Abstract
A 49- year old immunocompetent male presented with a right flank abscess and was found to have disseminated cryptococcal disease. Treatment was initiated with a one-week intravenous regime of amphotericin B and flucytosine based on recent trial data that this is as effective, and less toxic, than the standard two weeks. After completion of intravenous treatment he was discharged with oral anti-fungals and is making a good recovery with ongoing follow up.
Keywords: Disseminated cryptococcosis, Immunocompetent, Short course induction therapy
1. Introduction
Cryptococcus neoformans var grubii is an encapsulated yeast found worldwide, often in soil contaminated with bird faeces [1] and is one of the causative agents of cryptococcosis (the other being cryptococcus gattii). Route of infection is commonly by inhalation and in immunocompetent individuals it will typically not cause disease. In those who are immunocompromised, mostly patients with HIV, it can result in an opportunistic pulmonary infection [2] that can disseminate via the haematogenous route, typically to the central nervous system (CNS) [3]. Other common risk factors for dissemination include organ transplant, use of high dose steroids, haematological malignancy and sarcoidosis [4]. Disseminated disease in apparently immunocompetent hosts is uncommon [5] and here we detail the case of such a patient. Within this subset of patients, presentation with soft tissue abscess without osseous involvement, as described below, is even more uncommon [6]. The treatment of these patients is poorly defined and the approach to management varies greatly depending on the site and severity of the disease [7]. Based on evidence from trials in HIV positive patients the usual approach to treatment has been 2 weeks of intravenous (IV) amphotericin-based induction therapy. However, recent trial data from a large study of retroviral positive patients in Africa has shown that a shorter induction course of IV amphotericin, or an all oral combination of fluconazole and flucytosine, is as effective in Cryptococcal meningitis [8]. This research was driven by a need to find alternative management options in resource limited settings but as shown in this case the results can be successfully applied in other instances.
2. Case presentation
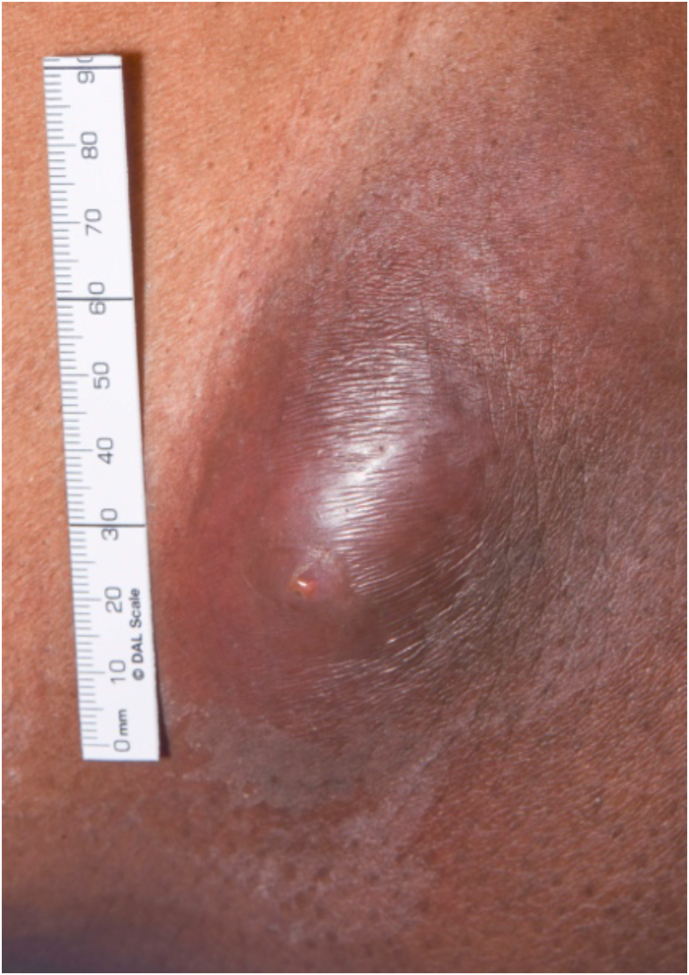
A 49-year-old Black Caribbean male, born and raised in the UK, presented to the ED (day 0) with a 3-week history of right flank pain and swelling which initially improved and then worsened in spite of antibiotics prescribed by his GP. He had no trauma to the area and denied any associated fevers, cough, night sweats, unintentional weight loss, headaches or visual changes. He had last travelled abroad, to Ghana, 6 months prior to presentation. His only past medical history was of hypertension. The patient denied smoking, alcohol or drug use and is employed as a senior safety system engineer but had been working from home since the onset of the Covid-19 pandemic. He is also employed as a Community Service Supervisor and as part of this role had been involved in gardening. He denied any exposure to birds in either his professional or personal life. On presentation he was afebrile with a heart rate of 62 beats per minute, blood pressure of 159/100 mmHg and oxygen saturations of 99% on air. Physical examination was unremarkable with the exception of a 2 × 7cm raised, erythematous, fluctuant area on the right flank (Fig. 1).
Fig. 1.
Right flank abscess prior to incision and drainage.
The patient's bloods tests on day 0 were largely normal with the exception of a C-reactive protein of 46.9mg/L. Chest X-ray was unremarkable. On day +1 he underwent ultrasound guided drainage of the right flank abscess; 30mls of pus was aspirated and a more solid component remained. Samples were sent for routine microscopy and culture and the patient was discharged home. No organisms were seen on the Gram stain, but on standard agar plates a faint growth was identified at 2 days (day +3). Cryptococcus neoformans var. grubii was identified by MALDI-TOF mass spectrometry with a score of 2.24. This is an accurate method for the classification of bacteria and fungi with a score of equal to or greater than 2 indicative of species identification. Acid fast bacilli (AFB) microscopy and AFB culture looking for the presence of Mycobacterium tuberculosis were both negative. On day +4 the patient was recalled for a CT chest abdomen pelvis with contrast and CT head without contrast to look for disseminated disease. This demonstrated a complex subcutaneous abscess which extended into the gluteus medius muscle in addition to numerous hepatic abscesses (up to 2.5cm) with bilateral pulmonary nodular changes and hilar and paratracheal lymphadenopathy highly suggestive of disseminated cryptococcosis. No abnormal intracranial findings were seen. On day +5 he was admitted and cryptococcal serum antigen (CrAg) titre was found to be positive at a titre of 1:80. Blood cultures taken at this time exhibited no growth after 5 days. In view of the limitations of CT in ruling out cerebral cryptococcal infection on day +8 an MRI head with gadolinium was also performed. This indicated no brain involvement and was confirmed by lumbar puncture on day +9 which revealed clear colourless cerebrospinal fluid (CSF) with white cell count <1, protein 0.33g/L (0.13–0.4) and glucose 3.3mmol/L (2.2–3.9).
Initial immunological screen was unremarkable including negative HIV1+2, HTLV 1 + 2, hepatitis B and hepatitis C antibodies. HbA1C was 38mmol/mol (20–42) suggesting no undiagnosed diabetes mellitus. Levels of C3 and C4, CD3, CD4 and CD8, serum ACE, B12 and folate and thyroid function were all within normal limits. ANCA screen was negative. Of interest, he was found to be covid-19 antibody positive; he described having had no symptoms of coronavirus but a member of his household had experienced a flu-like illness and anosmia several months earlier.
On day +5 the patient was initiated on IV liposomal amphotericin B (AmBisome) 4mg/kg OD and IV flucytosine (5FC) 25mg/kg QDS. Concurrently he underwent successful incision and drainage of his abscess. He was clinically well throughout his admission and after one week of IV AmBisome + 5FC treatment was fit for discharge. He was therefore converted to an all oral regime of fluconazole 1200mg OD and flucytosine 25 mg/kg QDS for a further week. He has now begun an 8-week course of fluconazole 800mg for the consolidation phase of his treatment with a plan to reduce to 200mg OD for maintenance therapy. He remains very well with no re-accumulation of the abscess and will continue on long term follow up with clinical status and repeat imaging used to monitor treatment response.
3. Discussion
Disseminated cryptococcal infection is prevalent in HIV positive patients worldwide and is one of the leading causes of death in AIDS patients, second only to tuberculosis [9]. It most commonly presents as subacute meningitis or meningoencephalitis but can affect almost any body part causing presentations such as cutaneous lesions, septic arthritis, hepatitis, osteomyelitis and prostatitis [10]. However, disseminated cryptococcosis is also seen, much more rarely, in immunocompetent patients. Of the species causing cryptococcosis Cryptococcus neoformans var. grubii is associated with infection in both immunocompromised and immunocompetent individuals while Cryptococcus gatti is more associated with disease in immunocompetent individuals [5]. Most reported cases of disseminated disease in those with no immunocompromise describe primary pulmonary and cutaneous infections in which patients present with symptoms such as fever, cough, dyspnoea, chest pain and skin lesions [2,3,11]. A much smaller number of case reports, to our knowledge only six, describe patients who have presented with abscesses. Of these, one describes a case of primary cutaneous cryptococcosis (in which the yeast was inoculated directly through the skin and there was no dissemination) [12] and two describe cases in which the patient was also found to have coinfection with tuberculosis [13,14]. In another, the patient presented with recurrent abscesses and after multiple negative specimen cultures was started on empirical anti-tuberculous treatment before Cryptococcus neoformans was later isolated [6]. Interestingly, in the remaining two cases the diagnosis was thought to be soft tissue sarcoma until aspirate was shown to contain yeast cells [1,10]. These cases highlight the challenges associated with diagnosis of disseminated cryptococcal disease, particularly when the primary symptom is soft tissue abscess, likely due to the rarity of this presentation and of this disease amongst immunocompetent patients. In this case the patient exhibited no respiratory symptoms but as there was no history of an inoculating injury the route of infection is assumed to have inhalation followed by haematogenous spread.
The treatment approach for cryptococcal meningitis in HIV positive patients is well defined by the World Health Organisation and is divided into three stages; induction, consolidation and maintenance [15]. Until recently the gold standard induction regime was two weeks of amphotericin B and flucytosine but the recent Advancing Cryptococcal Meningitis Treatment for Africa (ACTA) trial demonstrated that a one-week induction with the same anti-fungals was non-inferior in terms of disease clearance and resulted in lower 10 week mortality; 36.2% vs 39.7% for the one and two week patient cohorts respectively [8]. Additionally, the data showed that if amphotericin B is unavailable or cannot be safely administered an all oral induction regimen of 1200mg fluconazole and 100mg/kg/day flucytosine was non inferior in terms of mortality at 10 weeks but that disease clearance was slower. The driving force behind this research was the burden of disease in resource limited countries where drug availability is limited and it is challenging logistically, and expensive, to provide the biochemical monitoring needed with administration of IV amphotericin B. Monitoring of renal function, liver function and full blood counts is needed to prevent renal failure, hepatic failure, anaemia and secondary infections. Indeed, the reduction in mortality with a shorter period of administration may be attributed to the reduced risk of drug toxicity and sepsis [15].
However, the management of disseminated disease in immunocompetent, HIV negative, patients is less well defined. There is no randomised trial data for treatment of non-CNS cryptococcosis in HIV negative patients resulting in a variety of approaches as the optimum regime and duration of treatment have not been clearly established [16]. For example, in the case studies describing cryptococcal abscesses discussed earlier the management ranged from incision and drainage alone [12] to a combination of surgical intervention and varying regimes of amphotericin B, flucytosine, itraconazole and fluconazole [1,6,10,13,14]. Given the lack of evidence regarding the outcomes from different treatment approaches guidelines are often extrapolated from studies in HIV positive patients. The use of fluconazole for consolidation and maintenance in cryptococcal meningitis is one such example; randomised control trials have proven its efficacy in HIV positive patients but only retrospective case studies have evaluated this in HIV negative patients [7].
Given the limited data on treatment of non-CNS cryptococcosis in non-HIV patients, our management was influenced by data from the ACTA trial. This allowed for a shorter inpatient stay followed by an effective all oral regimen for the second week of induction therapy. This was a pragmatic approach given the patient was clinically well enough to be managed at home. It is, to our knowledge, the first occasion that a case report has detailed the use of a short IV induction regime followed by oral fluconazole and 5FC in an immunocompetent individual. However, given the patient's high burden of disease there is undoubtedly still a need for long term maintenance therapy. The necessary duration of this, as discussed above, is uncertain and will be guided by clinical response and repeat imaging. At the time of writing a second CT scan, performed 2.5 months after the first, indicated almost complete resolution of the right flank abscess and some reduction in the size of the hepatic abscesses and the hilar and paratracheal lymphadenopathy but showed fairly widespread ongoing disease. A third CT scan is awaited. CrAg titre is also being monitored but the clinical usefulness of this is uncertain. Previous studies, carried out on HIV positive patients with cryptococcal disease, have indicated that titres decrease over time in the majority of patients but that there is no significant correlation between serum CrAg levels and response to treatment or relapse [17,18].
Declaration of competing interest
There are none.
Acknowledgements
None.
References
- 1.Suchitha S., Sheeladevi C.S., Sunila R., Manjunath G.V. Disseminated cryptococcosis in an immunocompetent patient: a case report. Case Rep. Path. 2012:652351. doi: 10.1155/2012/652351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollam R., Yassin M., Phan T. Disseminated cryptococcosis in an immunocompetent patient. Resp. Med. Case Rep. 2020;30:101034. doi: 10.1016/j.rmcr.2020.101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkhihal B., Hasnaoui A., Ghfir I., Moustachi A., Aoufi S., Lyagoubi M. Disseminated cryptococcosis in an immunocompetent patient. J. Mycol. Med. 2015;5:208–212. doi: 10.1016/j.mycmed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 4.AlMutawa F., Leto D., Chagla Z. Disseminated cryptococcal disease in non-HIV, nontransplant patient. Case Rep. Infect. Dis. 2016:1725287. doi: 10.1155/2016/1725287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mada P., Nowack B., Cady B., Chandranesan A.S.J. Disseminated cryptococcosis in an immunocompetent patient. BMJ Case Rep. 2017 doi: 10.1136/bcr-2016-218461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q., Zhu Y., Chen S., Zhu L., Zhang S., Zhang W. Disseminated cryptococcosis with recurrent multiple abscesses in an immunocompetent patient: a case report and literature review. BMC Infect. Dis. 2017;17:1–6. doi: 10.1186/s12879-017-2459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas P.G., Perfect J.R., Cloud G.A., Larsen R.A., Pankey G.A., Lancaster D.J. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 8.Molloy S.F., Kanyama C., Heyderman R.F., Loyse A., Koufanfack Chanda D. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N. Engl. J. Med. 2018;278:1004–1017. doi: 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 9.Vechi H.T., Theodoro R.C., de Oliveira A.L., da Silva Gomes R.M.O., de Almeida Soares R.D., Freire M.G. Invasive fungal infection by Cryptococcus neoformans var. grubii with bone marrow and meningeal involvement in a HIV-infected patient: a case report. BMC Infect. Dis. 2019;19:1–8. doi: 10.1186/s12879-019-3831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaskill T., Payne D., Brigman B. Cryptococcal abscess imitating a soft-tissue sarcoma in an immunocompetent host: a case report. JBJS. 2010;92:1890–1893. doi: 10.2106/JBJS.I.01091. [DOI] [PubMed] [Google Scholar]
- 11.do Amaral D.M., Rocha R.D., Carneiro L.E.P., Vasconcelos D.M., de Abreu M.A.M.M. Disseminated cryptococcosis manifested as a single tumour in an immunocompetent patient, similar to the cutaneous primary forms. An. Bras. Dermatol. 2016;91:29–31. doi: 10.1590/abd1806-4841.20164582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz D., Held J., Goerke S., Wagner D., Tintelnot D., Henneke P. Primary cutaneous cryptococcosis in an eight-year-old immunocompetent child: how to treat? Klin. Pädiatr. 2015;227:41–44. doi: 10.1055/s-0034-1387775. [DOI] [PubMed] [Google Scholar]
- 13.Al-Tawfiq J.A., Ghandour J. Cryptococcus neoformans abscess and osteomyelitis in an immunocompetent patient with tuberculous lymphadenitis. Infection. 2007;35:377–382. doi: 10.1007/s15010-007-6109-9. [DOI] [PubMed] [Google Scholar]
- 14.Singh R., Xess I. Multiple osseous involvement in a case of disseminated cryptococcosis. Indian J. Orthop. 2010;44:336–338. doi: 10.4103/0019-5413.65158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organisation . 2018. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidation Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. [PubMed] [Google Scholar]
- 16.Saag M.S., Graybill R.J., Larsen R.A., Pappas P.G., Perfect J.R., Powderly W.G. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 2000;30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 17.Aberg J.A., Watson J., Segal M., Chang L.W. Clinical utility of monitoring serum cryptococcal antigen (sCRAG) titers in patients with AIDS-related cryptococcal disease. HIV Clin. Trials. 2000;1:1–6. doi: 10.1310/NQXR-ULMG-MM1B-3T2B. [DOI] [PubMed] [Google Scholar]
- 18.Powderly W.G., Cloud G.A., Dismukes W.E., Saag M.S. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 1994;18(5):789–792. doi: 10.1093/clinids/18.5.789. [DOI] [PubMed] [Google Scholar]