Graphical abstract
Keywords: Electronic cigarette, e-liquid, Nicotine, Pharmacokinetic
Highlights
-
•
Evaluating the pharmacokinetics of nicotine absorption from electronic cigarettes.
-
•
Nicotine absorption into the blood is influenced by several factors.
-
•
These include e-liquid composition, user behavior and device characteristics.
-
•
Any regulation of nicotine levels in e-liquids should reflect this heterogeneity.
Abstract
Several regulatory initiatives around the world restrict the amount of nicotine permitted in electronic cigarette liquids in an attempt to reproduce the nicotine delivery of combusted tobacco products, such as cigarettes, and or reduce the risk of consumers absorbing too much nicotine into their body at one time. Such an approach, however, assumes that (i) there is a strong correlation between the levels of nicotine in electronic cigarette liquids and nicotine intake into the body and (ii) that this correlation holds true across the various different types of electronic cigarette devices currently available on the market. In order to test these hypotheses, this study examines the available scientific literature on nicotine intake from electronic cigarettes, as measured by levels in the blood. Analysis of the published data reveals that nicotine absorption into the body is influenced by a combination of many factors, including electronic cigarette liquid composition, user behavior and device characteristics. Notably, it was observed that open-tank (refillable) electronic cigarettes, which often enable users to vary device power, can deliver high nicotine levels to consumers, sometimes at greater doses than a conventional tobacco cigarette, even at the lower nicotine liquid concentrations typically available. For electronic cigarettes to be viable alternative choices to smoking, they should provide consumers with an equally satisfying experience, including in terms of nicotine absorption into the body. Therefore, any regulation seeking to restrict the amount of nicotine in electronic cigarette liquids should take all the factors influencing nicotine intake into account.
1. Introduction
Electronic cigarettes are battery-powered devices which heat a liquid, usually containing nicotine, to produce an inhalable aerosol, colloquially known as electronic cigarette ‘vapor’. Over the past decade, electronic cigarettes have become firmly established as acceptable alternatives to combusted tobacco products among some smokers. However, as electronic cigarettes are a relatively novel product category and continue to evolve rapidly, both in terms of product design and performance, the scientific understanding of this product category is still incomplete. This is further complicated by the heterogeneity of the category, encompassing both closed (non-refillable) and open (refillable) devices as well as a broad range of electronic cigarette liquid (e-liquid) formulations, including recent formulations containing nicotine salts.
Understanding the science of electronic cigarettes, from both product design and product performance perspectives, is critical for any objective evidence-based regulation of the product category. It is also important that consumers have access to such information, in an understandable manner, to allow them to make informed choices about which products they may wish, or not wish, to use.
Most e-liquids contain nicotine at varying concentrations depending on the specific brand or sub-brand. The pharmacokinetics of nicotine delivery from electronic cigarettes has been proposed to be a key performance metric; in particular maximum blood concentration (Cmax), time to maximum blood concentration (Tmax), and overall blood nicotine exposure (as defined by the area under the concentration-time curve (AUC)) [1]. It is thought that the closer the pharmacokinetic profile is to that obtained through cigarette smoking, the more likely it is that electronic cigarettes will be accepted as satisfying alternatives to cigarettes among smokers [2].
The factors influencing nicotine delivery from different types of electronic cigarettes also have important implications for how they are proportionately regulated. When electronic cigarettes were first introduced, they represented novel consumer products that did not easily fit into any preexisting regulatory framework. Consequently, regulators at national and international levels often adopt differing approaches, from complete product category bans to no specific regulatory requirements [3].
Where electronic cigarette regulations do exist, upper limits on the nicotine concentration in e-liquids are common, often being introduced to try and emulate the nicotine delivery from combusted tobacco products and/or in response to concerns over possible nicotine toxicity and or addictiveness. For example, in 2014, the European Union (EU) adopted specific electronic cigarette regulations as part of the revised Tobacco Products Directive [4]. In doing so, the EU imposed a nicotine ceiling of 20 mg/mL in e-liquids to allow “for a delivery of nicotine that is comparable to the permitted dose of nicotine derived from a standard cigarette during the time needed to smoke such a cigarette” [5].
In contrast to the EU approach, Canada has deferred to existing chemicals regulation to set a nicotine limit in e-liquids of 66 mg/mL, based solely on the toxicological properties of the molecule [6]. Notably, prominent scientists in the field have criticized the European 20 mg/mL limit as too low to match the nicotine delivery of most cigarettes, suggesting that higher levels, in the range of 50 mg/mL, are needed to help improve acceptance of electronic cigarette use among smokers [7].
Since the implementation of these nicotine limits in e-liquids, an increasing number of studies have been published assessing nicotine uptake from electronic cigarettes in adult consumers [8]. The data gathered to date indicates that there are several factors which can influence nicotine uptake, including user experience, device type and e-liquid nicotine concentration [[8], [9], [10]]. However, the inter-relationships between each factor, as well as their relative contribution to nicotine pharmacokinetics, have yet to be fully elucidated. The purpose of this paper is thus to address these questions by presenting a pooled analysis of all identified studies on nicotine blood uptake from electronic cigarettes, as measured by Cmax and Tmax, to further tease out the specific associations between these factors and nicotine blood pharmacokinetics. The findings will not just be of interest from a scientific standpoint but will also be of value to regulatory authorities who are considering adopting, or revising existing, nicotine limits in e-liquids.
2. Materials and methods
2.1. Literature search strategy
The objective of the literature search strategy was to identify all relevant published scientific papers; namely those investigating the pharmacokinetics of nicotine delivery from electronic cigarettes. The search was conducted in four steps as follows:
-
1
Retrieval of relevant papers cited in previously published reviews on nicotine pharmacokinetics of electronic cigarettes, which included Voos, et al., [11], Fearon, et al. [8], and DeVito & Krishnan-Sarin [10].
-
2
PubMed® (https://www.ncbi.nlm.nih.gov/pubmed/) was used to carry out literature searches from 2010 to February 2020 with the following search terms: (electronic cigarettes / e-cigarettes / vaping) AND pharmacokinetic OR nicotine delivery. The year 2010 was chosen as an appropriately inclusive conservative date based on the report entitled E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General (2016), indicating that 2011 might be a reasonable cutoff date, noting that “[b]ecause e-cigarettes only became prevalent in the tobacco product marketplace in recent years, minimal data are available on their use before 2011” [12].
-
3
An additional literature search was carried in March 2020 on STN using the Medline and Embase databases. The STN basic index covers the title, abstract and index terms, which includes the Medline Mesh headings and Embase index terms. The search strategy applied is outlined in the Supplemental Information 1.
-
4
Bibliographies of published papers identified in Steps 1, 2, and 3 were interrogated to identify any additional potentially relevant papers published from 2010.
In Steps 2 and 3, abstracts were examined first, with full texts obtained only for papers which appeared likely to be relevant and included nicotine pharmacokinetic data. Inclusion criteria were: Articles in English, original articles providing data on nicotine pharmacokinetics, original articles directly related to the topic outlined in the review, peer reviewed original articles.
At each step, papers (or abstracts) examined for potential relevance were only those not previously considered. At the end of this process, a set of potentially relevant papers was obtained. Subsequently, more detailed examination of the full texts at the data entry stage revealed that some papers did not actually meet the inclusion criteria, leading to a reduction in the list of relevant papers.
2.2. Determination of key variables (device type, user experience, vaping regime)
For sub-category analyses, examining the influence of electronic cigarette device type (open or closed), user experience and vaping regime used, such information was obtained from the source paper. For device type, where the information was not explicitly stated in the source publications, it was obtained from the manufacturer web site or other publicly available information (e.g., marketing materials). Where a particular parameter could not be confirmed from the source paper or other publicly available sources, the respective paper was not included in the relevant sub-analysis.
2.3. Statistical analyses
Cmax and Tmax were the main parameters considered for statistical analyses. Publications showed heterogeneity in Cmax calculation and reporting depending on study designs. Therefore, all reported base-line adjusted Cmax (Cmaxb) were selected or were calculated using standard subtractive method when base-line concentration was also reported.
Data were pooled and appropriate methodologies dealing with publication’s weight and heterogeneity were applied.
Tmax and Cmaxb univariate distributions were summarized by way of weighted Tukey’s boxplots showing medians, interquartile ranges, whiskers and the data outside the whiskers. Furthermore, the bivariate distribution between Cmaxb and Tmax has been assessed by plotting both medians and interquartile ranges intervals.
The association between Cmaxb and nicotine concentration has been calculated using the weighted Pearson’s correlation statistics together with mixed-model analyses of variance to obtain corresponding average slope accounting for random variability across publications.
Comparisons between subgroup of data (e.g., conventional cigarettes vs. electronic cigarettes) were performed in an exploratory and visual manner showing boxplot of the respective distributions, as well as scatter plots with correlation measures and slopes estimates. Marker (dots) widths were adjusted according to the sample size of each study to reflect the relative weight of the study in the pooled data analysis.
Tmax and Cmaxb distributions between subgroup of data were statistically compared using the weighted Wilcoxon rank sum scores.
All statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc. SAS/STAT, Cary, NC, USA).
3. Results
3.1. Characteristics of included studies
The applied search strategy identified 192 studies, published between 2010 and 2020. Retrieved studies were examined for Cmax and Tmax data as well as the study conditions applied. Studies were excluded if no Cmax was reported or could be estimated from the provided data (Supplemental Information 2). In addition, studies lacking data on specific study variables, e.g., experience of electronic cigarette use, were excluded from further analyses where such information was required. The final analytical sample included 27 individual studies (Fig. 1, Table 1).
Fig. 1.
Flow chart of search strategy showing total number of papers identified, number of rejections, and final number included in analysis.
Table 1.
Summary of the 27 papers included in final analysis, including key metrics.
| Year | Paper | Total subjects | Subjects with Cmax | Subjects with adjusted Cmax | Subjects with Tmax |
|---|---|---|---|---|---|
| 2010 | Bullen, et al., (2010). Tobacco Control [13] | 17 | 17 | 17 | 17 |
| Eissenberg, (2010) Tobacco Control [14] | 96 | 96 | 0 (0.0 %) | 0 (0.0 %) | |
| Vansickel, et al., (2010). Cancer Epidemiology, Biomarkers & Prevention [15] | 96 | 96 | 96 | 0 (0.0 %) | |
| 2014 | Dawkins & Corcoran, (2014). Psychopharmacology [16] | 14 | 14 | 7 (50.0 %) | 0 (0.0 %) |
| Nides, et al., (2014). Am J Health Behav [17] | 32 | 32 | 16 (50.0 %) | 0 (0.0 %) | |
| 2015 | D'Ruiz, et al., (2015). BMC Public Health [18] | 278 | 278 | 278 | 139 (50.0 %) |
| 2016 | Dawkins, et al., (2016). Psychopharmacology [19] | 66 | 66 | 66 | 0 (0.0 %) |
| Lopez, et al., (2016). Nicotine & Tobacco Research [20] | 128 | 128 | 0 (0.0 %) | 0 (0.0 %) | |
| Ramoa, et al., (2016). Tobacco Control [21] | 128 | 128 | 64 (50.0 %) | 0 (0.0 %) | |
| St. Helen, et al., (2016). Addiction [22] | 13 | 13 | 13 | 13 | |
| St. Helen, et al., (2016). Tobacco Regulatory Science [23] | 13 | 13 | 13 | 13 | |
| Walele, et al., (2016). Regul Toxicol Pharmacol [24] | 72 | 72 | 0 (0.0 %) | 72 | |
| 2017 | Fearon, et al., (2017). Am J Health Behav [25] | 148 | 148 | 148 | 148 |
| Hajek, et al., (2017). Psychopharmacology [26] | 99 | 99 | 99 | 99 | |
| Hiler, et al., (2017). Experimental and Clinical Psychopharmacology [27] | 192 | 161 (83.9 %) | 161 (83.9 %) | 0 (0.0 %) | |
| Stiles, et al., (2017). Psychopharmacology [28] | 180 | 180 | 180 | 180 | |
| 2018 | Hajek, et al., (2018). Psychopharmacology [29] | 120 | 120 | 120 | 120 |
| Spindle, et al., (2018). Drug Alcohol Depend [30] | 60 | 60 | 30 (50.0 %) | 0 (0.0 %) | |
| Stiles, et al., (2018). Psychopharmacology [31] | 220 | 220 | 220 | 220 | |
| 2019 | Hiler, et al., (2019). Experimental and Clinical Psychopharmacology [32] | 256 | 224 (87.5 %) | 128 (50.0 %) | 0 (0.0 %) |
| O'Connell, et al., (2019). Internal and Emergency Medicine [33] | 86 | 86 | 86 | 86 | |
| St. Helen, et al., (2019). Addiction [34] | 66 | 66 | 66 | 66 | |
| Voos, et al., (2019). Psychopharmacology [35] | 108 | 108 | 108 | 108 | |
| Yingst, et al., (2019). Jama Network Open [36] | 6 | 6 | 6 | 6 | |
| Yingst, et al., (2019). PLOS One [37] | 24 | 10 (41.7 %) | 24 | 10 (41.7 %) | |
| 2020 | Hajek, et al., (2020). Addiction [2] | 40 | 40 | 40 | 40 |
| Maloney, et al., (2020). Tobacco Control [38] | 72 | 72 | 72 | 0 (0.0 %) | |
| Total | Total | 2630 | 2553 (97.1 %) | 2058 (78.3 %) | 1337 (50.8 %) |
A detailed analysis of the individual study protocols revealed high variability in terms of study design, devices nomenclature and reporting of final data. Study variables included, but were not limited to, electronic cigarette device type, e-liquid composition, number of participants and their vaping experience, study duration and sampling frequency, vaping topography, e.g., number of puffs, puff duration and puff interval. In addition, outcomes measures varied and included reporting of single value, median, arithmetic or geometric mean Cmax, Tmax and nicotine boost (Supplemental Information 3).
3.2. Cmax as a function of e-liquid nicotine concentration
Base-line adjusted Cmax (Cmaxb) was chosen as a key parameter in the analysis as it is a direct measure for how much nicotine is really delivered to the consumer. All Cmaxb values determined for electronic cigarettes were plotted against the respective e-liquid nicotine concentration. Where available, Cmaxb data from conventional cigarette control groups was included for comparative purposes (Fig. 2).
Fig. 2.
Association between Cmax and nicotine. All papers with available Cmax base-line adjusted values. Model with intercept and slope at random. Gray area representing Nicotine range from conventional cigarettes. The size of the marker reflects the relative weight of the study in the pooled data analysis.
Cmaxb determined for electronic cigarette users showed a wide distribution ranging from 1 ng/mL to approximately 44 ng/mL. The majority of reported values were, however, below 20 ng/mL. Analysis indicated a weak positive association between Cmaxb and electronic cigarette nicotine concentration (slope 0.21 ng.mL/mg.mL, Pearson r = 0.15). Thus, Cmaxb cannot be predicted by the e-liquid nicotine concentration. Cmaxb values for conventional cigarettes were more consistent, with both higher median and mean Cmaxb observed compared to electronic cigarettes (Fig. 3).
Fig. 3.
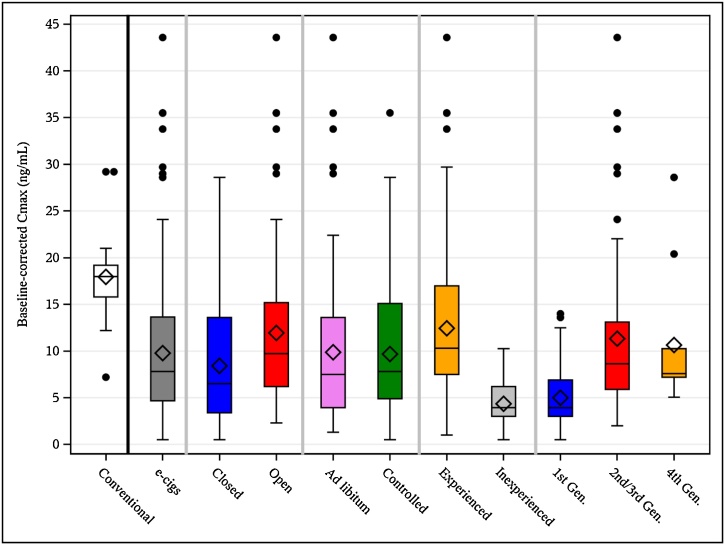
Base-line adjusted Cmax distributions. All papers with available Cmax base-line adjusted values. The boxes are representing the interquartile ranges (Q3-Q1), whiskers the 2.5 % and 97.5 % percentiles and extreme values (>1.5*IQR) are represented by dots. Horizontal lines within the boxes are representing the median and diamonds the means.
As the lack of a robust relationship between Cmaxb and e-liquid nicotine concentration in the total pooled data could simply reflect the high heterogeneity among the included studies, further sub-analyses were conducted wherein one or more study variables were fixed. To this end, the data set was analyzed based on select variables, such as device type, puffing behavior and user experience.
3.3. Nicotine Cmaxb from open (refillable) versus closed (non-refillable) electronic cigarettes
The total data set was categorized based on whether the electronic cigarettes used in each study were open or closed systems. To determine whether the specific electronic cigarette(s) used in each study were open or closed systems, information on each product was obtained from manufacturer web sites and or other publicly available sources (e.g., promotional materials)
Analysis of each group revealed a trend for open systems to achieve higher Cmaxb values compared to closed system devices (Fig. 4) when e-liquid nicotine concentration increased (open systems slope = 0.37 ng.mL/mg.mL, Pearson r = 0.33, closed systems slope = 0.16 ng.mL/mg.mL, Pearson r = 0.18). Similarly, the median Cmaxb was higher for open systems compared to closed system electronic cigarettes at 9.7 ng/mL and 6.5 ng/mL, respectively (Table 2).
Fig. 4.
Association between Cmax and nicotine by electronic cigarette type (open or closed systems). All papers with available Cmax base-line adjusted values. Models with intercept and slope at random. Gray area representing Nicotine range from conventional cigarettes. The size of the marker reflects the relative weight of the study in the pooled data analysis.
Table 2.
Base-line adjusted Cmax distribution (ng/mL). Population: all papers with available Cmax base-line adjusted values.
| Category | N | Mean | SD | Q3 | Median | Q1 |
|---|---|---|---|---|---|---|
| Conventional | 17 | 18 | 24.2 | 19.2 | 18 | 15.8 |
| e-cigs | 117 | 9.8 | 27 | 13.7 | 7.8 | 4.7 |
| Closed | 54 | 8.4 | 26.8 | 13.6 | 6.5 | 3.4 |
| Open | 60 | 11.9 | 26.6 | 15.2 | 9.7 | 6.2 |
| Ad libitum | 53 | 9.9 | 32.6 | 13.6 | 7.5 | 3.9 |
| Controlled | 64 | 9.7 | 21.6 | 15.1 | 7.8 | 4.9 |
| Experienced | 99 | 12.4 | 24.4 | 17 | 10.3 | 7.5 |
| Inexperienced | 18 | 4.4 | 12.3 | 6.2 | 3.9 | 3 |
| 1st Gen. | 33 | 5 | 13.5 | 6.9 | 3.9 | 3 |
| 2nd/3rd Gen. | 66 | 11.3 | 26.2 | 13.1 | 8.6 | 5.9 |
| 4th Gen. | 8 | 10.6 | 26.8 | 10.3 | 7.6 | 7.2 |
As closed system electronic cigarettes have evolved significantly over time, from the first-generation ‘cig-a-like’ devices to the more recent fourth-generation ‘pod’ devices, it was decided to compare these two sub-categories to see if the improved device performance typically associated with pod devices is reflected in their ability to deliver nicotine to the blood.
No significant trend could be observed for pod or cig-a-likes devices to reach higher Cmaxb values (Fig. 5) when nicotine increased (pod devices slope = 0.17 ng.mL/mg.mL, Pearson r = 0.51, cig-a-likes devices slope = 0.12 ng.mL/mg.mL, Pearson r = 0.30). The median Cmaxb for pod devices was 7.6 ng/mL and higher compared to the median Cmaxb for cig-a-likes at 3.9 ng/mL. Second-and third generation devices were analyzed as one category. Cmaxb values were widely distributed and a moderate positive association between e-liquid nicotine concentration and Cmaxb could be observed (slope = 0.36 ng.mL/mg.mL, Pearson r = 0.31).
Fig. 5.
Association between Cmax and nicotine by electronic cigarette device generation. All papers with available Cmax base-line adjusted values. Models with intercept and slope at random. Gray area representing Nicotine range from conventional cigarettes. The size of the marker reflects the relative weight of the study in the pooled data analysis.
3.4. Puffing behavior and nicotine Cmaxb
Data collected under controlled and ad libitum puffing conditions were analyzed separately and compared (Fig. 6). High Cmaxb distribution was observed in both data sets with a weak positive association between Cmaxb and e-liquid nicotine concentration (ad libitum slope 0.32 ng.mL/mg.mL, Pearson r = 0.07, controlled slope 0.13 ng.mL/mg.mL, Pearson r = 0.27). In addition, median Cmaxb were similar under the analyzed ad libitum and controlled puffing conditions, at 7.5 ng/mL and 7.8 ng/mL, respectively.
Fig. 6.
Association between Cmax and nicotine by puffing behaviour (ad libitum or controlled). All papers with available Cmax base-line adjusted values. Models with intercept and slope at random. Gray area representing Nicotine range from conventional cigarettes. The size of the marker reflects the relative weight of the study in the pooled data analysis.
3.5. User experience can influence nicotine Cmaxb
Scientific studies also suggest that smokers with no experience in using electronic cigarettes, i.e., inexperienced users, have a significantly different use behavior than experienced electronic cigarette users [9; 27]. Therefore, the impact of prior electronic cigarette use experience on nicotine uptake was assessed by comparing data from experienced and inexperienced users. A moderately positive association between Cmaxb and electronic cigarette nicotine concentration was observed for experienced electronic cigarette users (slope 0.28 ng.mL/mg.mL, Pearson r = 0.40) (Fig. 7). In contrast, the association between Cmaxb and electronic cigarette nicotine concentration for inexperienced users was very weak (slope 0.07 ng.mL/mg.mL, Pearson r = 0.63). Experienced electronic cigarette users also demonstrated a wider Cmaxb distribution with significantly higher median Cmaxb compared to inexperienced users, at 10.3 ng/mL and 3.9 ng/mL, respectively (Table 2).
Fig. 7.
Association between Cmax and nicotine by electronic cigarette user experience (experienced or inexperienced). All papers with available Cmax base-line adjusted values. Models with intercept and slope at random. Gray area representing Nicotine range from conventional cigarettes. The size of the marker reflects the relative weight of the study in the pooled data analysis.
3.6. Nicotine tmax distribution
Tmax was reported by 15 (56 %) of the studies and extracted for analysis. Conventional cigarettes showed low variability with the majority of Tmax values below 10 min and a median Tmax of 7 min (Fig. 8, Table 3). In contrast, electronic cigarettes demonstrated highly distributed Tmax values with a median of 10 min.
Fig. 8.
Tmax distributions. All papers with available Cmax base-line adjusted values. The boxes are representing the interquartile ranges (Q3-Q1), whiskers the 2.5 % and 97.5 % percentiles and extreme values (>1.5*IQR) are represented by dots. Horizontal lines within the boxes are representing the median and diamonds the means.
Table 3.
Tmax (min). Population: all papers with available Cmax base-line adjusted values.
| Category | N | Mean | SD | Q3 | Median | Q1 |
|---|---|---|---|---|---|---|
| Conventional | 13 | 11.9 | 93.6 | 8.1 | 7 | 5.4 |
| e-cigs | 73 | 16.1 | 53.8 | 24.2 | 10 | 6 |
| Closed | 39 | 17.4 | 64 | 24.2 | 15.1 | 6 |
| Open | 34 | 11.2 | 37.5 | 10 | 10 | 6 |
| Ad libitum | 40 | 16.6 | 65.8 | 21.8 | 10.1 | 6 |
| Controlled | 33 | 15.2 | 34.9 | 30 | 10 | 7 |
| Experienced | 61 | 15.5 | 56.2 | 30 | 6 | 6 |
| Inexperienced | 12 | 17 | 40.9 | 21.8 | 19.9 | 10.1 |
| 1st Gen. | 25 | 14.6 | 41.9 | 21.8 | 15.1 | 6 |
| 2nd/3rd Gen. | 37 | 15.9 | 61.3 | 10 | 6.9 | 6 |
| 4th Gen. | 6 | 6.6 | 6.7 | 7.9 | 7 | 6 |
Further analysis of Tmax revealed differences between the subcategories of electronic cigarette data (Fig. 8). Closed system devices showed wide Tmax distribution and a higher median at 15.1 min compared to open systems, which demonstrated more consistent Tmax values and median Tmax10 min (p < 0.01). Tmax data collected under ad libitum and controlled puffing conditions demonstrated similar median Tmax at 10.1 min and 10 min, respectively (NS). Finally, inexperienced electronic cigarette users had a high median Tmax at 19.9 min, while it was lower for experienced electronic cigarette users at 6 min (NS).
3.7. The relationship between Cmaxb and Tmax
The distributions of Cmaxb and Tmax were plotted for each category, including median, first and third quartile (Fig. 9). Comparison of the conventional cigarette and electronic cigarette data sets indicated important differences between the two product categories. Conventional cigarette data showed consistent values with greater Cmaxb and lower Tmax compared to electronic cigarettes, which showed high variability in both dimensions. When analyzed separately, open system electronic cigarettes demonstrated lower variability with higher Cmaxb values and predominately low Tmax values compared to closed systems.
Fig. 9.
Bivariate Cmax base-line adjusted and Tmax distributions. All papers with available Cmax base-line adjusted values. The markers are representing the medians and error bars the inter-quartile ranges (IQR).
4. Discussion
Over the past decade, electronic cigarettes have become increasingly popular among smokers as alternative choices to combusted tobacco products, such as cigarettes. In part, this growth has been driven by the potential of electronic cigarette use to reduce the health effects associated with continued smoking [[39], [40], [41], [42], [43], [44], [45]].
As most electronic cigarettes contain nicotine, understanding the pharmacokinetics of nicotine delivery among consumers is important from several perspectives. Users of electronic cigarettes may, for example, be interested in understanding how much nicotine they are taking up into their bodies compared to that obtained through their current or former use of cigarettes. Likewise, regulatory authorities who either already have regulated, or are considering regulating this product category will be eager to know that the delivery of nicotine from electronic cigarettes does not raise additional public health concerns.
This review has explored the relationship between nicotine content in e-liquids and uptake by users, as measured by blood nicotine Cmax and Tmax levels. In order to conduct this analysis, a literature search was conducted to identify papers published between 2010 (around the time electronic cigarettes became widely available) and 2020 reporting blood nicotine levels in electronic cigarette users.
Pooling the data for all product types showed a weak linear positive relationship between nicotine concentration in e-liquid and base-line adjusted Cmax (Fig. 2). The median base-line adjusted Cmax value is statistically lower for electronic cigarettes compared to cigarettes (7.8 ng/mL versus 18 ng/mL; Fig. 3, Table 2). These data strongly suggest that other factors play a role in determining nicotine uptake, such as electronic cigarette design, e-liquid composition and user behavior (including nicotine titration by users [19]).
The importance of device design can be seen when the available data is subdivided by whether the electronic cigarettes are open tank (i.e., refillable) or closed tank (i.e., non-refillable) systems. Under this sub-population analysis, it can be seen that the median base-line adjusted Cmax values are slightly higher in users of open versus closed systems (Fig. 3, Table 2). The reason behind this has yet to be fully elucidated but could reflect the fact that open tank products tend to be of higher power, often with larger heating coil areas, so more e-liquid is converted into aerosol per puff (and thus more nicotine is present in each puff).The associations with e-liquid nicotine content, however, remain weak (Fig. 4).
Independent US researchers affiliated with the FDA and the NIH recently published a study in which they analyzed the levels of nicotine and its major metabolites in the urine of electronic cigarette consumers [46]. The authors observed that the concentrations of nicotine and its metabolites was generally higher in users of open systems than in users of closed systems, suggesting higher nicotine exposure in the former group. However, levels in urine were similar when exclusive open and closed system users were stratified as daily or non-daily users. The authors conclude that, based on these findings, exclusive electronic cigarette users with similar use patterns are likely to receive comparable levels of nicotine, irrespective of device type used. This would be consistent with previous reports suggesting that some users at least partially self-titrate their nicotine absorption during electronic cigarette use to suit personal preferences [19].
The influence of user behavior on nicotine uptake is demonstrated when the data is interrogated according to user regime (ad libitum or controlled) and user experience with electronic cigarettes (inexperienced or experienced). Ad libitum, in which users are free to use the product as they wish, and controlled, in which users are instructed to use the product in a certain manner, usage conditions yielded similar results, both in terms of median base-line adjusted Cmax values (Fig. 3, Table 2) as well as association with e-liquid nicotine concentration (Fig. 6). As seen in the pooled electronic cigarette data set, there is a weak positive relationship under both conditions between Cmax and e-liquid nicotine. A stronger effect of user behavior is seen when the data is interrogated based on user experience with electronic cigarettes (inexperienced or experienced users). In this instance, the median base-line adjusted Cmax values were higher in the experienced group compared to the inexperienced group (Fig. 3, Table 2). In addition, there was a moderate positive association between base-line adjusted Cmax values and e-liquid nicotine content in the experienced group (Fig. 7). In contrast, there was only a very weak positive association in the inexperienced group. Taken together, these findings reveal that user behavior, most notably user experience, can influence nicotine intake from electronic cigarettes. This may be due to experienced users being more familiar with both the functioning of the device as well as the sensory aspects of inhaling the aerosol generated during use. The lack of any major influence seen under ad libitum or controlled vaping conditions suggests that differences in inter-puff interval may be compensated by users through, for example, adjusting puff duration.
Some of the studies identified in this investigation also provide information on the types of electronic cigarette used, allowing the relative effect of device type on nicotine intake to also be assessed. Electronic cigarettes can be broadly categorized into 4 ‘generations’; first-generation ‘cig-a-like’ devices, second-generation ‘cartomizer’ devices, third generation ‘mod’ devices and 4th generation ‘pod’ devices. Both first and fourth generation devices are typically non-refillable closed systems and are sold with pre-filled e-liquid cartridges which attach to the device. In contrast, second and third generation devices are typically refillable open systems, using either commercially available e-liquid refill bottles or user self-mixed liquids. For the purposes of this analyses, second and third generation devices have been pooled as it is not clear from the source papers which of the two generations an individual refillable product falls into.
The median base-line adjusted Cmax values are similar for the more advanced generations of electronic cigarette devices, with a trend towards second/third generation devices having higher values, followed by fourth generation and then first-generation devices (Fig. 3, Table 2). A similar trend is observed when comparing Cmax and e-liquid nicotine concentration (Fig. 5). A moderate positive association is seen for second and third generation devices whereas there are similar, very weak, positive associations for first and fourth generation devices. These findings are somewhat surprising in light of previous reports of higher nicotine dependence in users of fourth generation devices compared to users of other generation devices [47]. However, they are consistent with previous saliva cotinine measurements which indicated that a particular brand of fourth generation device exposes users to levels of nicotine similar to other electronic cigarette types [48]. The findings here may simply reflect the fact that second and third generation devices tend to be more powerful, and thus generate more aerosol, than first and fourth generation devices.
Analysis of the Tmax values across all the published studies included in this review reveals that the mean Tmax values for cigarettes are lower than those for all electronic cigarettes combined, but the difference was no significand due to high variability in the data, especially from the electronic cigarette studies (Fig. 8, Table 3). Further subcategorizing the electronic cigarette Tmax data reveals some interesting, albeit non-significant, trends. For example, mean Tmax values are lower for open systems compared to closed systems, again possibly reflecting the typically higher aerosol yield of open system devices. The mean Tmax values for second, third and fourth generation devices are also lower than that for first generation devices, and in a similar range to cigarette Tmax (Fig. 8, Table 3). As with the Cmax data, these differences are likely due to differences in product design / performance as well as user behavior.
It has been suggested previously that the speed of absorption of nicotine is related to product abuse liability [49]. The basis for this theory comes from studies on drugs of abuse, such as cocaine and heroin, which demonstrate that formulations and routes of administration that enhance the speed of drug delivery to the brain increase the propensity for addiction [50,51]. Indirect support for a similar mechanism with respect to nicotine comes from the observation that nicotine replacement therapies, which deliver nicotine to the brain more slowly than cigarettes, do not generate dependency, despite yielding comparable blood nicotine levels [52].
Plotting Cmax and Tmax together on the same graph shows that second, third and fourth generation devices, as well as experienced users, have the highest median base-line adjusted Cmax and shortest Tmax (Fig. 9, Supplemental Information 4). In contrast, first generation devices and the closed device group, of which first generation devices are an example, have the lowest median base-line adjusted Cmax and longest Tmax. None of the groups, however, had both similar Cmax and Tmax values to the cigarette group.
As previously mentioned, several countries around the world have chosen to regulate the nicotine content of e-liquids. The approaches taken, however, differ. The 20 mg/mL limit in the EU is designed to allow “for a delivery of nicotine that is comparable to the permitted dose of nicotine derived from a standard cigarette during the time needed to smoke such a cigarette” [5]. Canada, in contrast, has set a nicotine limit in e-liquids of 66 mg / ml, based on the toxicological properties of the molecule [6].
Virtually all life-threatening nicotine intoxication cases reported in the literature were attributed to e-liquid ingestion [53], i.e., due to accidental ingestion of e-liquid or resulting from suicide attempts using e-liquids. While the 60 mg value is still widely reported in textbooks, databases and safety sheets, the exact lethal dose remains undefined [53,54]. Maessen et al., noted, “there is no consensus on the lethal dose of nicotine. In this study, we provide a clear overview of nicotine plasma concentrations in survivors versus patients that died. In our dataset, the lethal nicotine concentration is between 800 and 1600 mg L−1, which is 4.4- to 8.9-fold higher than the generally accepted lethal oral dose of 60 mg or less that would lead to a plasma concentration of approximately 180 mg L−1" [53].
It is important to remember that the 20 mg/mL limit in the EU was formulated in the early 2010s, as the European Tobacco Directive was being amended. At this time, there was little data available on the nicotine uptake from electronic cigarettes to objectively assess whether this limit does indeed match the nicotine delivery from cigarettes. Furthermore, most electronic cigarettes on the market were first or second generation devices which, while they are still available, are not the predominant types of electronic cigarette on the market today, which is dominated by more efficient third and fourth generation devices [8,55].
Indeed, the basis for the 20 mg/mL nicotine limit in the EU was challenged in 2014 in a letter to the EU by a group of independent scientists. At least three communications were made by scientists to the Commission to point out the flawed reasoning underpinning the 20 mg/mL limit [7,56,57]. The authors contended that the science relied upon by the EU in determining the 20 mg/mL limit was misinterpreted and that “20 mg/mL e-liquid provides less than one-third of the nicotine delivered by one tobacco cigarette. 50 mg/mL is needed to roughly match a tobacco cigarette. All other existing studies confirm this. Some 20–30% of electronic cigarette users use liquids above 20 mg. Higher nicotine content liquids are typically used by the most dependent smokers, who have the highest risk of smoking-related damage, and who benefit most from switching to electronic cigarettes. Most such heavy smokers need more than 20 mg/mL to switch from smoking to vaping” [56].
Other experts in the area have also questioned the ongoing suitability of the EU limit based on the evolved understanding of the electronic cigarette category, arguing that it is over-simplistic to assume that e-liquid nicotine concentration is the sole determinant of nicotine yield from electronic cigarettes and uptake by users, as is the case in the EU regulation [27,58]. The experts point out that nicotine yield and uptake is dependent on several factors, including user-selected battery voltage, heater resistance as well as user puff topography. Similarly, O’Connell and colleagues recommend that the nicotine concentration limit in the EU should be reviewed in line with the latest scientific evidence to ensure that electronic cigarette use remains a viable alternative to cigarette smoking [33], a view shared by both Public Health England [39] and the Royal College of Physicians [40].
Overall, the analyses conducted in this review call into question the appropriateness of using e-liquid nicotine content as a general, and only, predictor of nicotine uptake by electronic cigarette users. It also brings into question the scientific rationale for establishing nicotine limits in e-liquids to try and mimic the nicotine delivery of cigarettes. There is no ‘standard electronic cigarette’; both changes in electronic cigarette device design and e-liquid composition can influence the nicotine uptake by users. Similarly, user behavior itself, such as puff duration and frequency, can also influence the amount of nicotine absorbed into the blood, allowing users to self-titrate their nicotine intake, even at higher e-liquid concentrations.
At a minimum, the analysis conducted here supports the conclusion by some experts in the field that current electronic cigarettes do not deliver nicotine to the same extent as conventional cigarettes [26,[59], [60], [61], [62]]. This in turn infers that the current 20 mg/mL nicotine ceiling for e-liquids in the EU is not necessarily sufficient to achieve the stated regulatory aim of delivering nicotine “that is comparable to the permitted dose of nicotine derived from a standard cigarette during the time needed to smoke such a cigarette” [5]. In order to reflect the heterogeneity of the category, as well as other contributing factors such as user experience, the 66 mg/mL limit stipulated in Canadian regulations would appear to be more appropriate, which is in line with the observation that “50 mg/mL is needed to roughly match a tobacco cigarette” [56].
Given the variability among current electronic cigarettes, it has previously been suggested that nicotine flux, that is the amount of nicotine emitted in the vapor of electronic cigarettes, could be a better target for setting regulatory limits [58]. The rationale is that by focusing on the nicotine levels in the emissions, many of the variables associated with e-liquid composition and device performance are controlled for. The data from the studies reviewed here, however, call into question such an approach. In particular, the difference in nicotine uptake seen between experienced and inexperienced vapers indicates that users may be titrating their nicotine exposure by, for example, adjusting puff duration, puff strength, inhalation pattern, etc. Such compensatory behavior in experienced users has been observed previously under ad libitum usage conditions, in particular among users of lower nicotine concentration e-liquids [19].
An alternative approach would be wider adoption of holistic product standards. To date, several countries have adopted standards for electronic cigarettes and e-liquids, either on a voluntary or mandatory basis (Supplemental information 5). There remains, however, heterogeneity in approach across countries, which can cause confusion among consumers, regulatory and manufacturers alike. There are initiatives under way at pan national levels to harmonize standards, but these have yet to be fully realized For example, in Europe, CEN, the European Standards Agency, is working on the development of a suite of electronic cigarette standards covering definitions, devices, e-liquids, emissions, leachables and child safety. At a broader level, ISO, the International Standards Organization, is developing voluntary testing standards to assay key constituents of e-liquids and electronic cigarette aerosol.
If manufacturers commit to meeting such product standards, including robust quality management systems to monitor and document the manufacture of e-liquid and devices, regulators would have reassurance about the quality and safety of the products on the market. This should, in turn facilitate the introduction of proportionate regulations which allow the category to continue to grow in a responsible manner. Product standards are also key from a consumer perspective. Although the category is expanding rapidly today there are nevertheless still many consumers who are unsure about the relative safety of electronic cigarettes. This was exemplified by the recent reports of ‘E-cigarette or Vaping product use-Associated Lung Injury’ (EVALI), a respiratory illness initially incorrectly attributed to the use of nicotine-containing electronic cigarettes. It was subsequently shown that the condition was caused by an additive in cannabinoid vaporizers [63,64]. Suitable product standards would help avoid such situations by giving consumers the necessary assurance that the products underwent a thorough product stewardship and safety assessment.
This study has several limitations. First, it is not intended or designed to be a systematic review, so it is possible that some relevant studies may not have been identified using the applied search strategy. Secondly, the search strategy utilized only targeted English language publications, so any relevant studies in other languages would not have been captured. Despite these limitations, this review provides one of the most comprehensive analyses to date of published nicotine pharmacokinetic data following electronic cigarette use and will be of interest to both the scientific community and policy makers.
5. Conclusions
In conclusion, vaping behavior is complex and uniquely individual; it cannot be explained simply by the psychopharmacological effects of nicotine, including the amount of nicotine a user of tobacco products and electronic cigarettes obtains. The sensory, social, tactile, and ritualistic aspects of electronic cigarette vaping are extremely important and likely represent key factors in the behavior of many individuals. No two electronic cigarette users behave in the same way. Consequently, not all users of electronic cigarettes will necessarily obtain an equivalent amount of nicotine as their usual consumption of cigarettes. Instead of focusing solely on the nicotine content of e-liquids, a more pragmatic approach to regulation of electronic cigarettes would be product standards which ensure the quality and relative safety of such products in order to provide an appropriate level of consumer protection.
Funding
This work was funded by Japan Tobacco International, Switzerland.
CRediT authorship contribution statement
Karin Jacobson: Investigation, Validation, Writing - review & editing. Javier Martinez: Conceptualization, Writing - original draft, Writing - review & editing. Sylvain Larroque: Validation, Visualization, Formal analysis. Ian W. Jones: Conceptualization, Writing - review & editing. Thilo Paschke: Investigation, Conceptualization, Supervision.
Declaration of Competing Interest
The authors of this scientific study are employees of Japan Tobacco International. The views and conclusions contained herein are solely those of the authors and do not necessarily represent the views and conclusions of Japan Tobacco International.
Acknowledgements
We would like to thank Paul Belcher at Japan Tobacco International for providing expertise and support in conducting the literature search.
Edited by Dr. A.M Tsatsaka
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.toxrep.2020.12.016.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Carter L.P., Stitzer M.L., Henningfield J.E., O’Connor R.J., Cummings K.M., Hatsukami D.K. Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol. Biomarkers Prev. 2009;18(12):3241–3262. doi: 10.1158/1055-9965.EPI-09-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajek P., Pittaccio K., Pesola F., Myers Smith K., Phillips-Waller A., Przulj D. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction. 2020;115(6):1141–1148. doi: 10.1111/add.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etter J.F., Bullen C., Flouris A.D., Laugesen M., Eissenberg T. Electronic nicotine delivery systems: a research agenda. Tob. Control. 2011;20(3):243–248. doi: 10.1136/tc.2010.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Parliament and the Council of the European Union . 2014. Directive 2014/40/EU.https://ec.europa.eu/health/sites/health/files/tobacco/docs/dir_201440_en.pdf [Google Scholar]
- 5.European Parliament and the Council of the European Union . 2014. Directive 2014/40/EU.https://ec.europa.eu/health/sites/health/files/tobacco/docs/dir_201440_en.pdf Recital 38. [Google Scholar]
- 6.Canada Minister of Justice . 2019. Vaping Products Labellling and Packaging Regulations, SOR/2019-353.https://laws-lois.justice.gc.ca/PDF/SOR-2019-353.pdf [Google Scholar]
- 7.Farsalinos K. 2014. The European Commission Has Misinterpreted My Scientific Research on Nicotine in e-cigarettes.http://www.ecigarette-research.com/web/index.php/2013-04-07-09-50-07/147-misinterpreted-research [Google Scholar]
- 8.Fearon I.M., Eldridge A.C., Gale N., McEwan M., Stiles M.F., Round E.K. Nicotine pharmacokinetics of electronic cigarettes: a review of the literature. Regul. Toxicol. Pharmacol. 2018;100:25–34. doi: 10.1016/j.yrtph.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Blank M.D., Pearson J., Cobb C.O., Felicione N.J., Hiler M.M., Spindle T.R., Breland A. What factors reliably predict electronic cigarette nicotine delivery? Tob. Control. 2019 doi: 10.1136/tobaccocontrol-2019-055193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVito E.E., Krishnan-Sarin S. E-cigarettes: Impact of E-Liquid Components and Device Characteristics on Nicotine Exposure. Curr. Neuropharmacol. 2018;16(4):438–459. doi: 10.2174/1570159X15666171016164430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voos N., Goniewicz M.L., Eissenberg T. What is the nicotine delivery profile of electronic cigarettes? Expert Opin. Drug Deliv. 2019;16(11):1193–1203. doi: 10.1080/17425247.2019.1665647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services . 2016. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General.https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/pdfs/2016_sgr_entire_report_508.pdf [Google Scholar]
- 13.Bullen C., McRobbie H., Thornley S., Glover M., Lin R., Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob. Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 14.Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob. Control. 2010;19(1):87–88. doi: 10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vansickel A.R., Cobb C.O., Weaver M.F., Eissenberg T.E. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomarkers Prev. 2010;19(8):1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawkins L., Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl) 2014;231(2):401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- 17.Nides M.A., Leischow S.J., Bhatter M., Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am. J. Health Behav. 2014;38(2):265–274. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- 18.D’Ruiz C.D., Graff D.W., Yan X.S. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health. 2015;15:991. doi: 10.1186/s12889-015-2349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawkins L.E., Kimber C.F., Doig M., Feyerabend C., Corcoran O. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (Berl) 2016;233(15-16):2933–2941. doi: 10.1007/s00213-016-4338-2. [DOI] [PubMed] [Google Scholar]
- 20.Lopez A.A., Hiler M.M., Soule E.K., Ramoa C.P., Karaoghlanian N.V., Lipato T., Breland A.B., Shihadeh A.L., Eissenberg T. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine Tob. Res. 2016;18(5):720–723. doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramoa C.P., Hiler M.M., Spindle T.R., Lopez A.A., Karaoghlanian N., Lipato T., Breland A.B., Shihadeh A., Eissenberg T. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tob. Control. 2016;25(e1):e6–9. doi: 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St Helen G., Havel C., Dempsey D.A., Jacob P., 3rd, Benowitz N.L. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535–544. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Helen G., Ross K.C., Dempsey D.A., Havel C.M., Jacob P., 3rd, Benowitz N.L. Nicotine delivery and vaping behavior during ad libitum E-cigarette access. Tob. Regul. Sci. 2016;2(4):363–376. doi: 10.18001/TRS.2.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walele T., Sharma G., Savioz R., Martin C., Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part A: Pharmacokinetics. Regul. Toxicol. Pharmacol. 2016;74:187–192. doi: 10.1016/j.yrtph.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Fearon I.M., Eldridge A., Gale N., Shepperd C.J., McEwan M., Camacho O.M., Nides M., McAdam K., Proctor C.J. E-cigarette nicotine delivery: data and learnings from pharmacokinetic studies. Am. J. Health Behav. 2017;41(1):16–32. doi: 10.5993/ajhb.41.1.2. http://www.ncbi.nlm.nih.gov/pubmed/27935787 [DOI] [PubMed] [Google Scholar]
- 26.Hajek P., Przulj D., Phillips A., Anderson R., McRobbie H. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology (Berl) 2017;234(5):773–779. doi: 10.1007/s00213-016-4512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiler M., Breland A., Spindle T., Maloney S., Lipato T., Karaoghlanian N., Shihadeh A., Lopez A., Ramoa C., Eissenberg T. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp. Clin. Psychopharmacol. 2017;25(5):380–392. doi: 10.1037/pha0000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stiles M.F., Campbell L.R., Graff D.W., Jones B.A., Fant R.V., Henningfield J.E. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl) 2017;234(17):2643–2655. doi: 10.1007/s00213-017-4665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajek P., Przulj D., Phillips-Waller A., Anderson R., McRobbie H. Initial ratings of different types of e-cigarettes and relationships between product appeal and nicotine delivery. Psychopharmacology (Berl) 2018;235(4):1083–1092. doi: 10.1007/s00213-017-4826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spindle T.R., Talih S., Hiler M.M., Karaoghlanian N., Halquist M.S., Breland A.B., Shihadeh A., Eissenberg T. Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug Alcohol Depend. 2018;188:193–199. doi: 10.1016/j.drugalcdep.2018.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiles M.F., Campbell L.R., Jin T., Graff D.W., Fant R.V., Henningfield J.E. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl.) 2018;235(7):2077–2086. doi: 10.1007/s00213-018-4904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiler M., Karaoghlanian N., Talih S., Maloney S., Breland A., Shihadeh A., Eissenberg T. Effects of electronic cigarette heating coil resistance and liquid nicotine concentration on user nicotine delivery, heart rate, subjective effects, puff topography, and liquid consumption. Exp. Clin. Psychopharmacol. 2019 doi: 10.1037/pha0000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connell G., Pritchard J.D., Prue C., Thompson J., Verron T., Graff D., Walele T. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern. Emerg. Med. 2019;14(6):853–861. doi: 10.1007/s11739-019-02025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St Helen G., Nardone N., Addo N., Dempsey D., Havel C., Jacob P., 3rd, Benowitz N.L. Differences in nicotine intake and effects from electronic and combustible cigarettes among dual users. Addiction. 2020;115(4):757–767. doi: 10.1111/add.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voos N., Smith D., Kaiser L., Mahoney M.C., Bradizza C.M., Kozlowski L.T., Benowitz N.L., O’Connor R.J., Goniewicz M.L. Effect of e-cigarette flavors on nicotine delivery and puffing topography: results from a randomized clinical trial of daily smokers. Psychopharmacology (Berl.) 2020;237(2):491–502. doi: 10.1007/s00213-019-05386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yingst J.M., Hrabovsky S., Hobkirk A., Trushin N., Richie J.P., Jr., Foulds J. Nicotine absorption profile among regular users of a pod-based electronic nicotine delivery system. JAMA Netw. Open. 2019;2(11) doi: 10.1001/jamanetworkopen.2019.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yingst J.M., Foulds J., Veldheer S., Hrabovsky S., Trushin N., Eissenberg T.T., Williams J., Richie J.P., Nichols T.T., Wilson S.J., Hobkirk A.L. Nicotine absorption during electronic cigarette use among regular users. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0220300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloney S., Eversole A., Crabtree M., Soule E., Eissenberg T., Breland A. Acute effects of JUUL and IQOS in cigarette smokers. Tob. Control. 2020 doi: 10.1136/tobaccocontrol-2019-055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNeill A., Brose L.S., Calder R., Hitchman S.C., Hajek P., McRobbie H. 2015. E-cigarettes: An Evidence Update, a Report Commissioned by Public Health England, PHE Publications Gateway Number: 2015260.https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update [Google Scholar]
- 40.Royal College of Physicians . 2016. Nicotine Without Smoke: Tobacco Harm Reduction, London. ISBN 978-1-86016-600-606. [Google Scholar]
- 41.Kavvalakis M.P., Stivaktakis P.D., Tzatzarakis M.N., Kouretas D., Liesivuori J., Alegakis A.K., Vynias D., Tsatsakis A.M. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J. Anal. Toxicol. 2015;39(4):262–269. doi: 10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- 42.Flouris A.D., Poulianiti K.P., Chorti M.S., Jamurtas A.Z., Kouretas D., Owolabi E.O., Tzatzarakis M.N., Tsatsakis A.M., Koutedakis Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. 2012;50(10):3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 43.van der Toorn M., Koshibu K., Schlage W.K., Majeed S., Pospisil P., Hoeng J., Peitsch M.C. Comparison of monoamine oxidase inhibition by cigarettes and modified risk tobacco products. Toxicol. Rep. 2019;6:1206–1215. doi: 10.1016/j.toxrep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akomolafe O.R., Imafidon C.E., Olukiran O.S., Oladele A.A., Akanji B.O. Sub-acute administration of lower doses of nicotine caused sex-dependent improvement of renal function in Wistar rats. Toxicol. Rep. 2017;4:535–542. doi: 10.1016/j.toxrep.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrams D.B., Glasser A.M., Pearson J.L., Villanti A.C., Collins L.K., Niaura R.S. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu. Rev. Public Health. 2018;39:193–213. doi: 10.1146/annurev-publhealth-040617-013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rostron B.L., Coleman B., Cheng Y.C., Kimmel H.L., Oniyide O., Wang L., Chang C.M. Nicotine exposure by device type among adult electronic nicotine delivery system users in the population assessment of tobacco and health study, 2015-2016. Cancer Epidemiol. Biomarkers Prev. 2020 doi: 10.1158/1055-9965.EPI-20-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boykan R., Goniewicz M.L., Messina C.R. Evidence of nicotine dependence in adolescents who use Juul and similar pod devices. Int. J. Environ. Res. Public Health. 2019;16(12) doi: 10.3390/ijerph16122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nardone N., Helen G.S., Addo N., Meighan S., Benowitz N.L. JUUL electronic cigarettes: nicotine exposure and the user experience. Drug Alcohol Depend. 2019;203:83–87. doi: 10.1016/j.drugalcdep.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samaha A.N., Robinson T.E. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol. Sci. 2005;26(2):82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Gossop M., Griffiths P., Powis B., Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br. J. Addict. 1992;87(11):1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 51.Hatsukami D.K., Fischman M.W. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA. 1996;276(19):1580–1588. http://www.ncbi.nlm.nih.gov/pubmed/8918856 [PubMed] [Google Scholar]
- 52.Henningfield J.E., Keenan R.M. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 1993;61(5):743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- 53.Maessen G.C., Wijnhoven A.M., Neijzen R.L., Paulus M.C., van Heel D.A.M., Bomers B.H.A., Boersma L.E., Konya B., van der Heyden M.A.G. Nicotine intoxication by e-cigarette liquids: a study of case reports and pathophysiology. Clin. Toxicol. Phila. (Phila) 2020;58(1):1–8. doi: 10.1080/15563650.2019.1636994. [DOI] [PubMed] [Google Scholar]
- 54.Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch. Toxicol. 2014;88(1):5–7. doi: 10.1007/s00204-013-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams M., Talbot P. Design features in multiple generations of electronic cigarette atomizers. Int. J. Environ. Res. Public Health. 2019;16(16) doi: 10.3390/ijerph16162904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Etter J.-F., Farsalinos K., Hajek P., Le Houezec J., McRobbie H., Bullen C., Kozlowski L.T., Nides M., Kouretas D., Polosa R., Fagerström K., Jarvis M., Dawkins L.E., Caponnetto P., Foulds J. 2014. Scientific Errors in the Tobacco Products Directive: a Letter Sent by Scientists to the European Union.http://www.ecigarette-research.org/research/index.php/whats-new/whatsnew-2014/149-tpd-errors [Google Scholar]
- 57.Dawkins L.E. 2014. Please Do Not Distort My Words to Justify Your Policy.https://www.clivebates.com/guest-blog-lynne-dawkins-puts-the-commission-straight/ [Google Scholar]
- 58.Shihadeh A., Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob. Res. 2015;17(2):158–162. doi: 10.1093/ntr/ntu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biener L., Song E., Sutfin E.L., Spangler J., Wolfson M. Electronic cigarette trial and use among young adults: reasons for trial and cessation of vaping. Int. J. Environ. Res. Public Health. 2015;12(12):16019–16026. doi: 10.3390/ijerph121215039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farsalinos K.E., Yannovits N., Sarri T., Voudris V., Poulas K. Nicotine delivery to the aerosol of a heat-not-Burn tobacco product: comparison with a tobacco cigarette and E-Cigarettes. Nicotine Tob. Res. 2018;20(8):1004–1009. doi: 10.1093/ntr/ntx138. [DOI] [PubMed] [Google Scholar]
- 61.Yong H.H., Borland R., Cummings K.M., Gravely S., Thrasher J.F., McNeill A., Hitchman S., Greenhalgh E., Thompson M.E., Fong G.T. Reasons for regular vaping and for its discontinuation among smokers and recent ex-smokers: findings from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction. 2019;114(Suppl 1):35–48. doi: 10.1111/add.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver S.R., Heath J.W., Ashley D.L., Huang J., Pechacek T.F., Eriksen M.P. What are the reasons that smokers reject ENDS? A national probability survey of U.S. Adult smokers, 2017-2018. Drug Alcohol Depend. 2020;211 doi: 10.1016/j.drugalcdep.2020.107855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doukas S.G., Kavali L., Menon R.S., Izotov B.N., Bukhari A. E-cigarette or vaping induced lung injury: a case series and literature review. Toxicol. Rep. 2020;7:1381–1386. doi: 10.1016/j.toxrep.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiPasquale M., Gbadamosi O., Nguyen M.H.L., Castillo S.R., Rickeard B.W., Kelley E.G., Nagao M., Marquardt D. A mechanical mechanism for vitamin e acetate in E-cigarette/Vaping-Associated lung injury. Chem. Res. Toxicol. 2020;33(9):2432–2440. doi: 10.1021/acs.chemrestox.0c00212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.