Abstract
Cervical cancer is the second most common cause of cancer-related death among women worldwide, especially in developing countries. Oxidative stress has been associated with cervical cancer. Many studies demonstrated that the low level of antioxidants induces the production of free radicals that cause lipid peroxidation, DNA, and protein damage leading to mutations that favors malignant transformation. This is a case-control institutional study conducted to evaluate the level of oxidative stress in cervical cancer patients and the age-matched healthy controls. We measured level of TBARS expressed as MDA, activity of SOD and GSH level by the spectrophotometric method, and level of 8-OHdG was estimated using a competitive sandwich ELISA assay. Our results showed a significant increase in the level of lipid peroxidation in group IV when compared to the control, group II and group III (p < 0.001). The activity of SOD was also significantly higher in group IV when compared to the control group (p < 0.001), group II (p < 0.001), and group III (p < 0.001). The level of GSH was also significantly lower in group IV when compared to the control group (p < 0.01), group II (p < 0.01), and group III (p < 0.01). The level of 8-OHdG was significantly higher in group IV than in the other groups (p < 0.01). The results suggest that oxidative stress is involved in the pathogenesis of cervical cancer, which is demonstrated by an increased level of lipid peroxidation and higher levels of 8-OHdG and an altered antioxidant defense system.
Keywords: Cervical cancer, Oxidative stress, FIGO stage, Antioxidant, Free radicals
Highlights
-
•
The level of MDA, 8-OHdG and SOD activity increased in all the cases when compared to healthy control.
-
•
A significant decline in GSH level was observed in cases when compared to healthy control.
-
•
The alterations in the level of these parameters reflect the oxidative stress in cervical carcinoma.
-
•
This becomes more pronounced in advanced stages due to the increased tumor burden.
1. Introduction
Cervical cancer is one of the most common causes of female malignancy, representing a major global health challenge. In 2018, an estimated 569,847 new cases of cervical cancer were diagnosed, and 311,365 deaths occurred worldwide due to cervical malignancy [1]. According to the National Institute of Cancer Prevention and Research (NICPR) India, Cervical Cancer is the second most common cancer-causing death in women in the country. In India, around 96,922 women are newly diagnosed, and 60,078 women die of cervical cancer every year as per GLOBOCAN 2018 [1]. Considering the current incidence rates, the annual load of new cases is expected to increase to 225,000 by 2025 in India [2,3]. Almost all cervical cancers are caused by high-risk subtypes of the human papillomavirus (HPV), and HPV screening and vaccination programs are effective strategies in disease prevention [4]. HPV causes cervical cancer by damaging DNA, but recent data revealed that oxidative stress plays a role in its development [5]. Chemoradiation is known to improve the survival of patients with cervical cancer. Oxidative stress is an imbalance between the prooxidant-antioxidant system. A decrease in the level of antioxidants generated free radicals, which leads to DNA damage, causing dysfunction and disease [6]. It is caused by a disturbed oxidant-antioxidant balance in favor of oxidants, leading to excessive generation of free radicals, particularly reactive oxygen species (ROS), and biological damages. Superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (• OH) are kinds of ROS that are produced by partial reduction of atmospheric O2 [7].
Malondialdehyde (MDA) is one of the most common markers of oxidative stress and is an oxidant-antioxidant adduct in cancer patients. Lipid peroxidation is initiated by the reactive oxygen species (ROS), which is produced from different processes leading to the production of excessive MDA, which in turn changes the normal cell functioning and causes cancer [8]. The high level of MDA concentration can be ascribed to a higher production of ROS due to a rise in the oxidative damage in patients of uterine cancer. In the progression of disease, the production oxygen radical also increases, which in turn increases lipid peroxidation. This process results in the damage or degeneration of cell membrane and DNA [9]. The increase in free radical generation leads to excessive lipid peroxidation, indicated by a rise in serum MDA. Free radicals may cause evident changes to cell membrane function and the structural organization of DNA, thereby leading to mutations. Therefore, it can be stated that the product of lipid peroxidation could be one of the possible causes of cancer progression [9,10].
In almost all cancers, the high level of ROS has been detected, making it one of the best-characterized phenotype in cancer. The level of oxidative stress can be assessed by the highly unstable metabolites like 8-hydroxy 2-deoxyguanosine (8-OHdG). It is one of the most abundant oxidatively modified lesions in DNA, and it represents a marker of oxidative stress. 8-OHdG is a product of oxidative damage to 2′-deoxyguanosine. Among the bases, guanine is the most susceptible DNA target, which can be mispaired with adenine. Therefore, in the next replication adenine will pair with thymine causing G:C- > T:A transversion. 8-OHdG may be induced by hydroxyl radical or singlet oxygen [11].
Glutathione (GSH) is considered to be one of the leading detox agents. It is known that the amount of GSH in the cell influences the sensitivity of cells to anticancer treatment and toxicity (a decrease in GSH level increases drug toxicity). Therefore, the determination of the GSH level is crucial to foresee whether the cancerous cells will be sensitive to the effect of the drug, or if the effect of the drug toward normal cells will be innocuous. It is defined that, in comparison with a partial response, patients who have cervical cancer have a significantly decreased level of GSH in the blood and tumor, given a complete response to the treatment [12,13].
Our study aimed to assess the activity of SOD and level of non-enzymatic antioxidant GSH included in the antioxidant defense of the subjects and to determine the level of lipid peroxidation in the patients' serum, as well as the level of 8-OHdG in women with different stages of cervical cancer and healthy women. There are limited studies that have established any correlations between damage caused by free radicals and different FIGO (International Federation of Obstetrics and Gynecology) staging system of cervical cancer patients. Therefore, the present study was designed to evaluate the possible involvement of oxidative stress during the progression of cervical carcinoma in different FIGO stages.
2. Materials and methods
2.1. Ethical statement
Ethical clearance was obtained from the institutional ethical committee of our institution before starting the study (reference no: ECR/Bhu/Inst/UP/2013/Re-registration-2017 dt.January 31, 2017). Written informed consent from all the patients and healthy individuals are obtained.
2.2. Subjects
The study was conducted between May 2017 and Sept 2019.100 patients with histopathologically proven carcinoma cervix who visited the Department of Obs. & Gynae, Sir Sunderlaal Hospital, Banaras Hindu University were recruited for the study. Clinical staging of hundred patients was done by gynaecologist and these are recruited into four groups as per cervical cancer FIGO stages I, II, III & IV. Subjects with age-matched healthy women (n = 28) visiting the hospital for a routine health check-up, hospital staffs, and lab members willing to participate in the study were taken as healthy controls. Informed consent was taken from each subject and control purely for research purposes.
Inclusion criteria for the control group were: patients of age-group 25–45 and histopathologically proved and clinically diagnosed patients of cervical cancer were included for the study. Exclusion criteria were: patients with any other genital malignancy, other causes of vaginal bleeding, and patients with a history of radiotherapy or chemotherapy were excluded from the study.
2.3. Sample collection and processing
Approximately 5 mL of blood was obtained through venipuncture under aseptic condition in a clean, plain, red topped dry vial. Blood was kept for 10 min before centrifugation. Serum was separated from the blood through centrifugation at 3000 RPM for 10 min and stored in an eliquote at −20 °C until required for analysis. In both groups, MDA, SOD, and GSH were measured using a modified spectrophotometric method; 8-OHdG was estimated using a competitive sandwich ELISA assay. Besides, these parameters were estimated in patients with cervical cancer before chemoradiation.
2.4. Laboratory analysis
All the chemicals used in this study were of analytical grade and purchased from SRL Mumbai and Human ELISA kit for 8-OHdG was purchased from QAYYEE-BIO (LoT: 04/2018, QY-E05181).
Lipid peroxidation was estimated by the formation of Thiobarbituric acid reactive substances (TBARS) described by Satoh K, 1978 [14]. The samples were deproteinized with 15% TCA (Trichloroacetic acid) and then treated with 0.375% TBA (Thiobarbituric Acid). The mixture was heated in a boiling water bath for 15 min. It was then cooled to room temperature and centrifuged at 3500 rpm for 10 min, and formed TBARS was measured at 535 nm. Total thiobarbituric acid-reactive materials (TBARS) was expressed as MDA. The value is expressed as nmol/ml.
8-OHdG was performed using human 8-OHdG competitive sandwich ELISA Kit (LoT: 04/2018, QY-E05181), which is based on the antigen-antibody reaction in samples. Standard, test samples, and HRP (Horse Radish Peroxidase) labeled 8-OHdG antibody (Ab) was added to enzyme wells, which is pre-coated with 8-OHdG Ab, then incubation was carried out, and uncombined enzymes were removed after washing. Upon the addition of Chromogen solutions A and B, the color of the liquid was changed into blue, and the reaction with the acid caused the color to become yellow. The intensity of color and concentration of 8-OHdG samples were positively correlated. The final values were expressed in ng/ml.
The test of GSH was based on development of a yellow color when 5′5′ dithiobis (2- nitrobenzoic acid) was added to sulphhydryl compounds. The color thus developed was fairly stable for about 10 min and the reaction was read at 412 nm. Two tenth of whole blood was added to 1.8 ml distilled water. 3 ml of the precipitating solution was mixed with the hemolysate. The mixed was allowed to stand for approx. 5 min and the filtered. 2 ml of the filtrate was added to 8 ml of phosphate solution in a testtube. 1 ml of DTNB solution was added. A blank was prepared with 8 ml of the phosphate solution, 2 ml of diluted precipitating solution and 1 ml of the DTNB reagent. Absorbance was taken within 30 s at 412 nm. The value is expressed in nM/L. (Ellman's method. 1959) [15].
SOD assay by the pyrogallol autoxidation method was carried out following the procedure of Marklund and Marklund (1974). The reaction was initiated by the addition of 100 μl of freshly prepared 2·6 mM pyrogallol solution in 10 mM HCl to attain a final concentration of pyrogallol of 0·13 mM in the assay mixture.
2.5. Statistical analysis
Statistical analysis was performed using the SPSS IBM Statistics software version 23.0. The statistical analysis included the parametric method: Student's t-test, analysis of variance, and Pearson's correlation. Since the variables are normally distributed, the comparison between groups was performed by analysis of variance. Comparisons of the control group with the patients were done using students t-test. Pearson's correlation was done to observe the correlation of 8-OHdG with GSH and MDA with GSH. Two-tailed p-values less than 0.05 were considered statistically significant and p-values less than 0.0001 were considered to be highly significant. All the results were expressed in Mean ± SD.
3. Results
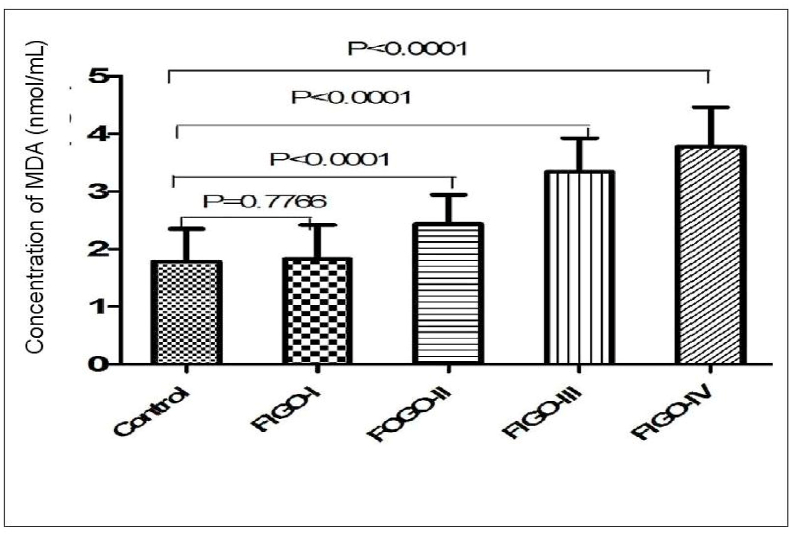
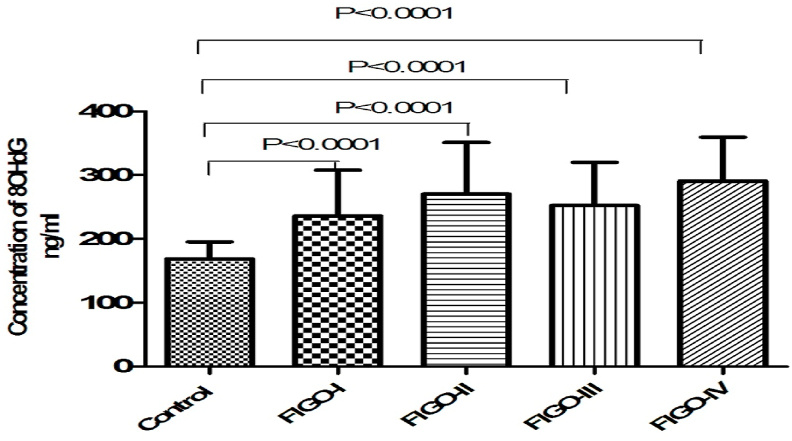
Mean age of control and four FIGO groups included in the study were expressed in Table 1. Data obtained in this study demonstrated moderate elevation in the level of MDA in FIGO II and FIGO III as compared to FIGO I and FIGO II. This result follows the extent of the severity of the stages. Statistically, significant differences are between the control group and FIGO IV (p < 0.001) as well as between FIGO II & FIGO IV (p < 0.001) (Fig. 1). We observed statistically significant differences in the level of 8-OHdG between the control group and group IV (FIGO IV) (p < 0.01) but no significant differences between FIGO II and FIGO III (Fig. 2).
Table 1.
Characteristics of patients and controls included in the study. Age (Mean ± SD).
| Characteristics of subjects | Number of subjects | Mean Age± SD |
|---|---|---|
| FIGO I | 21 | 37.66 ± 5.83 |
| FIGO II | 25 | 37.88 ± 4.56 |
| FIGO III | 26 | 38.03 ± 4.97 |
| FIGO IV | 28 | 37.96 ± 5.39 |
| Control | 28 | 34.46 ± 5.82 |
Fig. 1.
Bar diagram showing level of MDA (nmol/ml) in four patients group of FIGO I, II, III, IV stage and healthy control group with significant p-value (p < 0.001). Student's T-test.
Fig. 2.
Bar diagram showing level of serum 8OHdG (ng/ml) in four patients group of FIGO I, II, III, IV stage and healthy control group with highly significant p-value (p < 0.0001), Student's T-test.
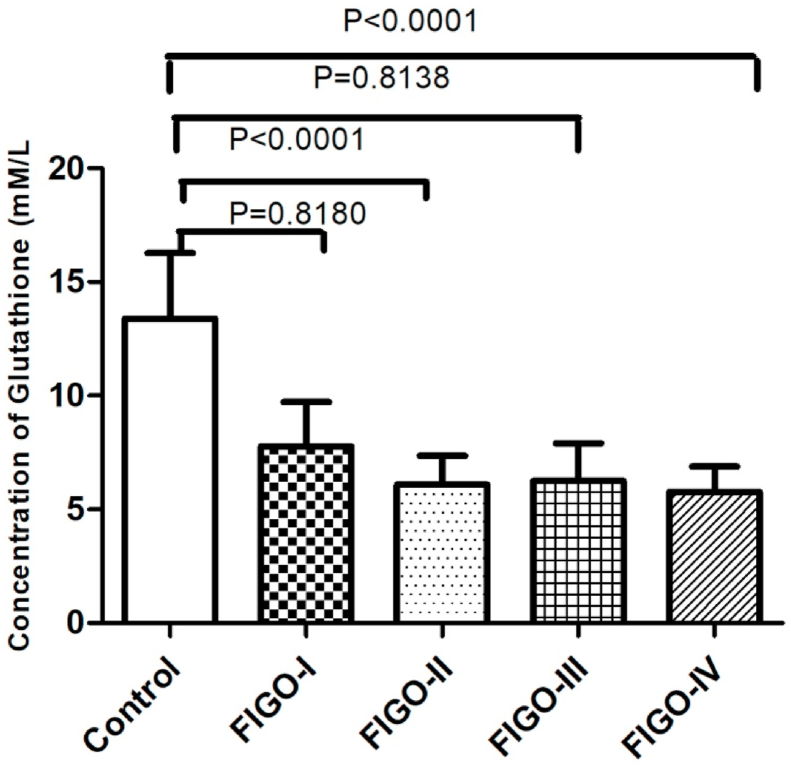
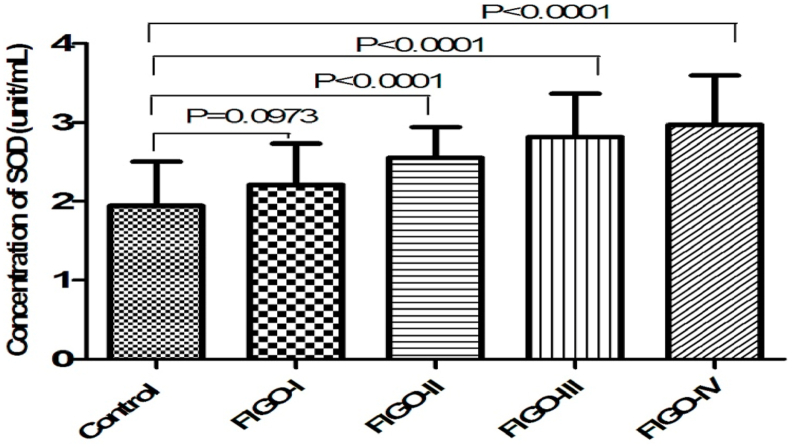
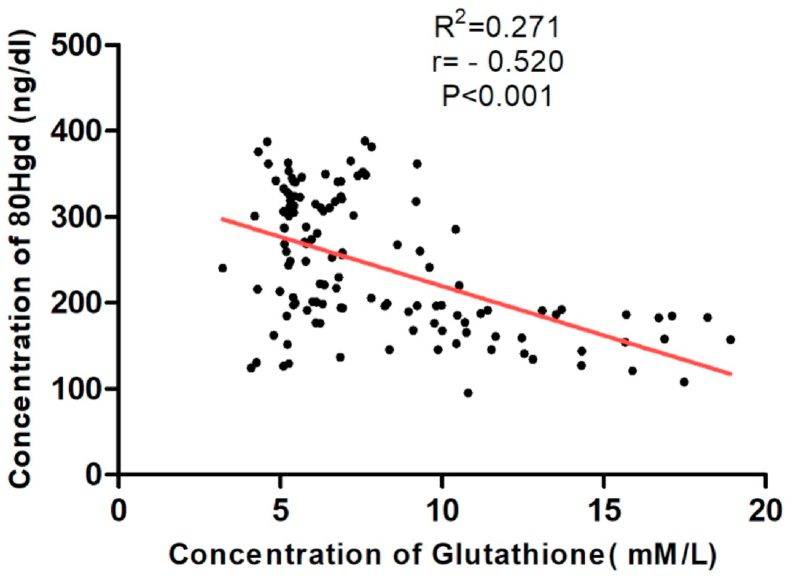
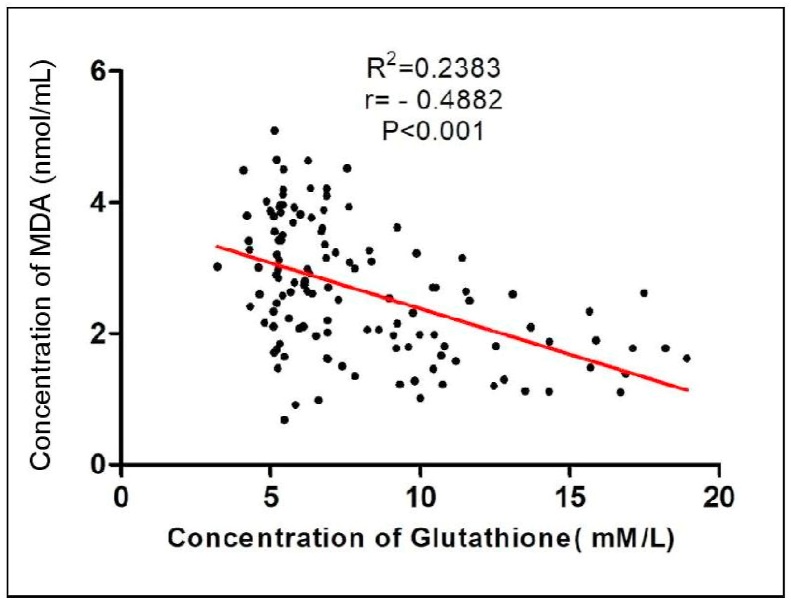
We observed a statistically significant decrease in the level of non-enzymatic antioxidant GSH (p < 0.001) in patients with cervical cancer compared to healthy subjects. (Table 2) (Fig. 3). Tumor progression is followed by an increase in SOD activity resulting in significant differences when FIGO IV is compared with the other three groups and with the control group (p < 0.001) (Fig. 4). Pearson's rank correlation Fig. 5 showed that serum GSH was significantly and negatively correlated with 8-OHdG (p < 0.001). Significant negative correlations was observed between GSH and MDA (p < 0.001) in Fig. 6.
Table 2.
Level of serum MDA (mg/dL), SOD (unit/ml), Glutathione (mM/L) and 8OHdG (ng/ml) data in mg/dl in control (N = 28) and Carcinoma cervix patients (N = 100) with grade (FIGO I, II,III, IV).
| Parameters | Grade | Cases (N = 100) (Mean ± SD) | Control (N = 28) (Mean ± SD) | P-value | Inter group comparison One Way ANOVA |
|---|---|---|---|---|---|
| Concentration of MDA (nmol/ml) | FIGO I (N = 21) | 1.82 ± 0.59 | 0.777ns | ||
| FIGO II (N = 25) | 2.42 ± 0.50 | <0.001 | |||
| FIGO III (N = 26) | 3.34 ± 0.58 | <0.001 | |||
| FIGO IV (N = 28) | 3.77 ± 0.67 | 1.78 ± 0.56 | <0.001 | F = 61.07 P < 0.001 | |
| Concentration of SOD (unit/ml) | FIGO I (N = 21) | 2.20 ± 0.52 | 0.097 | ||
| FIGO II (N = 25) | 2.55 ± 0.39 | <0.001 | |||
| FIGO III (N = 26) | 2.81 ± 0.54 | <0.001 | |||
| FIGO IV (N = 28) | 2.96 ± 0.62 | 1.94 ± 0.56 | <0.001 | F = 16.64 P < 0.001 | |
| Concentration of Reduced Glutathione (mM/L) | FIGO I (N = 21) | 7.77 ± 1.94 | <0.001 | ||
| FIGO II (N = 25) | 6.09 ± 1.26 | <0.001 | |||
| FIGO III (N = 26) | 6.25 ± 1.65 | <0.001 | |||
| FIGO IV (N = 28) | 5.76 ± 1.11 | 13.39 ± 2.87 | <0.001 | F = 78.66 P < 0.001 | |
| Concentration of 8OHdG (ng/ml) | FIGO I (N = 21) | 236.02 ± 71.73 | <0.001 | ||
| FIGO II (N = 25) | 270.54 ± 80.49 | <0.001 | |||
| FIGO III (N = 26) | 252.35 ± 67.77 | <0.001 | |||
| FIGO IV (N = 28) | 290.90 ± 68.53 | 168.78 ± 26.83 | <0.001 | F = 14.25 P < 0.001 |
Fig. 3.
Bar graph showing level of serum GSH (mM/L) in four patient group of FIGO I, II, III, IV stages and healthy control group with significant p-values (p < 0.001). Student's T-test.
Fig. 4.
Bar graph showing activity of serum SOD (unit/ml) in four patient group of FIGO I, II, III, IV stages and healthy control group with highly significant p-value (p < 0.001). Student's T-test.
Fig. 5.
Graph showing negative correlation between GSH(mM/L) and 8-OHdG (ng/ml) (p < 0.001) with r = −0.520 and R2 = 0.271 using Pearson's Correlation Test.
Fig. 6.
Graph showing negative correlation between GSH(mM/L) and MDA (nmol/ml) (p < 0.001) with r = −0.4882 and R2 = 0.2383 using Pearson’ Correlation Test.
4. Discussion
Free radicals are involved in most of the cell process regulation especially active form of these free radicals tends to indicate oxidative stress in a cell. The antioxidative system prevents the organism from the damage caused by oxidative stress. Imbalance between oxidants-anitioxidant system have been investigated in many cancers including cervical cancer. But there is a lack of data that shows how oxidative stress is important in prognosis and predictive aspect of the disease.
The predominant factors in the etiopathogenesis of cervical cancer are chronic inflammation due to cervical trauma, bacteria, and viruses, especially human papillomavirus (HPV). Chronic inflammation results in the activation of the monocyte/macrophage system, which produces high levels of ROS. It is well demonstrated that excess production of free radicals can cause oxidative damage to the cells. The generation of ROS also causes genetic damage to the cervical epithelium, leading to transformation into malignant cells and initiation of cervical cancer. Furthermore, promotion and progression of these transformed cells are also related to the oxidant/antioxidant milieu in humans. Therefore, HPV infection may induce oxidative stress in cervical cancer [16].
Malondialdehyde, an end product of lipid peroxidation, is highly cytotoxic, acts as a tumor promoter. The level of MDA in cervical cancer patients is significantly increased. There is a significant rise in the serum MDA concentration in patients with cervical cancer [17]. Moreover, previous studies also demonstrated that MDA levels in the blood of different types of cancer patients were significantly higher when it was compared with healthy controls [[18], [19], [20]]. Similarly the result of this study showed that level of lipid peroxidation was significantly higher in serum of the FIGO stages of all the four groups as compared to the healthy control group. Similar trend was observed in plasma MDA in patients with cervical intraepithelial neoplasia (CIN) [21]. Therefore, oxidative stress is considered to be a predominant factor even in the initial stage of carcinogenesis. We consider oxidative injury as a prominent factor even in the early steps of carcinogenesis. This stress increases manifold in malignant cells since the cancer cells themselves produce oxidants, deplete antioxidants thus setting up a vicious cycle. This becomes more pronounced in advanced stages due to the increased tumor burden. Therefore, this study supports the hypothesis that the disturbance in the oxidant-antioxidant level leads to lipid oxidative damage, that provides a mechanistic basis for initiation and progression of cancer.
Glutathione peroxidase (GPx) catalyzed the conversion of glutathione (GSH) to glutathione disulphide (GSSH) while its reduction is catalyzed by glutathione reductase from GSSH to GSH [22,23]. They act as secondary antioxidants and protects the cell from many cytotoxic and carcinogenic agents by scavenging reactive oxygen species. A high GSH level is needed to restore the sufficient concentration of antioxidants and to stimulate the scavenger enzymes indispensable to counteract the damaging actions of free radicals [26]. Mukund et al. demonstrated the level of glutathione in patients with cervical cancer and a control healthy women. The results showed that the level of GSH in patients with cervical cancer were significantly lower than the healthy control women [27]. We also observed a significant decline in GSH level in patients of cervical cancer when compared to healthy control group which could be considered as an adaptive response to oxidative stress.
SOD is one of the primary antioxidant enzyme which is widely distributed in all the cells but present in higher amount in red blood cells. SOD protects the cell against lipid peroxidation and catalyzes the dismutation of superoxide anions into oxygen and hydrogen peroxide. SOD enhanced its activity compensatively when there is increased production of superoxide anions. Inflammation in the cervix increases the production of SOD which in turn increases intracellular hydrogen peroxide, thus creating an environment favorable for DNA damage and for initiation and progression of cancer. Our study demonstrated that activity of SOD in blood of all the examined patients group were significantly increased compared to healthy control group. Our result showed statistical significance for patients in the advanced disease group as compared to healthy subjects. Demirci et al. [28] also showed an increase in SOD activity in cervical cancer patients, compared to the control group. Decreased SOD activity was reported by Balasubramaniyan et al. [27] in cervical tissues [29] and by Srivastava et al. [30] in blood.
We demonstrated that level of 8-OHdG tends to increase as we move from FIGO I to FIGO IV stage of cervical cancer i.e., higher stage of the disease, thereby suggesting its role in progression of cervical carcinogenesis. A similar study was done by Sgambato et al. [31], they observed progressive significant rise in the level of 8-OHdG from LSIL (Low-grade squamous intraepithelial lesions) to HSIL (high-grade squamous intraepithelial lesions) to invasive carcinomas. They suggested that alteration of this parameter at early stages of the process might help to predict patients at high risk of progression [31].
5. Conclusion
Our data suggested an imbalance between oxidant-antioxidant status of patients in all the four groups of cases when compared to healthy controls. This imbalance plays an important role in the pathogenesis and progression of cervical cancer though the involvement of these parameters is altered in oxidative stress. In summary, altered antioxidative enzymes activity, higher lipid peroxidation and higher level of 8-OHdG reflect the oxidative stress in cervical carcinoma, and this becomes more pronounced in advanced stages due to the increased tumor burden. The supplementation of antioxidants may reduce the progression of disease in cervical cancer patients.
Author contribution
Kulsoom Zahra designed the study and drafted the manuscript. Surendra Pratap Mishra supervised and finalize the drafting of the manuscript. Sandeep Patel performed all the statistical analysis. Kulsoom Zahra and Tulika Dey performed the experiment. Uma Pandey co-supervised and finalize the editing of the manuscript. All the authors approved the final submission of the manuscript and final drafting of the article.
Funding
This experiment did not receive any kind of extramural research grant from any agency.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like extend our sincere gratitude to all the participants involved in this study.
Contributor Information
Kulsoom Zahra, Email: kulsoom.zahra19@gmail.com.
Sandeep Patel, Email: sandeeppatelsa@gmail.com.
Tulika Dey, Email: dtulika9@gmail.com.
Uma Pandey, Email: uma.pandey2006@gmail.com.
Surendra Pratap Mishra, Email: drsurendram2@gmail.com.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018 Nov;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sreedevi A., Javed R., Dinesh A. Epidemiology of cervical cancer with special focus on India. Int. J. Wom. Health. 2015;7:405. doi: 10.2147/IJWH.S50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnan S., Madsen E., Porterfield D., Varghese B. Advancing cervical cancer prevention in India: implementation science priorities. Oncol. 2013 Dec;18(12):1285. doi: 10.1634/theoncologist.2013-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crosbie E.J., Einstein M.H., Franceschi S., Kitchener H.C. Human papillomavirus and cervical cancer. Lancet. 2013 Sep 7;382(9895):889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 5.Naidu M.S., Suryakar A.N., Swami S.C., Katkam R.V., Kumbar K.M. Oxidative stress and antioxidant status in cervical cancer patients. Indian J. Clin. Biochem. 2007 Sep 1;22(2):140–144. doi: 10.1007/BF02913333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgescu S.R., Mitran C.I., Mitran M.I., Caruntu C., Sarbu M.I., Matei C., Nicolae I., Tocut S.M., Popa M.I. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress. Journal of immunology research. 2018:2018. doi: 10.1155/2018/5315816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2015 Dec;15(1):71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruk J., Y Aboul-Enein H. Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev. Med. Chem. 2017 Jul 1;17(11):904–919. doi: 10.2174/1389557517666170228115324. [DOI] [PubMed] [Google Scholar]
- 9.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN oncology. 2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelić M., Mandić A., Kladar N., Sudji J., Božin B., Srdjenović B. Lipid peroxidation antioxidative defense and level of 8-hydroxy-2-deoxyguanosine in cervical cancer patients. J. Med. Biochem. 2018;37:336–345. doi: 10.1515/jomb-2017-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakabeppu Y., Sakumi K., Sakamoto K., Tsuchimoto D., Tsuzuki T., Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol. Chem. 2006 Apr 1;387(4):373–379. doi: 10.1515/BC.2006.050. [DOI] [PubMed] [Google Scholar]
- 12.Peklak-Scott C., Townsend A.J., Morrow C.S. Dynamics of glutathione conjugation and conjugate efflux in detoxification of the carcinogen, 4-nitroquinoline 1-oxide: contributions of glutathione, glutathione S-transferase, and MRP1. Biochemistry. 2005 Mar 22;44(11):4426–4433. doi: 10.1021/bi047810y. [DOI] [PubMed] [Google Scholar]
- 13.Lyakhovich V.V., Vavilin V.A., Zenkov N.K., Menshchikova E.B. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc.) 2006 Sep 1;71(9):962–974. doi: 10.1134/s0006297906090033. [DOI] [PubMed] [Google Scholar]
- 14.Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978 Nov 15;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 15.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959 May 1;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 16.Nirmala J.G., Narendhirakannan R.T. Detection and genotyping of high-risk HPV and evaluation of antioxidant status in cervical carcinoma patients in Tamil Nadu State, India-a case control study. Asian Pac. J. Cancer Prev. APJCP. 2011 Jan 1;12(10):2689–2695. [PubMed] [Google Scholar]
- 17.Manju V., Sailaja J.K., Nalini N. Circulating lipid peroxidation and antioxidant status in cervical cancer patients: a case-control study. Clin. Biochem. 2002 Nov 1;35(8):621–625. doi: 10.1016/s0009-9120(02)00376-4. [DOI] [PubMed] [Google Scholar]
- 18.Hristozov D., Gadjeva V., Vlykova T., Dimiitrov G. Evaluation of oxidative stress in patients with cancer. Arch. Physiol. Biochem. 2001;109:331–336. doi: 10.1076/apab.109.4.331.4248. [DOI] [PubMed] [Google Scholar]
- 19.Skrzydlewska E., Sulkowski S., Koda M., Zalewski B., Kanczuga-Koda L., Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol.: WJG. 2005 Jan 21;11(3):403. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumaraguruparan R., Subapriya R., Kabalimoorthy J., Nagini S. Antioxidant profile in the circulation of patients with fibroadenoma and adenocarcinoma of the breast. Clin. Biochem. 2002 Jun 1;35(4):275–279. doi: 10.1016/s0009-9120(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 21.Lee G.J., Chung H.W., Lee K.H., Ahn H.S. Antioxidant vitamins and lipid peroxidation in patients with cervical intraepithelial neoplasia. J. Kor. Med. Sci. 2005 Apr 1;20(2):267–272. doi: 10.3346/jkms.2005.20.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhabak K.P., Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Accounts Chem. Res. 2010 Nov 16;43(11):1408–1419. doi: 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- 23.Sharma R., Yang Y., Sharma A., Awasthi S., Awasthi Y.C. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxidants Redox Signal. 2004 Apr 1;6(2):289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- 26.Balasubramaniyan N., Subramanian S., Govindasamy S. Status of antioxidant systems in human carcinoma of uterine cervix. Canc. Lett. 1994 Dec 9;87(2):187–192. doi: 10.1016/0304-3835(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 27.Mukundan H., Bahadur A.K., Kumar A., Sardana S., Naik S.L., Ray A., Sharma B.K. Glutathione level and its relation to radiation therapy in patients with cancer of uterine cervix. Indian J. Exp. Biol. 1999;37:858–864. [PubMed] [Google Scholar]
- 28.Demirci S., Ozsaran Z., Celik H.A., Aras A.B., Aydin H.H. The interaction between antioxidant status and cervical cancer: a case control study. Tumori Journal. 2011 May;97(3):290–295. doi: 10.1177/030089161109700306. [DOI] [PubMed] [Google Scholar]
- 29.Nirmala J.G., Narendhirakannan R.T. Detection and genotyping of high-risk HPV and evaluation of antioxidant status in cervical carcinoma patients in Tamil Nadu State, India-a case control study. Asian Pac. J. Cancer Prev. APJCP. 2011 Jan 1;12(10):2689–2695. [PubMed] [Google Scholar]
- 30.Srivastava S., Natu S.M., Gupta A., Pal K.A., Singh U., Agarwal G.G., Singh U., Goel M.M., Srivastava A.N. Lipid peroxidation and antioxidants in different stages of cervical cancer: prognostic significance. Indian J. Canc. 2009 Oct 1;46(4):297. doi: 10.4103/0019-509X.55549. [DOI] [PubMed] [Google Scholar]
- 31.Sgambato A., Zannoni G.F., Faraglia B., Camerini A., Tar quini E., Spada D. Decreased expression of the CDK in hibitor p27Kip1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gynecol. Oncol. 2004;92(3):776–783. doi: 10.1016/j.ygyno.2003.12.008. [DOI] [PubMed] [Google Scholar]