated.
In the claims categorized as Misleading some valid piece of information was often utilized, but it was interpreted wrongly or exaggerated to support the marketing. For example, silver is to some extent still used in today’s medicine but uses besides topical use are not supported in the medical field, contrary to the claims found (Table 3). Also, WHO has determined a NOAEL for lifetime exposure to silver, and this was presented as a warranty for safe doses of colloidal silver use (Table 4). However, NOAEL cannot guarantee absence of adverse effects, and the value is not meant to be used as such in recommendations to consumers.
In the websites information provided by the authorities about the possible dangers of colloidal silver use were discredited by stating that the authorities are referring to a wrong form of silver, or that the studied products had been manufactured incorrectly, distorting the results. Some more blatant statements were also made in which scientific competence of the authorities and their ability to make a difference between true and false data were questioned, and their ability to do what is best for the lay people harshly criticized. The whole health care system and pharmaceutical industry were claimed to be corrupted and untrustworthy, and only to be interested in increasing their incomes on the expense of the lay peoples’ health.
3.1.2. Changes on the websites
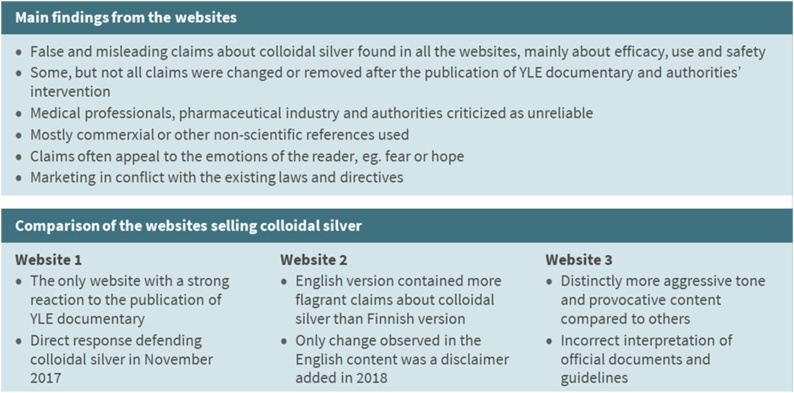
Changes were observed in the contents on both of the analyzed Finnish websites during the studied timeframe (Table 5, Table 6). The Yle documentary was not shown in Sweden and no changes were observed on the website 3. No data from 2017 were available for the website 2, but differences were found on the websites between the years 2016 and 2018 (Table 6). In 2016, there was more information provided, and the misleading claims were more blatant and common. After the Yle documentary and intervention of the authorities in 2017, the websites were revised by removing whole pages about the use and effects of colloidal silver, and misleading claims were revised or removed from the websites. In 2018, the revision of contents of the websites continued. However, questionable and false claims were still found. In some cases, the promotion of the effectiveness of colloidal silver was substantiated by advocating the long-term worldwide use of colloidal silver products. In some cases, misleading claims were removed from the front page of the website, but the information could still be found on other parts of the pages. Summary of the analysis of the websites marketing colloidal silver is shown in Fig. 2.
Table 5.
Changes observed in claims during 2016-2018 on the Website 1.
| 2016 | 2017 | 2018 |
|---|---|---|
| “Silver is the nature’s own antibiotic.” | “Silver is the nature’s own “antibiotic”.” | Statement removed. |
| “Colloidal silver is excellent for treating different inconveniences in animals and humans, for example scratches, wounds, ringworm, rashes, acne, warts, eye and ear infections, sun burned skin, insect bites, oral ulcers, sore throat, and it can be used to prevent cough and common cold.” | “Only external use of colloidal silver as a disinfectant on skin and different surfaces is accepted in EU. It can be used in animals and humans as a mouthwash, hand sanitizer, and to treat scratches and wounds, burns, rashes, sun burned skin, insect bites and to disinfect mouth, gums and throat.” | Page about the medical use of colloidal silver removed. |
| “The healing effect of silver has been known for centuries.” | “The healing effect of silver has been known for centuries. Colloidal silver was sold for both internal and external use for over 100 years before 2014, when EU removed colloidal silver from the supplement catalogue.” | “There are about 10 million colloidal silver water users in North America and 100 million users worldwide.” |
| “Colloidal silver water helps the immune system to fight against microbes and to prevent diseases. If you get sick, colloidal silver destroys microbes and can hasten healing.” | “Colloidal silver water can be used as a disinfectant on the skin and other surfaces. It kills microbes and can prevent illnesses.” | “Colloidal silver water can be used as a disinfectant on the skin and other surfaces. It kills microbes and the use as a disinfectant can prevent illnesses.” |
Table 6.
Changes observed in claims during 2016-2018 on the Website 2.
| 2016 | 2018 |
|---|---|
| “Colloidal silver can be used for wounds, acne, burns, chafes, herpes, genital herpes, herpes zoster, insect stings and bites, rashes, psoriasis and fungal infections like athlete’s foot, hepatitis and yeast fungus syndrome.” | Lists of use were removed from all other pages except the English version of the colloidal silver –page. |
| “The silver in fish is the reason why Japanese people live for so long.” | Removed from the Finnish colloidal silver –page, but remained in the English version. |
| “The silver in colloidal silver water is harmless for humans as pure colloidal metal and as ions. The salts of silver are dangerous.” | Removed from the website. |
Fig. 2.
Summary of the analysis of websites 1, 2 and 3 marketing colloidal silver.
3.2. Facebook groups
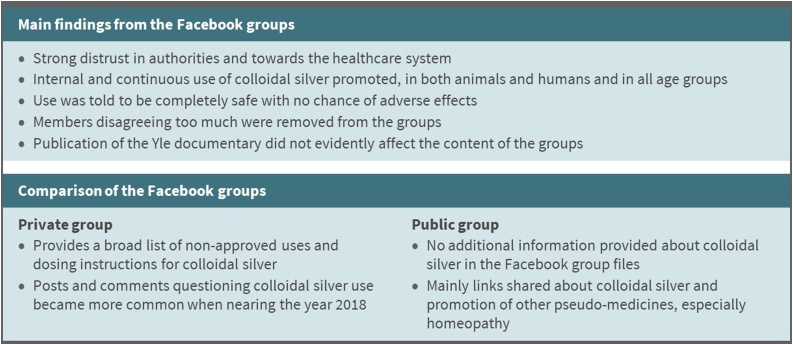
In both of the analyzed Facebook groups, the discussion topics and opinions about colloidal silver were similar to those found on the websites. The most significant differences were based on the fact that the websites are regulated by authorities and the Facebook groups are not. After November 2017, some of the misleading claims on the Finnish websites selling colloidal silver were removed or changed, but the discussion in the Facebook groups did not change. The advocation of colloidal silver use was distinctly more aggressive in the Facebook groups compared to the websites. Criticism of the authorities and promotion of internal use of colloidal silver, from newborns to elderly, from common cold to cancer treatments, and from single to everyday use were ordinary topics in posts made by the group members on Facebook. Although there were increasingly more people questioning colloidal silver towards 2018, asking about possible adverse effects and seeking for assurance of safe use, they were clearly a minority, and these people were in some cases removed from the groups. Summary of the analysis of the Facebook groups are shown in Fig. 3.
Fig. 3.
Summary of the analysis of the Private and Public Facebook groups.
4. Discussion
We observed that the marketing of colloidal silver products on the analyzed websites was in conflict with the EU regulations (BPR, EU Regulation 528/2012). They were marketed similarly to food supplements, even though colloidal silver is classified as a disinfectant in the EU. Furthermore, even if colloidal silver was classified as a supplement, the marketing would not be in compliance with the EU Regulation on health and nutrition claims (No 1924/2006). Medical claims, as well as false and misleading claims not based on scientific data were commonly used.
Distrust against the authorities of the health care system and medical field was distinctly present on all the websites, e.g. discrediting the authorities’ guidance concerning the use of colloidal silver or questioning the motives of the pharmaceutical industry. On the Finnish websites, changes were made, especially in the contents of the front pages, after intervention by the authorities. However, questionable claims still remained in other parts of the websites. In the analyzed Facebook groups, promotion of internal use of colloidal silver in order to treat diseases is still very common and is recommended regardless of the age or health issue of the customer.
Previous studies on the marketing of pseudo-medicines and the structures of quackery remedies have shown, that typically, the marketing appeals to the emotions of the reader, especially to fear [24], and creates provocative conspiracy theories about the pharmaceutical industry [2,25]. The results of the present study are consistent with these findings. The fear tactic is used e.g. when claiming that colloidal silver can protect against biological weapons (Table 2). False suggestions that natural drugs would be superior to synthetic drugs (Table 2) also utilize the fear in the consumer, aiming to assure the safety of the product. The possible health risks are then belittled by referring to them as being purely cosmetic, e.g. claiming that silver temporarily affects only epidermis (Table 4), while it is known that argyria is permanent [16].
Also claims about untrustworthy authorities and corrupted big pharma were found. The claims accused the pharmaceutical companies of only being interested in profits (Table 2) while omitting the fact that sales are also essential for small colloidal silver producing companies. Claiming that research about colloidal silver is inadequate because of the lack of financial value (Table 4) omits the fact that enough studies exist about its negative health effects when given orally. It was also indirectly claimed that EU restrictions are too strict and that colloidal silver is used safely in other parts of the world also internally (Table 5). It was thus indirectly suggested that being known, being sold for a long lime, or being used for a large number of people guarantees the safety of oral silver, while the only way to prove efficacy and safety are preclinical and clinical studies [26].
According to our knowledge, similar studies assessing the validity of marketing claims of a specific quackery product at different time points, and evaluation of the actions taken in the marketing after intervention by authorities have not been conducted before. However, with regard to medicines, a content analysis of false and misleading claims in television advertisements of prescription and nonprescription drugs indicated that 57 % of the most emphasized claims were classified as Misleading and 10 % False [22]. Content analysis studies on Facebook posts about health claims are scarce. Recently, a study on vaccine-related Facebook posts showed that misinformative posts are more popular and spread easier than evidence-based posts [27]. Altogether, findings of the present study are in accordance with the outcome of these papers.
Short-term exposure to colloidal silver does not necessarily lead to immediate adverse effects. In a 14-day human study daily oral doses of 100 μg or 480 μg colloidal silver resulted in detectable silver concentrations in serum [28], but a wide range of clinical tests revealed no significant changes. However, when cytotoxicity of five commercial colloidal silver sprays was assessed in vitro, each of the products significantly decreased cell viability in a rat intestinal epithelial cell (IEC-6) model [29]. In addition, many people use colloidal silver for much longer periods, with known side-effects, such as argyria, argyrosis and organ failures (TREFs). Also, when consumers are using colloidal silver products, there is a possibility that the contents of the products, e.g. concentrations do not correspond to what is shown in the label, since the manufacturing is not strictly regulated. This was observed in an analysis of 22 commercial colloidal silver spray supplements [30]. There was a high degree of variation between claimed and measured concentrations of total silver, and silver contents both below and over the claimed concentrations were observed. The claimed total silver concentrations were from 10 mg/l to 500 mg/l, but the measured total silver varied from 0.54 mg/l to 960 mg/l. There was also variation in other parameters, such as relative percentages of particulate vs. soluble silver. Silver containing nanoparticles were found in all the selected products.
Facebook is a social media platform where people can create a profile and share their thoughts and all kinds of information with other people whom they are personally connected with. In addition, people can join open, closed or secret groups dedicated to discussion about specific subjects. Social media has become an important source of health information, especially for young people [31]. In Facebook groups people can share their opinions with other people they identify with, and these opinions may be different from the consensus shared by authorities, physicians, and governmental agencies. Information from trusted peers who share the same experiences is often perceived as more credible than that of authorities [36], which enhances the spread of misleading recommendations in the groups. The members of these Facebook groups, and their posts promoting colloidal silver, create a similar phenomenon to a powerful marketing strategy utilizing the loyal and dedicated customers, also called marketing advocates or brand advocates [32]. The advocates uncritically support the product they use, endorse it and defend it to other consumers, which provides the product and the company influential advertising.
To oppose the spread of false information on social media without limiting freedom of speech is difficult. Facebook has been known to be reluctant to ban or remove content, saying that public discourse should not be stifled and that it would be difficult to distinguish between false information and satire [33]. Currently, the Facebook community standards do not ban or address false health claims. However, Facebook has announced recently that they will start to reduce the visibility of sensational or misleading health claims. A machine learning model will identify posts that contain phrases typical of exaggerated or misleading claims, as well as posts that promote services or products with the help of health-related claims and show them lower in the users’ news feed.
The information available about products, such as colloidal silver, is most often from the sources that market the products. The information is likely to be biased and no warning about the possible adverse effects is given [34]. It is difficult for lay people to make a difference between the provocative marketing of the products and evidence-based data about the subject. It can be seen from the results that even after authority intervention the marketing can still contain false information about the product. When this is combined with the social environment of Facebook groups endorsing the use of these products and discrediting authorities, there is a possibility that people start using colloidal silver instead of medical treatments. Colloidal silver itself can cause adverse effects, and the use may delay or prevent proper medical care with severe consequences. The authorities should provide sufficient information about quackery remedies, such as oral colloidal silver, in an understandable form to the consumer. Also, through more strict regulation of marketing it could be ensured that the lay people would be less exposed to false and misleading information.
In conclusion, since the marketing of colloidal silver still provided false information about the product after intervention by the authorities, more strict regulation of marketing of products, such as colloidal silver, is needed. In the current Finnish legislation, many important safety aspects of health-related claims cannot be addressed. At the moment there is no specific legislation regarding quackery remedies, and the regulation is adapted from other legislation, e.g. legislation on consumer protection or the Criminal Code of Finland [35]. The phenomena of quackery have repeated itself with varying products frequently throughout history and has always been difficult to control. Nowadays with the lay peoples’ free access to internet and enormous amount of data, the regulation of quackery is even more challenging, but also more important than ever.
In social media, discussion topics and opinions on colloidal silver were similar to those on the websites marketing colloidal silver, but they were more blatant and internal use was highly endorsed. The results highlight the importance of interaction between the authorities and the lay people. In order to give information about products, such as colloidal silver, to lay people, authorities should focus on using understandable language and suitable platforms to spread the information, especially social media where the discussion about the subject takes place. Also, lay people should be encouraged to report any suspicious activity or marketing regarding such products as colloidal silver to the authorities.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Veera Leino: Writing - original draft, Investigation. Riikka Airaksinen: Writing - review & editing, Visualization. Matti Viluksela: Writing - review & editing. Kirsi Vähäkangas: Writing - review & editing, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.12.021.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Cassileth B.R., Yarett I.R. Cancer quackery: the persistent popularity of useless, irrational’ alternative’ treatments. Oncology (Williston Park, N Y) 2012;26(8):754–758. [PubMed] [Google Scholar]
- 2.Widder R.M., Anderson D.C. The appeal of medical quackery: a rhetorical analysis. Res. Soc. Adm. Pharm. 2015;11(2):288–296. doi: 10.1016/j.sapharm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Fung M.C., Weintraub M., Bowen D.L. Colloidal silver proteins marketed as health supplements. JAMA. 1995;274(15):1196–1197. doi: 10.1001/jama.1995.03530150020017. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.S., Kuk E., Yu K.N. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Quang Van Quy, Anh-Tuan Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci: Nanosci. Nanotechnol. 2013;4(3) [Google Scholar]
- 6.Akter M., Sikder M.T., Rahman M.M. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J. Adv. Res. 2018;9:1–16. doi: 10.1016/j.jare.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks) European commission; 2014. Opinion on Nanosilver: Safety, Health and Environmental Effects and Role in Antimicrobial Resistance.https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_039.pdf [Google Scholar]
- 8.Kędziora A., Speruda M., Krzyżewska E. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018;19(2):444. doi: 10.3390/ijms19020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqi K.S., Husen A., Rao R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnology. 2018;16(1):14. doi: 10.1186/s12951-018-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrivastava S., Bera T., Roy A. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18(22) doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]
- 11.Lansdown A.B.G. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010;2010 doi: 10.1155/2010/910686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordberg G.F., Fowler B.A., Nordberg M. 4th ed. Elsevier Science; Burlington: 2014. Handbook on the Toxicology of Metals. [Google Scholar]
- 13.Gaillet S., Rouanet J. Silver nanoparticles: their potential toxic effects after oral exposure and underlying mechanisms – a review. Food Chem. Toxicol. 2015;77:58–63. doi: 10.1016/j.fct.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Cameron S.J., Hosseinian F., Willmore W.G. A current overview of the biological and cellular effects of nanosilver. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19072030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadrup N., Lam H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver – a review. Regul. Toxicol. Pharmacol. 2014;68(1):1–7. doi: 10.1016/j.yrtph.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Drake P.L., Hazelwood K.J. Exposure-related health effects of silver and silver compounds: a review. Ann. Occup. Hyg. 2005;49(7):575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- 17.SCCS (Scientific Committee on Consumer Safety) European Commission; 2018. Opinion on Colloidal Silver.https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_219.pdf SCCS/1596/2018. [Google Scholar]
- 18.FDA Over-the-counter drug products containing colloidal silver ingredients or silver salts, final rule. Nat. Arch. Rec. Administration. 1999;64(158):44653–44658. [PubMed] [Google Scholar]
- 19.Therapeutic Goods Administration . Australian Government Department of Health; 2004. Colloidal Silver & Related Products.https://www.tga.gov.au/colloidal-silver-related-products (Accessed Sep 5, 2019) [Google Scholar]
- 20.EFSA Panel on Food Additive and Nutrient Sources added to Food (ANS) European Food Safety Authority (EFSA), Parma, Italy. Scientific opinion on the re-evaluation of silver (E 174) as food additive. EFSA J. 2016;14:4364. [Google Scholar]
- 21.Gardberg A. 2017. Spotlight | Silvervatten - Bot Eller Hot? Yle.https://arenan.yle.fi/1-3797225 [Google Scholar]
- 22.Faerber A., Kreling D. Content analysis of false and misleading claims in television advertising for prescription and nonprescription drugs. J. Gen. Intern. Med. 2014;29(1):110–118. doi: 10.1007/s11606-013-2604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization . 4th ed. World Health Organization; Geneva: 2011. Guidelines for Drinking-water Quality.https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf?sequence=1 [Google Scholar]
- 24.Bernard V.W. Medical quackery. Am. J. Public Health Nations Health. 1965;55(8):1142–1147. doi: 10.2105/ajph.55.8.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyerstein B.L. Alternative medicine and common errors of reasoning. Acad. Med. 2001;76(3):230. doi: 10.1097/00001888-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Brodniewicz-Proba T. The scope and requirements related to preclinical and clinical studies of a new medicinal product, including biotechnological and biosimilar products. Acta Pol. Pharm. 2008;65(6):641–645. [PubMed] [Google Scholar]
- 27.Gandhi C.K., Patel J., Zhan X. Trend of influenza vaccine Facebook posts in last 4 years: a content analysis. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.01.010. Epub ahead of print https://www.ajicjournal.org/article/S0196-6553(20)30037-7/fulltext (Accessed 12 March 2020) [DOI] [PubMed] [Google Scholar]
- 28.Munger M.A., Radwanski P., Hadlock G.C. In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomedicine. 2014;10(1):1–9. doi: 10.1016/j.nano.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers K.R., Henson T.E., Navratilova J. In vitro intestinal toxicity of commercially available spray disinfectant products advertised to contain colloidal silver. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers K.R., Navratilova J., Stefaniak A. Characterization of engineered nanoparticles in commercially available spray disinfectant products advertised to contain colloidal silver. Sci. Total Environ. 2018;619-620:1375–1384. doi: 10.1016/j.scitotenv.2017.11.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klassen K.M., Douglass C.H., Brennan L. Social media use for nutrition outcomes in young adults: a mixed-methods systematic review. Int. J. Behav. Nutr. Phys. Act. 2018;15(1):70. doi: 10.1186/s12966-018-0696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Constantinides E. Foundations of social media marketing. Proc. - Soc. Behav. Sci. 2014;148:40–57. [Google Scholar]
- 33.Facebook . 2020. Community Standards, False News.https://www.facebook.com/communitystandards/false_news (Accessed Mar 10,2020) [Google Scholar]
- 34.Ritvo P., Irvine J., Katz J. The patient’s motivation in seeking complementary therapies. Patient Educ. Couns. 1999;38(2):161–165. doi: 10.1016/s0738-3991(99)00063-4. [DOI] [PubMed] [Google Scholar]
- 35.Makkonen K., Paaso K. 2020. The Ministry of Social Affairs and Health Is to Start Preparing Legislation on Alternative Treatments.https://stm.fi/en/article/-/asset_publisher/stm-kaynnistaa-valmistelun-vaihtoehtohoitoja-koskevasta-lainsaadannosta (Accessed Mar 10,2020) [Google Scholar]
- 36.Eysenbach G. credibility of health information and digital media: New perspectives and implications for youth. digital media, youth, and credibility. In: Metzger Miriam J., Flanagin Andrew J., The John D., Catherine T., editors. MacArthur Foundation Series on Digital Media and Learning. The MIT Press; Cambridge, MA: 2008. pp. 123–154. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.